Abstract
Helicobacter pylori can produce a persistent infection in the human stomach, where chronic and active inflammation, including the infiltration of phagocytes such as neutrophils and monocytes, is induced. H. pylori may have a defense system against the antimicrobial actions of phagocytes. We studied the defense mechanism of H. pylori against host-derived peroxynitrite (ONOO−), a bactericidal metabolite of nitric oxide, focusing on the role of H. pylori urease, which produces CO2 and NH3 from urea and is known to be an essential factor for colonization. The viability of H. pylori decreased in a time-dependent manner with continuous exposure to 1 μM ONOO−, i.e., 0.2% of the initial bacteria remained after a 5-min treatment without urea. The bactericidal action of ONOO− against H. pylori was significantly attenuated by the addition of 10 mM urea, the substrate for urease, whereas ONOO−-induced killing of a urease-deficient mutant of H. pylori or Campylobacter jejuni, another microaerophilic bacterium lacking urease, was not affected by the addition of urea. Such a protective effect of urea was potentiated by supplementation with exogenous urease, and it was almost completely nullified by 10 μM flurofamide, a specific inhibitor of urease. The bactericidal action of ONOO− was also suppressed by the addition of 20 mM NaHCO3 but not by the addition of 20 mM NH3. In addition, the nitration of l-tyrosine of H. pylori after treatment with ONOO− was significantly reduced by the addition of urea or NaHCO3, as assessed by high-performance liquid chromatography with electrochemical detection. These results suggest that H. pylori-associated urease functions to produce a potent ONOO− scavenger, CO2/HCO3−, that defends the bacteria from ONOO− cytotoxicity. The protective effect of urease may thus facilitate sustained bacterial colonization in the infected gastric mucosa.
Nitric oxide (NO) is known to play an important role in host defense against a variety of microbes (1, 12, 15, 20, 36, 37), although NO itself does not show sufficient antimicrobial activity (24, 55). Some metabolites of NO, such as peroxynitrite (ONOO−), are considered to be responsible for the antimicrobial as well as the pathogenic effects of NO. NO and superoxide (O2−) react in a diffusion-limited manner, forming ONOO− (5), a strong oxidant and nitrating agent (4, 5, 23) that exhibits potent bactericidal activity (22, 57) as well as cytotoxicity for mammalian cells in vitro and in vivo (4, 5). It has been reported that both NO and O2− that are simultaneously produced in local areas of infection are critically involved in antimicrobial defense in murine salmonellosis (Salmonella enterica serovar Typhimurium infection), possibly through formation of ONOO− (49).
Helicobacter pylori can infect human gastric mucosa chronically; such infection is known to be associated with gastritis, peptic gastric ulcer, duodenal ulcer, and an increased risk for gastric cancer (3, 6, 21, 45, 52). A unique feature of H. pylori infection is its persistence, which causes prolonged active inflammation, including infiltration of neutrophils and monocytes in gastric mucosa (11, 39). Increased expression of the inducible type of NO synthase (iNOS) (16–18, 30, 42, 47) and elevated formation of nitrotyrosine (17, 30) are also observed in the gastric mucosae of patients with H. pylori infection. However, the mechanism of the persistent infection of H. pylori, despite the production of highly bactericidal ONOO− and other reactive nitrogen species, is not clear.
Several investigations have suggested a role for H. pylori urease in the survival and pathogenesis of the bacteria (29, 31, 35, 46). Urease catalyzes the hydrolysis of urea to form carbon dioxide (CO2) and ammonia (NH3). It is reported that urease functions in H. pylori infection to neutralize gastric acid by producing NH3 (31). Enhanced production of NH3 also may facilitate the formation of NH3-derived compounds, such as monochloramine, which shows cytotoxic effects on host cells (46). Enhancement of bacterial motility (35) and inhibition of phagocytic clearance of bacteria (29) were also reported as functions of urease. The pathogenic potential of urease is so far mainly attributed to NH3 produced by the enzymatic reaction. In contrast, little attention has been paid to the roles of CO2/HCO3− produced in the same process. It is noteworthy that the chemical reactivity of ONOO− is reported to be modulated by CO2/HCO3− (26, 28, 54). Specifically, ONOO− reacts rapidly with CO2, and through the formation of ONOOCO2−, not only is isomerization of ONOO− to NO3− accelerated (27, 50), but also the nitration potency of ONOO− is significantly enhanced and the oxidation potential is markedly attenuated (54, 56). For example, CO2/HCO3− facilitates ONOO−-induced nitration of aromatic compounds, such as tyrosine and guanine (guanosine); however, it suppresses their oxidation (26, 54, 56). In addition, the in vitro bactericidal activity of ONOO− on Escherichia coli was reduced by the addition of NaHCO3 (22, 57).
Therefore, the purpose of this study was to clarify the role of urease in persistent colonization of H. pylori, especially to examine the protective effects of CO2 produced by urease against the bactericidal activity of ONOO− in vitro.
MATERIALS AND METHODS
Bacteria.
H. pylori ATCC 43504 was obtained from the American Type Culture Collection (Manassas, Va.). H. pylori HPK5 and its isogenic ureB mutant HPT209 (lacking urease), which was produced by allelic exchange mutagenesis, were generously provided by T. Nakazawa, Department of Microbiology, Yamaguchi University School of Medicine, Ube, Japan (35). Campylobacter jejuni isolated from a clinical source was also used in this study. These bacteria were routinely grown in brucella broth (Becton Dickinson & Co., Cockeysville, Md.) supplemented with 10% fetal calf serum (Intergen Co., Purchase, N.Y.) in the presence (H. pylori HPT209) or absence (H. pylori ATCC 43504, H. pylori HPK5, and C. jejuni) of 7 μg of kanamycin sulfate/ml under microaerobic conditions maintained in a GasPak jar (Becton Dickinson & Co.) with an H2- and CO2-generating agent, CampyPak (Becton Dickinson & Co.).
Reagents.
ONOO− was prepared from nitrite and hydrogen peroxide in a quenched-flow reactor as previously described (5). The NO-liberating agent propylamine NONOate (CH3N[N(O)NO]−(CH2)3NH2+CH3, 1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazine) (P-NONOate) which has a half-life of 7.6 min in aqueous solutions at a neutral pH under our experimental conditions, was obtained from Dojindo Laboratories, Kumamoto, Japan. Urea, NaHCO3, and an aqueous solution of ammonia (NH4OH) were from Wako Pure Chemicals Co. Ltd., Osaka, Japan. Urease from Bacillus pasteurii, 3-nitro-l-tyrosine (nitrotyrosine), and l-tyrosine were purchased from Sigma Chemical Co., St. Louis, Mo. Dihydrorhodamine 123 (DHR) was purchased from Molecular Probes, Inc., Eugene, Oreg. Two urease inhibitors, N-(diaminophosphinyl)-4-fluorobenzamide (flurofamide) and acetohydroxamic acid (AHX), were from ICN Biomedicals Inc., Aurora, Ohio, and Nacalai Tesque Inc., Kyoto, Japan, respectively. Pronase was obtained from Calbiochem-Novobiochem Co., La Jolla, Calif. All other reagents were from commercial sources.
Bactericidal assays.
We used 0.5 M phosphate buffer (pH 7.6) containing 0.15 M NaCl (0.5 M phosphate-buffered saline [PBS]) for the bactericidal assays of ONOO− and other effector substances to minimize changes in pH during the reaction because of infusion of the alkaline solution of ONOO− (in 10 mM NaOH) and production of NH3 and HCO3−, which might have affected the pH of the reaction mixture. Suspensions of H. pylori cultured for 36 to 48 h and of C. jejuni cultured for 24 h were washed with and resuspended in 0.5 M PBS immediately before use. Bacterial suspensions were kept on ice until use. Urease activity and bacterial motility and morphology were checked before each use. The constant-flux infusion method (7, 40) was used to treat the bacteria with steady concentrations of ONOO−. In the constant-flux infusion process, the effective and constant concentration of ONOO− is maintained by balancing infusion and decomposition of ONOO− in the system. The concentrations of ONOO− maintained constant were estimated by the DHR oxidation assay, as described earlier (8). Specifically, DHR (28 μM) was added to the reaction mixture of ONOO− without bacteria; simultaneously, the ONOO− infusion was stopped, and the amount of the oxidized product rhodamine was measured fluorometrically. The concentration of ONOO− was then estimated by using a standard curve of the amount of rhodamine generated as a function of ONOO−, which was prepared separately by reaction of DHR with a bolus of ONOO− injected into 0.5 M PBS. As a result, by infusion 10, 100, and 1,000 μM ONOO− in 10 mM NaOH into 0.5 M PBS (1.2 ml) at a flow rate of 240 μl/min, the concentrations of ONOO− remained constant at 0.3, 1, and 3 μM, respectively. H. pylori or C. jejuni (108 CFU/ml each) samples were treated constantly with 1 μM ONOO− by infusing 100 μM ONOO− in the absence or presence of urea, NaHCO3, or NH4OH as described above. Aliquots (120 μl) were removed from the reaction mixture at 30-s intervals and were immediately diluted with nutrient broth (Eiken Chemical Co. Ltd., Tokyo, Japan) and seeded on brucella agar plates containing 5% lysed horse blood (Nippon Bio-Test Laboratories Inc., Tokyo, Japan) for the colony-forming assay. After cultivation for 5 days (H. pylori) or 2 days (C. jejuni) under microaerobic conditions, the number of colonies formed was determined.
To examine the bactericidal effect of NO on H. pylori, P-NONOate at 1, 10, and 100 μM was added to the suspension of H. pylori ATCC 43504 (108 CFU/ml) in 0.5 M PBS. After incubation for 3, 5, and 10 min, aliquots of the bacterial suspension were diluted with nutrient broth and seeded on brucella agar plates containing 5% lysed horse blood. Viable H. pylori organisms were quantified by the colony-forming assay, as just described.
Measurement of nitrotyrosine.
Suspensions of H. pylori (5 × 108 CFU/ml) treated continuously with 1 μM ONOO− for 3 min in the presence or absence of 20 mM NaHCO3 or 10 mM urea, as described above, were centrifuged at 1,600 × g and then resuspended in 10 mM potassium phosphate buffer (pH 7.4) containing 0.15 M NaCl (250 μl). Aliquots (100 μl) were treated with 60 μg of pronase/ml for 18 h at 50°C. The bacterial suspension became clear and no precipitate was seen in the resultant reaction mixture even after centrifugation at 10,000 × g, indicating that the bacterial cells were completely digested by the pronase treatment. After filtration through a centrifugal filter unit (Ultrafree-MC with a 10,000 nominal molecular weight limit; Millipore Corp., Bedford, Mass.), the filtrate was processed by high-performance liquid chromatography (HPLC) coupled to electrochemical detection with 12 CoulArray electrode cells (ESA, Inc., Chelmsford, Mass.) (9, 19). Nitrotyrosine recovered from the bacterial cells was separated on a reverse-phase column (4.6 by 250 mm) (TSKgel ODS-80Ts; Tosoh Co., Tokyo, Japan) and eluted with 50 mM sodium acetate buffer (pH 4.7) containing 5% methanol at a flow rate of 0.8 ml/min. The CoulArray electrode array detector was operated with applied potentials at 200, 250, 300, 350, 400, 450, 500, 550, 600, 650, 700, and 750 mV. Peaks of nitrotyrosine and l-tyrosine were identified and quantified based on comigration with known concentrations of authentic standards and their electrochemical activation profiles. Identification of nitrotyrosine was confirmed by the disappearance of the peak after reduction of nitrotyrosine to aminotyrosine by 20 mM sodium dithionite. The amounts of nitrotyrosine and tyrosine were quantified from the peak areas obtained at 750 and 600 mV, respectively.
Statistical analysis.
Statistical analyses were done with the two-tailed t test for unpaired data.
RESULTS
Bactericidal effect of ONOO− on H. pylori.
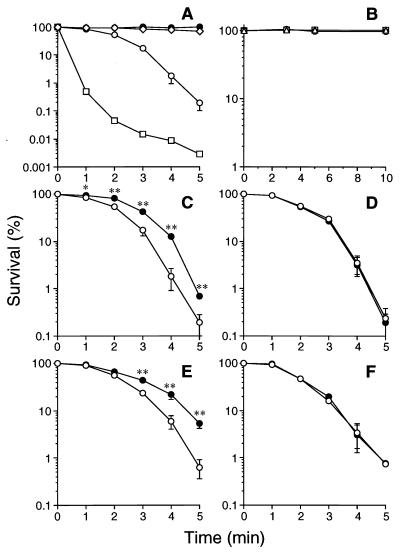
It is now known that ONOO− is a key intermediate in the NO-dependent bactericidal effect. Induction of iNOS and formation of nitrotyrosine, an indicator of ONOO− formation, in H. pylori-infected stomach have also been documented (16–18, 30, 42, 47). Therefore, we examined the bactericidal activity of authentic ONOO− (5) by using the constant-flux infusion method (7, 40). The number of viable bacteria expressed as CFU declined after exposure to ONOO− in a dose- and time-dependent manner (Fig. 1A). Products of ONOO− decomposition, mainly nitrate anion (28), showed no bactericidal activity against H. pylori (Fig. 1A).
FIG. 1.
(A) Bactericidal effect of ONOO− on H. pylori ATCC 43504. Decomposed ONOO− (equivalent to 1 mM ONOO−) (●) or ONOO− at 10 μM (◊), 100 μM (○), or 1 mM (□) in 10 mM NaOH was infused into 1.2 ml of bacterial suspension (108 CFU/ml) at a flow rate of 240 μl/min. Every 30 s, 120-μl aliquots were removed from the reaction mixture, and the number of viable bacteria was determined by the colony-forming assay. Concentrations of ONOO− in the reaction mixture during infusion of 10 μM, 100 μM, and 1 mM ONOO− were assumed to be maintained at constant 0.3, 1, and 3 μM concentrations, respectively. Data are means ± SD from three independent experiments. (B) Survival of H. pylori after exposure to the NO-donor P-NONOate. H. pylori ATCC 43504 organisms (108 CFU/ml) were incubated for the indicated periods with 1 (○), 10 (□), or 100 (▵) μM P-NONOate, followed by colony-forming assay. (C and D) Effects of urea on bactericidal action of ONOO− on H. pylori ATCC 43504 (C) and C. jejuni (D). (E and F) Effects of urea on the bactericidal action of ONOO− on H. pylori HPK5 (E) and its isogenic ureB mutant, HPT209 (F). Bacteria (108 CFU/ml) were exposed to a constant concentration of ONOO− (1 μM) in the presence (●) or absence (○) of 10 mM urea for the indicated periods, and the number of viable bacteria at each time point was determined. Data from three independent experiments are expressed as means ± SD. ∗ and ∗∗, P < 0.05 and P < 0.01 versus control without urea, respectively.
The NO-liberating agent P-NONOate was examined by incubation with H. pylori ATCC 43504 (108 CFU/ml) in 0.5 M PBS (pH 7.6) for up to 10 min. P-NONOate at 1, 10, or 100 μM did not affect the viability of the bacteria (Fig. 1B). It is known that one molecule of P-NONOate releases two molecules of NO with a half-life of 7.6 min at a neutral pH (25, 40). Hence, an appreciable concentration of NO (up to 200 μM) did not kill this bacterium, nor was a sufficient amount of bactericidal metabolites of NO, such as ONOO−, formed during the reaction period. In any event, these results indicate that NO per se exhibits very little bactericidal action, which is a great contrast to ONOO−.
Effect of urea on bactericidal action of ONOO− on H. pylori and C. jejuni.
As shown in Fig. 1C, in the presence of a physiological concentration (10 mM) of urea, survival of H. pylori was significantly increased. Because urea did not affect the decomposition rate of ONOO− at the pH range 7.0 to 10.0, as assessed by measuring absorbance at 302 nm (data not shown), direct detoxification of ONOO− by urea itself was not plausible. When clinically isolated C. jejuni, another microaerophilic bacteria lacking urease activity, was treated with ONOO− in the same experimental settings in the presence or absence of urea, the susceptibility of C. jejuni to ONOO− was not affected by the addition of urea (Fig. 1D), suggesting that the contribution of urease produced by H. pylori to the suppression of the cytotoxicity of ONOO− was required. To further verify this notion, the bactericidal action of ONOO− against H. pylori HPK5 and its isogenic mutant HPT209, lacking urease, was examined with or without the addition of 10 mM urea. The strains showed similar sensitivities to ONOO− in the absence of urea (Fig. 1E and F). In contrast, urea attenuated the bactericidal effect of ONOO− on the wild-type strain, HPK5 (Fig. 1E), but it did not affect the bacterial killing of ONOO− for the mutant with the urease gene disruption, HPT209 (Fig. 1F). This result indicates again that urease activity is required for urea-dependent attenuation of the ONOO− cytotoxicity.
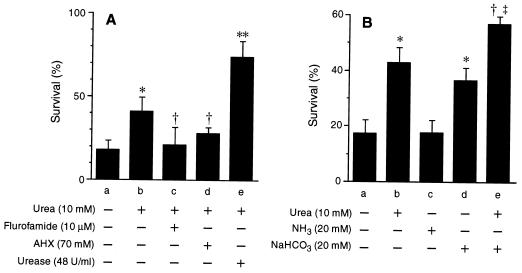
The role of urease was further investigated with two urease inhibitors. It has been reported that flurofamide is a specific inhibitor of extracellular urease and that AHX is effective on both intracellular and extracellular urease (35). In the presence of 10 μM flurofamide or 70 mM AHX, the protective effect of urea was almost completely nullified (Fig. 2A), while in the absence of urea, these urease inhibitors did not affect the bactericidal action of ONOO− on H. pylori (data not shown). Flurofamide seemed to be more effective than AHX, so extracellular urease localized on the surface of bacterial cells plays an important role in suppressing the bactericidal action of ONOO−. In contrast to urease inhibitors, the addition of urease derived from B. pasteurii augmented the protective effect of urea (Fig. 2A). These data indicate that the bactericidal effect of ONOO− against H. pylori is diminished by bacterial urease activity.
FIG. 2.
(A) Effects of urease inhibitors and additional urease on the bactericidal action of ONOO− on H. pylori. H. pylori ATCC 43504 organisms (108 CFU/ml) were exposed to a constant concentration of ONOO− (1 μM) for 3 min in the absence (a) or presence (b to e) of 10 mM urea. Reactions were performed in the presence of 10 μM flurofamide (c), 70 mM AHX (d), or 48 U of B. pasteurii urease per ml (e). (B) Effects of NaHCO3 and NH3 on bactericidal action of ONOO− on H. pylori. H. pylori ATCC 43504 organisms (108 CFU/ml) were exposed to a constant concentration of ONOO− (1 μM) for 3 min in the presence of 10 mM urea (b), 20 mM NH3 (c), 20 mM NaHCO3 (d), or 10 mM urea plus 20 mM NaHCO3 (e) or in the absence of all compounds (a), and the colony-forming assay was performed. Data from three independent experiments are expressed as means ± SD. ∗, P < 0.01 versus a; †, P < 0.05 versus b; ∗∗, P < 0.005 versus b; and ‡, P < 0.005 versus d.
Effects of NaHCO3 and NH3 on bactericidal action of ONOO− on H. pylori.
We examined the effects of the products of the urea-urease reaction, CO2 and NH3, on the bactericidal activity of ONOO−. NaHCO3 (20 mM) suppressed bacterial killing by ONOO− to the same degree as 10 mM urea, whereas NH4OH (20 mM) did not (Fig. 2B). Furthermore, urea (10 mM) plus NaHCO3 (20 mM) showed an additive protective effect for the survival of H. pylori exposed to ONOO− (Fig. 2B), suggesting that urease increases bacterial survival in in vivo situations in which physiological concentrations of HCO3− and urea are close to those used in this experiment, i.e., about 20 and 10 mM, respectively (38).
A change in the pH of the media might affect the chemical reactivity of ONOO− (26, 27, 56). In our experimental settings, however, NH3 released after urea hydrolysis by H. pylori urease did not alter the pH of the reaction mixture. The pH values of the suspension of 108 CFU of H. pylori ATCC 43504 per ml in 0.5 M PBS after 0, 1, 2, 3, 4, and 5 min of infusion of 100 μM ONOO− at a flow rate of 240 μl/min in the absence of urea were 7.57 ± 0.01, 7.60 ± 0.01, 7.63 ± 0.01, 7.66 ± 0.02, 7.68 ± 0.02, and 7.69 ± 0.01, respectively, and those obtained in the presence of 10 mM urea were 7.57 ± 0.01, 7.60 ± 0.01, 7.63 ± 0.02, 7.65 ± 0.02, 7.67 ± 0.02, and 7.69 ± 0.01 (means ± standard deviations [SD] of three independent experiments). In addition, as shown in Fig. 1A, infusion of an alkaline solution alone (decomposed ONOO− in 10 mM NaOH) did not affect the viability of H. pylori. Also, NH3 per se had no appreciable effect on the bactericidal action of ONOO− (Fig. 2B). We therefore deduced that the protective effect of urease against ONOO− is dependent on its CO2 production but is not dependent on NH3 release or the change in pH.
Nitrotyrosine formation in H. pylori after treatment with ONOO−.
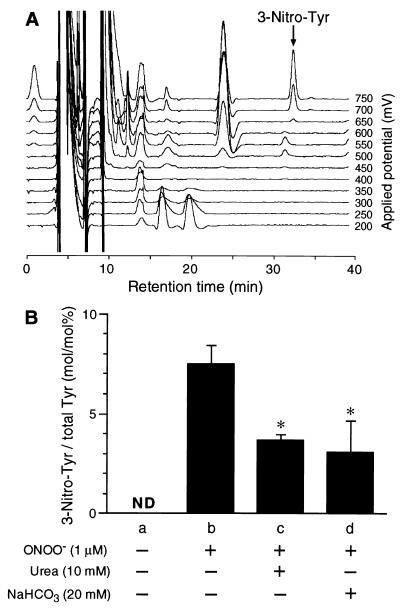
ONOO− is known to nitrate aromatic compounds, including tyrosine (4, 23). To assess the effect of urease activity on the chemical reactivity of ONOO− with the bacterial components, we quantified nitrotyrosine in H. pylori cells by using HPLC coupled to electrochemical detection with 12 electrodes (Fig. 3A). The amount of nitrotyrosine in the bacterial cells exposed to 1 μM ONOO− for 3 min was 267 ± 22 pmol/108 CFU, or 7.48% ± 1.2% of the total tyrosine (Fig. 3B). Nitrotyrosine was not detected (less than 0.1 pmol/108 CFU) in the control bacterial cells (no exposure to ONOO−). In contrast, we could not detect any appreciable amount of nitrotyrosine in the bacterial cells treated with P-NONOate (data not shown), indicating that ONOO−, but not NO, exhibits a strong tyrosine-nitrating potential in H. pylori. The addition of 10 mM urea or 20 mM NaHCO3 to the reaction mixture of ONOO− lowered the formation of nitrotyrosine by 50% (Fig. 3B). Since CO2 accelerates decomposition of ONOO− (27, 50), it is plausible that CO2/HCO3− added or formed by bacterial urease might increase the decomposition rate of ONOO− and thus suppress the reactivity of ONOO− with the bacteria.
FIG. 3.
Nitrotyrosine formation in H. pylori cells after treatment with ONOO−. 3-Nitro-l-tyrosine (3-Nitro-Tyr) was quantified by HPLC coupled to electrode detectors, with the pronase digests of non-ONOO−-treated H. pylori ATCC 43504 cells (a) or those exposed to 1 μM ONOO− (b to d) for 3 min in the absence (b) or presence of 10 mM urea (c) and 20 mM NaHCO3 (d). (A) Elution profile of the pronase digest of H. pylori exposed to 1 μM ONOO− in the absence of urea or NaHCO3. (B) The amounts of 3-Nitro-Tyr formed in H. pylori were expressed as the ratio of 3-Nitro-Tyr to total l-tyrosine (Tyr) recovered from bacterial cells. ND, 3-Nitro-Tyr was not detected (<0.1 pmol/108 CFU). ∗, P < 0.05 versus b. Data are means ± SD of three independent experiments.
DISCUSSION
H. pylori produces a large quantity of urease, which amounts to 5% of the total protein of the bacterium (14). Urease genes in the H. pylori genome are composed of two gene clusters: ureAB genes and ureIEFGH genes (10). Colonization of H. pylori mutants whose ureA, ureB, ureG, or ureI gene was disrupted in experimental animals was known to be suppressed (13, 44, 48, 53). In addition, proton pump inhibitors used for treatment of H. pylori infection inhibit bacterial urease in an irreversible fashion (33). All these studies imply that H. pylori urease is essential for H. pylori colonization in the stomach.
Several studies were carried out to elucidate the roles of H. pylori urease in bacterial colonization in the stomach. Neutralization of gastric acid with NH3 produced by the enzyme might allow the bacterium to survive in the acidic milieu (31). It is reported that the motility of H. pylori, which is known to be an important characteristic of the bacterium in the colonization of experimental animals, is enhanced by the urea-urease reaction, particularly in a viscous environment (35). Inhibition of neutrophil function by NH3 was also proposed as a pathogenic mechanism of this enzyme (32).
In addition to these possible roles of urease, the results obtained in this study clearly demonstrate that H. pylori urease functions as a part of the defense system of the bacteria themselves against ONOO− (Fig. 1 and 2).
In previous work, elevated generation of ONOO− in vivo and its involvement in antimicrobial host defense were reported for a murine salmonellosis model. Results indicated that suppressing ONOO− generation by inhibiting either NO or O2− production or by scavenging these radicals accelerated the growth of S. enterica serovar Typhimurium in the liver and further augmented its pathogenicity, as evidenced by the increased mortality of infected mice (49). It was thus suggested that ONOO− effectively clears bacteria from sites of infection in vivo (1, 49). In recent years, increased expression of iNOS mRNA and its product has been confirmed in H. pylori-infected gastric tissues of patients and experimental animals (16–18, 30, 42, 47). Formation of ONOO− and/or other reactive nitrogen species produced by the NO2−-H2O2-myeloperoxidase system at sites of infection by H. pylori is also suggested by the immunohistochemical detection of nitrotyrosine (17, 30, 51). Furthermore, it has recently been reported that not only phagocytic inflammatory cells but also H. pylori itself produce O2− (34), which indicates that ONOO− may be formed in and around the bacteria in vivo, where production of NO and O2− is simultaneously elevated as described above. Consequently, ONOO− may function as a major bactericidal effector for H. pylori in the stomach. In a separate experiment, however, no significant difference was found between the number of H. pylori organisms colonizing iNOS-knockout mice and that in wild-type mice (unpublished observation). In this context, it is quite reasonable that H. pylori has evolved with the system, such as urease, that is capable of detoxifying ONOO−, and hence steady and sustained colonization in the infected stomach is facilitated.
A high concentration of ONOO− was used in the present study so that we could obtain reproducible results and clearly demonstrate the bactericidal action of ONOO−. The bacteria were directly exposed to a 1 μM effective concentration of ONOO− in vitro, which is considered to be an extremely severe condition for the bacteria compared with the in vivo setting in infected foci containing various endogenous substances that affect the reactivity of ONOO− (2). Even under such conditions, the physiological concentration of urea increased the survival fractions of two strains of H. pylori (ATCC 43504 and HPK5) 3.7- to 8.4-fold after exposure to ONOO− for 5 min (Fig. 1C and E). Therefore, it is conceivable that the urease could function efficiently as a protective factor of H. pylori against ONOO− produced in vivo.
Although it is reported that ONOO−-dependent nitration of aromatic compounds, including tyrosine, is enhanced in the presence of CO2 (26, 54), formation of nitrotyrosine in H. pylori was suppressed by the addition of urea or NaHCO3 (Fig. 3B). Recently, Romero et al. reported that CO2 shortened the half-life and the diffusion distance of ONOO− and hence inhibited the oxidation of oxyhemoglobin in red blood cells by ONOO− (43). Therefore, the results obtained in this study suggest that CO2 formed by bacterial urease inhibits the reactivity of ONOO− with the bacterial components and accelerates its decomposition outside the bacterial cells. It is of great importance, then, that H. pylori urease is localized not only in the cytoplasm but also on the surface of the bacteria (41). In our experimental settings, surface-bound urease seemed to play an important role in the decomposition of ONOO− (Fig. 2A).
In conclusion, urease of H. pylori plays a role in the defense against the toxicity of ONOO− via production of CO2, and it may confer the capacity for sustained infection in vivo. Improved understanding of the pathogenic role of urease, in view of a host-pathogen interaction, will help in the exploration of effective therapeutic treatments for H. pylori infection and its related gastric diseases, including gastric cancer.
ACKNOWLEDGMENTS
We thank Judith B. Gandy for editing and Rie Yoshimoto for typing the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research from Monbusho (Ministry of Education, Science and Culture) of Japan.
REFERENCES
- 1.Akaike T, Suga M, Maeda H. Free radicals in viral pathogenesis: molecular mechanisms involving superoxide and NO. Proc Soc Exp Biol Med. 1998;217:64–73. doi: 10.3181/00379727-217-44206. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez B, Ferrer-Sueta G, Freeman B A, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 3.Asaka M, Takeda H, Sugiyama T, Kato M. What role does Helicobacter pylori play in gastric cancer? Gastroenterology. 1997;113(6 Suppl.):S56–S60. doi: 10.1016/s0016-5085(97)80013-3. [DOI] [PubMed] [Google Scholar]
- 4.Beckman J S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 5.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 7.Castro L, Alvarez M N, Radi R. Modulatory role of nitric oxide on superoxide-dependent luminol chemiluminescence. Arch Biochem Biophys. 1996;333:179–188. doi: 10.1006/abbi.1996.0379. [DOI] [PubMed] [Google Scholar]
- 8.Crow J P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 9.Crow J P. Measurement and significance of free and protein-bound 3-nitrotyrosine, 3-chlorotyrosine, and free 3-nitro-4-hydroxyphenylacetic acid in biologic samples: a high-performance liquid chromatography method using electrochemical detection. Methods Enzymol. 1999;301:151–160. doi: 10.1016/s0076-6879(99)01078-2. [DOI] [PubMed] [Google Scholar]
- 10.Cussac V, Ferrero R L, Labigne A. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J Bacteriol. 1992;174:2466–2473. doi: 10.1128/jb.174.8.2466-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon M F. Pathophysiology of Helicobacter pylori infection. Scand J Gastroenterol. 1994;201(Suppl.):7–10. [PubMed] [Google Scholar]
- 12.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–1989. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans D J, Jr, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 15.Fang F C. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S, Ramanujam K S, Wong A, Fantry G T, Drachenberg C B, James S P, Meltzer S J, Wilson K T. Increased expression and cellular localization of inducible nitric oxide synthase and cycloxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 17.Goto T, Haruma K, Kitadai Y, Ito M, Yoshihara M, Sumii K, Hayakawa N, Kajiyama G. Enhanced expression of inducible nitric oxide synthase and nitrotyrosine in gastric mucosa of gastric cancer patients. Clin Cancer Res. 1999;5:1411–1415. [PubMed] [Google Scholar]
- 18.Hahm K-B, Lee K-J, Choi S-Y, Kim J-H, Cho S-W, Yim H, Park S-J, Chung M-H. Possibility of chemoprevention by the eradication of Helicobacter pylori: oxidative DNA damage and apoptosis in H. pylori infection. Am J Gastroenterol. 1997;92:1853–1857. [PubMed] [Google Scholar]
- 19.Hensley K, Maidt M L, Pye Q N, Stewart C A, Wack M, Tabatabaie T, Floyd R A. Quantitation of protein-bound 3-nitrotyrosine and 3,4-dihydroxyphenylalanine by high-performance liquid chromatography with electrochemical array detection. Anal Biochem. 1997;251:187–195. doi: 10.1006/abio.1997.2281. [DOI] [PubMed] [Google Scholar]
- 20.Hibbs J B, Taintor R R, Varin Z, Rachlin E M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 21.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 22.Hurst J K, Lymar S V. Toxicity of peroxynitrite and related reactive nitrogen species toward Escherichia coli. Chem Res Toxicol. 1997;10:802–810. doi: 10.1021/tx970008v. [DOI] [PubMed] [Google Scholar]
- 23.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan S S, Lancaster J R, Jr, Basford R E, Simmons R L. Effect of nitric oxide on staphylococcal killing and interactive effect with superoxide. Infect Immun. 1996;64:69–76. doi: 10.1128/iai.64.1.69-76.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keefer L K, Nims R W, Davies K M, Wink D A. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 26.Lemercier J-N, Padmaja S, Cueto R, Squadrito G L, Uppu R M, Pryor W A. Carbon dioxide modulation of hydroxylation and nitration of phenol by peroxynitrite. Arch Biochem Biophys. 1997;345:160–170. doi: 10.1006/abbi.1997.0240. [DOI] [PubMed] [Google Scholar]
- 27.Lymar S V, Hurst J K. Rapid reaction between peroxynitrite ion and carbon dioxide: implications for biological activity. J Am Chem Soc. 1995;117:8867–8868. [Google Scholar]
- 28.Lymar S V, Hurst J K. CO2-catalyzed one-electron oxidations by peroxynitrite: properties of the reactive intermediate. Inorg Chem. 1998;37:294–301. [Google Scholar]
- 29.Makristathis A, Rotika E, Labigne A, Willinger B, Rotter M L, Hirschl A M. Highly significant role of Helicobacter pylori urease in phagocytosis and production of oxygen metabolites by human granulocytes. J Infect Dis. 1998;177:803–806. doi: 10.1086/517814. [DOI] [PubMed] [Google Scholar]
- 30.Mannick E E, Bravo L E, Zarama G, Realpe J L, Zhang X-J, Ruiz B, Fontham E T H, Mera R, Miller M J S, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 31.Marshall B J, Barret L J, Prakash C, McCallum R W, Guerrant R L. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology. 1990;99:697–702. doi: 10.1016/0016-5085(90)90957-3. [DOI] [PubMed] [Google Scholar]
- 32.Mayo K, Held M, Wadstrom T, Megraud F. Helicobacter pylori-human polymorphonuclear leukocyte interaction in the presence of ammonia. Eur J Gastroenterol Hepatol. 1997;9:457–461. doi: 10.1097/00042737-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Nagata K, Satoh H, Iwahi T, Shimoyama T, Tamura T. Potent inhibitory action of the gastric proton pump inhibitor lansoprazole against urease activity of Helicobacter pylori: unique action selective for H. pylori cells. Antimicrob Agents Chemother. 1993;37:769–774. doi: 10.1128/aac.37.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata K, Yu H, Nishikawa M, Kashiba M, Nakamura A, Sato E F, Tamura T, Inoue M. Helicobacter pylori generates superoxide radicals and modulates nitric oxide metabolism. J Biol Chem. 1998;273:14071–14073. doi: 10.1074/jbc.273.23.14071. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura H, Yoshiyama H, Takeuchi H, Mizote T, Okita K, Nakazawa T. Urease plays an important role in the chemotactic motility of Helicobacter pylori in a viscous environment. Infect Immun. 1998;66:4832–4837. doi: 10.1128/iai.66.10.4832-4837.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Investig. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan C F, Hibbs J B. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 38.Neithercut W D, el Nujumi A M, McColl K E. Measurement of urea and ammonium concentrations in gastric juice. J Clin Pathol. 1993;46:462–464. doi: 10.1136/jcp.46.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen H, Andersen L P. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology. 1992;103:1747–1753. doi: 10.1016/0016-5085(92)91430-c. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 41.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pignatelli B, Bancel B, Estève J, Malaveille C, Calmels S, Correa P, Patricot L M, Laval M, Lyandrat N, Ohshima H. Inducible nitric oxide synthase, anti-oxidant enzymes and Helicobacter pylori infection in gastritis and gastric precancerous lesions in humans. Eur J Cancer Prev. 1998;7:439–447. doi: 10.1097/00008469-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Romero N, Denicola A, Souza J M, Radi R. Diffusion of peroxynitrite in the presence of carbon dioxide. Arch Biochem Biophys. 1999;368:23–30. doi: 10.1006/abbi.1999.1272. [DOI] [PubMed] [Google Scholar]
- 44.Skouloubris S, Thiberge J-M, Labigne A, De Reuse H. The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infect Immun. 1998;66:4517–4521. doi: 10.1128/iai.66.9.4517-4521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–2069. [PubMed] [Google Scholar]
- 46.Suzuki M, Miura S, Suematsu M, Fukumura D, Kurose I, Suzuki H, Kai A, Kudoh Y, Ohashi M, Tsuchiya M. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol. 1992;263:G719–G725. doi: 10.1152/ajpgi.1992.263.5.G719. [DOI] [PubMed] [Google Scholar]
- 47.Tatemichi M, Ogura T, Nagata H, Esumi H. Enhanced expression of inducible nitric oxide synthase in chronic gastritis with intestinal metaplasia. J Clin Gastroenterol. 1998;27:240–245. doi: 10.1097/00004836-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Tsuda M, Karita M, Mizote T, Morshed M G, Okita K, Nakazawa T. Essential role of Helicobacter pylori urease in gastric colonization: definite proof using a urease-negative mutant constructed by gene replacement. Eur J Gastroenterol Hepatol. 1994;6(Suppl. 1):S49–S52. [PubMed] [Google Scholar]
- 49.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uppu R M, Squadrito G L, Pryor W A. Acceleration of peroxynitrite oxidations by carbon dioxide. Arch Biochem Biophys. 1996;327:335–343. doi: 10.1006/abbi.1996.0131. [DOI] [PubMed] [Google Scholar]
- 51.van der Vliet A, Eiserich J P, Shigenaga M K, Cross C E. Reactive nitrogen species and tyrosine nitration in the respiratory tract. Epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med. 1999;160:1–9. doi: 10.1164/ajrccm.160.1.9807044. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 53.Wirth H P, Beins M H, Yang M, Tham K T, Blaser M J. Experimental infection of Mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect Immun. 1998;66:4856–4866. doi: 10.1128/iai.66.10.4856-4866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yermilov Y, Yoshie Y, Rubio J, Ohshima H. Effects of carbon dioxide/bicarbonate on induction of DNA single-strand breaks and formation of 8-nitroguanine, 8-oxoguanine and base-propenal mediated by peroxynitrite. FEBS Lett. 1996;399:67–70. doi: 10.1016/s0014-5793(96)01288-4. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida K, Akaike T, Doi T, Sato K, Ijiri S, Suga M, Ando M, Maeda H. Pronounced enhancement of ·NO-dependent antimicrobial action by an ·NO-oxidizing agent, imidazolineoxyl N-oxide. Infect Immun. 1993;61:3552–3555. doi: 10.1128/iai.61.8.3552-3555.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Squadrito G L, Pryor W A. The mechanism of the peroxynitrite-carbon dioxide reaction probed using tyrosine. Nitric Oxide. 1997;1:301–307. doi: 10.1006/niox.1997.0130. [DOI] [PubMed] [Google Scholar]
- 57.Zhu L, Gunn C, Beckman J S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]