Abstract
Did Beringian environments represent an ecological barrier to humans until less than 15 000 years ago or was access to the Americas controlled by the spatial–temporal distribution of North American ice sheets? Beringian environments varied with respect to climate and biota, especially in the two major areas of exposed continental shelf. The East Siberian Arctic Shelf (‘Great Arctic Plain’ (GAP)) supported a dry steppe-tundra biome inhabited by a diverse large-mammal community, while the southern Bering-Chukchi Platform (‘Bering Land Bridge’ (BLB)) supported mesic tundra and probably a lower large-mammal biomass. A human population with west Eurasian roots occupied the GAP before the Last Glacial Maximum (LGM) and may have accessed mid-latitude North America via an interior ice-free corridor. Re-opening of the corridor less than 14 000 years ago indicates that the primary ancestors of living First Peoples, who already had spread widely in the Americas at this time, probably dispersed from the NW Pacific coast. A genetic ‘arctic signal’ in non-arctic First Peoples suggests that their parent population inhabited the GAP during the LGM, before their split from the former. We infer a shift from GAP terrestrial to a subarctic maritime economy on the southern BLB coast before dispersal in the Americas from the NW Pacific coast.
Keywords: Beringia, Quaternary, palaeogenomics, archaeology, migration
1. Introduction
Recent palaeogenomics research has confirmed the hypothesis, proposed more than two centuries ago, that the Indigenous peoples of the Western Hemisphere are derived from a population in Northern Asia. Palaeogenomics also confirms the thesis that the ancestral Native American population migrated from Asia to North America via the Bering Strait region, where large areas of continental shelf were exposed during periods of cold climate by lowered sea level [1,2].
In 1937, Eric Hultén proposed the palaeogeographic label Beringia for the exposed shelf areas in the Bering Strait region, which he hypothesized, based on modern plant distribution, had provided a refugium for arctic and subarctic plants during the cold-climate periods [3]. There are two such areas of continental shelf, both of which now are fully submerged. One is the Bering-Chukchi Platform, which adjoins Chukotka and western Alaska and often is equated with the ‘Bering Land Bridge' (BLB). The other is the East Siberian Arctic Shelf, which extends from the Chukchi Sea to the western shore of the Laptev Sea and the Taimyr Peninsula (i.e. across the central and eastern Siberian Arctic). Although all the Bering-Chukchi Platform falls within the geographical definition of Beringia, the East Siberian Arctic Shelf occupies a larger region that lies outside both the original and later, expanded definitions of Beringia [4,5] (figure 1).
Figure 1.
Map of Beringia, showing location of sites mentioned in the text.
In this review, we address the question of the role of Beringia in the peopling of the Western Hemisphere. The Beringian environment often has been viewed as the critical variable in the timing of migration(s) from Northern Asia to the Americas. Specifically, Beringia is widely seen as having represented an ecological barrier to human populations due to cold-climate effects on plant and animal productivity. According to this view, climate warming during the final millennia of the Pleistocene or after 15 000 years ago (15 ka) rendered the exposed Bering-Chukchi Platform habitable for humans, allowing movement of a North Asian population into Alaska and rapid dispersal throughout the Western Hemisphere [6,7]. An alternative view is that the spatial and temporal pattern of deglaciation after 19 ka controlled the timing and routes of human dispersal in mid-latitude North America and farther south.
The Beringian environment is linked to another major problem in the peopling of the Western Hemisphere: explaining the primary source of biological variation among living First Peoples. The principal divisions among Native Americans have been explained as a product of either (a) multiple migrations from Northern Asia to the Americas or (b) regional diversification of a single population after its arrival in the Western Hemisphere. A multiple migration thesis was proposed in 1986 by Greenberg et al. based on identification of three major linguistic and biological subdivisions (‘Eskimo-Aleut,’ Na-Dene and ‘Amerind’) among First Peoples [8]. Their ‘three-wave model’ was resuscitated in 2012 by Reich et al. based on the first whole-genome analysis of the Native American population [9].
Alternatively, Szathmary and others argued that Native American population biology was more parsimoniously explained by a single migration from Asia (before the Last Glacial Maximum (LGM), which began 26.5 ka) and subsequent diversification in the Western Hemisphere [10,11]. This view was supported by mtDNA studies of living populations during the 1990s suggesting an early divergence of ancestral First Peoples from their East Asian source population [11,12]. In 2007, Tamm et al. proposed, also based on mtDNA analyses, that the Native American founder population had been isolated in Beringia during the LGM (‘Beringian standstill’ model) [13]. The standstill model rested on the assumption that Beringian environments represented a potential refugium—not an ecological barrier—for ancestral First Peoples before their dispersal throughout the Western Hemisphere about 15 ka [14,15].
2. Climate, sea level and glaciation
(a) . Before the Last Glacial Maximum (59–26.5 ka)
A new history of global sea level recently has been proposed for the period preceding the LGM (Marine Isotope Stage 3 or MIS 3 [59–29 ka]), based on dated beach ridges on the Atlantic coast of North America and sediment cores from East Asia. Pico et al. postulate significantly higher sea level than previously thought for this period, peaking at 40 m below that of the present day approximately 45 ka [16]. This implies a reduced volume of continental ice, and the proposed revision of sea-level history has been applied to reconstruction of a smaller Laurentide ice sheet (LIS) in Canada during 45–30 ka [17,18].
Higher sea levels and a reduced LIS during the period preceding the LGM have implications for human settlement of Beringia and the Western Hemisphere. As sea level rose above −53 m, the Bering-Chukchi Platform would have been flooded, severing a land connection between Asia and North America. A smaller LIS during MIS 3 would have provided a wider ice-free corridor between the LIS and the Cordilleran ice sheet in the Canadian Rockies, enhancing interior access from Beringia to mid-latitude North America before the LGM. It also implies a slower and later closing of the corridor, as both ice sheets expanded and eventually coalesced in response to colder climates after 40 ka (i.e. providing prolonged access to mid-latitude North America).
The revised sea-level history and reconstruction of the LIS have been disputed, based on both geochronology and climate-stratigraphy, and the issue currently is subject to debate among Quaternary geologists [19,20]. As a result, there is uncertainty about the presence of a land connection between Asia and North America and the spatial-temporal dimensions of the ice-free corridor before the LGM. There is agreement however between the revised and earlier proposed sea-level histories that global sea level fell rapidly after 40 ka (i.e. the beginning of cold Heinrich Event 4 or HE 4) reaching a minimum of at least −120 m at the beginning of the LGM (approx. 26.5 ka) [16,20]. There also is consensus that re-advance of the LIS and Cordilleran ice sheet began during HE 4, eventually blocking both interior (i.e. ice-free corridor) and NW Pacific coastal access from Beringia to mid-latitude North America [17,18,20] (figure 2).
Figure 2.
Palaeoclimate record for the past 60 000 years, based on the NGRIP oxygen-isotope core, calibrated with the IntCal20 radiocarbon calibration curve [21], showing Heinrich Events 5–1 (HE 5–HE 1) and interstadials mentioned in the text (GI 6, etc.) (adapted from OxCal v4.4.4 https://c14.arch.ox.ac.uk/oxcal/OxCal.html).
(b) . The Last Glacial Maximum (26.5–19 ka)
There currently is less uncertainty about sea-level history and glacial chronology for the LGM, although the timing of the coalescence of the LIS and Cordilleran ice sheet remains unclear. While Dalton et al. [17] concluded that the ice-free corridor closed during the early LGM (≥ 25 ka) bison genetics suggest that it may have remained open as late as 23 ka [22]. Also relevant to Beringia and its role in the peopling of the Western Hemisphere is the discovery that the Verkhoyansk Mountains were not glaciated during the LGM [23]. Because these mountains extend above the Arctic Circle, where they sometimes exceed 1900 m in elevation, the absence of glaciers 26.5–19 ka can be explained only by limited moisture supply, underscoring the extreme aridity of the region (East Siberian Arctic Shelf) during the LGM (discussed below).
(c) . After the Last Glacial Maximum (19–11 ka)
New dates are available for both the deglaciation of the NW Pacific coast and the reopening of the interior ice-free corridor in western Canada. Deglaciation of the NW Pacific coast began as early as 18 ka and a viable coastal route between southern Beringia and mid-latitude North America may have been available by 17 ka (although probably requiring some use of watercraft) [24]. Also pertinent to human settlement is early retreat of the ice on the southern coast of Alaska (less than 18 ka), which would have facilitated movement from the southern BLB to the NW Pacific coast before the rapid rise in sea level (less than 16 ka) [25].
The reopening of the corridor between the Cordilleran ice sheet and LISs now appears to have taken place after 14 ka. A narrow ice-free corridor apparently extended from the Yukon to southern Alberta (roughly 2000 km in length) at 13.8 ± 0.5 ka and might have allowed limited movement of people from the interior of Beringia to the Northern Plains at this time (although movement of bison through the corridor is thought to have occurred less than 13.4 ka) [22,26]. The late dating of the post-LGM ice-free corridor indicates that the rapid dispersal of First Peoples in mid-latitude North America that began before 14.5 ka (based on a group of reliably dated archaeological sites in mid-latitude North America) probably began on the NW Pacific coast, which was largely deglaciated at this time [2,24,25].
3. Reconstructing Beringian environments
(a) . Bering-Chukchi platform (‘Bering Land Bridge’)
Debate and controversy have attended the reconstruction of BLB environments for many decades. Hultén postulated a refugium for ‘oceanic plants’ in the southern region of Beringia based on the distribution of living taxa [3]. During the 1960s, Colinvaux analysed pollen cores from islands in the Bering Sea and localities in western Alaska, concluding that the BLB supported an ‘herbaceous tundra like that of modern Barrow’ although possibly with some ‘steppe characteristics’ [27]. Guthrie disputed this reconstruction, based on the analysis of the mammalian fauna, which suggested a northern grassland (i.e. exhibited a predominance of grazers, such as bison and horse) [28].
During the 1990s, the analysis of cores retrieved from the former surface of the land bridge indicated a mesic tundra, and Guthrie and others later proposed that the low-lying BLB supported a mesic tundra belt that acted as an ecological barrier to at least some of the steppe-adapted taxa (including invertebrates) on opposing sides of the Bering Strait [29–31]. Both the oceanic influence of the North Pacific on the southern land bridge and poor drainage conditions created by the lack of topographic relief on the BLB would have promoted a mesic tundra environment. The debate has continued, nevertheless. Several researchers postulated an LGM refugium for some arboreal taxa on the land bridge (based on pollen data) [32]. New palaeo-botanical studies (including ancient DNA data) on one of the Pribilof Islands indicated that this was unlikely, however, at least during the post-LGM period [7,33].
Regardless of the net primary productivity (NPP) levels during the LGM and later, terrestrial habitat on the land bridge probably had a low carrying capacity for humans. Even at the most southerly latitudes of the BLB, virtually none of their diet would have been derived directly from plant foods, while the terrestrial mammalian biomass for mesic tundra probably was lower than that of high-latitude steppe-tundra of the LGM and post-LGM (including arctic Beringia, as described below). Many large grazers, such as bison and horse, that inhabited arctic Beringia may have been rare or absent on the southern land bridge, where extensive peat formation took place during the LGM [34], although mammoth, which survived after its inundation on one of the Pribilofs, was present. A seasonal resource in the form of migratory waterfowl, attracted to ponds and wetlands on the poorly drained BLB, also may have existed [35] (figure 3).
Figure 3.
Glaciation in the Northern Hemisphere during the LGM, showing the extent of the peat region and location of sites in northern Eurasia mentioned in the text (adapted from Lindgren et al. fig. 1 [34]).
(b) . East Siberian Arctic Shelf (‘Great Arctic Plain’)
By contrast, there is a consensus regarding the climate and biota of the exposed East Siberian Arctic Shelf, which supported a dry steppe-tundra biome populated by a diverse large mammalian fauna. The critical variable was the lack of moisture, intensified during intervals of extreme cold (cryo-aridity), as falling sea levels moved the Arctic Ocean coast hundreds of kilometres north and the north Eurasian ice sheet reduced the flow of moisture from the west [4]. As a result, conditions were too dry for formation of the wet tundra soils and peat bogs found in the Arctic today. In their place, warmer and more productive soils, similar to those found in the northern steppe zone, developed across the exposed shelf areas, as well as over the adjoining lowland basins of northeastern Siberia (e.g. Kolyma Basin) and the North Slope of Alaska. Schirrmeister et al. labelled this landscape the ‘Great Arctic Plain’ (GAP) [31,36,37].
Recent efforts to reconstruct the vegetation of the GAP, described as ‘steppe-tundra’ or ‘cryo-arid steppe’ without modern analogue, have drawn from a large palaeogenomic plant database extracted from sample localities across the circumpolar zone. The vegetation was dominated by forbs, graminoids and willow shrubs, reaching a peak in diversity at the beginning of the LGM (26.5 ka), followed by a marked decrease in diversity during the coldest phases of the LGM [38]. The mammalian fauna, reconstructed from hundreds of radiocarbon-dated bones recovered from localities on the northeastern Siberian lowlands, arctic Alaska/Yukon and islands in the East Siberian Sea, included typical northern steppe dwellers, such as bison, horse and woolly rhinoceros, along with tundra species, such as reindeer and musk ox. Woolly mammoth, which enjoyed a wide dietary niche, was especially common and survived on island remnants of both the GAP and southern BLB into the mid Holocene [4,31,36,39].
The large mammal populations apparently fluctuated significantly, probably in response to climate change and its effects on plant productivity. Major changes in population size are inferred from variations in numbers of radiocarbon-dated bones, as well as from genetic bottlenecks in the ancient DNA data. Mann et al. hypothesized that populations peaked at the beginning of interstadials when plant productivity rose in response to increased temperature and moisture, but before wet tundra soils could develop [36]. Several researchers report a major decline in the mammoth population at the end of the LGM (ca 20–15 ka), while bison numbers seem to decline sharply in eastern Beringia during the same period [36,40]. It currently is unclear what relationship these fluctuations may have had to any human occupants of arctic Beringia.
4. Human settlement of northern Eurasia
The settlement of latitudes above 45° North in Eurasia by anatomically modern humans and their predecessors provides an essential context for understanding the peopling of Beringia and the Western Hemisphere. Although Neanderthals and Denisovans adapted to some cold and relatively unproductive (i.e. low plant and animal productivity) environments in northern Eurasia, only modern humans appear to have occupied the Arctic and Beringia. The critical variable was the capacity of modern humans for designing technology of unprecedented structural and functional complexity, including mechanical instruments and devices.
(a) . Neanderthals/Denisovans
The Neanderthals inhabited southwest Europe (including during glacial periods) where climates generally are mild and plant and animal productivity are relatively high, but their spatial/temporal distribution in eastern Europe and northern Asia, where climates are more continental and biological productivity lower, was limited. The Denisovans are found only in Asia. Both taxa were present in southwest Siberia (Altai Mountains) during the earlier Late Pleistocene. They appear to have been confined largely to places where natural shelters and wood were available [41,42].
Little is known about Denisovan anatomy or diet, but the Neanderthals are represented by a large sample of skeletal remains, which exhibit various anatomical adaptations to low temperature (e.g. thick chest, large head, shortened extremities) [41,43]. It may be assumed that their caloric demands were high, based on their body mass and climate setting. Stable isotope analyses and zooarchaeology indicate a high animal protein and fat diet (i.e. a northern diet) with an emphasis on large mammals [44]. Given their presence in southwest Europe during periods of extreme cold, low biological productivity may have been a more severe limiting factor than winter temperature. Despite their anatomical and dietary adaptations to northern environments, Neanderthal genetics reveal small populations with high inbreeding coefficients, subject to periodic local extinctions [41,45]. In the Altai region, a 2020 whole-genome analysis from Chagyrskaya Cave indicates an inbred population of less than 60 individuals [46].
(b) . Anatomically modern humans
New discoveries in southern France suggest an early presence of modern humans in southwest Europe during a warm interval before 50 ka [47], while evidence for their presence in the more continental regions of northern Eurasia post-dates cold HE 5 (less than 47 ka) [21,48]. A combination of dated human remains yielding ancient DNA (e.g. Ust’-Ishim in western Siberia) and dated artefact assemblages assigned to the Initial Upper Palaeolithic (IUP) industry (associated with modern human remains at Bacho Kiro in Bulgaria) suggests a rapid spread across central/eastern Europe and Siberia by 45 ka [21,48–50]. Modern humans were present, at least on a seasonal basis, in the European Arctic before 40 ka and probably on a year-round basis in arctic Siberia and Beringia by 33 ka [51,52]. The basal Eurasian lineages reflect significant genetic admixture from local Neanderthal/Denisovan populations [53,54].
Despite the genomic contribution from the local Neanderthal population, modern humans in northern Eurasia seem to have acquired few if any of the evolved anatomical adaptations to cold climate of the former. While only fragmentary skeletal remains have been recovered from time periods before 40 ka, complete skeletons from later periods before the LGM indicate retention of warm-climate anatomy (e.g. high brachial index) from lower latitudes, which would have rendered them susceptible to hypothermia and cold injury [55,56]. Stable isotope analyses and zooarchaeology reveal a northern diet comparable to that of the Neanderthals (i.e. significant niche overlap) but also a dietary breadth expanded to smaller vertebrates [57,58], while their genetics reflect adaptation to a diet high in polyunsaturated fatty acids (PUFAs) [59]. Palaeodemographic information for eastern Europe and arctic Siberia/Beringia suggest relatively large hunter–gatherer populations with little inbreeding [60,61].
Modern humans were an invasive species in northern Eurasia, hampered by an anatomical pattern better suited to the tropics. They nevertheless drove the local Neanderthal and Denisovan populations to extinction within a few thousand years, and occupied habitats and climate zones beyond the range of their predecessors. The explanation for this phenomenon lies in the fact that modern humans—including the basal Eurasian lineages—were equipped with most of the technologies found among recent hunter–gatherers in high latitudes [62].
Mechanical projectile weaponry is inferred from the morphometrics of Levallois points in the IUP and indicated by diagnostic impact fractures in southwest Europe approximately 45 ka [63,64]. Indirect evidence for snaring/trapping small mammals is reported from the East European Plain greater than 40 ka, while traces of mechanical rotary drills (which suggest fire-making technology) are found in both Eastern Europe (Kostenki) and southwest Siberia (Denisova Cave) in this time range. Evidence for sewn clothing (eyed needles) is dated to approximately 45 ka in the IUP level at Denisova Cave [62,65,66]. Reliable traces of artificial shelters date to 32 ka at the latest [67].
Equipped with these technologies, which appear to have been absent among Neanderthals and Denisovans (e.g. [68]), modern humans increased their foraging efficiency and success rate and harvested resources unavailable to the former in north Eurasian habitats where plant and animal productivity was low (especially during cold-climate periods). With tailored insulated clothing, artificial shelters, fire-making devices and alternative fuels (e.g. fresh bone), they not only survived extreme winter temperatures, but foraged effectively in cold weather and expanded their range into areas devoid of natural shelters and adequate wood fuel [48,62].
By 40 ka, palaeogenomics and archaeology indicate that regional populations had emerged or replaced the modern human meta-population in northern Eurasia. The East Asian lineage represented by the living Han population is identified at Tianyuan Cave in northern China at this time based on a whole-genome analysis of human remains [69]. The same lineage is present in the Amur River Basin at 33 ka and 19 ka (i.e. before and at the end of the LGM) [70]. It reflects early admixture with the Denisovans and is the primary ancestor (60–70%) of the Indigenous peoples of the Americas (arctic and non-arctic peoples) [61,71,72].
As described below, a suite of genetic adaptations to high latitudes (including low UV radiation) found today among both the arctic and non-arctic populations of the Western Hemisphere indicates that ancestral First Peoples inhabited the Arctic before the split between the two populations, which is estimated to have taken place during the LGM [73]. The ancestral group, which is designated Ancient Palaeo-Siberians (APS) in palaeogenomics, diverged from its East Asian parent lineage approximately 30 ka [95% CI 36.4–26.8 ka] [61,72]. APS is present in subarctic Siberia (59° North latitude) at Khaiyrgas Cave at 17 ka [74]. At this site, as well as at younger sites in Siberia and Beringia, APS is associated with a diagnostic set of stone artefacts comprising small wedge-shaped cores and microblades. These artefacts are found in older levels at Khaiyrgas Cave dating to the later LGM (approx. 24 ka) [74–76].
A second regional population with roots in western Eurasia (Ancient North Eurasians (ANE)) expanded into Northeast Asia before 30 ka and contributed to the East Asian genome and—both indirectly and directly—to the Native American genome. Genetic admixture with the west Eurasian lineage is evident at 34 ka in the woman from Salkhit in northern Mongolia [77]. The ANE population is present in southern Siberia during middle and late Upper Palaeolithic times at Mal'ta (24 ka) and Afontova Gora (17 ka), and their immediate ancestors (see below) are found in the Arctic and western Beringia as early as 33 ka [39,61,78].
5. The settlement of Beringia
(a) . Before the Last Glacial Maximum (35–26.5 ka)
Traces of a human presence in Beringia before 35 ka are limited and problematic, but given the evidence described above for occupation of the colder and drier parts of northern Eurasia 45–35 ka and the large mammal resources available on the exposed East Siberian Arctic Shelf during warm interstadials of this period, one or more episodes of modern human settlement in Beringia before 35 ka seems plausible, if not likely. The earliest well-dated archaeological sites and human remains are represented by a cluster of open-air sites near the mouth of the Yana River that have yielded thousands of stone and non-stone artefacts, and a large quantity of associated faunal remains, including steppe bison, reindeer, hare and mammoth. Calibration of a large sample of radiocarbon dates with the IntCal20 curve shows a concentration of ages around 34.5–31.5 ka, coinciding with two brief interstadials (Greenland Interstadial 6 [GI 6] and GI 5) [21,39,52].
The Yana River sites are tied to exploitation of the GAP and probably represent a winter occupation on its southern upland margin. It is unclear whether the sites simply indicate a human presence in western arctic Beringia at this time or reflect a peak in human population numbers. Following the Mann et al. model [36], large mammal populations should have experienced one or more increases during the two brief interstadials. Whole-genome analyses of two human teeth recovered from the sites indicate a robust hunter–gatherer population with an estimated effective population size (Ne) of 500 and low inbreeding coefficient (i.e. compare with Chagyrskaya Cave Neanderthal population described earlier) [61].
The ancient DNA analyses also reveal that the Yana River site occupants represent the west Eurasian lineage (designated Ancient North Siberians (ANS)) recently arrived in Northeast Asia. They carried a genetic adaptation to a northern diet (ancestral allele for expression of the FADS1 gene, advantageous for a diet rich in PUFAs) which presumably reflects a shift from a low-latitude diet containing a relatively high proportion of plant foods [59]. They apparently did not develop any genetic adaptations to vitamin D deficiency related to low UV radiation during the winter months [70,79]. Conceivably, there were dietary sources of vitamin D in western arctic Beringia during the interstadials that preceded the LGM (e.g. mushrooms, eggs).
There is evidence for people in eastern Beringia in the form of biomarker data (faecal sterol molecular markers) from two lakes in arctic Alaska. Sediment cores from Burial Lake and Lake E-5 in the Brooks Range yield recurring peaks of a high coprostanol : stigmastanol ratio (greater than 0.18) in the 34.5–31.5-ka time range and later. Although the faecal steroids are not species specific, the ratio values suggest a human presence relative to dominant background inputs of large herbivores. The cores also contain evidence for burning in the form of polycyclic aromatic hydrocarbon (PAH) fluxes and charcoal, another possible indicator of human activity in a region with limited lightning ignitions [80]. However, a 2021 field reconnaissance of Lake E-5 failed to turn up any archaeological remains (including evidence of Holocene occupation) [81].
Evidence for a human presence in mid-latitude North America (i.e. south of the Canadian ice sheets) and farther south before 15 ka is limited and controversial. The recently reported discovery of footprints in sediments dating to 23–21 ka on an ancient lakeshore in New Mexico provides an unusually strong case for people in the Western Hemisphere during the LGM [82], although the dating has been disputed (e.g. [83]). If people moved into mid-latitude North America before closure of the ice-free corridor, they conceivably represent either the west Eurasian lineage in Beringia before the LGM or the East Asian lineage in Beringia during the early LGM (see below). In either case, the size and long-term stability of any population in mid-latitude North America may have been severely constrained by the spatial–temporal dimensions of the ice-free corridor between 35 ka and its closure during the early LGM (25–23 ka or earlier).
Beringia itself may have been abandoned or have witnessed a severe decline in the human population after the GI 5 interstadial, which was followed by an episode of extreme cold climate (HE 3) at ca 30 ka [39]. The large-mammal biomass most likely decreased significantly at this time (and dietary sources of vitamin D may have disappeared altogether) due to increased aridity and reduced primary productivity.
(b) . During the Last Glacial Maximum (26.5–19 ka)
The LGM was a period of sustained cold and glacial re-advance in the Northern Hemisphere that reduced the land area of the Arctic to an arid zone between the North Eurasian and coalesced North American ice sheets (figure 3). An initial phase of extreme cold was interrupted by a brief interstadial (GI 2) at 24–23 ka, followed by another interval of low temperatures that were slightly milder than the initial phase [21]. Despite the extreme, sustained cryo-arid climate, which lowered plant and animal biomass, new research indicates that people were present in the colder and drier regions of northern Eurasia, including the central East European Plain and subarctic Siberia, although their numbers may have been significantly reduced [76,84].
A suite of high-latitude genetic adaptations (‘arctic signal’) found throughout the Indigenous peoples of the Western Hemisphere suggests that their immediate ancestors occupied the Arctic during the LGM [73,85]. The adaptations include the ancestral allele for FADS1, already common in the north Eurasian population, which became fixed not only in arctic and non-arctic First Peoples but also in related East Asian groups [59]. They also include a variant of EDAR V370A, which was under strong positive selection pressure during the LGM and appears to be an adaptation to vitamin D deficiency (due to low UV radiation) related to nursing infants [70,79]. In a recent paper, Niedbalski & Long attributed more than 20 000 high frequency alleles among living Native American populations to high latitude adaptation, including genes related to diet, cardiac processes and production of melanin in skin, hair and eyes [86]. The arctic signal also is evident in dental morphology: the distribution of three-rooted first molars in the Western Hemisphere suggests an arctic origin for the living First Peoples of mid-latitude North America and South America [87].
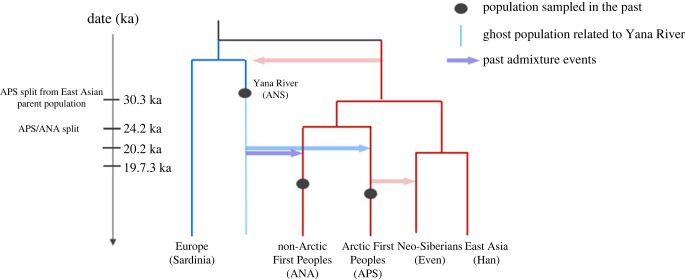
As described earlier, the primary ancestor of living First Peoples (APS) diverged from its East Asian parent population approximately 30 ka [61,72]. The estimated time of divergence between APS and the First Peoples of mid-latitude North America and South America (designated Ancient Native Americans (ANA)) provides a minimum date for the presence of APS in the Arctic, based on the genetic ‘arctic signal’ in both populations. (In our view, the ANA designation is misleading because the APS population, which includes the living arctic peoples of North America, also contains Native American groups.) Sikora et al. concluded that the APS population split into two groups approximately 24 ka, one of which (designated Ancient Beringians (AB)) was the immediate source of the ANA population approximately 20–19 ka [61]. The AB are represented by aDNA from human remains in Alaska dating to 11.5 ka and 9 ka, respectively [72,88]. It now appears, however, that the ‘Ancient Beringians’ were a later offshoot of the North American ANA population [71]. Thus, the estimated time of APS/ANA divergence apparently lies somewhere between the GI 2 interstadial (approx. 24 ka) and the end of the LGM (19 ka) (figure 4).
Figure 4.
Palaeogenomic framework for the peopling of the Western Hemisphere, illustrating early split between west Eurasian lineage (left) and East Asian lineage (right) (adapted from Sikora et al. fig. 2b [61]).
The inferred presence of the APS population—or at least a portion of it—in the Arctic during the LGM has implications for the settlement history of Beringia. Most terrestrial habitat above the Arctic Circle was glaciated during 26.5–19 ka, excepting the region that lay between the north Eurasian and North American ice sheets. Most of this region was occupied by a dry plain that supported a relatively uniform steppe-tundra biome (i.e. the GAP) at least with respect to the fauna, as described previously [36,37,39]. We assume that any LGM human inhabitants of the GAP would have ranged widely across this environment, including areas that lie within conventional definitions of Beringia. We also assume, following the Mann et al. [36] model, that conditions for human occupation were most favourable during the brief GI 2 interstadial approximately 24 ka.
Evidence for a human presence in Beringia during the LGM is limited in comparison to the preceding interval [39,52]. The biomarker record from Burial Lake and Lake E-5 in eastern arctic Beringia suggests that people were present, at least periodically, during the LGM, however and the Burial Lake record exhibits a sustained peak for both the coprostanol : stigmastanol ratio and PAH fluxes at approximately 24–23 ka [80]. At Bluefish Caves (northern Yukon), humanly modified mammoth remains and radiocarbon-dated bones with tool cut marks suggest that humans occupied the site during the LGM [89], although their interpretation has been disputed [90].
(c) . After the Last Glacial Maximum (19–11 ka)
While climates warmed slightly after 19 ka, this interval was followed by another cold period (HE 1) during 15.7–14.5 ka [21]. Significant warming took place after HE 1, during the GI 1 (or Bølling/Allerød) interstadial 14.5–12.9 ka. Global sea levels began to rise gradually after approximately 16 ka although the BLB was not completely flooded until approximately 11 ka [91]. The dating of archaeological sites and human remains in mid-latitude North America shows that the ANA population dispersed rapidly in areas south of the Canadian ice sheets after 15 ka, reaching the southern tip of South America before 13 ka [2].
As described earlier, the relatively late opening of a traversable corridor between the retreating Cordilleran ice sheet and LIS (less than 14 ka) indicates that most, if not all, of the population that spread throughout the Western Hemisphere was derived from the NW Pacific coast. This conclusion helps explain the rapid growth and dispersal of the ANA population [14,92] because marine sediment cores from the North Pacific reveal a peak in marine productivity during the GI 1 interstadial [93]. Although the earliest traces of people on the NW Pacific coast currently date to ca 13 ka [94], they probably were present there before 15 ka.
The inferred presence of the ANA population on the NW Pacific coast before 15 ka has implications for the settlement history of Beringia. Before 15 ka, the NW Pacific was accessible to people in Beringia only from the southern BLB [24,25]. Because the narrow coastal zone restricted its human inhabitants to a diet based largely on marine resources, its earliest occupants probably arrived with a developed maritime economy (and some watercraft). Accordingly, we conclude that the ANA population probably adapted to coastal habitat on the southern BLB before 15 ka. We also conclude—based on the genetic ‘arctic signal’ found throughout arctic and non-arctic First Peoples—that the ANA population on the BLB was recently derived from the Arctic, most likely the GAP/arctic Beringia (not the NE Asian maritime zone) [73,86,95].
There is no supporting archaeological evidence for people on the southern BLB and we can only speculate on the process by which people from arctic Beringia developed a maritime economy on a subarctic coast (apparently abandoning the microblade technology of the APS along the way). The shift from terrestrial to coastal diet may have been driven by several factors. Population pressure on the inhabitants of the GAP (a consequence of elevated plant and animal productivity during the GI 2 interstadial approximately 24 ka?) conceivably promoted expansion into the subarctic zone, where—as discussed earlier—the large-mammal biomass and human carrying capacity of the terrestrial habitat must have been lower than that of the arctic plain (due to widespread tundra soil and peat formation). The marginal character of the terrestrial subarctic habitat would have encouraged greater dependance on marine resources along the southern coast (which began to retreat after 16 ka). Driftwood derived from a source (pine, alder) in the southern NW Pacific may have been available after 16 ka [96]. Perhaps cold HE 1, which began approximately 17 ka, was a catalyst for a shift to a marine diet at a time when terrestrial resources would have declined, while accelerated glacial run-off from eastern Beringia is thought to have fertilized waters on the southern coast [97].
A large and growing population on the NW Pacific coast during the GI 1 interstadial also has implications for the settlement history of Beringia. While most expansion of the ANA population apparently took place in mid-latitude North America and farther south, the geography of the narrow coastal zone probably promoted expansion northward (i.e. back into Beringia) as well. We suggest that most of the Beringian archaeological record for the GI 1 interstadial (14.5–12.9 ka), including the oldest occupations at Ushki (Kamchatka) and assemblages assigned to the Nenana complex in central Alaska [98], conceivably represent the northward expansion of the ANA population from the NW Pacific coast.
6. Conclusion and future research
We conclude that the distribution of North American ice sheets during and after the LGM was the critical variable in the peopling of the Western Hemisphere. Movement of both the APS and ANA populations (which diverged before 19 ka) from the interior of Beringia was blocked by the coalesced LIS and Cordilleran ice sheet until after 14 ka (by which time the ANA population already had dispersed widely in mid-latitude North America). Early deglaciation of the NW Pacific coast, which began approximately 18 ka, allowed the ANA population to occupy this region from the southern coast of the BLB before 15 ka and subsequently to disperse, from this region, throughout the unglaciated Western Hemisphere. An ice-free corridor between the two ice sheets was present before the LGM and may have allowed limited movement of people in the arctic or interior regions of Beringia to mid-latitude North America.
Our conclusion is bolstered by evidence from various spatial and temporal contexts in northern Eurasia that modern humans occupied most climate zones and habitats of the Northern Hemisphere after 45 ka, including the LGM during 26.5–19 ka [76,84]. Rather than presenting an ecological barrier, the large mammal populations of the GAP—much of which lies within arctic Beringia—probably attracted modern humans to high latitudes, although genetic adaptation to low UV radiation, especially in the absence of dietary sources of vitamin D, may have been an important factor in the settlement history of the GAP [79]. Marine resources along the southern coast of Beringia and adjoining areas of the NW Pacific, which became especially rich after 14.5 ka, also must have attracted human settlement.
We conclude that the variation among living First Peoples is more likely to be a product of regional differentiation of populations after their divergence from their source population in Northeast Asia, and not the result of multiple migrations to the Western Hemisphere [10,11]. This conclusion is based on a synthesis of recent research in geology and genetics suggesting that: (a) the ANA population dispersed from the NW Pacific coast, which must have been accessed from the southern BLB; and (b) the immediate ancestors of the ANA population (i.e. APS) occupied the Arctic, including arctic Beringia, before the APS/ANA split. We conclude that the primary division among living First Peoples (i.e. arctic versus non-arctic) is the result of an event that took place in Beringia before 15 ka, i.e. divergence of arctic interior (GAP) and subarctic coastal (southern BLB) adaptations and peoples.
Although these conclusions are broadly consistent with the 2007 ‘Beringian standstill’ model, which postulated an extended period of isolation for the Native American founder population in Beringia [13–15], we now view the term ‘standstill’ as a mischaracterization of what probably took place during and after the LGM. The founder population did not retreat into a refugium during the LGM but expanded into an immense arctic plain where extreme climate and lowered sea level promoted steppe-tundra flora and fauna. During or after the LGM, the population spread into a less productive subarctic environment and eventually developed a northern coastal economy, which facilitated further expansion into the NW Pacific and massive population growth and dispersal during the final interstadial of the Pleistocene.
By linking the divergence of arctic and non-arctic First Peoples to development of a coastal adaptation in southern Beringia that subsequently gave rise to the large NW Pacific coast population, our conclusions may be reconciled with the multiple migration model, at least in part. The early NW Pacific coast peoples conceivably represent the ancestral Dené [99], who constitute a major linguistic and biological group within the Indigenous peoples of the Western Hemisphere [8–11,87]. The multiple migrations of the 1986 ‘three-wave model’ (see introduction) may be equated with the three steps of geographical expansion postulated here (i.e. Beringia, NW Pacific coast and mid-latitude North America and South America).
In our view, the most promising avenue of future research on the questions addressed in this review lies in those areas of northeastern Siberia and northern Alaska/Yukon that represent the former southern and eastern upland margins of the GAP, including islands in the East Siberian Sea. Ongoing efforts to reconstruct fluctuations in large herbivore populations will shed light on the resources available to people on the GAP before, during, and after the LGM [36,40].
We are pursuing field research on both sides of the Bering Strait, including western arctic Beringia, which yields evidence for the presence of the west Eurasian lineage before the LGM, but has yet to produce definitive evidence for the East Asian lineage greater than 10 ka, although a credible archaeological proxy for APS (microblades) dates to approximately 15 ka [39,52]). New research at Bluefish Caves (northern Yukon) should resolve uncertainties regarding the stratigraphic context and dating of the stone artefacts and modified bones [89]. Another focus of research in eastern Beringia is the small number of lakes (e.g. Burial Lake) that did not evaporate during the LGM, several of which have yielded biomarker evidence for people before and during the LGM [80,81].
Acknowledgements
The authors are grateful to Lilian Hoffecker, who prepared figures 1,2, and 3.
Data accessibility
This article has no additional data.
Authors' contributions
J.F.H.: writing—original draft; S.A.E.: writing—review and editing; G.R.S.: writing—review and editing; D.H.O.: writing—review and editing; L.J.H.: writing—review and editing; O.P.: writing—review and editing; V.P.: writing—review and editing; E.P.: writing—review and editing; L.B.: writing—review and editing; R.S.V.: writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no conflict of interests.
Funding
V.P. and E.P. are grateful to the Russian Science Foundation for support of their work (projects 16-18-10265 and 21-18-00457).
References
- 1.Willerslev E, Meltzer DJ. 2021. Peopling of the Americas as inferred from ancient genomics. Nature 594, 356-364. ( 10.1038/s41586-021-03499-y) [DOI] [PubMed] [Google Scholar]
- 2.Waters MR. 2019. Late Pleistocene exploration and settlement of the Americas by modern humans. Science 365, eaat5447. ( 10.1126/science.aat5447) [DOI] [PubMed] [Google Scholar]
- 3.Hultén E. 1937. Outline of the history of Arctic and boreal biota during the Quaternary period. New York, NY: Lehre J Cramer. [Google Scholar]
- 4.Sher AV, Kuzmina SA, Kuznetsova TV, Sulerzhitsky LD. 2005. New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals. Q. Sci. Rev. 24, 533-569. ( 10.1016/j.quascirev.2004.09.007) [DOI] [Google Scholar]
- 5.Hopkins DM. 1959. Cenozoic history of the Bering Land Bridge. Science 129, 1519-1528. ( 10.1126/science.129.3362.1519) [DOI] [PubMed] [Google Scholar]
- 6.Meiri M, et al. 2014. Faunal record identified Bering isthmus conditions as constraint to end-Pleistocene migration to the New World. Proc. R. Soc. B 281, 20132167. ( 10.1098/rspb.2013.2167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wooller MJ, Saulnier-Talbot É, Potter BA. 2018. A new terrestrial palaeoenvironmental record from the Bering Land Bridge and context for human dispersal. R. Soc. Open Sci. 5, 180145. ( 10.1098/rsos.180145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg JH, Turner CG, Zegura SL. 1986. The settlement of the Americas: a comparison of the linguistic, dental, and genetic evidence. Curr. Anthropol. 27, 477-497. ( 10.1086/203472) [DOI] [Google Scholar]
- 9.Reich D, et al. 2012. Reconstructing Native American population history. Nature 488, 370-374. ( 10.1038/nature11258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szathmary EJE. 1993. Genetics of aboriginal North Americans. Evol. Anthropol. 1, 202-220. ( 10.1002/evan.1360010606) [DOI] [Google Scholar]
- 11.Bonatto SL, Salzano FM. 1997. A single and early migration for the peopling of the Americas supported by mitochondrial DNA sequence data. Proc. Natl Acad. Sci. USA 94, 1866-1871. ( 10.1073/pnas.94.5.1866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster P, Harding R, Torroni A, Bandelt H-J. 1996. Origin and evolution of Native American mtDNA variation: a reappraisal. Am. J. Hum. Genet. 59, 935-945. [PMC free article] [PubMed] [Google Scholar]
- 13.Tamm E, et al. 2007. Beringian standstill and spread of Native American founders. PLoS ONE 9, e829. ( 10.1371/journal.pone.0000829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulligan CJ, Kitchen A, Miyamoto MM. 2008. Updated three-stage model for the peopling of the Americas. PLoS ONE 3, e3199. ( 10.1371/journal.pone.0003199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffecker JF, Elias SA, O'Rourke DH. 2014. Out of Beringia? Science 343, 979-980. ( 10.1126/science.1250768) [DOI] [PubMed] [Google Scholar]
- 16.Pico T, Creveling JR, Mitrovica JX. 2017. Sea-level records from the U.S. mid-Atlantic constrain Laurentide Ice Sheet extent during Marine Isotope Stage 3. Nat. Commun. 8, 15612. ( 10.1038/ncomms15612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton AS, Stokes CR, Batchelor CL. 2020. Evolution of the Laurentide and Innuitian ice sheets prior to the Last Glacial Maximum (115 ka to 25 ka). Earth-Sci. Rev. 224, 103875. ( 10.1016/j.earscirev.2021.103875) [DOI] [Google Scholar]
- 18.Batchelor CL, et al. 2019. The configuration of Northern Hemisphere ice sheets through the Quaternary. Nat. Commun. 10, 3713. ( 10.1038/s41467-019-11601-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller GH, Andrews JT. 2019. Hudson Bay was not deglaciated during MIS-3. Quat. Sci. Rev. 225, 105944. ( 10.1016/j.quascirev.2019.105944) [DOI] [Google Scholar]
- 20.Gowan EJ, et al. 2021. A new global ice sheet reconstruction for the past 80 000 years. Nat. Commun. 12, 1199. ( 10.1038/s41467-021-21469-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimer PJ, et al. 2020. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725-757. ( 10.1017/RDC.2020.41) [DOI] [Google Scholar]
- 22.Heintzman PD, et al. 2016. Bison phylogeography constrains dispersal and viability of the Ice Free Corridor in western Canada. Proc. Natl Acad. Sci. USA 113, 8057-8063. ( 10.1073/pnas.1601077113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stauch G, Lehmkuhl F. 2010. Quaternary glaciations in the Verkhoyansk Mountains, Northeast Siberia. Quat. Res. 74, 145-155. ( 10.1016/j.yqres.2010.04.003) [DOI] [Google Scholar]
- 24.Lesnek AJ, Briner JP, Lindqvist C, Baichtal JF, Heaton TH. 2018. Deglaciation of the Pacific coastal corridor directly preceded the human colonization of the Americas. Sci. Adv. 4, eaar5040. ( 10.1126/sciadv.aar5040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalton AS, et al. 2020. An updated radiocarbon-based ice margin chronology for the last deglaciation of the North American Ice Sheet Complex. Quat. Sci. Rev. 234, 106223. ( 10.1016/j.quascirev.2020.106223) [DOI] [Google Scholar]
- 26.Clark J, et al. 2022. The age of the opening of the Ice-Free Corridor and implications for the peopling of the Americas. Proc. Natl Acad. Sci. USA 119, e2118558119. ( 10.1073/pnas.2118558119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colinvaux PA. 1967. Quaternary vegetational history of arctic Alaska. In The Bering Land Bridge (ed. Hopkins DM), pp. 207-231. Stanford, CA: Stanford University Press. [Google Scholar]
- 28.Guthrie RD. 1968. Paleoecology of the large-mammal community in interior Alaska during the late Pleistocene. Am. Midl. Nat. 79, 346-463. ( 10.2307/2423182) [DOI] [Google Scholar]
- 29.Elias SA, Short SK, Nelson CH, Birks HH. 1996. Life and times of the Bering Land Bridge. Nature 382, 60-63. ( 10.1038/382060a0) [DOI] [Google Scholar]
- 30.Elias SA, Crocker B. 2008. The Bering Land Bridge: a moisture barrier to the dispersal of steppe-tundra biota? Quat. Sci. Rev. 27, 2473-2483. ( 10.1016/j.quascirev.2008.09.011) [DOI] [Google Scholar]
- 31.Guthrie RD. 2001. Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammoth tooth pits, buckles, and inside-out Beringia. Q. Sci. Rev. 20, 549-574. ( 10.1016/S0277-3791(00)00099-8) [DOI] [Google Scholar]
- 32.Brubaker LB, Anderson PM, Edwards ME, Lozhkin AV. 2005. Beringia as a glacial refugium for boreal trees and shrubs: new perspectives from mapped pollen data. J. Biogeogr. 32, 833-848. ( 10.1111/j.1365-2699.2004.01203.x) [DOI] [Google Scholar]
- 33.Wang Y, Heintzman PD, Newsom L, Bigelow NH, Wooller MJ, Shapiro B, Williams JW. 2017. The southern coastal Beringian land bridge: cryptic refugium or pseudorefugium for woody plants during the Last Glacial Maximum? J. Biogeogr. 44, 1559-1571. ( 10.1111/jbi.13010) [DOI] [Google Scholar]
- 34.Lindgren A, Hugelius G, Kuhry P. 2018. Extensive loss of past permafrost carbon but a net accumulation into present-day soils. Nature 560, 219-222. ( 10.1038/s41586-018-0371-0) [DOI] [PubMed] [Google Scholar]
- 35.Hoffecker JF, Elias SA, Potapova O. 2020. Arctic Beringia and Native American origins. PaleoAmerica 6, 158-168. ( 10.1080/20555563.2020.1725380) [DOI] [Google Scholar]
- 36.Mann DH, Groves P, Reanier RE, Gaglioti BV, Kunz ML, Shapiro B. 2015. Life and extinction of megafauna in the ice-age Arctic. Proc. Natl Acad. Sci. USA 112, 14 301-14 306. ( 10.1073/pnas.1516573112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirrmeister L, Hubberten HW, Rachold V, Grosse G. 2005. Lost world—Late Quaternary environment of periglacial Arctic shelves and coastal lowlands in NE-Siberia. In 2nd Int. Alfred Wegener Symp., Bremerhaven, Germany, 30 October–2 November. (doi:10013/epic.26457) [Google Scholar]
- 38.Wang Y, et al. 2021. Late Quaternary dynamics of arctic biota from ancient environmental genomics. Nature 600, 86-92. ( 10.1038/s41586-021-04016-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitulko VV, Pavlova EY, Nikolskiy PA. 2017. Revising the archaeological record of the Upper Pleistocene Arctic Siberia: human dispersal and adaptations in MIS 3 and 2. Quat. Sci. Rev. 165, 127-148. ( 10.1016/j.quascirev.2017.04.004) [DOI] [Google Scholar]
- 40.Murchie TJ, et al. 2021. Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA. Nat. Commun. 12, 7120. ( 10.1038/s41467-021-27439-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Churchill SE. 2014. Thin on the ground: Neandertal biology, archeology, and ecology. Ames, IA: John Wiley & Sons. [Google Scholar]
- 42.Gokhman D, Mishol N, de Manuel M, de Juan D, Shuqrun J, Meshorer E, Marques-Bonet T, Rak Y, Carmel L. 2019. Reconstructing Denisovan anatomy using DNA methylation maps. Cell 179, 180-192. ( 10.1016/j.cell.2019.08.035) [DOI] [PubMed] [Google Scholar]
- 43.Trinkaus E. 1981. Neanderthal limb proportions and cold adaptation. In Aspects of human evolution (ed. Stringer C), pp. 187-224. London, UK: Taylor & Francis. [Google Scholar]
- 44.Bocherens H. 2011. Diet and ecology of the Neanderthals: implications from C and N isotopes: insights from bone and tooth biogeochemistry. In Neanderthal lifeways, subsistence, and technology: one hundred and fifty years of neanderthal study (eds Conard NJ, Richter J), pp. 73-85. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 45.Sánchez-Quinto F, Lalueza-Fox C. 2014. Almost 20 years of Neanderthal palaeogenetics: adaptation, admixture, diversity, demography, and extinction. Phil. Trans. R. Soc. B 370, 20130374. ( 10.1098/rstb.2013.0374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mafessoni F, et al. 2020. A high-coverage Neandertal genome from Chagyrskaya Cave. Proc. Natl Acad. Sci. USA 117, 15 132-15 136. ( 10.1073/pnas.2004944117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slimak L, et al. 2022. Modern human incursion into Neanderthal territories 54 000 years ago at Mandrin, France. Sci. Adv. 8, eabj9496. ( 10.1126/sciadv.abj9496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffecker JF. 2017. Modern humans: their African origin and global dispersal. New York, NY: Columbia University Press. [Google Scholar]
- 49.Fu Q, et al. 2014. Genome sequence of a 45 000-year-old modern human from western Siberia. Nature 514, 445-449. ( 10.1038/nature13810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hublin JJ, et al. 2020. Initial Upper Palaeolithic Homo sapiens from Bacho Kiro, Bulgaria. Nature 581, 299-302. ( 10.1038/s41586-020-2259-z) [DOI] [PubMed] [Google Scholar]
- 51.Pavlov P, Roebroeks W, Svendsen JI. 2004. The Pleistocene colonization of northeastern Europe: a report on recent research. J. Hum. Evol. 47, 3-17. ( 10.1016/j.jhevol.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 52.Pavlova EY, Pitulko VV. 2020. Late Pleistocene and early Holocene climate changes and human habitation in the arctic western Beringia based on revision of palaeobotanical data. Quat. Internatl. 549, 5-25. ( 10.1016/j.quaint.2020.04.015) [DOI] [Google Scholar]
- 53.Fu Q, et al. 2015. An early modern human from Romania with a recent Neanderthal ancestor. Nature 524, 216-h219. ( 10.1038/nature14558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sankararaman S, Mallick S, Patterson N, Reich D. 2016. The combined landscape of Denisovan and Neanderthal ancestry in present-day humans. Curr. Biol. 26, 1241-1247. ( 10.1016/j.cub.2016.03.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holliday TW. 1997. Body proportions in Late Pleistocene Europe and modern human origins. J. Hum. Evol. 32, 423-448. ( 10.1006/jhev.1996.0111) [DOI] [PubMed] [Google Scholar]
- 56.Hoffecker JF. 2002. Desolate landscapes: ice-age settlement of Eastern Europe. New Brunswick, NJ: Rutgers University Press. [Google Scholar]
- 57.Wißing C, et al. 2019. Stable isotopes reveal patterns of diet and mobility in the last Neandertals and first modern humans in Europe. Sci. Rep. 9, 4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drucker DG, et al. 2017. Isotopic analyses suggest mammoth and plant in the diet of the oldest anatomically modern humans from far southeast Europe. Sci. Rep. 7, 6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathieson I. 2020. Limited evidence for selection at the FADS locus in Native American populations. Mol. Biol. Evol. 37, 2029. ( 10.1093/molbev/msaa064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sikora M, et al. 2017. Ancient genomes show social and reproductive behavior of early Upper Paleolithic foragers. Science 358, 659-662. ( 10.1126/science.aao1807) [DOI] [PubMed] [Google Scholar]
- 61.Sikora M, et al. 2019. The population history of northeastern Siberia since the Pleistocene. Nature 570, 182-188. ( 10.1038/s41586-019-1279-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffecker JF, Hoffecker IT. 2017. Technological complexity and the global dispersal of modern humans. Evol. Anthropol. 26, 285-299. ( 10.1002/evan.21553) [DOI] [PubMed] [Google Scholar]
- 63.Shea JJ, Sisk ML. 2010. Complex projectile technology and Homo sapiens dispersal into western Eurasia. PaleoAnthropol. 2010, 100-122. [Google Scholar]
- 64.Sano K, et al. 2019. The earliest evidence for mechanically delivered projectile weapons in Europe. Nat. Ecol. Evol. 3, 1409-1414. ( 10.1038/s41559-019-0990-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derevianko AP, Shunkov MV, Markin SV. 2014. Dinamika paleoliticheskikh industrii v Afrike i Evrazii v pozdnem pleistotsene i problema formirovaniya Homo sapiens. Novosibirsk, Russia: SB RAS Press. [Google Scholar]
- 66.Douka K, et al. 2019. Age estimates for hominin fossils and the onset of the Upper Palaeolithic at Denisova Cave. Nature 565, 640-644. ( 10.1038/s41586-018-0870-z) [DOI] [PubMed] [Google Scholar]
- 67.Stepanchuk VN, et al. 2004. Bogatosharova stoyanka Mira na Sredn'omu Dnipri: osnoni rezul'tati razkopok 2000 roku pp. 62-98. Kiev, Ukraine: Kam'yana doba Ukraini. [Google Scholar]
- 68.Sandgathe DM, Dibble HL, Goldberg P, McPherron SP, Turq A, Niven L, Hodgkins J. 2011. On the role of fire in Neandertal adaptations in western Europe: evidence from Pech de l'Aze IV and Roc de Marsal, France. PaleoAnthropol. 2011, 216-242. [Google Scholar]
- 69.Yang MA, et al. 2017. 40 000-year-old individual from Asia provides insight into early population structure in Eurasia. Curr. Biol. 27, 3202-3208. ( 10.1016/j.cub.2017.09.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao X, et al. 2021. The deep population history of northern East Asia from the Late Pleistocene to the Holocene. Cell 184, 1-11. ( 10.1016/j.cell.2020.12.019) [DOI] [PubMed] [Google Scholar]
- 71.Ning C, et al. 2020. The genomic formation of First American ancestors in East and Northeast Asia. bioRxiv. ( 10.1101/2020.10.12.336628) [DOI] [Google Scholar]
- 72.Moreno-Mayar JV, et al. 2018. Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. Nature 553, 203-207. ( 10.1038/nature25173) [DOI] [PubMed] [Google Scholar]
- 73.Amorim CEG, Nunes K, Meyer D, Comas D, Bortolini MC, Salzano FM, Hünemeier T. 2017. Genetic signature of natural selection in first Americans. Proc. Natl Acad. Sci. USA 114, 2195. ( 10.1073/pnas.1620541114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kılınç GM, et al. 2021. Human population dynamics and Yersinia pestis in ancient northeast Asia. Sci. Adv. 7, eabc4587. ( 10.1126/sciadv.abc4587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stepanov AD, et al. 2003. Peshchera Khaiyrgas na srednei Lene (rezultaty issledovanyi 1998–1999 gg.). In Drevnie kultury severo-vostochnoi Azii. Astroarkheologiya. Paleoinformatika (ed. AN Alekseev), pp. 98-113. Novosibirsk, Russia: "Nauka". [Google Scholar]
- 76.Kuzmin YV, Kosintsev PA, Stepanov AD, Boeskorov GG, Cruz RJ. 2017. Chronology and faunal remains of the Khaiyrgas Cave (eastern Siberia, Russia). Radiocarbon 59, 575-582. ( 10.1017/RDC.2016.39) [DOI] [Google Scholar]
- 77.Massilani D, et al. 2020. Denisovan ancestry and population history of early East Asians. Science 370, 579-583. ( 10.1126/science.abc1166) [DOI] [PubMed] [Google Scholar]
- 78.Raghavan M, et al. 2013. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505, 87-91. ( 10.1038/nature12736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hlusko LJ, et al. 2018. Environmental selection during the last ice age on the mother-to-infant transmission of vitamin D and fatty acids through breast milk. Proc. Natl Acad. Sci. USA 115, E4426. ( 10.1073/pnas.1711788115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vachula RS, Huang Y, Russell JM, Abbott MB, Finkenbinder MS, O'Donnell JA. 2020. Sedimentary biomarkers reaffirm human impacts on northern Beringian ecosystems during the Last Glacial period. Boreas 49, 514-525. ( 10.1111/bor.12449) [DOI] [Google Scholar]
- 81.Goebel T, Hoffecker JF, Graf KE, Vachula RS. 2022. Archaeological reconnaissance at Lake E5 in the Brooks Range, Alaska and implications for the early human biomarker record of Beringia. Q. Sci. Rev. 286, 107553. ( 10.1016/j.quascirev.2022.107553) [DOI] [Google Scholar]
- 82.Bennett MR, et al. 2021. Evidence of humans in North America during the Last Glacial Maximum. Science 373, 1528-1531. ( 10.1126/science.abg7586) [DOI] [PubMed] [Google Scholar]
- 83.Oviatt CG, Madsen DB, Rhode D, Davis LG. 2022. A critical assessment of claims that human footprints in the Lake Otero basin, New Mexico date to the Last Glacial Maximum. Quat. Res . 1-10. ( 10.1017/qua.2022.38) [DOI] [Google Scholar]
- 84.Pryor AJE, Beresford-Jones DG, Dudin AE, Ikonnikova EM, Hoffecker JF, Gamble C. 2020. Chronology and function of a new circular mammoth bone structure from Kostenki 11. Antiquity 94, 323-341. ( 10.15184/aqy.2020.7) [DOI] [Google Scholar]
- 85.Fumagalli M, et al. 2015. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 349, 1343. ( 10.1126/science.aab2319) [DOI] [PubMed] [Google Scholar]
- 86.Niedbalski SD, Long JC. 2022. Novel alleles gained during the Beringian isolation. Sci. Rep. 12, 4289. ( 10.1038/s41598-022-08212-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scott GR, Turner CG, Townsend GC, Martinón-Torres M. 2018. The anthropology of modern human teeth: dental morphology and its variation in recent and fossil Homo sapiens, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 88.Moreno-Mayar JV, et al. 2018. Early human dispersals within the Americas. Science 362, eaav2621. ( 10.1126/science.aav2621) [DOI] [PubMed] [Google Scholar]
- 89.Bourgeon L, Burke A, Higham T. 2017. Earliest human presence in North America dated to the Last Glacial Maximum: new radiocarbon dates from Bluefish Caves, Canada. PLoS ONE 12, e0169486. ( 10.1371/journal.pone.0169486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krasinski KE, Blong JC. 2020. Unresolved questions about site formation, provenience, and the impact of natural processes on bone at the Bluefish Caves, Yukon Territory. Arctic Anthropol. 57, 1-21. ( 10.3368/aa.57.1.1) [DOI] [Google Scholar]
- 91.Lambeck K, Rouby H, Purcell A, Sun Y, Sambridge M. 2014. Sea level and global ice volumes from the Last Glacial Maximum to the Holocene. Proc. Natl Acad. Sci. USA 111, 15 296-15 303. ( 10.1073/pnas.1411762111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Llamas B, et al. 2016. Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Sci. Adv. 2, e1501385. ( 10.1126/sciadv.1501385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohfeld KE, Chase Z. 2011. Controls on deglacial changes in biogenic fluxes in the North Pacific Ocean. Q. Sci. Rev. 30, 3350-3363. ( 10.1016/j.quascirev.2011.08.007) [DOI] [Google Scholar]
- 94.McLaren D, Fedje D, Dyck A, Mackie Q, Gauvreau A, Cohen J. 2018. Terminal Pleistocene epoch human footprints from the Pacific coast of Canada. PLoS ONE 13, e0193522. ( 10.1371/journal.pone.0193522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott GR, et al. 2021. Peopling the Americas: not “out of Japan”. PaleoAmerica 7, 309-332. ( 10.1080/20555563.2021.1940440) [DOI] [Google Scholar]
- 96.Hebda CFG, et al. 2022. Late Pleistocene palaeoenvironments and a possible glacial refugium on northern Vancouver Island, Canada: evidence for the viability of early human settlement on the northwest coast of North America . Q. Sci. Rev. 279, 107388. ( 10.1016/j.quascirev.2022.107388) [DOI] [Google Scholar]
- 97.Wang R, Kuhn G, Gong X, Biskaborn BK, Gersonde R, Lembke-Jene L, Lohmann G, Tiedemann R, Diekmann B. 2021. Deglacial land–ocean linkages at the Alaskan continental margin in the Bering Sea. Front. Earth Sci. 9, 712415. ( 10.3389/feart.2021.712415) [DOI] [Google Scholar]
- 98.Hoffecker JF, Elias SA. 2007. Human ecology of Beringia. New York, NY: Columbia University Press. [Google Scholar]
- 99.Lindo J, et al. 2017. Ancient individuals from the North American Northwest coast reveal 10 000 years of regional continuity. Proc. Natl Acad. Sci. USA 114, 4093-4098. ( 10.1073/pnas.1620410114) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.