Abstract
Background
Stomatitis is one of the main reasons to discontinue everolimus in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2–) metastatic breast cancer (mBC). To decrease stomatitis and subsequently early treatment discontinuations or dose reductions, the DESIREE trial investigated the use of a stepwise dose-escalation schedule of everolimus (EVE esc).
Patients and methods
DESIREE is a phase II, multicentre, randomised, double-blind, placebo-controlled trial in patients with HR+/HER2– mBC and progression/relapse after nonsteroidal aromatase inhibitor treatment. Patients were randomised to EVE esc (2.5 mg/day, week 1; 5 mg/day, week 2; 7.5 mg/day, week 3; 10 mg/day, weeks 4-24) or everolimus 10 mg/day (EVE 10mg) for 24 weeks plus exemestane. The primary endpoint was the incidence of stomatitis episodes grade ≥2 within 12 weeks of treatment. The secondary endpoints included toxicity, relative total dose intensity (RTDI) and quality of life (QoL).
Results
A total of 160 patients were randomised and 156 started treatment (EVE esc: 80; EVE 10mg: 76). The median age of patients was 64 years (range 33-85), 56.3% patients in the EVE esc arm versus 42.1% in the EVE 10mg arm had liver metastasis (P = 0.081) and 62.5% versus 51.3% received over one metastatic therapy line (P = 0.196). Within 12 weeks, the incidence of stomatitis episodes grade ≥2 was significantly lower in the EVE esc arm compared with the EVE 10mg arm (28.8% versus 46.1%; odds ratio 0.47, 95% confidence interval 0.24-0.92; P = 0.026). Toxicity was in line with the known safety profile without new safety concerns. The median RTDI was 91.1% in the EVE esc arm versus 80.0% in the EVE 10mg arm (P = 0.329). Discontinuation rate in the first 3 weeks was 6.3% versus 15.8%, respectively (P = 0.073). QoL was comparable between the two treatment arms.
Conclusions
A dose-escalation schema of everolimus over 3 weeks can be successfully used to reduce the incidence of high-grade stomatitis in the first 12 weeks of treatment in patients with HR+/HER2– mBC.
Trial registration
ClinicalTrials.govNCT02387099; https://clinicaltrials.gov/ct2/show/NCT02387099
Key words: everolimus, exemestane, metastatic breast cancer, stomatitis
Highlights
-
•
Stomatitis is a common side-effect of everolimus which is used to treat HR+ mBC.
-
•
To decrease stomatitis, DESIREE investigated a stepwise dose-escalation versus standard schedule of everolimus.
-
•
Everolimus dose escalation over 3 weeks reduced the incidence of high-grade stomatitis.
-
•
The everolimus dose-escalation schedule can be used as an alternative when everolimus treatment is initiated.
Introduction
Significant progress has been made in recent years regarding the outcome of patients with metastatic breast cancer (mBC). However, mBC remains an incurable disease,1 and therefore the balance between potential severe side-effects, control of the disease and symptoms plays an important role. A consensus in the national and international guidelines is that an endocrine-based therapy should be primarily used in patients with metastatic hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative mBC, unless the patient’s clinical symptoms or preferences suggest other treatments.1, 2, 3
However, it is clinical reality that an endocrine resistance develops in the majority of patients with advanced BC.4 In HR-positive/HER2-negative advanced BC, the phosphatidylinositol 3-kinase (PI3K) pathway plays an essential role.5 The mammalian target of rapamycin (mTOR) protein kinase complex is a key downstream effector of the PI3K/protein kinase B (AKT)/mTOR pathway which is an important mechanism of endocrine resistance.4 Indeed, the mTOR inhibitor everolimus, in combination with the steroidal aromatase inhibitor (AI) exemestane, is an approved and often used therapeutic option after treatment failure with a nonsteroidal AI (NSAI). In the BOLERO-2 study, after a median follow-up of 18 months, progression-free survival (PFS) was increased to 11 months with everolimus plus exemestane from 4.1 months with placebo plus exemestane [central review, HR 0.38, 95% confidence interval (CI) 0.31-0.48; log-rank P = 0.0001].6
The most common grade 3 or 4 adverse event (AE) in the BOLERO-2 trial was stomatitis (8% everolimus plus exemestane versus 1% placebo plus exemestane).7 A similar rate of high-grade stomatitis was reported in other studies investigating everolimus plus exemestane in patients with HR-positive/HER2-negative advanced BC.8, 9, 10 Real-world safety and efficacy data on everolimus in combination with exemestane were also consistent with the results from BOLERO-2.11,12
Importantly, everolimus-related side-effects such as stomatitis often led to dose reductions or treatment discontinuations. The rate of everolimus-related stomatitis in patients with solid tumours was 67% in a meta-analysis.13 Most stomatitis events were grade 1/2, with grade 3/4 events reported in only 9% of the patients. Nevertheless, even grade 2 stomatitis is distressing for patients and might have a negative impact on quality of life (QoL) and treatment adherence. However, in the BOLERO-2 study a time to definitive deterioration analysis showed that median time to definitive deterioration was longer in the everolimus plus exemestane group compared with the placebo plus exemestane group.14
Therefore, strategies to prevent everolimus-induced stomatitis have been investigated. The single-arm phase II SWISH study examined the prevention of everolimus-related stomatitis with dexamethasone mouthwash in patients with advanced HR-positive/HER2-negative BC.15 This strategy reduced the incidence of grade 2 or worse stomatitis to 2%.
In the phase II randomised DESIREE study, we looked for another approach to reduce stomatitis in patients with advanced BC treated with everolimus plus exemestane. Postmenopausal patients with HR-positive/HER2-negative advanced BC were randomised to either start with the approved 10 mg/day dosage of everolimus (EVE 10mg) plus exemestane or a dose-escalation schema of everolimus (EVE esc) plus exemestane.
Methods
Patients
DESIREE (NCT02387099) is a phase II, multicentre, randomised, double-blind and placebo-controlled trial in postmenopausal patients with HR-positive/HER2-negative mBC and progression or relapse after NSAI treatment. The aim of the DESIREE study was to compare the incidence of stomatitis episodes grade ≥2 within 12 weeks after treatment start using a conventional and a dose-escalation schema of everolimus in combination with exemestane.
The main eligibility criteria were postmenopausal women with locally advanced or mBC not amenable to curative treatment by surgery or radiotherapy alone and without indication for chemotherapy (e.g. symptomatic visceral metastasis); histologically confirmed HR-positive status (estrogen receptor and/or progesterone receptor) defined as >1% stained cells16 and HER2-negative status defined as either immunohistochemistry 0-1 or 2+ with in situ hybridisation ratio <2.017; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0-2; recurrence or disease progression during or after NSAI treatment; adequate hematologic and organ function, glycaemia and blood lipids. Exclusion criteria were a life expectancy of <3 months and not adequately controlled brain metastases. The study protocol was approved by an independent ethics committee, institutional review boards and the competent authority. All patients provided written informed consent for trial participation and biomaterial collection.
Study design and treatment
Patients were randomised in a 1:1 ratio to receive either a dose escalation of everolimus 2.5 mg/day at week 1, 5 mg/day at week 2, 7.5 mg/day at week 3 and 10 mg/day at weeks 4-24 or everolimus 10 mg/day for 24 weeks in combination with exemestane 25 mg daily. In both arms, study medication was given until 24 weeks of treatment completeness, disease progression, unacceptable toxicity of the study drug or withdrawal of consent of the patient (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100601). According to the study protocol, poststudy treatment was at the investigator’s discretion and should be documented until first subsequent chemotherapy or end of study. Follow-up was not part of the study.
Outcomes and study assessments
The primary endpoint was the rate of stomatitis episodes grade ≥2 within 12 weeks of treatment. Patients in whom the occurrence of stomatitis cannot be completely assessed during the first 12 weeks because of premature treatment discontinuation due to AE, patient’s decision or investigator’s decision was considered as having an episode of stomatitis (stomatitis grade <2 and premature treatment discontinuation). The assessment of stomatitis grade was performed using the World Health Organisation (WHO)’s Oral Toxicity Scale.18
The secondary endpoints were incidence of stomatitis episodes grade ≥2 within 24 weeks of treatment start, cumulative rate of stomatitis episodes of any grade within 12 weeks and 24 weeks of treatment start, clinical benefit rate (CBR), relative total dose intensity (RTDI), time to onset of stomatitis episodes grade ≥2, safety and QoL.
Safety evaluations were based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
RTDI was defined as a total dose intensity within the entire treatment achieved by a patient relative to intended dose intensity based on the planned schedule of the treatment and was expressed as a percentage.
Time to onset of grade ≥2 stomatitis was defined as the time from randomisation to first episode of grade ≥2 stomatitis.
CBR was defined as complete response, partial response or stable disease without any sign of tumour progression according to RECIST version 1.119 and was assessed at 24 weeks after treatment start.
QoL was assessed using version 4 of the Functional Assessment of Cancer Therapy (FACT-B) questionnaire.20 Subscales (Physical well-being, Social/Family well-being, Emotional well-being, Functional well-being and Additional Concerns) and the FACT-B Trial Outcome Index (FACT-B TOI), the FACT-G total score and the FACT-B total score were calculated.
Statistical analysis
Sample size calculation was based on the primary endpoint. Overall, 156 patients (78 in each arm) were required to detect a clinically relevant difference of 20% in the rate of stomatitis episode grade ≥2 between treatment arms at 12 weeks (40% and 20% estimated in the EVE 10mg and EVE esc arms, respectively) using a two-sided continuity-corrected χ2 test with a significance level of α = 0.20 and a power of 90%.
The primary and secondary endpoint analyses were performed on the modified intention-to-treat (mITT) analysis set including all randomised patients who started therapy. The rate of stomatitis episodes grade ≥2 was calculated together with the 80% (due to design α of 0.2) and additional 95% CI21 for each treatment arm and overall, and compared between treatment arms using a continuity-corrected χ2 test (α = 0.20). Furthermore, odds ratios (ORs) from the univariate logistic regression were displayed with the 80% and 95% CI.
The significance level for all secondary analyses was set to a two-sided α = 0.05 with 95% CIs; adjustment for multiple testing was not planned. Multivariate logistic regression analysis adjusted for the parameters age (>65 versus ≤65), ECOG PS (1-2 versus 0), body mass index (≥25 versus <25 kg/m2) and number of previous therapy lines for mBC (>1 versus 0/1) was post hoc conducted for binary outcomes to report adjusted ORs with 95% CI.
Time to onset of stomatitis was displayed as a cumulative incidence curve and compared between arms using the Gray test.22 Discontinuation of study treatment due to AEs, patients’ or investigators’ decision, progression or death without stomatitis grade ≥2 were considered competing events.
All further safety and compliance analyses were conducted in the safety analysis set which included all patients from the mITT set, except one patient who was randomised to the EVE esc arm but received the complete dose during the first 3 weeks of treatment. Therefore, this patient was analysed in the EVE 10mg arm. Of note, one patient in the EVE 10mg arm was excluded from the safety analysis due to uncompleted safety documentation (missing data). Additional AEs, excluding stomatitis events (primary endpoint), were summarised by frequency and percentage of patients within the AE category of interest, by treatment arm and overall. Fisher’s exact test was used to compare frequencies of AEs and compliance parameters between treatment arms (P values are to be considered descriptive).
The CBR was assessed in the mITT set with 95% CI and compared between treatment arms using a two-sided Fisher’s exact test.
QoL was analysed in the safety analysis set using repeated-measures mixed-effects models with main effect terms ‘treatment’ and ‘time’, the interaction term ‘treatment-by-time’ and baseline values as covariate.
All statistical analyses were performed using SAS version 9.4 with SAS Enterprise Guide Version 7.1 on Microsoft Windows 10 Enterprise. Data cut-off was 5 July 2021.
Results
Baseline
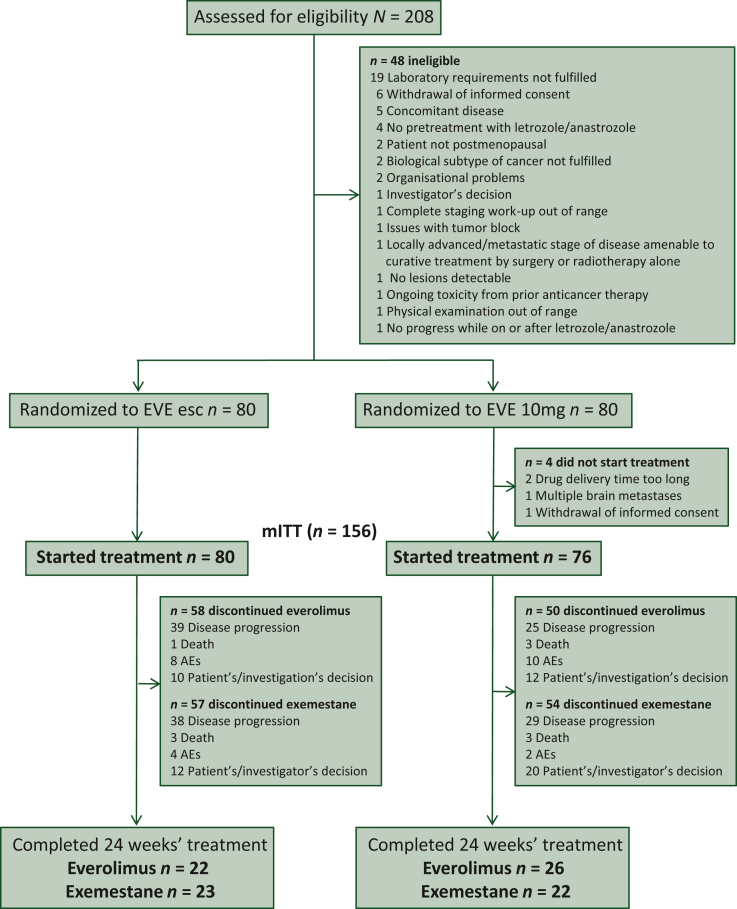
Between June 2015 and October 2020, 208 patients were screened at 29 sites in Germany, 160 were randomised and 156 started treatment (EVE esc: 80 patients, EVE 10mg: 76 patients; Figure 1). The median age at study entry was 64 years (range 33-85) with a significant difference in the predefined age groups (P = 0.014). More patients received adjuvant treatment in the EVE esc arm than in the EVE 10mg arm (60.0% versus 44.7%; P = 0.046); 56.3% of patients in the EVE esc versus 42.1% in the EVE 10mg arm had liver metastasis (P = 0.081). The other baseline characteristics were equally distributed between the two treatment arms (Table 1). Previous therapies for metastatic disease were endocrine therapy (100%), targeted therapy (81.4%) and chemotherapy (75.6%).
Figure 1.
CONSORT diagram.
AE, adverse event; esc, escalated; EVE, everolimus.
Table 1.
Baseline characteristics
| Parameter | Category | EVE esc n = 80 | EVE 10mg n = 76 | Overall n = 156 | P value |
|---|---|---|---|---|---|
| Age, years | Median (range) | 64.5 (33.0-85.0) | 63.0 (41.0-81.0) | 64.0 (33.0-85.0) | 0.538 |
| <40 | 2 (2.5) | 0 (0.0) | 2 (1.3) | 0.014 | |
| 40-<50 | 11 (13.8) | 2 (2.6) | 13 (8.3) | ||
| 50-<65 | 27 (33.8) | 39 (51.3) | 66 (42.3) | ||
| ≥65 | 40 (50.0) | 35 (46.1) | 75 (48.1) | ||
| BMI, kg/m2 | <25 | 35 (43.8) | 39 (51.3) | 74 (47.4) | 0.423 |
| ≥25 | 45 (56.3) | 37 (48.7) | 82 (52.6) | ||
| Menopausal status | Pre/perimenopausala | 1 (1.3) | 0 (0.0) | 1 (0.6) | >0.99 |
| Postmenopausal | 79 (98.8) | 76 (100) | 155 (99.4) | ||
| ECOG PS | 0 | 57 (71.3) | 63 (82.9) | 120 (76.9) | 0.202 |
| 1 | 20 (25.0) | 12 (15.8) | 32 (20.5) | ||
| 2 | 3 (3.8) | 1 (1.3) | 4 (2.6) | ||
| Stomatitis | No | 77 (96.3) | 73 (96.1) | 150 (96.2) | 0.949 |
| Grade 1 | 3 (3.8) | 3 (3.9) | 6 (3.8) | ||
| Histological tumour type | Ductal or ductal lobular invasive | 54 (67.5) | 52 (68.4) | 106 (67.9) | 0.677 |
| Lobular invasive | 19 (23.8) | 20 (26.3) | 39 (25.0) | ||
| Other | 7 (8.8) | 4 (5.3) | 11 (7.1) | ||
| Number of metastatic sites | 1 | 22 (27.5) | 28 (36.8) | 50 (32.1) | 0.512 |
| 2 | 30 (37.5) | 28 (36.8) | 58 (37.2) | ||
| 3 | 19 (23.8) | 15 (19.7) | 34 (21.8) | ||
| ≥4 | 9 (11.3) | 5 (6.6) | 14 (9.0) | ||
| Selected metastatic sitesb | Bone | 55 (68.8) | 57 (75.0) | 112 (71.8) | 0.477 |
| Liver | 45 (56.3) | 32 (42.1) | 77 (49.4) | 0.081 | |
| Lung | 21 (26.3) | 17 (22.4) | 38 (24.4) | 0.582 | |
| Pleura | 12 (15.0) | 7 (9.2) | 19 (12.2) | 0.331 | |
| Number of previous metastatic regimens | 0 | 6 (7.5) | 7 (9.2) | 13 (8.3) | 0.849 |
| 1-2 | 59 (73.8) | 53 (69.7) | 112 (71.8) | ||
| >2 | 15 (18.8) | 16 (21.1) | 31 (19.9) | ||
| Disease setting at first diagnosis | Neoadjuvant | 8 (10.0) | 18 (23.7) | 26 (16.7) | 0.046 |
| Adjuvant | 48 (60.0) | 34 (44.7) | 82 (52.6) | ||
| Advanced | 24 (30.0) | 24 (31.6) | 48 (30.8) | ||
| Previous therapy in advanced setting | Chemotherapyc | 61 (76.3) | 57 (75.0) | 118 (75.6) | >0.99 |
| Endocrine therapyd | 80 (100) | 76 (100) | 156 (100) | n.a. | |
| Targeted therapye | 66 (82.5) | 61 (80.3) | 127 (81.4) | 0.837 |
Data are n (%).
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; esc, escalated; EVE, everolimus; n.a., not applicable; PS, performance status.
Protocol deviation.
Some patients had more than one metastatic site.
Chemotherapy included a combination of taxane and anthracycline or a combination of nontaxane and anthracycline or anthracycline alone, or taxane alone.
Endocrine therapy included anastrozole or letrozole or exemestane or fulvestrant or tamoxifen.
Targeted therapy included palbociclib or ribociclib or bisphosphonates or denosumab.
Stomatitis
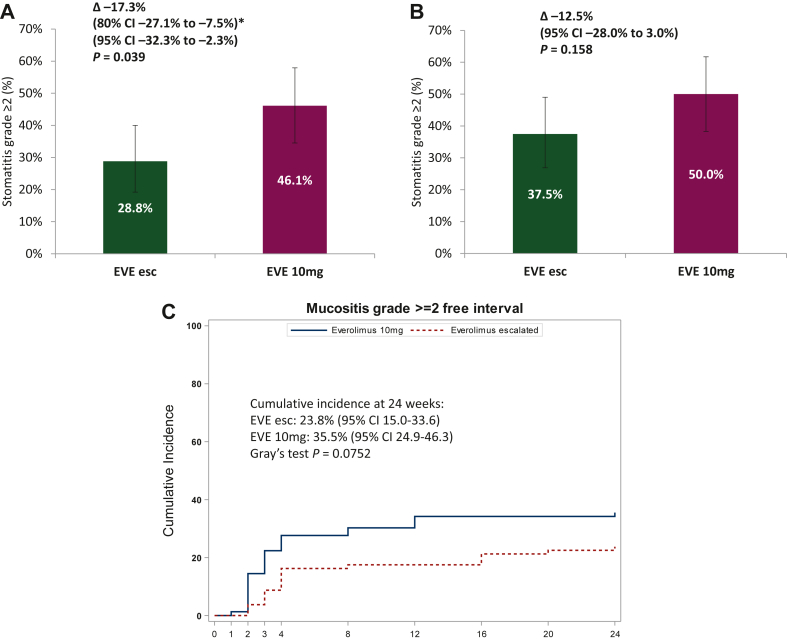
Within 12 weeks of treatment, the incidence of stomatitis episodes grade ≥2 was 28.8% (95% CI 19.2% to 40.0%) in the EVE esc arm compared with 46.1% (95% CI 34.5% to 57.9%) in the EVE 10mg arm (P = 0.039) with an OR of 0.47 (95% CI 0.24-0.92; P = 0.026; Figure 2A). The rate of stomatitis grades ≥2 without considering premature discontinuations was 18.8% (95% CI 10.9% to 29.0%) versus 35.5% (95% CI 24.9% to 47.3%), respectively (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100601). A post hoc multivariate analysis adjusted for age, ECOG PS, body mass index and number of previous therapy lines for mBC confirmed that patients in the EVE esc arm had a lower chance to experience a stomatitis event grade ≥2 at 12 weeks (OR 0.40, 95% CI 0.20-0.81; P = 0.011; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100601). There was no significant difference in the incidence of any grade stomatitis episodes between the two arms (62.5% in the EVE esc arm versus 73.7% in the EVE 10mg arm; P = 0.185; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100601).
Figure 2.
Stomatitis episodes grade ≥2 within (A) 12 and (B) 24 weeks of treatment as well as (C) time to onset of stomatitis grade ≥2 within 24 weeks. (A) Incidence of stomatitis episodes grade ≥2 within 12 weeks considering stomatitis grade <2 and premature treatment discontinuation due to adverse events, patient’s decision or investigator’s decision. ∗Primary endpoint design: type I error 20%. (B) Incidence of stomatitis episodes grade ≥2 within 24 weeks considering stomatitis grade <2 and premature treatment discontinuation due to adverse events, patient’s decision or investigator’s decision. (C) Time to onset of stomatitis grade ≥2 within 24 weeks was assessed post hoc. Competing events were defined as discontinuation of study treatment due to adverse event, patient’s decision or investigator’s decision, progression or death without stomatitis grade ≥2.
CI, confidence interval; esc, escalated; EVE, everolimus; OR, odds ratio.
At 24 weeks of treatment the incidence of stomatitis episodes grade ≥2 was numerically lower but without statistical significance between the EVE esc and EVE 10mg arms (37.5% versus 50.0%; OR 0.60, 95% CI 0.32-1.14; P = 0.117; Figure 2B). The incidence of mucositis grade ≥2 without considering premature discontinuations was 23.8% in the EVE esc arm versus 35.5% in the EVE 10mg arm (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100601). A post hoc multivariate analysis showed that patients in the EVE esc arm had a significantly lower risk to experience stomatitis episodes grade ≥2 (OR 0.49, 95% CI 0.25-0.98; P = 0.043; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100601). There was no significant difference in the incidence of any grade stomatitis between the two arms (67.5% in the EVE esc arm versus 77.6% in the EVE 10mg arm; P = 0.216; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100601).
Competing risk analysis showed that the cumulative incidence of stomatitis grade ≥2 at 24 weeks was 23.8% (95% CI 15.0% to 33.6%) in the EVE esc versus 35.5% (95% CI 24.9% to 46.3%) in the EVE 10mg arm (P = 0.075; Figure 2C).
Other safety analyses
All patients evaluable for safety analysis experienced at least one AE, 91.6% experienced haematological and 100% nonhaematological AEs (Table 2). Toxicity (excluding stomatitis) was not significantly different between treatment arms. The most frequently reported any grade haematological AEs in the EVE esc arm versus the EVE 10mg arm were anaemia (74.7% versus 81.6%; P = 0.336) and leukopenia (67.1% versus 67.1%). The most frequently reported any grade nonhaematologic AEs in the EVE esc arm versus the EVE 10mg arm were high-density lipoprotein cholesterol increase (96.2% versus 93.4%; P = 0.489), serum cholesterol increase (77.2% versus 85.5%; P = 0.219), aspartate aminotransferase increase (79.7% versus 72.4%; P = 0.347), alanine aminotransferase increase (67.1% versus 53.9%; P = 0.103), hypertriglyceridaemia (73.4% versus 72.4%; P >0.99), hyperglycaemia (57.0% versus 60.5%; P = 0.745), fatigue (53.2% versus 52.6%; P >0.99) and low-density lipoprotein cholesterol increase (51.9% versus 72.4%; P = 0.013). Among grade 3-4 haematological AEs, anaemia (3.8% versus 6.6%; P = 0.489), leukopenia (3.8% versus 2.6%; P >0.99) and neutropenia (3.8% versus 5.3%; P = 0.716) were the most frequent. The most frequently reported grade 3-4 nonhaematological AEs were aspartate aminotransferase evaluation (8.9% versus 2.6%; P = 0.168), alanine aminotransferase evaluation (5.1% versus 1.3%; P = 0.367) and hyperglycaemia (5.1% versus 9.2%; P = 0.362). No significant difference in pneumonitis (adverse event of special interest) was observed between the arms (any grade: 7.6% versus 7.9%; grade 3-4: 1.3% only in the EVE esc arm; Table 2).
Table 2.
Predefined adverse events excluding stomatitis events (primary endpoint) with an incidence of ≥10% regardless of causality in both arms based on the safety population (n = 155) at 24 weeks
| AEs | Any grade |
High-grade (grade 3-4) |
||||
|---|---|---|---|---|---|---|
| EVE esc n = 79, n (%) | EVE 10mg n = 76, n (%) | P value | EVE esc n = 79, n (%) | EVE 10mg n = 76, n (%) | P value | |
| Summary of all AEs | ||||||
| Any AE | 79 (100) | 76 (100) | n.a. | 37 (46.8) | 33 (43.4) | 0.747 |
| Any haematological AE | 70 (88.6) | 72 (94.7) | 0.247 | 8 (10.1) | 8 (10.5) | >0.99 |
| Any nonhaematological AE | 79 (100) | 76 (100) | n.a. | 35 (44.3) | 29 (38.2) | 0.514 |
| Other AEs | 75 (94.9) | 68 (89.5) | 0.240 | 25 (31.7) | 17 (22.4) | 0.210 |
| At least one SAE | 23 (29.1) | 22 (28.9) | >0.99 | n.a. | n.a. | — |
| AESI (pneumonitis) | 6 (7.6) | 6 (7.9) | >0.99 | 1 (1.3) | 0 (0.0) | >0.99 |
| Predefined AEs | ||||||
| Anaemia | 59 (74.7) | 62 (81.6) | 0.336 | 3 (3.8) | 5 (6.6) | 0.489 |
| Leukopenia | 53 (67.1) | 51 (67.1) | >0.99 | 3 (3.8) | 2 (2.6) | >0.99 |
| Thrombocytopenia | 29 (36.7) | 36 (47.4) | 0.196 | 1 (1.3) | 1 (1.3) | >0.99 |
| Neutropenia | 33 (41.8) | 29 (38.2) | 0.743 | 3 (3.8) | 4 (5.3) | 0.716 |
| Blood AP increased | 41 (51.9) | 36 (47.4) | 0.631 | 2 (2.5) | 1 (1.3) | >0.99 |
| ASAT increased | 63 (79.7) | 55 (72.4) | 0.347 | 7 (8.9) | 2 (2.6) | 0.168 |
| ALAT increased | 53 (67.1) | 41 (53.9) | 0.103 | 4 (5.1) | 1 (1.3) | 0.367 |
| Blood creatinine increased | 24 (30.4) | 31 (40.8) | 0.184 | 0 (0.0) | 0 (0.0) | n.a. |
| Fatigue | 42 (53.2) | 40 (52.6) | >0.99 | 2 (2.5) | 1 (1.3) | >0.99 |
| Diarrhoea | 28 (35.4) | 19 (25.0) | 0.167 | 2 (2.5) | 2 (2.6) | >0.99 |
| Decreased appetite | 22 (27.8) | 16 (21.1) | 0.355 | 1 (1.3) | 2 (2.6) | 0.615 |
| Nausea | 23 (29.1) | 26 (34.2) | 0.604 | 1 (1.3) | 0 (0.0) | >0.99 |
| Cough | 24 (30.4) | 21 (27.6) | 0.727 | 0 (0.0) | 0 (0.0) | n.a. |
| Headache | 24 (30.4) | 17 (22.4) | 0.279 | 0 (0.0) | 1 (1.3) | 0.490 |
| Weight decreased | 16 (20.3) | 22 (28.9) | 0.263 | 0 (0.0) | 0 (0.0) | n.a. |
| Dyspnoea | 16 (20.3) | 23 (30.3) | 0.195 | 1 (1.3) | 2 (2.6) | 0.615 |
| Arthralgia | 18 (22.8) | 22 (28.9) | 0.463 | 0 (0.0) | 0 (0.0) | n.a. |
| Epistaxis | 9 (11.4) | 6 (7.9) | 0.589 | 0 (0.0) | 0 (0.0) | n.a. |
| Vertigo | 11 (13.9) | 8 (10.5) | 0.627 | 0 (0.0) | 0 (0.0) | n.a. |
| Hypertriglyceridemia | 58 (73.4) | 55 (72.4) | >0.99 | 1 (1.3) | 1 (1.3) | >0.99 |
| Hypoglycaemia | 16 (20.3) | 9 (11.8) | 0.192 | 0 (0.0) | 0 (0.0) | n.a. |
| Hyperglycaemia | 45 (57.0) | 46 (60.5) | 0.745 | 4 (5.1) | 7 (9.2) | 0.362 |
| Serum cholesterol increased | 61 (77.2) | 65 (85.5) | 0.219 | 1 (1.3) | 2 (2.6) | 0.615 |
| LDL cholesterol increaseda | 41 (51.9) | 55 (72.4) | 0.013 | n.a. | n.a. | n.a. |
| HDL cholesterol increaseda | 76 (96.2) | 71 (93.4) | 0.489 | n.a. | n.a. | n.a. |
AEs are not mutually exclusive. One patient in the EVE 10mg arm was excluded due to uncompleted safety documentation (missing data) and one patient who was randomised to the EVE esc arm received a full dose of 10 mg everolimus during the escalation phase, and therefore was analysed in the EVE 10mg arm (EVE esc: n = 79 and EVE 10mg n = 76).
AE, adverse event; AESI, adverse event of special interest; ALAT, alanine aminotransferase; AP, alkaline phosphatase; ASAT, aspartate aminotransferase; EVE, everolimus; esc, escalated; HDL, high-density lipoprotein; LDL, low-density lipoprotein; n.a., not applicable; SAE, serious adverse event.
No grading available.
In addition to the predefined AEs, the most reported any grade other (not predefined) AEs in the EVE esc arm versus the EVE 10mg arm were other nervous system disorders (26.6% versus 26.3%; P >0.99), rash (22.8% versus 11.8%; P = 0.091), other infections and infestations (21.5% versus 11.8%; P = 0.134) and other gastrointestinal disorders (20.3% versus 18.4%; P = 0.840; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100601, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100601).
Overall, 45 patients had at least one SAE (23 in EVE esc and 22 in EVE 10mg; Table 2). During the 24 weeks of study treatment four patients died (one in the EVE esc and three in the EVE 10mg arm) due to disease progression.
Compliance
Median duration of everolimus exposure during 24 weeks of treatment was 12.7 weeks (range 0.1-24.0) in the EVE esc arm and 16.0 weeks (range 0.7-24.0) in the EVE 10mg arm (P = 0.592). Median RTDI was 91.1% (range 0.2-100) in the EVE esc arm versus 80.0% (range 1.2-100) in the EVE 10mg arm (P = 0.329; Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100601).
During the first 3 weeks of treatment (escalation phase), only 6.3% of patients in the EVE esc arm compared with 15.8% in the EVE 10mg arm (P = 0.073) discontinued everolimus mainly due to AEs in both arms (1.3% versus 6.6%). The rate of patients who discontinued everolimus prior to week 12 was 46.3% in the EVE esc arm versus 32.9% in the EVE 10mg arm (P = 0.103). The most common reason for everolimus discontinuation was disease progression in both arms (31.3% versus 9.2%, respectively) and toxicity in the EVE 10mg arm (6.3% versus 9.2%, respectively). Subsequently at 24 weeks, everolimus was discontinued in 72.5% of patients in the EVE esc arm and 65.8% in the EVE 10mg arm (P = 0.390) mainly due to disease progression (48.8% versus 32.9%, respectively; Table 3).
Table 3.
Summary of treatment discontinuation, dose reduction and interruption during study treatment
| Status/reason | EVE esc n = 79 n (%) | EVE 10mg n = 77 n (%) | Overall n = 156 n (%) | P value |
|---|---|---|---|---|
| Discontinued everolimus | ||||
| Within the first 3 weeks (escalation phase) | 5 (6.3) | 12 (15.8) | 17 (10.9) | 0.073 |
| Disease progression | 1 (1.3) | 1 (1.3) | 2 (1.3) | |
| Death | 0 (0.0) | 2 (2.6) | 2 (1.3) | |
| AE | 1 (1.3) | 5 (6.6) | 6 (3.8) | |
| Patient’s or investigator’s decision | 3 (3.8) | 4 (5.2) | 7 (4.5) | |
| Within the first 12 weeks | 37 (46.3) | 25 (32.9) | 62 (39.7) | 0.103 |
| Disease progression | 25 (31.3) | 7 (9.2) | 32 (20.5) | |
| Death | 0 (0.0) | 3 (3.9) | 3 (1.9) | |
| AE | 5 (6.3) | 7 (9.2) | 12 (7.7) | |
| Patient’s or investigator’s decision | 7 (8.8) | 8 (10.5) | 15 (9.6) | |
| Within 24 weeks | 58 (72.5) | 50 (65.8) | 108 (69.2) | 0.390 |
| Disease progression | 39 (48.8) | 25 (32.9) | 64 (41.0) | |
| Death | 1 (1.3) | 3 (3.9) | 4 (2.6) | |
| AE | 8 (10.0) | 10 (13.2) | 18 (11.5) | |
| Patient’s or investigator’s decision | 10 (12.6) | 12 (15.8) | 22 (14.1) | |
| Discontinued exemestane | 23 (28.8) | 22 (28.9) | 45 (28.8) | >0.99 |
| Disease progression | 38 (47.5) | 29 (38.2) | 67 (42.9) | |
| Death | 3 (3.8) | 3 (3.9) | 6 (3.8) | |
| AE | 4 (5.0) | 2 (2.6) | 6 (3.8) | |
| Patient’s or investigator’s decision | 12 (15.0) | 20 (26.3) | 32 (20.5) | |
| Dose reduction everolimus | ||||
| Patients with everolimus reduced to 5 mg | 23 (31.5) | 24 (36.4) | 47 (33.8) | 0.593 |
| Haematological AE related to study medication | 4 (5.1) | 2 (2.6) | 6 (3.8) | 0.681 |
| Nonhaematological AE related to study medication | 16 (20.3) | 14 (18.2) | 30 (19.2) | 0.840 |
| AE not related to study medication | 0 (0.0) | 3 (3.9) | 3 (1.9) | 0.118 |
| Other reason | 1 (1.3) | 1 (1.3) | 2 (1.3) | >0.99 |
| Unknown reason | 3 (3.8) | 4 (5.2) | 7 (4.5) | 0.718 |
| Interruption everolimus | ||||
| Patients with at least one treatment interruption | 47 (59.5) | 43 (55.8) | 90 (57.7) | 0.746 |
| Haematological AE related to study medication | 6 (7.6) | 6 (7.8) | 12 (7.7) | >0.99 |
| Nonhaematological AE related to study medication | 26 (32.9) | 29 (37.7) | 55 (35.3) | 0.616 |
| AE not related to study medication | 10 (12.7) | 14 (18.2) | 24 (15.4) | 0.381 |
| Patient’s noncompliance | 7 (8.9) | 6 (7.8) | 13 (8.3) | >0.99 |
| Organisational reason | 5 (6.3) | 1 (1.3) | 6 (3.8) | 0.210 |
| Other reason | 9 (11.4) | 6 (7.8) | 15 (9.6) | 0.589 |
Note that one patient who was randomised to the EVE esc arm received 10mg everolimus during the escalation phase of 3 weeks, and was therefore analysed in the EVE 10mg arm.
AE, adverse event; EVE, everolimus; esc, escalated.
Overall, 31.5% of patients in the EVE esc arm versus 36.4% in the EVE 10mg arm (P = 0.593) required dose reduction of everolimus to 5 mg, mainly due to treatment-related nonhaematological AEs (20.3% versus 18.2%, respectively; P = 0.840). Everolimus interruptions were required in 59.5% of patients in the EVE esc arm and 55.8% in the EVE 10mg arm (P = 0.746) mostly due to treatment-related nonhaematological AEs (Table 3). The median duration of cumulative dose interruptions was 12 days (range 1-44) in the EVE esc arm compared with 15 days (range 1-48) in the EVE 10mg arm (P = 0.009). Exemestane treatment was interrupted in 19.0% and 15.6% of patients in the EVE esc and EVE 10mg arms, respectively (P = 0.674).
Efficacy
The rate of patients with partial response was 10.0% in the EVE esc arm versus 6.6% in the EVE 10mg arm, whereas the rate of patients with progressive disease was 58.8% in the EVE esc arm compared with 44.7% in the EVE 10mg arm. The CBR was 23.8% in the EVE esc arm versus 31.6% in the EVE 10mg arm (P = 0.288; absolute difference –7.8%) at 24 weeks of treatment (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100601). The post hoc multivariate analysis adjusted for the presence of liver metastases at baseline and number of previous therapy lines for mBC confirmed this observation (OR 0.75; 95% CI 0.36-1.55; P = 0.436; Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100601).
Quality of life
Prospectively captured QoL, assessed using the FACT-B questionnaire, was not significantly different in the EVE esc arm compared with the EVE 10mg arm. The mean FACT-B total score was generally high in both treatment arms but without statistically significant and clinically meaningful differences between arms (105.5 versus 103.5, respectively; P = 0.706) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100601).
Poststudy treatment
Data on poststudy treatment was available for 71 patients (EVE esc n = 30; EVE 10mg n = 41). Overall, 57.7% received everolimus and exemestane beyond the study, 18.3% other endocrine therapy, 5.6% CDK4/6 inhibitor in combination with endocrine therapy and 18.3% chemotherapy (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2022.100601).
Discussion
The DESIREE study demonstrated that the dose-escalation schema of everolimus in combination with exemestane significantly reduced the incidence of stomatitis grade ≥2 within the first 12 weeks of treatment compared with conventionally administered everolimus (10 mg) plus exemestane (28.8% versus 46.1%; P = 0.039).
Oral toxicity such as stomatitis is a class effect of mTOR inhibitors, although the exact mechanism remains unclear.23 However, it might be related to the inhibition of basal layer cells of the oral epithelium. In addition, mTOR inhibitors are immunosuppressive and increase the risk of infection when the oral mucosa is damaged. In clinical practice, however, it is of great importance not only to treat stomatitis, but also to avoid it whenever possible. An analysis on occurrence and time course of stomatitis in the BOLERO-2 study showed that more than one-third of all stomatitis grade ≥2 was reported in the first weeks.24 To address this issue, we designed the DESIREE trial, investigating if a dose-escalation schedule of everolimus in combination with exemestane could significantly reduce the incidence of stomatitis grade ≥2 in the first 12 weeks compared with conventionally administered everolimus (10 mg) plus exemestane.
In the BOLERO-2 study, the incidence of all grade stomatitis and related oral AEs (including mouth ulceration, aphthous stomatitis, glossodynia, gingival pain, lip ulceration, and glossitis) was 67%.7 Although these events were largely reversible and 98% of patients with grade 2 stomatitis had complete resolution after a median of 16 days, 24% of patients required everolimus dose interruptions and/or adjustments, and 3% of patients discontinued treatment with the combination regimen because of stomatitis or related events. None of the patients included in the BOLERO-2 trial had previously received a CDK4/6 inhibitor. Recently, the use of CDK4/6 inhibitors in combination with NSAI has become standard first-line therapy in metastatic HR-positive/HER2-negative breast cancer. A retrospective study investigating the clinical benefit of everolimus plus exemestane in patients who have progressed on combination therapy of an NSAI and a CDK4/6 inhibitor versus NSAI alone showed that a previous treatment with CDK4/6 inhibitor had no impact on survival outcomes for everolimus plus exemestane.25 Overall, of the 43 included patients, 9 (20.9%) required everolimus dose reduction (11.8% in the CDK4/6 inhibitor arm versus 26.9% in the control arm; P = 0.281) and 19 (44.2%) patients developed stomatitis, of whom 15 (78.9%) had no documentation of receiving prophylactic dexamethasone mouthwash, which was not necessarily standard therapy at that time. Hence, a dose-escalation schedule of everolimus might be considered for reducing the incidence of stomatitis in this setting.
Within 24 weeks of treatment, the incidence of stomatitis episodes grade ≥2 was numerically lower in the EVE esc arm, but there was no significant difference anymore. These findings support the need for a close monitoring especially in the early treatment phase to better prevent or manage oral side-effects.
During the first 12 weeks of treatment, the rate of 18.8% of stomatitis grade ≥2 without considering premature discontinuations in the EVE esc arm was comparable to the rates of 18% and 12% seen in patients receiving two different steroid-containing mouth rinses from a randomised phase II trial, which is a different strategy to reduce/prevent everolimus-induced stomatitis. This study showed that the prophylactic therapy with two different steroid-containing mouth rinses substantially reduced incidences of stomatitis any grade and grade ≥2 during the first 12 weeks of treatment in patients with mBC receiving everolimus plus an AI.26 In the SWISH trial, a prophylactic dexamethasone-based mouthwash reduced the incidence of grade ≥2 stomatitis to 2% by 8 weeks at the cost of a minimal risk of oral candidiasis (2 patients) in patients with advanced HR-positive/HER2-negative breast cancer treated with exemestane plus everolimus.15 Based on this single-arm phase II trial, the 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5) guidelines recommend the use of prophylactic steroid mouthwash as a standard measure for the prevention of stomatitis induced by mTOR inhibitors.1
Toxicity reported in the DESIREE study was in line with the known safety profile of everolimus and exemestane, without new safety concerns. The use of a dose-escalation regimen did not lead to significant differences in dose reductions, interruptions and discontinuations, resulting in a similar median RTDI between two arms. In the UNIRAD study, dose reductions of everolimus were less common in patients starting with 5 mg compared with full dose (28.4% versus 46.8%).27
In the BOLERO-2 study, time to definitive deterioration of the global QoL was significantly longer in patients treated with exemestane plus everolimus than with exemestane plus placebo, despite a higher incidence of grade 3 or 4 toxicities reported in the exemestane plus everolimus group.14 In the DESIREE study, the mean FACT-B total score was generally high in both treatment arms without statistically significant and clinically meaningful differences between arms.
It is important to note that there was a numerical, but not statistically significant difference of –7.8% in the CBR favouring the standard everolimus administration schedule. More patients had progressive diseases in the EVE esc arm at 24 weeks. This might be partially explained by differences in the patient characteristics: more patients in the EVE esc arm had an ECOG PS >1, more metastatic sites and more liver metastases. Although this numerical difference in favour of EVE 10mg was not statistically significant, we cannot exclude a lower efficacy of the EVE esc arm. However, in the noninterventional, real-word BRAWO study evaluating safety and efficacy of the approved combination of everolimus plus exemestane in HR-positive/HER2-negative advanced BC, most patients started with the standard dose everolimus of (10 mg), while about one-third of the patients started with everolimus 5 mg. The starting dose, however, did not impact progression-free survival.9,28 These results suggest that patients in routine clinical practice are sometimes treated with a dose-escalation schema of everolimus to allow the patient’s organism to adapt to the therapy.
A major strength of the study is that we used a randomised, double-blind, placebo-controlled design that minimises imbalances between the two study arms. Furthermore, to the best of our knowledge, DESIREE is the only randomised phase II trial in postmenopausal HR-positive/HER2-negative patients with advanced breast cancer investigating whether a dose escalating schedule of everolimus can reduce the incidence of severe stomatitis in the first 12 weeks. A limitation of the DESIREE trial is that the information collected on use of dexamethasone-based mouthwashes as a prophylaxis of stomatitis as well as previous treatment with CDK4/6 inhibitors was inconsistent, which might have influenced the results. Another limitation was the fixed study treatment of 24 weeks resulting in an extended recruitment of patients.
However, the DESIREE met its primary endpoint and demonstrated that a dose-escalation schema of everolimus over 3 weeks can be successfully implemented to reduce the incidence of high-grade stomatitis in the first 12 weeks of treatment. This could be an alternative strategy for reduction of everolimus-related stomatitis as the use of a steroid-based mouthwash has a small but real risk of developing oral candidiasis.
In conclusions, these results have the potential to improve the toxicity profile of patients treated with everolimus. As stomatitis is a common side-effect of targeted therapies for breast cancer, it may be worthwhile to investigate if the use of escalating dose regimens might improve the tolerability of other new targeted agents.
Acknowledgements
The authors thank all patients and their families participating in the trial, the investigators and their teams (Supplementary material) and the team at the GBG Headquarters, especially Dr Ioannis Gkantiragas for managing the study, Sabine Kleinefeld as the responsible data manager and Dr Valentina Vladimirova for editorial assistance.
Funding
Drug and financial support was provided by Novartis, Germany (no grant number). The funders had no role in the collection, analysis, or interpretation of the data, and had no access to the study data. The study statisticians (NB and VN) had access to the raw data. The report was reviewed by all authors. The sponsor (GBG Forschungs GmbH) has developed the study design and written the report together with the members of the palliative subboard of the German Breast Group (GBG). The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosure
VM reports personal fees from Amgen, Astra Zeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva, Seagen, GSK, Gilead; personal fees from Genomic Health, Gilead, Hexal, Roche, Pierre Fabre, Amgen, Clin Sol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, GSK, Gilead, other from Novartis, Roche, Seagen, Genentech, outside the submitted work. MS reports grants or contracts to the Institution from AstraZeneca, BioNTech, Eisai, German Breast Group, Genentech, Novartis, Pantarhei Bioscience, Pfizer, Pierre Fabre, Roche; consulting fees from AstraZeneca, BioNTech, Eisai, Daiichi Sankyo, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Pierre Fabre, Roche, Seagen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Roche, Seagen; support for attending meetings and/or travel from Pfizer and Roche; patents planned, issued or pending: EP 2951317 B1 and EP 2390370 B1; participation on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Lilly, Novartis, Pantarhei Bioscience, Pfizer, Pierre Fabre, Roche, Seagen; receipt of equipment, materials, drugs, medical writing, gifts or other services from Roche. MR reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Pfizer; support for attending meetings and/or travel from Novartis, Pfizer. TD reports consulting fees from Novartis Adboard, iOMEDICO adboard. TL reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Roche, Clovis, MSD, Novartis, Pfizer, Lilly, GSK, Gilead, AstraZeneca; support for attending meetings and/or travel from MSD, Celgene, Clovis, Gilead, Daiichi Sankyo, Pfizer; participation on a data safety monitoring board or advisory board for Amgen, MSD, Roche, Tesaro, Pfizer, Lilly, Myriad, Eisai, GSK, Daiichi Sankyo. KL reports payment for participation on Advisory Board from Seagen, Novartis, AstraZeneca, Genomic Health, Roche, Daiichi Sankyo, Lilly, MSD, Eisai; payment or honoraria for lectures from Novartis, Genomic Health, Roche, Lilly. MT reports trial funding, payment to the institution from Exact Sciences, Endomag; consulting fees or Advisory board from Agendia, Amgen, AstraZeneca, Becton/Dickinson, ClearCut, Clovis, Daiichi Sankyo, Eisai, Exact Sciences, Gilead, Grünenthal, GSK, Lilly, MSD, Neodynamics, Onkowissen, Organon, Pfizer, Pfm medical, Pierre-Fabre, Roche, Seagen, Sirius Pintuition, Sysmex; payment or honoraria for lectures, manuscript writing from Amgen, AstraZeneca, Art tempi, ClearCut, Clovis, Connect Medica, Daiichi Sankyo, Eisai, Gilead, Hexal, Exact Sciences, Eickeler, Onkowissen, Sysmex, Vifor, Viatris, I-Med-Institute, Lilly, MSD, Novartis, Pfizer, pfm medical, Roche, Seagen, Servier; support for attending meetings and/or travel from Amgen, Art tempi, AstraZeneca, Clearcut, Clovis, Connect medica, Daiichi Sankyo, Exact Sciences, I-Med-Institute, Lilly, MSD, Novartis, Pfizer, pfm medical, Neodynamics, Seagen; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from AWOgyn. MvM reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, AstraZeneca, Daiichi Sankyo, Genomic Health, Gilead, GSK, Lilly, Molecular Health, Mylan, Novartis, Pfizer, Pierre Fabre, Roche, Seagen; support for attending meetings and/or travel from Lilly. SL reports honoraria for Advisory board, lectures and research grant paid to the institution from Novartis, AstraZeneca, Daiichi-Sankyo, Pfizer, Roche; advisory board and research grant paid to the institution from AbbVie, BMS (Celgene), Gilead; honoraria for advisory board paid to the institution from Amgen, EirGenix, GSK, Lilly, Merck KGaA, Pierre-Fabre, Sanofi; honoraria for Advisory board and medical writing paid to the institution, and nonfinancial support from Seagen; patent issued and royalties (Digital Ki67 Evaluator) VM Scope GmbH paid to the institution; patents pending: EP14153692.0 (immunosignature in TNBC), EP21152186.9 (signature for CDK4/6 inhibitor), EP19808852.8. (GeparNUEVO) paid to the institution; patent issued: EP15702464.7 (Predicting response to an anti-HER2 containing therapy) paid to the institution. No other potential conflict of interest relevant to this article was reported.
Data sharing
| Will individual participant data be available (including data dictionaries)? | Yes |
| What data in particular will be shared? | Individual participant data that underlie the results reported in this article, after final analysis and publication of all secondary efficacy endpoints |
| What other documents will be available? | Study protocol; statistical report (if necessary for the project) |
| When will data be available (start and end dates)? | Beginning after final analysis and publication of all secondary efficacy endpoints; no end date |
| With whom? | Researchers who provide translational research proposals. Proposals should be approved by the GBG scientific board. |
| For what types of analyses? | To achieve aims in the approved proposal |
| By what mechanism will data be made available? | Proposals should be directed to http://www.gbg.de/de/forschung/translationale-forschung.php; to gain access, data requestors will need to sign a data transfer agreement |
Supplementary data
References
- 1.Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rugo H.S., Rumble R.B., Macrae E., et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 3.Thill M., Friedrich M., Kolberg-Liedtke C., et al. AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2021. Breast Care (Basel) 2021;16:228–235. doi: 10.1159/000516420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy C.G., Dickler M.N. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer. 2016;23:R337–R352. doi: 10.1530/ERC-16-0121. [DOI] [PubMed] [Google Scholar]
- 5.Miricescu D., Totan A., Stanescu-Spinu, Badoiu S.C., Stefani C., Greabu M. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects. Int J Mol Sci. 2020;22:173. doi: 10.3390/ijms22010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yardley D.A., Noguchi S., Pritchard K.I., et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baselga J., Campone M., Piccart M., et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im Y.H., Karabulut B., Lee K.S., et al. Safety and efficacy of everolimus (EVE) plus exemestane (EXE) in postmenopausal women with locally advanced or metastatic breast cancer: final results from EVEREXES. Breast Cancer Res Treat. 2021;188:77–89. doi: 10.1007/s10549-021-06173-z. [DOI] [PubMed] [Google Scholar]
- 9.Lüftner D., Schuetz F., Schneeweiss A., et al. Abstract P6-18-08: Everolimus + exemestane for HR+ advanced breast cancer in routine clinical practice – final results from the non-interventional trial, BRAWO. In Poster Session Abstracts: American Association for Cancer Research. Cancer Res. 2019;79 P6-18-08. [Google Scholar]
- 10.Tesch H., Stoetzer O., Decker T., et al. Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: results of the single-arm, phase IIIB 4EVER trial. Int J Cancer. 2019;144:877–885. doi: 10.1002/ijc.31738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciruelos E., Jerusalem G., Martin M., et al. Everolimus plus exemestane in hormone-receptor-positive, HER2-negative locally advanced or metastatic breast cancer: incidence and time course of adverse events in the phase IIIb BALLET population. Clin Transl Oncol. 2020;22:1857–1866. doi: 10.1007/s12094-020-02327-5. [DOI] [PubMed] [Google Scholar]
- 12.Steger G.G., Egle D., Bartsch R., et al. Efficacy and safety of everolimus plus exemestane in patients with HR+, HER2– advanced breast cancer progressing on/after prior endocrine therapy in routine clinical practice: primary results from the non-interventional study. STEPAUT. Breast. 2020;50:64–70. doi: 10.1016/j.breast.2020.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo H.S., Hortobagyi G.N., Yao J., et al. Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Ann Oncol. 2016;27:519–525. doi: 10.1093/annonc/mdv595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burris H.A., Lebrun F., Rugo H.S., et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 15.Rugo H.S., Seneviratne L., Beck J.T., et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18:654–662. doi: 10.1016/S1470-2045(17)30109-2. [DOI] [PubMed] [Google Scholar]
- 16.Allison K.H., Hammond M.E.H., Dowsett M., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 17.Wolff A.C., Hammond M.E.H., Hicks D.G., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 18.WHO handbook for reporting results of cancer treatment. 48. WHO offset publication, Geneva: WHO; 1979.
- 19.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.European Organisation for Research and Treatment of Cancer Quality of Life. FACT-B. https://www.facit.org/measures/FACT-B Available at.
- 21.Clopper C.J., Pearson E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 22.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Zhang Y., Yan H., Xu Z., Yang B., Luo P., He Q. Molecular basis for class side effects associated with PI3K/AKT/mTOR pathway inhibitors. Expert Opin Drug Metab Toxicol. 2019;15:767–774. doi: 10.1080/17425255.2019.1663169. [DOI] [PubMed] [Google Scholar]
- 24.Rugo H.S., Pritchard K.I., Gnant M., et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25:808–815. doi: 10.1093/annonc/mdu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook M.M., Al Rabadi L., Kaempf A.J., Saraceni M.M., Savin M.A., Mitri Z.I. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021;26:101–106. doi: 10.1002/onco.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones V.E., McIntyre K.J., Paul D., et al. Evaluation of miracle mouthwash plus hydrocortisone versus prednisolone mouth rinses as prophylaxis for everolimus-associated stomatitis: a randomized phase II study. Oncologist. 2019;24:1153–1158. doi: 10.1634/theoncologist.2018-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachelot T., Dalenc F., Chabaud S., et al. Efficacy of everolimus in patients with HR+/HER2– high risk early stage breast cancer. Ann Oncol. 2021;32:574–575. [Google Scholar]
- 28.Fasching P.A., Grischke E.M., Schuetz F., et al. Analysis of everolimus starting dose as prognostic marker in HR+ mBC patients treated with everolimus (EVE) + exemestane (EXE): Results of the 3rd interim analysis of the non-interventional trial BRAWO. J Clin Oncol. 2017;35:1061. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.