Abstract
Background
Treatment options are limited for participants with microsatellite stable (MSS) metastatic colorectal cancer (mCRC) that progressed after two or more prior therapies. Studies have shown that blockade of both lymphocyte-activation gene 3 (LAG-3) and programmed cell death protein 1 (PD-1) can improve antitumor activity. Here, we evaluate the antitumor activity of the LAG-3 antibody favezelimab alone or in combination with pembrolizumab in participants with MSS mCRC.
Patients and methods
Eligible participants with MSS PD-1/programmed death-ligand 1 (PD-L1) treatment-naive mCRC that progressed on two or more prior therapies received 800 mg favezelimab, 800 mg favezelimab plus 200 mg pembrolizumab, or 800 mg favezelimab/200 mg pembrolizumab co-formulation, every 3 weeks. The primary endpoint was safety, the secondary endpoint was objective response rate (ORR), and exploratory endpoints included duration of response, progression-free survival (PFS), and overall survival (OS).
Results
At the data cut-off date of 23 October 2020, a total of 20 participants received favezelimab alone, 89 received favezelimab plus pembrolizumab (including as favezelimab/pembrolizumab co-formulation); 48 had PD-L1 combined positive score (CPS) ≥1 tumors. At this interim analysis median follow-up was 5.8 months with favezelimab and 6.2 with favezelimab plus pembrolizumab. Treatment-related adverse events (TRAEs) were 65% with favezelimab and 65.2% with favezelimab plus pembrolizumab. Grade ≥3 TRAEs were 15% with favezelimab and 20% with favezelimab plus pembrolizumab. No grade 5 TRAEs occurred. Common TRAEs (≥15%) included fatigue (20.0%), nausea (15.0%) with favezelimab, and fatigue (16.9%) with favezelimab plus pembrolizumab. Confirmed ORR was 6.3% with favezelimab plus pembrolizumab, with median duration of response of 10.6 months (range 5.6-12.7 months), median OS of 8.3 months (95% confidence interval 5.5-12.9 months), and median PFS of 2.1 months (1.9-2.2 months). In an exploratory analysis of PD-L1 CPS ≥1 tumors, the confirmed ORR was 11.1%, median OS was 12.7 months (4.5 to not reached), and median PFS was 2.2 months (1.8-4.2 months) with favezelimab plus pembrolizumab.
Conclusions
Favezelimab with or without pembrolizumab had a manageable safety profile, with no treatment-related deaths. Promising antitumor activity was observed with combination therapy, particularly in participants with PD-L1 CPS ≥1 tumors.
Key words: LAG-3, PD-1, colorectal cancer, advanced
Highlights
-
•
Response rates were higher with favezelimab plus pembrolizumab in participants with MSS mCRC with PD-L1 CPS ≥1 tumors.
-
•
Favezelimab plus pembrolizumab vs pembrolizumab improved exploratory efficacy outcomes of survival and duration of response in MSS mCRC.
-
•
The pharmacokinetics of favezelimab were similar with favezelimab plus pembrolizumab in combination and in co-formulation.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer-related death in the United States, although CRC-related mortality rates have been on the decline.1 The emergence of anti-programmed cell death protein 1 (anti-PD-1) and other checkpoint inhibitor therapies has led to major advances in the treatment of participants with microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic CRC (mCRC) who represent ∼5% of participants with mCRC. Despite these advances, standard-of-care for the majority of those diagnosed with non-MSI-H/dMMR or microsatellite stable (MSS) mCRC remains fluoropyrimidine-based chemotherapy combination regimens and monoclonal antibodies targeting vascular endothelial growth factor and epidermal growth factor receptor (in participants with RAS wild-type tumors). Some studies suggest that programmed death-ligand 1 (PD-L1) expression in a subpopulation of participants with mCRC may indicate poor prognosis.2 Moreover, participants with previously treated MSS mCRC that has progressed following treatment with two or more prior therapies have limited treatment options.3,4 Therapies such as regorafenib or TAS-102 provide limited durability of clinical benefit in previously treated MSS mCRC, with median overall survival (OS) ranging from 6 to 9 months.5,6 There remains an unmet need for immunotherapy-based combination regimens that provide durable clinical benefit for those with previously treated MSS mCRC.
Lymphocyte-activation gene 3 (LAG-3) is an immunomodulatory receptor that regulates Teff homeostasis, proliferation, and activation and has a role in Treg cell suppressor activity.7 LAG-3 is co-expressed with PD-1 on anergic T cells and preclinical studies show that blockade of both LAG-3 and PD-1 potentiates T-cell activity and results in reversal of T-cell anergy.8, 9, 10
Recent data from the phase III RELATIVITY-047 study showed that the fixed-dose combination of the anti-LAG-3 antibody relatlimab and nivolumab versus nivolumab alone in participants with advanced, untreated melanoma resulted in statistically significant improvement in progression-free survival (PFS) [median 10.1 months versus 4.6 months; hazard ratio 0.75, 95% confidence interval (CI) 0.6-0.9; P = 0.0055].11
MK-4280 (favezelimab) is a humanized, immunoglobulin G4, anti-LAG-3 monoclonal antibody that prevents binding of LAG-3 to its ligand, major histocompatibility complex class II. The MK-4280-001 phase I study (NCT02720068) is a two-part study of the safety and pharmacokinetics (PK) of favezelimab as monotherapy and in combination with pembrolizumab either in sequential administration or as the 800 mg favezelimab/200 mg pembrolizumab co-formulation in adults with metastatic solid tumors. Co-formulation may reduce the number of required injections, result in fewer side-effects, and lead to increased therapeutic efficacy compared with sequential treatment administration. Previously presented data from the dose-escalation phase (part A) of this first-in-human multicohort study showed that the anti-LAG-3 antibody favezelimab was well tolerated alone and with pembrolizumab across all dose levels.12
In this current analysis from the dose-confirmation phase (part B) of the MK-4280-001 study, we present results of an interim analysis of the safety and efficacy of favezelimab alone or in combination with pembrolizumab in participants with advanced MSS mCRC that had progressed on available therapies.
Methods
Patients
Eligible participants were aged ≥18 years with histologically or cytologically confirmed metastatic solid tumors for which there is no beneficial therapy in the dose-escalation (part A) phase. In the dose-confirmation phase (part B) participants had to have MSS locally advanced or mCRC originating in either the colon or rectum that progressed on all available standard-of-care therapies including fluoropyrimidine, oxaliplatin, and irinotecan (3L+) without prior anti-PD-1/PD-L1 therapy. Selection criteria also included measurable disease per immune-related Response Evaluation Criteria in Solid Tumors version 1.1 (irRECIST v1.1), Eastern Cooperative Oncology Group performance status of 0 or 1, adequate organ function, submission of a newly-obtained or archival tumor sample for PD-L1 analysis, and provision of written, informed consent. Participants were excluded if they had received chemotherapy, radiation, or biological cancer therapy or had not recovered to Grade 0-1 from adverse events (AEs) due to cancer therapies administered within 4 weeks of study treatment, had received prior anti-LAG-3, anti-PD-1/PD-L1, or anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) therapy, or had known MSI-H/dMMR mCRC. Full eligibility criteria are in the study protocol (Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100639).
Trial design and treatment
In part B of this open-label, phase I study, 20 participants with 3L+ MSS mCRC were enrolled to receive the recommended phase II dose of 800 mg favezelimab every 3 weeks (Q3W), with an additional 40 participants each enrolled to receive 800 mg favezelimab plus 200 mg pembrolizumab administered sequentially, or the 800 mg favezelimab/200 mg pembrolizumab co-formulation, each intravenously Q3W. Participants receiving 800 mg favezelimab could cross over to receive 800 mg favezelimab plus 200 mg pembrolizumab after confirmed disease progression (irRECIST, v1.1). Enrollment occurred via an interactive voice response system (IVRS)/integrated web response system. Participants with 3L+ CRC were enrolled to receive first favezelimab monotherapy, followed by 800 mg favezelimab plus 200 mg pembrolizumab either in sequential administration or as the favezelimab/pembrolizumab co-formulation. If at any time more than one arm was enrolling participants, they were assigned in an alternating fashion across the open arms at study level through IVRS.
Assessments
Tumor response was assessed per RECIST v1.1 by investigator review by computed tomography scan or magnetic resonance imaging at 9 weeks after first dose and every 9 weeks thereafter. During follow-up, survival was assessed every 12 weeks. In part B, PD-L1 was assessed (but not required to be completed) during screening using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Inc., Carpinteria, CA). For patients with CRC, MSI-H or dMMR status was determined locally by PCR or immunohistochemistry (IHC) before or during screening. Tumors were MSI-H if at least two of five microsatellite markers changed compared with controls as detected by PCR, and dMMR if expression of at least one of four marker proteins were lost compared with controls as detected by IHC. AEs were graded according to National Cancer Institute CTCAE, version 4.0. Toxicities were characterized for seriousness, causality, and toxicity grade.
PK
The PK of favezelimab were assessed at all doses from 7 mg to 800 mg. At the recommended phase II dose (RP2D) of 800 mg favezelimab in participants with 3L+ MSS mCRC, PK were assessed as monotherapy, in combination with pembrolizumab, and as the favezelimab/pembrolizumab co-formulation. Serum samples for PK assessments were drawn at multiple timepoints across the Q3W dosing interval over multiple dosing cycles. LAG-3 target engagement of favezelimab in systemic circulation was also assessed by measuring total soluble LAG-3 (sLAG-3). Serum samples for sLAG-3 were drawn at multiple timepoints across the Q3W dosing interval over multiple dosing cycles.
Study oversight
The study was designed by academic investigators and employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc., Rahway, NJ, USA. The protocol was approved by the appropriate institutional review boards or ethics committee at each participating institution. All authors attest that the trial was conducted in accordance with the protocol and all its amendments and Good Clinical Practice standards. All participants provided written informed consent.
Endpoints
The primary objective was safety and tolerability of favezelimab alone and in combination with pembrolizumab as sequential therapy and when administered as a co-formulated product. Secondary objectives included characterization of the pharmacokinetic profile of favezelimab in all participants, alone or in combination with pembrolizumab as sequential therapy, and when administered as a co-formulated product, and objective response rate (ORR) per RECIST v1.1 by investigator review of favezelimab alone or in combination with pembrolizumab as sequential therapy and when administered as a co-formulated product. Exploratory objectives included duration of response (DOR) and PFS per RECIST v1.1 by investigator review, OS, and investigation of the relationship between efficacy biomarkers (e.g. PD-L1) and antitumor activity of favezelimab alone, or in combination with pembrolizumab and when administered as a co-formulated product. All exploratory endpoints are detailed in the protocol (Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100639).
Statistical analyses
The safety analysis population included all participants with at least one dose of study treatment including participants who crossed over from favezelimab alone to favezelimab plus pembrolizumab, and the PK analysis population included participants per protocol. The efficacy analysis population included the full analysis set (FAS) of all participants with a baseline scan with measurable disease by investigator assessment who received at least one dose of study treatment regardless of dose level (not including participants who crossed over from favezelimab only to favezelimab plus pembrolizumab). Summary statistics were provided for safety endpoints as appropriate. The point estimate and 95% CIs were provided for ORR. Kaplan–Meier estimates of PFS and OS were provided by PD-L1 status.
Results
Participants
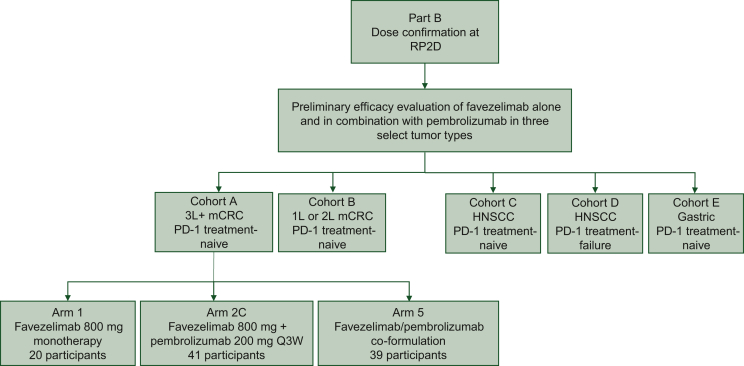
Between May and September 2019, 20 participants were enrolled to receive 800 mg favezelimab alone and 89 were enrolled to receive 800 mg favezelimab plus 200 mg pembrolizumab (41 as combination therapy plus 9 participants who crossed over from favezelimab alone, 39 from favezelimab/pembrolizumab co-formulation) (Figure 1). Participants who received favezelimab plus pembrolizumab either sequentially or as favezelimab/pembrolizumab co-formulation are referred to as the favezelimab plus pembrolizumab arm going forward. Participants who received favezelimab only had a median age of 61.5 years (range 36-80 years) and those who received favezelimab plus pembrolizumab had a median age of 58 years (range 32-81 years), with 36 having PD-L1 combined positive score (CPS) ≥1 and 35 having PD-L1 CPS <1 (Table 1). All participants receiving favezelimab monotherapy discontinued treatment due to clinical and disease progression in 19 (95%) participants, and participant withdrawal in 1 (5%). In addition, 77 of 80 (96%) participants receiving favezelimab plus pembrolizumab [excluding 9 participants who crossed over (FAS)] discontinued treatment due to clinical [11 (14%)] and disease progression [50 (62%)] in 61 (76%) participants, participant [10 (13%)] and physician withdrawal [1 (1%)] in 11 (14%) participants, and AEs in 5 (6%) participants. At the data cut-off date of 23 October 2020, the median duration of follow-up was 5.8 months in the favezelimab monotherapy arm and 6.2 months in the favezelimab plus pembrolizumab arm.
Figure 1.
Study diagram. Study flow for part B cohort A in participants with PD-1/PD-L1 treatment-naive MSS mCRC that progressed on ≥2 prior therapies.
HNSCC, head and neck squamous cell carcinoma; mCRC, metastatic colorectal cancer; MSS, microsatellite stable; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; Q3W, every 3 weeks; RP2D, recommended phase II dose.
Table 1.
Baseline characteristics in participants with MSS mCRC
| Characteristics, n (%) | Favezelimab N = 20 |
Favezelimab + pembrolizumab N = 80 |
|---|---|---|
| Age, years, median (range) | 61.5 (36–80) | 58.0 (32–81) |
| >65 years, n (%) | 6 (30.0) | 19 (23.8) |
| Male, n (%) | 9 (45.0) | 54 (67.5) |
| ECOG PSa 1 | 14 (70.0) | 49 (61.3) |
| PD-L1 CPS ≥1 | 12 (60.0) | 36 (45.0) |
| PD-L1 CPS <1 | 8 (40.0) | 35 (43.8) |
| PD-L1 missing | 0 | 9 (11.2) |
| Prior lines of therapy, n (%) | ||
| Adjuvant | 0 | 2 (2.5) |
| 1 | 0 | 4 (5.0) |
| 2 | 3 (15.0) | 16 (20.0) |
| ≥3 | 17 (85.0) | 58 (72.5) |
CPS, combined positive score; mCRC, metastatic colorectal cancer; MSS, microsatellite stable; PD-L1, programmed death-ligand 1.
ECOG PS, Eastern Cooperative Oncology Group performance status.
Safety
AEs of any cause occurred in 19 of 20 (95.0%) participants who received favezelimab only and 87 of 89 (97.8%) participants in the safety analysis population who received favezelimab plus pembrolizumab. Treatment-related events occurred in 13 (65.0%) and 58 (65.2%) participants, respectively, with grade ≥3 treatment-related events reported in 3 (15.0%) and 18 (20.2%), participants, respectively. No grade 5 treatment-related events were reported. The most commonly reported any grade treatment-related AEs (TRAEs) included fatigue in 4 (20.0%) and nausea in 3 (15.0%) participants who received favezelimab alone, and fatigue in 15 (16.9%), hypothyroidism in 13 (14.6%), and infusion-related reactions and decreased appetite in 6 (6.7%) participants each who received favezelimab plus pembrolizumab (Table 2). A total of 2 (10.0%) participants who received favezelimab alone and 32 (36.0%) who received favezelimab plus pembrolizumab had an immune-mediated AE. The most commonly reported immune-mediated events included hypothyroidism in 1 (5.0%) participant who received favezelimab and 14 (15.7%) who received favezelimab plus pembrolizumab, infusion-related reactions in 1 (5.0%) and 6 (6.7%), and hyperthyroidism and pneumonitis in 0 (0%) and 5 (5.6%) participants each, respectively (Table 2). No incidence of myocarditis was reported. Grade ≥3 immune-mediated events included pneumonitis occurring in two (2.2%) participants who received favezelimab plus pembrolizumab.
Table 2.
Summary of adverse events in patients with MSS mCRC
| Adverse events (AE) | Favezelimab N = 20 |
Favezelimab + pembrolizumab N = 89a |
||
|---|---|---|---|---|
| Any AE | 19 (95.0) | 87 (97.8) | ||
| Any treatment-related AE | 13 (65.0) | 58 (65.2) | ||
| Grade 3-4 | 3 (15.0) | 18 (20.2) | ||
| Led to discontinuation | 0 | 5 (5.6) | ||
| Immune-mediated AEs | 2 (10.0) | 32 (36.0) | ||
| Events ≥5% in any armb | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Nausea | 3 (15.0) | 0 | 4 (4.5) | 0 |
| Fatigue | 4 (20.0) | 1 (5.0) | 15 (16.9) | 2 (2.2) |
| Influenza-like illness | 2 (10.0) | 0 | 0 | 0 |
| Hypothyroidism | 1 (5.0) | 0 | 13 (14.6) | 0 |
| Diarrhea | 1 (5.0) | 0 | 3 (3.4) | 0 |
| Vomiting | 1 (5.0) | 0 | 1 (1.1) | 0 |
| Pruritus | 1 (5.0) | 0 | 7 (7.9) | 0 |
| Maculopapular rash | 1 (5.0) | 0 | 6 (6.7) | 1 (1) |
| Rash | 1 (5.0) | 0 | 5 (5.6) | 0 |
| Lipase increased | 1 (5.0) | 1 (5.0) | 0 | 0 |
| Thyroid function test abnormal | 1 (5.0) | 0 | 0 | 0 |
| Syncope | 1 (5.0) | 1 (5.0) | 0 | 0 |
| Arthralgia | 1 (5.0) | 0 | 2 (2.2) | 0 |
| Back pain | 1 (5.0) | 0 | 1 (1.1) | 0 |
| Muscle tightness | 1 (5.0) | 0 | 0 | 0 |
| Chills | 1 (5.0) | 0 | 2 (2.2) | 0 |
| Infusion-related reaction | 1 (5.0) | 0 | 6 (6.7) | 1 (1.1) |
| ALT increased | 1 (5.0) | 0 | 3 (3.4) | 2 (2.2) |
| Hyperphosphatemia | 1 (5.0) | 0 | 0 | 0 |
| Cough | 1 (5.0) | 0 | 4 (4.5) | 0 |
| Dermatitis acneiform | 1 (5.0) | 0 | 0 | 0 |
| Photosensitivity reaction | 1 (5.0) | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 5 (5.6) | 2 (2.2) |
| Hyperthyroidism | 0 | 0 | 5 (5.6) | 0 |
There were no grade 5 treatment-related events.
ALT, alanine aminotransferase; mCRC, metastatic colorectal cancer; MSS, microsatellite stable.
Includes nine patients who crossed over from favezelimab monotherapy.
Treatment-related adverse events.
PK
Favezelimab concentrations peaked at the end of the intravenous infusion followed by a decrease over the Q3W dosing interval (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100639). Geometric mean and 95% CI estimates for peak concentrations (Cmax), overall exposure (AUC0-3weeks), and day 21 trough concentration (Ctrough) of favezelimab in cycle 1 are shown in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100639. In participants receiving favezelimab plus pembrolizumab, estimated overall favezelimab exposure (AUC0-3weeks) was 1244 μg/ml/day (95% CI 1096-1412 μg/ml/day), Cmax was 241 μg/ml (95% CI 224-261 μg/ml), and Ctrough was 5 μg/ml (95% CI 3-9 μg/ml). These are consistent with the PK observed in participants who received the favezelimab/pembrolizumab co-formulation with mean favezelimab AUC0-3weeks of 1187 μg/ml/day (95% CI 1045-1349 μg/ml/day), Cmax of 235 μg/ml (95% CI 218-253 μg/ml) and Ctrough of 9 μg/ml (95% CI 6-15 μg/ml). The estimated median apparent half-life (T1/2) of favezelimab was 6.2 days either as monotherapy, or in combination with pembrolizumab in sequential administration or as favezelimab/pembrolizumab co-formulation. Total sLAG-3 in serum increased from baseline after favezelimab dosing and is reflective of target engagement, as has previously been shown for soluble targets binding to monoclonal antibodies.13,14 This increase in total sLAG-3 at 800 mg favezelimab RP2D was sustained over the 3-week dosing interval, irrespective of whether participants received favezelimab monotherapy, in combination with pembrolizumab in sequential administration or as favezelimab/pembrolizumab co-formulation (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100639).
Efficacy
No participant (0%) who received favezelimab alone had a confirmed or unconfirmed objective response. In participants who received favezelimab plus pembrolizumab in the FAS, 5 of 80 (6.3%) had a confirmed objective response [1 complete response (CR), 4 partial response (PR)]. Among participants with PD-L1 CPS ≥1 tumors, 4 of 36 (11.1%) had a response (1 CR, 3PR) compared with 1 of 35 (2.9%) participants with PD-L1 CPS <1 tumors (Table 3). A total of nine participants had a missing PD-L1 status and were not included. The median DOR was 10.6 months (range 5.6-12.5 months).
Table 3.
Antitumor activity with favezelimab plus pembrolizumab in participants with MSS mCRC by PD-L1 status
| Best response,an (%) | Favezelimab + pembrolizumab N = 80 |
|
|---|---|---|
| PD-L1 CPS ≥1 N = 36 |
PD-L1 CPS<1 N = 35 |
|
| Objective response rate | 4 (11.0) | 1 (2.9) |
| Best overall response | ||
| Complete response | 1 (2.8) | 0 (0.0) |
| Partial response | 3 (8.3) | 1 (2.9) |
| Stable disease | 9 (25.0) | 4 (11.4) |
| Progressive disease | 15 (41.7) | 24 (68.6) |
| DCR (CR + PR + SD) | 13 (36.1) | 5 (14.3) |
| Median duration of response, months (range) | 10.6 (5.6-12.5) |
|
CPS, combined positive score; CR, complete response; DCR, disease control rate; mCRC, metastatic colorectal cancer; MSS, microsatellite stable; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease.
15 Patients were non-assessable or had no assessment.
Median PFS was 2.1 months (95% CI 1.9-2.2 months) among participants in the FAS who received favezelimab plus pembrolizumab. Among those with PD-L1 CPS ≥1 tumors, median PFS was 2.2 months (95% CI 1.8-4.2 months) compared with 2.0 months (95% CI 1.9-2.1 months) in participants with PD-L1 CPS <1 tumors. The 12-month PFS rates were 7% and 12% among all participants and participants with PD-L1 CPS ≥1 tumors, respectively, and was not estimable in participants with PD-L1 CPS <1 tumors (Figure 2A).
Figure 2.
Kaplan–Meier estimates of (A) progression-free survival (PFS) and (B) overall survival (OS) with favezelimab plus pembrolizumab in patients with MSS mCRC by PD-L1 status.
CI, confidence interval; CPS, combined positive score; mCRC, metastatic colorectal cancer; MSS, microsatellite stable; PD-L1, programmed death-ligand 1.
The median OS was 8.3 months (95% CI 5.5-12.9 months) among participants in the FAS who received favezelimab plus pembrolizumab. In participants with PD-L1 CPS ≥1 tumors, median OS was 12.7 months (95% CI 4.5 months to not reached) compared with 6.7 months (95% CI 3.8-11.0 months) in participants with PD-L1 CPS <1 tumors. The 12-month OS rate was 41% in all participants, and 51% and 30% in participants with PD-L1 CPS ≥1 and PD-L1 CPS <1 tumors, respectively (Figure 2B).
Discussion
In this first interim report of the safety and antitumor activity of the LAG-3 inhibitor favezelimab in participants with metastatic solid tumors, favezelimab alone or in combination with pembrolizumab had a manageable safety profile, with similar incidence of grade ≥3 TRAEs observed in the monotherapy and combination therapy arms. No treatment-related deaths were observed in participants with MSS mCRC. In addition, promising antitumor activity was observed with combination therapy, including with the co-formulation (favezelimab/pembrolizumab), compared with monotherapy most notably in patients with PD-L1 CPS ≥1 tumors.
At this interim analysis, the most common TRAE observed was fatigue in 20% of participants who received favezelimab monotherapy and 16.9% of patients who received favezelimab plus pembrolizumab. Treatment-related events observed with monotherapy and combination therapy were similar to those previously observed with pembrolizumab across multiple tumor types.15, 16, 17 In addition, the incidence of treatment-related events was consistent with that observed with a similar combination of anti-LAG-3 and anti-PD-1 therapies.18 No incidence of myocarditis was observed in the current study. Pharmacokinetic parameters were similar for patients who received favezelimab plus pembrolizumab either in sequential administration or as the favezelimab/pembrolizumab co-formulation.
After a median follow-up of 5.8 months, the ORR was 0% for participants who received monotherapy with the RP2D of 800 mg favezelimab. In contrast, an ORR of 6.3% was observed in all participants who received favezelimab plus pembrolizumab, for an overall study ORR of 6.3%. Antitumor activity increased in participants with PD-L1 CPS ≥1 tumors where higher response rates and prolonged OS were observed. Specifically, ORR was 11.1% in participants with PD-L1 CPS ≥1 tumors and median OS was 12.7 months in participants with PD-L1 CPS ≥1 tumors compared with 6.7 months in participants with PD-L1 CPS <1 tumors. The ORR of 11.1% observed in patients with PD-L1 CPS ≥1 tumors compares favorably with the ORR observed with therapies such as regorafenib (1.0%)5 and TAS-102 (1.6%) in patients with previously treated mCRC, although participant PD-L1 CPS tumor expression was not a factor in the latter. The current data are in contrast to the lack of antitumor activity observed following pembrolizumab as monotherapy in participants with MSS mCRC.19,20 In the phase II KEYNOTE-016 study, an ORR of 40% with a 20-week PFS rate of 78% were observed in participants with dMMR mCRC compared with an ORR of 0% with 20-week PFS rate of 11% in participants with mismatch repair proficient mCRC.19 Similarly, in the multicohort KEYNOTE-028 study, no response (0 of 22) was observed in participants with non-MSI-H/dMMR PD-L1-positive mCRC. The increased antitumor activity observed in participants with PD-L1 CPS ≥1 tumors in the current study is consistent with the mechanism of action of immune checkpoint inhibition as PD-L1 expression correlates with other markers of immune infiltration.21 A limitation of the current study is that it is a single-arm, non-randomized study with a small patient population. An ongoing phase III study (NCT05064059) is evaluating the efficacy and safety of the favezelimab/pembrolizumab co-formulation in patients with PD-L1 CPS ≥1 MSS mCRC.
In summary, these data suggest that favezelimab plus pembrolizumab, including as the favezelimab/pembrolizumab co-formulation, is a promising treatment option for participants with MSS mCRC.
Acknowledgements
This study and assistance with medical writing were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD). We thank the participants and their families and caregivers for participating in the study, all primary investigators and their site personnel, Scot Ebbinghaus, MD (MSD) for critical review, and Luana Atherly-Henderson, PhD, CMPP (MSD) for medical writing assistance.
Funding
This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (no grant number).
Disclosure
EG reports research funding to the institution for clinical studies from Merck Sharp & Dohme (MSD), Novartis, AstraZeneca/MedImmune, Roche, Thermo Fisher Scientific, Taiho Oncology, Affimed Gmbh, Amgen SA, Anaveon AG, AstraZeneca AB, Biontech Gmbh, Catalym Gmbh, Cytomx, F. Hoffmann La Roche Ltd, F-Star Beta Limited, Genentech Inc., Genmab B.V., Hutchison, Medipharma Limited, Icon, Imcheck Therapeutics, Immunocore Ltd, Janssen-Cilag SA, MedImmune LLc, Merck Kgga, S.A – Peptomyc, Ribon Therapeutics, Roche Farma SA, Seattle Genetics Inc., Symphogen A/S, fees for consulting and advisory roles from Roche, Ellipses Pharma, NeoMed, Janssen, Boehringer Ingelheim, Seattle Genetics, TFS, Alkermes, Thermo Fisher Scientific, Bristol Myers Squibb (BMS), mAb Discovery, Anaveon, fees for speakers bureau from MSD, Roche, Thermo Fisher Scientific, Lilly, reimbursement for travel from BMS, Menarini, Glycotope GmbH, MSD; AS reports research funding to the institution from MSD, stock in BMS, Merck, fee for speakers bureau from Merck, Eisai; NJL, AP, YYL, TG report research funding to the institution for clinical studies from MSD; SAI reports research funding to the institution for clinical studies from MSD, AstraZeneca, Eisai, Boryung Pharm, Daewon Pharm, Roche, Pfizer, fees for consulting or advisory role from AstraZeneca, Daiichi Sankyo, Eisai, GlaxoSmithKline, Lilly, MSD, Novartis, Pfizer, Roche; RG reports research funding to the institution for clinical studies from MSD, Novartis, honoraria from BMS, Lilly, Medison, Roche, Novartis, Janssen, Takeda, MSD, Pfizer, Merck, fees for consulting or advisory role from Eisai, AstraZeneca, Bayer, MSD, Novartis, Boehringer Ingelheim, BOL Pharma, Roche, reimbursement for travel from Merck, Bayer, BMS, Medison; MDM reports research funding to the institution from MSD, Roche, Sanofi, PharmaMar, AbbVie, Janssen, Faron, Genentech, MacroGenics, Menarini, Nektar, Novartis; MW reports research funding to the institution for clinical studies, fees for consulting or advisory roles from BMS, Novartis, Kite, Heidelberg Pharma, Roche, Boehringer Ingelheim, honoraria from BMS, Merck, Roche, Novartis, Kite, Boehringer Ingelheim, AstraZeneca, reimbursement for travel from Glenmark, BMS, AstraZeneca; JP, SJ, MC, AA, JH are employees of MSD LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) and hold stock in Merck & Co., Inc., Rahway, NJ, USA; GSF reports research funding to the institution for clinical studies from 3-V Biosciences, Abbisko, AbbVie, ABL Bio ADC Therapeutics, Aileron, American Society of Clinical Oncology, Amgen, ARMO/Eli Lilly, Artios, AstraZeneca, BeiGene, BioAtla, BioInvent, Biothera, Bicycle, Boehringer Ingelheim, Celldex, Celgene, CicloMed, Curegenix, Curis, Cyteir, Daiichi, DelMar, eFFECTOR, Eli Lilly, EMD Serono, Epizyme, Erasca, Exelixis, Freenome, Fujifilm, Genmab, GlaxoSmithKline, Hutchison MediPharma, IGM Biosciences, Ignyta, ImmunoGen/MacroGenics, Incyte, Jacobio, Jounce, Kolltan, Loxo/Bayer, MedImmune, Millennium, Merck, miRNA Therapeutics, National Institutes of Health, Navire, NiKang, Novartis, OncoMed, Oncorus, Oncothyreon, Poseida, Precision Oncology, Prelude, PureTech, Pyramid, RasCal, Regeneron, Rgenix, Ribon, Samumed, Sapience, Silicon, Strategia, Syndax, Synthorx/Sanofi, Taiho, Takeda, Tarveda, Teneobio, Tesaro, Tocagen, Turning Point Therapeutics, U.T. MD Anderson Cancer Center, Vegenics, Xencor, fees for consulting or advisory role from Fujifilm, Silicon, Navire, Turning Point, Predicine, EMD Serono, reimbursement for travel from BMS, EMD Serono, Fujifilm, Millenium, Sarah Cannon Research Institute, speakers honoraria from Total Health Conferencing, Rocky Mountain Oncology Society.
Data sharing
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Supplementary Material
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Yang L., Xue R., Pan C. Prognostic and clinicopathological value of PD-L1 in colorectal cancer: a systematic review and meta-analysis. OncoTargets Ther. 2019;12:3671–3682. doi: 10.2147/OTT.S190168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel A., Hofheinz R.D., Kubicka S., et al. Treatment decisions in metastatic colorectal cancer – Beyond first and second line combination therapies. Cancer Treat Rev. 2017;59:54–60. doi: 10.1016/j.ctrv.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E., Cervantes A., Adam R., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A., van Cutsem E., Sobrero A., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 6.Yoshino T., Mizunuma N., Yamazaki K., et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 7.Maruhashi T., Sugiura D., Okazaki I.M., Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datar I., Sanmamed M.F., Wang J., et al. Expression analysis and significance of PD-1, LAG-3, and TIM-3 in human non–small cell lung cancer using spatially resolved and multiparametric single-cell analysis. Clin Cancer Res. 2019;25(15):4663–4673. doi: 10.1158/1078-0432.CCR-18-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris-Bookman S., Mathios D., Martin A.M., et al. Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int J Cancer. 2018;143(12):3201–3208. doi: 10.1002/ijc.31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo S.R., Turnis M.E., Goldberg M.V., et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawbi H.A., Schadendorf D., Lipson E.J., et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakhani B., Bauer T., Abraham A. The anti-LAG-3 antibody MK-4280 as monotherapy and in combination with pembrolizumab for advanced solid tumors: first-in-human phase 1 dose-finding study. J Immunother Cancer. 2018;6(suppl 1):115. abstract O126. [Google Scholar]
- 13.Lachmann H.J., Lowe P., Felix S.D., et al. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206(5):1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng K., Wang Y., Siradze K., et al. Measurement of IL-17AA and IL-17FF as pharmacodynamic biomarkers to demonstrate target engagement in the phase I study of MCAF5352A. AAPS J. 2018;21(1):9. doi: 10.1208/s12248-018-0280-z. [DOI] [PubMed] [Google Scholar]
- 15.Garon E.B., Rizvi N.A., Hui R., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 16.Shah M.A., Kojima T., Hochhauser D., et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 keynote-180 study. JAMA Oncol. 2019;5:546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A., Puzanov I., Dummer R., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipson E.J., Tawbi H.A.-H., Schadendorf D., et al. Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: primary phase III results from RELATIVITY-047 (CA224-047) J Clin Oncol. 2021;39(suppl 15) 9503. [Google Scholar]
- 19.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neil B.H., Wallmark J.M., Lorente D., et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayers M., Lunceford J., Nebozhyn M., et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.