Abstract
Background
BRAFV600E mutations occur in 8%-12% of metastatic colorectal cancer (mCRC) cases and are associated with poor survival. European guidelines recommend combination (doublet or triplet) chemotherapy plus bevacizumab in first line. However, an unmet need remains for more effective treatments for these patients.
Patients and methods
CAPSTAN CRC is a European, retrospective, multicenter, observational study evaluating real-world treatment practices for patients with BRAFV600E-mutant mCRC treated between 1 January 2016 and 31 January 2020. The primary objective was to describe first-line treatment patterns. Secondary objectives included describing baseline demographics, mutational testing procedures, treatment effectiveness, and safety.
Results
In total, 255 patients (median age 66.0 years; 58.4% female) with BRAFV600E-mutant unresectable mCRC from seven countries were included. Most had right-sided tumors (52.5%) and presented with synchronous disease at diagnosis (66.4%). Chemotherapy plus targeted therapy (68.7%) was preferred at first line over chemotherapy alone (31.3%). The main first-line treatments were FOLFOX plus bevacizumab (27.1%) and FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin, irinotecan) with/without bevacizumab (27.1%/19.2%). Median duration of first-line treatment was 4.9 months. Overall, 52.5% received second-line treatment. Across all first-line regimens, progression-free survival (PFS) and overall survival were 6.0 [95% confidence interval (CI) 5.3-6.7] months and 12.9 (95% CI 11.6-14.1) months, respectively. Triplet plus targeted therapy was associated with more adverse events (75.0%) compared with triplet chemotherapy alone (50.0%) and doublet chemotherapy alone (36.1%). Multivariate analysis identified low body mass index and presence of three or more metastatic sites as significant prognostic factors for PFS.
Conclusions
This study is, to date, the largest real-world analysis of patients with BRAFV600E-mutant mCRC, providing valuable insights into routine first-line treatment practices for these patients. The data highlight the intrinsic aggressiveness of this disease subgroup, confirming results from previous real-world studies and clinical trials, and stressing the urgent need for more effective treatment options in this setting.
Key words: metastatic colorectal cancer, observational, BRAF mutation, treatment practices, targeted therapy, real world
Highlights
-
•
Most patients with BRAF-mutant mCRC received doublet chemotherapy with or without targeted therapy at first line.
-
•
The triplet chemotherapy + targeted therapy group had the highest ORR (52.5%) versus the other treatment groups.
-
•
Median PFS was 6.0 months (95% CI 5.3-6.7) and median OS was 12.9 months (95% CI 11.6-14.1) across all regimens.
-
•
About 52.5% of patients were able to receive a potentially effective second-line therapy.
-
•
This study highlights the unmet need for effective treatment strategies for patients with BRAF-mutant mCRC.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide.1 Approximately 20% of patients with CRC present with metastatic disease at the time of initial diagnosis, with 5-year survival rates <20% for metastatic CRC (mCRC) across Europe.2,3 The identification of different tumor genomic mutations and profiles in recent years has proved to be pivotal in understanding tumor heterogeneity, guiding treatment and improving survival.4
Mutations in the BRAF gene occur in 8%-12% of mCRC cases, >95% of which are BRAFV600E.5,6 BRAFV600E mutations represent an aggressive phenotype associated with a poor prognosis and resistance to standard chemotherapy regimens.5, 6, 7, 8 Given the importance of BRAF as a prognostic and predictive biomarker, the European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network guidelines recommend the assessment of BRAF status alongside RAS at diagnosis of metastatic disease to better guide treatment decisions.5,9
Current international guidelines recommend treating patients with BRAFV600E-mutant mCRC in first line using either a doublet or triplet combination chemotherapy regimen with or without the vascular endothelial growth factor (VEGF) inhibitor, bevacizumab, based on a small subgroup analysis of patients in the TRIBE studies and retrospective series.5,9,10 The benefits of using anti-VEGF inhibitors, including aflibercept and ramucirumab, for patients with BRAFV600E-mutant mCRC were initially reported in post hoc analyses of randomized trials in second line.11,12 The results of individual patient data meta-analysis showed no increased benefit with folinic acid, 5-fluorouracil, oxaliplatin, irinotecan (FOLFOXIRI) plus bevacizumab versus doublet regimen plus bevacizumab among patients with BRAF-mutant tumors.10
Studies have suggested that BRAFV600E is a predictive marker for limited response to epidermal growth factor receptor (EGFR) inhibitors in patients with mCRC.13, 14, 15 Although the role of EGFR inhibitors in combination with chemotherapy is controversial in the first-line setting, the EGFR inhibitor cetuximab recently received approval in combination with the BRAF inhibitor encorafenib for use in the second-line setting after prior systemic therapy in BRAFV600E-mutant mCRC based on results from the pivotal BEACON CRC study.16, 17, 18, 19, 20
It is still unclear, however, what the optimal first-line treatment strategy is for this specific population, with local treatment practices and associated guidelines varying widely. Furthermore, although clinical trials provide valuable information regarding the safety and efficacy of therapies, a significant knowledge gap persists with regard to the real-world treatment practices for BRAFV600E-mutant mCRC, and their effectiveness and safety in routine clinical practice.5,6,9,17 Observational studies help to close this gap, complementing randomized controlled trials (RCTs) by putting clinical trial findings in real-world context.
CAPSTAN CRC (NCT04317599) is the largest observational study that collected real-world data to describe the baseline characteristics, treatment patterns, and treatment effectiveness and safety for patients with BRAFV600E-mutant mCRC across Europe.
Methods
Study design
The CAPSTAN CRC study (NCT04317599) is a retrospective, multicenter, observational study conducted in Europe for BRAFV600E-mutant mCRC adult patients (aged ≥18 years). The study was approved by the independent ethics committee or institutional review board at each site and was conducted in accordance with the requirements of the regulatory authorities of each country and in accordance with the Declaration of Helsinki. All patients were informed of their data collection or a waiver was obtained, according to applicable regulations.
The study index date was defined as the date of initiation of first-line treatment for BRAFV600E-mutant mCRC between 1 January 2016 and 31 December 2018. Patients were observed until death, loss to follow-up, or study cut-off date (31 January 2020), whichever occurred first.
Patient population and sample selection
Eligible patients had histologically or cytologically confirmed metastatic and unresectable CRC, with presence of a BRAFV600E mutation confirmed by a local tissue assay. Patients must have been ≥18 years at the time of mCRC diagnosis and started a registered first-line treatment for mCRC in their respective country during the study index period. Patients were excluded if they had concomitant cancer at the time of mCRC diagnosis (occurring <5 years since diagnosis) or participated in interventional trials on investigational drugs at the time of initiation of first-line treatment.
To obtain a representative sample of real-world patients undergoing treatment for BRAFV600E-mutant mCRC in Europe, and to minimize selection bias, the eligibility criteria were kept broad, and sites were targeted across different medical specialties (oncology and gastroenterology), countries, and hospital type (academic and non-academic). Stratified random sampling was used to select 62 representative sites and an additional 12 backup sites if needed. For each participating site, all eligible patients’ medical records were identified, and an enrollment list was pseudo-randomly generated (see Supplementary materials, available at https://doi.org/10.1016/j.esmoop.2022.100603).
Key study endpoints
The primary objective was to describe first-line treatment patterns in adult patients with BRAFV600E-mutant mCRC, including the duration of treatment. First-line treatment for BRAFV600E-mutant mCRC was defined as the systemic anticancer therapy initiated at first occurrence of unresectable, metastatic disease and received until first documented disease progression, treatment discontinuation, or treatment switch (whichever occurred first).
Secondary objectives included baseline demographic and clinical characteristics of patients with BRAFV600E-mutant mCRC, mutational testing procedures, effectiveness [progression-free survival (PFS), overall survival (OS), and overall response rate (ORR)], and safety of first-line therapies (according to MedDRA 23.0). Treatments received after first disease progression (second- and third-line therapies) were further exploratory objectives. Definitions of effectiveness outcomes can be found in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100603. Safety was described according to the proportion of patients with at least one relevant adverse event during first-line treatment and the frequencies of the most common events reported, both overall and by treatment regimen. A relevant adverse event was defined as an adverse event leading to first-line treatment modification, dose adaptation or discontinuation, or death.
Statistical analyses
To allow a 95% confidence interval (CI) precision of ±2.5%, ±3.4%, and ±4.5% for 5%, 10%, and 20% of patients, respectively, a target sample of 300 patients was set. The full analysis set (FAS) containing all patients who fulfilled the eligibility criteria was analyzed.
Analyses were descriptive, as no formal hypotheses were tested. Continuous variables were summarized by the median. Categorical variables were summarized by percentages and 95% CIs were provided as a measure of error. Where patients had missing data for a particular variable, they were excluded from that analysis. Median PFS and OS were assessed and Kaplan–Meier curves were generated. The reverse Kaplan–Meier method provided a measure of median follow-up, with corresponding 95% CIs estimated.21,22
Univariate analyses were carried out to identify prognostic factors for PFS and OS. Multivariate analyses for PFS and OS were then carried out, retaining factors at a P < 0.15 level from the univariate model. Cox proportional hazards models with a stepwise procedure were used to estimate the hazard ratio and corresponding 95% CIs.23
Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline site and patient characteristics
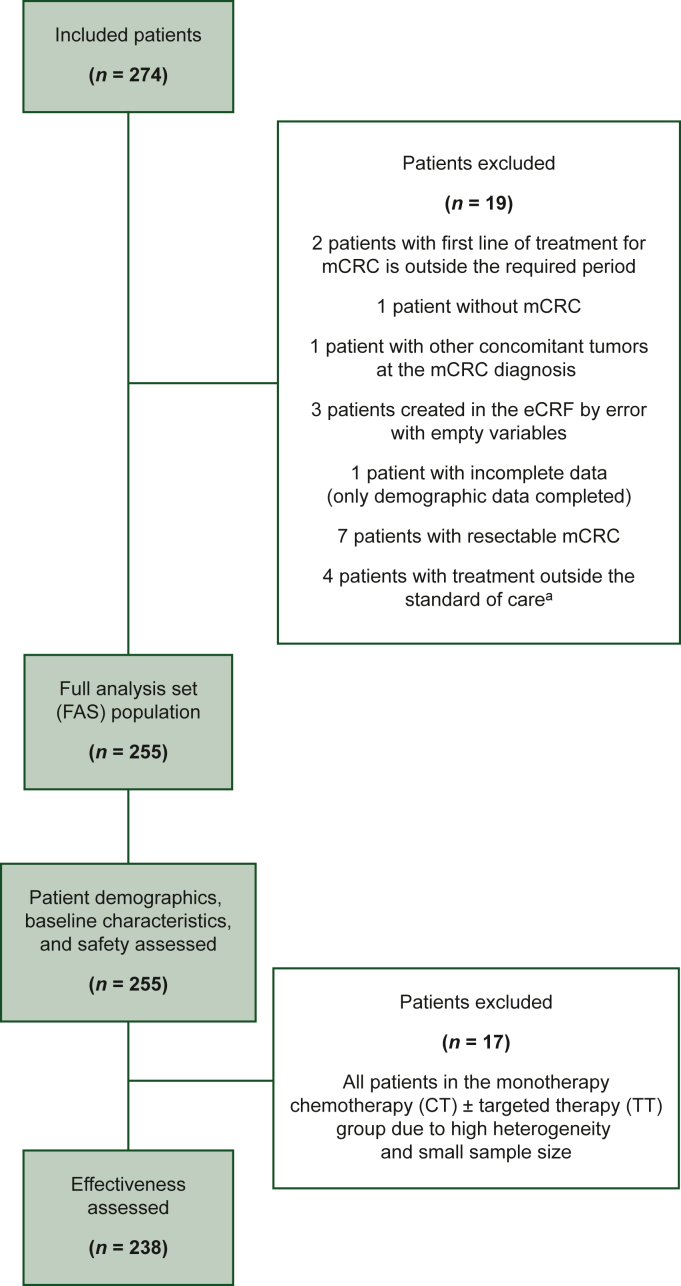
Of the 74 sites approached, there were 13 refusals, 7 dropouts, 4 unable to participate due to COVID-19 restrictions, and 4 unable to participate due to conflicting ongoing data collection programs. A further 12 sites either could not be activated or included no patients. The final sample consisted of 34 medical centers in seven European countries: Austria, Belgium, France, Germany, Italy, Spain, and the United Kingdom. There was a relatively even distribution across academic (n = 14) and nonacademic (n = 20) institutions, and a dominance of medical oncology units (n = 30) over gastroenterology units (n = 4). Of the targeted 300 patients, 274 were included initially, of which 19 were proven ineligible, leaving 255 patients included in the FAS (Figure 1).
Figure 1.
Population flow chart.
eCRF, electronic case report form; mCRC, metastatic colorectal cancer.
aThree patients in the monotherapy group were treated with irinotecan alone, bevacizumab alone, and panitumumab alone and one patient in the doublet chemotherapy group was treated with etoposide–carboplatin.
Of the 255 patients, 58.4% were female, with a median age at the start of first-line treatment of 66.0 years (range 27.0-89.0 years) across all regimens (Table 1). Most patients had right-sided primary tumors (52.5%) and presented with stage IV disease at diagnosis (66.4%). The median number of metastatic sites was two (32.2%) and the most common location of metastasis was the liver (58.0%), followed by the peritoneum (43.9%), lymph nodes (34.9%), and lungs (26.7%). The majority (63.9%) of patients had at least one prior surgery for either primary tumor (61.4%) or metastatic site (13.8%).
Table 1.
Baseline demographic and clinical characteristics of patients
| Variable | Monotherapy CT ± TT |
Doublet CT |
Doublet CT + TT |
Triplet CT |
Triplet CT + TT |
Total |
|---|---|---|---|---|---|---|
| n = 17 | n = 72 | n = 118 | n = 8 | n = 40 | N = 255 | |
|
Median age at start of first-line treatment n Median (min–max), years |
16 82.0 (69.0-89.0) |
72 69.0 (29.0-88.0) |
117 67.0 (27.0-87.0) |
8 61.0 (46.0-70.0) |
40 58.0 (34.0-74.0) |
253 66.0 (27.0-89.0) |
|
Sex Female, n (%) |
10 (58.8) | 42 (58.3) | 72 (61.0) | 5 (62.5) | 20 (50.0) | 149 (58.4) |
|
TNM stage at initial diagnosis of CRC n I/II/III/IV, % |
15 0/20.0/26.7/53.3 |
59 1.7/11.9/16.9/69.5 |
113 1.8/8.8/23.0/66.4 |
6 0/0/33.3/66.7 |
33 3.0/12.1/18.2/66.7 |
226 1.8/10.6/21.2/66.4 |
|
ECOG assessment n 0/1/2/3, % |
10 20.0/70.0/10.0/0.0 |
40 42.5/42.5/10.0/5.0 |
67 55.2/35.8/6.0/3.0 |
2 50.0/50.0/0.0/0.0 |
31 74.2/22.6/3.2/0.0 |
150 53.3/37.3/6.7/2.7 |
|
Primary tumor location n Left ± rectum, % Right only, % Left + right/transverse or right + rectum or not applicable, % |
17 11.8 76.5 11.8 |
72 23.6 58.3 18.1 |
118 35.6 49.2 15.3 |
8 87.5 12.5 0 |
40 37.5 50.0 12.5 |
255 32.5 52.5 14.9 |
|
At least one prior surgery for primary CRC n Yes, % |
17 70.6 |
71 62.0 |
118 58.5 |
8 75.0 |
40 62.5 |
254 61.4 |
|
At least one prior surgery for mCRC n Yes, % |
17 0 |
71 16.9 |
118 9.3 |
8 37.5 |
40 22.5 |
254 13.8 |
|
Number of metastatic sites Median (min–max) 1/2/≥3, % |
1.0 (1.0-3.0) 64.7/29.4/5.9 |
1.0 (1.0-5.0) 51.4/23.6/25.0 |
2.0 (1.0-6.0) 42.4/29.7/28.0 |
1.5 (1.0-2.0) 50.0/50.0/0 |
2.0 (1.0-4.0) 32.5/52.5/15.0 |
2.0 (1.0-6.0) 45.1/32.2/22.7 |
|
Location of metastasis Liver, % Liver only, % Peritoneum, % Lung, % Lymph nodes, % |
35.3 11.8 41.2 29.4 29.4 |
54.2 25.0 55.6 20.8 23.6 |
58.5 16.9 44.1 28.8 39.0 |
50.0 12.5 37.5 12.5 37.5 |
75.0 17.5 25.0 32.5 45.0 |
58.0 18.8 43.9 26.7 34.9 |
|
MSI tested n Yes, % MSI high, % of all tested |
11 64.7 54.5 |
36 50.0 27.8 |
69 58.5 20.3 |
6 75.0 16.7 |
31 77.5 16.1 |
153 60.0 23.5 |
CRC, colorectal cancer; CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; mCRC, metastatic colorectal cancer; MSI, microsatellite instability; TNM, tumor–node–metastasis; TT, targeted therapy.
BRAF mutational testing
The majority (95.7%) of patients were tested for BRAF mutations once, with 89.8% tested before the start of first-line treatment, 9.3% during first-line treatment, 0.4% after first- but before second-line treatment, and 0.4% during second-line treatment. A small number of patients were tested two times (4.2%). Most of the testing procedures for BRAF mutational status used tissue tumor samples (98.8%), with only 1.2% utilizing a blood test to detect circulating tumor DNA. Most tumor samples were archival (76.8%) versus fresh (23.2%). PCR (46.0%) and next-generation sequencing (38.1%) were the most frequently used methods to detect BRAF mutations.
About 60% of patients were also tested locally for microsatellite instability (MSI), with the highest testing rate occurring in France (82.4%) and Belgium (82.1%). Of those tested, 24.4% were mismatch repair deficient.
RAS testing was carried out for 91.8% of patients at first-line treatment, with the lowest testing rate reported in Spain (77.1%). As expected, most of these patients were RAS wild type (92.7%); however, 6.8% had a RAS mutation, an unexpectedly high rate, which may be due to the heterogeneity of CRC, and 0.4% had an unknown status. Next-generation sequencing was the predominant testing method used in France (52.1%), Germany (50.0%), and Austria (50.0%), whereas PCR was used more frequently in Spain (84.0%) and Belgium (52.0%).
Treatment patterns by line of therapy
For first-line therapy, 74.5% of patients received doublet chemotherapy, either alone (28.2%) or in combination with a targeted therapy (46.3%). When combined with a targeted therapy, 38.8% of patients received anti-VEGF and 7.5% received anti-EGFR. Only 6.7% of patients received monotherapy with or without targeted therapy. Of the 18.8% who received triplet chemotherapy, most received anti-VEGF (14.9%) and 0.8% received anti-EGFR. The main first-line treatments were FOLFOX plus bevacizumab (27.1%), FOLFOX alone (19.2%), and FOLFOXIRI plus bevacizumab (13.3%). The median duration of treatment for the overall population was 4.9 months (95% CI 4.0-5.3 months).
Subsequently, 52.5% and 30.2% received second- and third-line treatments, respectively. The most frequently used second-line regimens were folinic acid, 5-fluorouracil, irinotecan (FOLFIRI) plus bevacizumab (14.9%), FOLFIRI alone (14.2%), and BRAF inhibitor/mitogen-activated protein kinase kinase (MEK) inhibitor/cetuximab (11.9%). BRAF inhibitor plus cetuximab was given to 5.2% of patients at second line. The most common third-line treatment regimens were FOLFIRI plus bevacizumab (11.7%), BRAF inhibitor/MEK inhibitor/EGFR inhibitor combination (7.8%), FOLFOX plus bevacizumab (6.5%), and BRAF inhibitor/cetuximab/irinotecan (6.5%). BRAF inhibitor plus cetuximab was given to 2.6% of patients at third line. Together, regorafenib, trifluridine plus tipiracil (TAS-102), anti-programmed cell death protein 1 immunotherapy, and vinorelbine formed 26.0% of all third-line options prescribed.
Treatment patterns by country
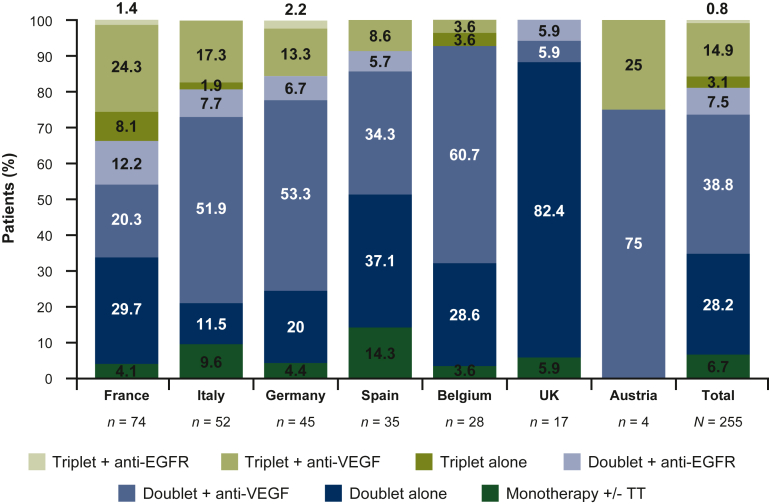
Patient and tumor characteristics were similar between countries; however, first-line treatment patterns differ. In Belgium, Germany, and Italy, the main treatment received was doublet chemotherapy plus anti-VEGF (60.7%, 53.3%, and 51.9%, respectively; Figure 2). In France and Spain, doublet chemotherapy (29.7% and 37.1%, respectively) and doublet chemotherapy plus targeted therapy (32.4% and 40.0%) were equally common as a first-line treatment.
Figure 2.
First-line mCRC treatment regimens by country.
The proportion of patients per country and across all countries (N = 255) in the full analysis set receiving each treatment type. The proportion of patients per treatment regimen is provided within each bar. +, with; +/-, with or without; EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer; TT, targeted therapy; VEGF, vascular endothelial growth factor.
Effectiveness
Effectiveness objectives were assessed on a subset of the FAS population (n = 238). The monotherapy chemotherapy ± targeted therapy group (n = 17) was excluded due to the high heterogeneity and low number of patients who received this regimen (Figure 1). For the effectiveness analysis, the two chemotherapy alone groups [triplet (n = 8) and doublet (n = 72)] were combined due to the small number of patients.
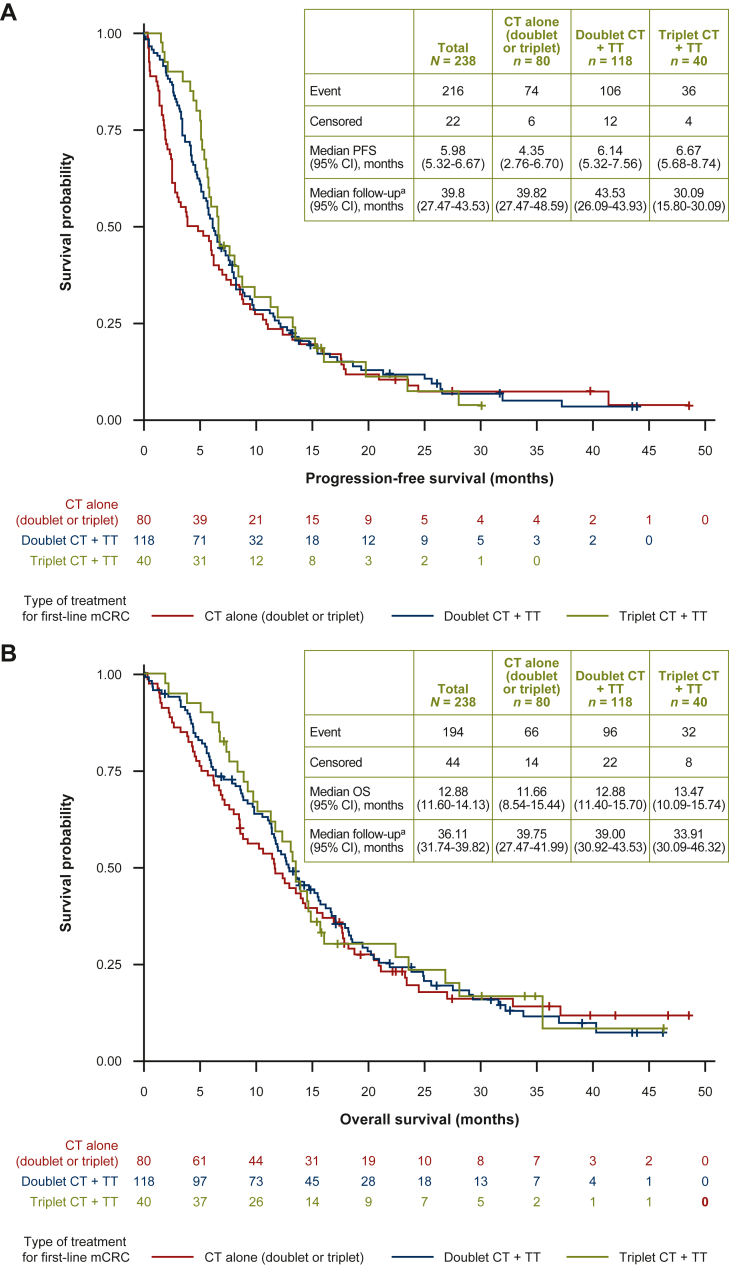
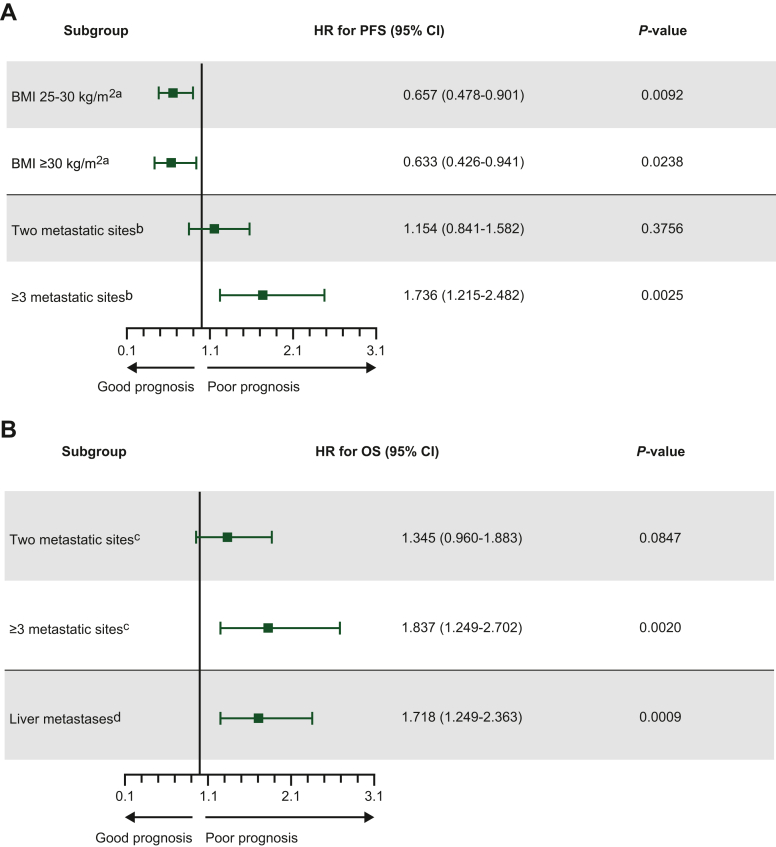
Across all first-line regimens (n = 238), median PFS was 6.0 months (95% CI 5.3-6.7 months; Figure 3A). The median PFS was similar across all three treatment groups [4.4 (95% CI 2.8-6.7) months, 6.1 (95% CI 5.3-7.6) months, and 6.7 (95% CI 5.7-8.7) months for the chemotherapy alone, doublet chemotherapy plus targeted therapy, and triplet chemotherapy plus targeted therapy groups, respectively]. Subsequent multivariate analysis found that body mass index (both 25-30 and ≥30 kg/m2) and the presence of ≥3 metastatic sites were significant prognostic factors of PFS (P = 0.0092, 0.0238, and 0.0025, respectively; Figure 4A). Interestingly, factors that may affect body mass index were not significant, including age (P = 0.8597) and peritoneal carcinomatosis (P = 0.2130), whereas performance status was not reliable due to missing data. Treatment choice was not assessed as a variable due to the dataset not being sufficiently robust. Univariate analysis data are provided in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100603.
Figure 3.
Kaplan–Meier estimates for (A) PFS and (B) OS according to first-line mCRC treatment (N = 238).
+, with; CI, confidence interval; CT, chemotherapy; mCRC, metastatic colorectal cancer; PFS, progression-free survival; OS, overall survival; TT, targeted therapy.aFollow-up was calculated using the reverse Kaplan–Meier method.
Figure 4.
Multivariate analysis for (A) PFS and (B) OS according to first-line mCRC treatment (P < 0.15; N = 238).
BMI, body mass index; CI, confidence interval; HR, hazard ratio; mCRC, metastatic colorectal cancer; OS, overall survival; PFS, progression-free survival. aReference group was BMI <25 kg/m2; bReference group was one metastatic site. cReference group was one metastatic site; dReference group was the absence of liver metastasis.
The median OS across all regimens was 12.7 months (95% CI 11.6-14.1 months; Figure 3B). The median OS was similar for all three treatment groups [11.7 (95% CI 8.5-15.4) months, 12.9 (95% CI 11.4-15.7) months, and 13.5 (95% CI 10.1-15.7) months for the chemotherapy alone, doublet chemotherapy plus targeted therapy, and triplet chemotherapy plus targeted therapy groups, respectively]. By multivariate analysis, the presence of three or more metastatic sites and liver metastases were found to be significant predictors of OS (P = 0.0020 and 0.0009, respectively), each increasing risk of death by 84% and 72%, respectively (Figure 4B and Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100603).
ORR was 32.9% (95% CI 26.9-38.9; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100603). The observed response rate was 52.5% in patients who received triplet chemotherapy plus targeted therapy (95% CI 37.0%-68.0%), 31.4% with doublet chemotherapy plus targeted therapy (95% CI 23.0%-39.7%), and 25.3% with doublet/triplet chemotherapy alone (95% CI 15.7%-34.9%). The complete response rate for all regimens was 2.1% and partial response rate was 30.8%, compared with stable disease (30.4%) and progressive disease (23.2%).
Exploratory analysis of the efficacy of first-line treatments was conducted by grouping patients who received doublet chemotherapy ± targeted therapy (n = 190) and comparing with patients who received triplet chemotherapy ± targeted therapy (n = 48; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100603). Median PFS was similar across both treatment groups [5.78 (95% CI 4.83-6.47) months versus 6.70 (95% CI 5.68-9.86) months]. A similar trend was seen for median OS [12.58 (95% CI 11.40-14.36) months versus 13.47 (95% CI 11.30-16.03) months]. A higher response rate was observed in patients who received triplet chemotherapy ± targeted therapy (50.0%; 95% CI 35.9%-64.1%), versus doublet chemotherapy ± targeted therapy (28.6%; 95% CI 22.1%-35.0%). However, limited conclusions can be drawn from these exploratory analyses due to the large difference in sample sizes between the two groups.
Additional exploratory analysis compared patients that received chemotherapy plus anti-EGFR, chemotherapy plus anti-VEGF, and chemotherapy alone. For patients who received chemotherapy plus anti-EGFR (n = 21) or chemotherapy alone (n = 80), median PFS was comparable (3.7; 95% CI 3.4-6.3 months versus 4.4; 95% CI 2.8-6.7 months). Median PFS was numerically higher for patients who received chemotherapy with anti-VEGF (n = 137; 6.7; 95% CI 5.8-8.0 months), with overlapping CIs for the three groups. Similarly, median OS was comparable across the chemotherapy plus anti-EGFR and chemotherapy alone groups (10.1; 95% CI 5.6-11.4 months versus 11.7; 95% CI 8.5-15.4 months), whereas median OS was higher for patients who received chemotherapy with anti-VEGF (13.7; 95% CI 12.6-16.0 months). CIs overlapped for all comparisons except for the chemotherapy plus anti-EGFR and chemotherapy plus anti-VEGF groups.
Safety
Safety objectives were assessed on the full FAS (n = 255). A total of 131 (51.4%) patients experienced at least one relevant adverse event during first-line treatment. For 49.0% of patients, at least one relevant adverse event was related to the mCRC therapy received during first-line treatment. The highest rate of adverse events was associated with triplet chemotherapy plus targeted treatment (75.0%) compared with triplet chemotherapy alone (50.0%), doublet chemotherapy alone (36.1%), doublet chemotherapy plus targeted treatment (49.2%), and monotherapy chemotherapy with or without targeted treatment (41.2%).
Across all first-line treatment regimens, the most frequent relevant adverse events were diarrhea (12.2%), peripheral neuropathy (7.5%), asthenia (7.1%), and neutropenia (7.1%). Triplet chemotherapy with targeted treatment resulted in higher rates of diarrhea (25.0%), peripheral neuropathy (25.0%; potentially due to oxaliplatin), and neutropenia (15.0%) compared with other first-line treatments. Rates of asthenia were higher for the triplet chemotherapy alone group.
Discussion
To the best of our knowledge, CAPSTAN CRC is the first study to assess treatment patterns for BRAFV600E-mutant mCRC in routine clinical practice across Europe. Although ESMO provides guidelines for the treatment of this subset of patients, these remain limited in scope and lack a clear consensus on therapeutic strategy in the first-line setting.6,24 Furthermore, current treatment recommendations are based largely on evidence from RCTs including <100 patients with BRAFV600E-mutant mCRC, except BEACON CRC (n = 665) and FIRE 4.5 (n = 108).5,6,9,20,25, 26, 27 BRAFV600E-mutant mCRC is a rare disease with an aggressive phenotype, making recruitment and patient retention challenging and preventing firm conclusions regarding best practices.5 Therefore, characterization of real-world first-line treatment patterns for patients with BRAFV600E-mutant mCRC in CAPSTAN CRC represents an important step.
The predominance of right-sided tumors and the high proportion of patients with metastatic disease at diagnosis, at >66% markedly higher than the 20% previously reported for CRC overall,2 emphasizes the aggressive biology of the BRAFV600E phenotype and the importance of early detection.
CAPSTAN CRC found most patients received doublet chemotherapy (with or without targeted therapy) in the first-line setting, with the most prescribed regimen being FOLFOX plus bevacizumab, followed by FOLFOX alone, and FOLFOXIRI plus bevacizumab. Just over half of patients received a second-line therapy. The notable variety of different treatments, in both first and second lines, perhaps reflects the heterogeneity of patients and complexity of the disease in real-world clinical practice.
It is unclear whether doublet or triplet chemotherapy regimens are superior for BRAFV600E-mutant mCRC in first line, or what the optimal treatment sequence is. In CAPSTAN CRC, ORR was higher with first-line triplet chemotherapy plus targeted therapy compared with doublet chemotherapy plus targeted therapy. However, the nature of the study and the possibility of selection bias preclude definitive conclusions. In the phase III TRIBE2 study, sequential administration of doublet chemotherapies plus bevacizumab was compared with triplet FOLFOXIRI plus bevacizumab in patients with previously untreated mCRC.28 Although triplet therapy improved survival overall, subgroup analyses of patients with BRAFV600E or RAS mutations revealed no indisputable benefit of triplet over doublet chemotherapies. Similarly, a meta-analysis of five RCTs showed no benefit of FOLFOXIRI plus bevacizumab over doublet plus bevacizumab in BRAF-mutant mCRC.10 Thus, the optimal choice of frontline chemotherapy regimen for these patients remains to be established.
In CAPSTAN CRC, most patients received first-line combination chemotherapy with bevacizumab, in line with European guidelines for BRAFV600E-mutant mCRC.5,9 Adding bevacizumab improved survival compared with chemotherapy alone in the subgroup analyses of patients with BRAFV600E-mutant mCRC in previous clinical trials and a recent analysis of pooled individual patient data from the Analysis and Research in CAncers of the Digestive system (ARCAD) database.8,29,30 Cetuximab and panitumumab have also demonstrated survival benefit in combination with FOLFIRI and FOLFOX for treatment-naive mCRC17; however, there is increasing evidence that the addition of cetuximab to chemotherapy is not effective in the first-line setting for patients with a BRAFV600E mutation.31 The recent phase II FIRE-4.5 study, which assessed FOLFOXIRI plus bevacizumab versus FOLFOXIRI plus cetuximab as first-line treatment for BRAFV600E-mutant mCRC, showed a trend toward improved survival with bevacizumab in patients with right-sided tumors.20 This further supports the use of bevacizumab in combination with chemotherapy in the first-line setting for these patients.
The median PFS in CAPSTAN CRC was 6.0 (95% CI 5.3-6.7) months and the median OS was 12.9 (95% CI 11.6-14.1) months across all first-line regimens. Median PFS and OS were similar for all three treatment groups. However, longer survival was seen in patients selected for clinical trials compared with our real-world population. FIRE 4.5 reported an OS of 17.1 months with first-line FOLFOXIRI plus bevacizumab versus 15.2 months with FOLFOXIRI plus cetuximab in BRAFV600E-mutant mCRC.20 The single-arm, phase II ANCHOR CRC study in patients with previously untreated BRAFV600E-mutant mCRC showed an OS of 17.2 months with encorafenib, binimetinib, and cetuximab.32
This study underscores the unmet need for more effective treatment strategies to improve the prognosis of patients with BRAFV600E-mutant mCRC and allow more patients to receive subsequent lines of treatment. Unfortunately, only a very modest proportion were able to receive second- (52.5%) and third-line (30.2%) treatments, reaffirming that rapid disease progression is common. Therefore, optimization of first-line treatment is especially relevant in this patient population.33
As both BRAF and RAS mutations are significant negative prognostic factors in CRC, it was encouraging to observe that most patients were tested for each (89.8% and 91.8%, respectively) prior to first-line treatment.5,9 However, while BRAF testing is standard of care in most European regions, with rates as high as 97% in Northern and Western Europe, testing at initial diagnosis (20%) trails behind East Asia (41%), Australasia (41%), and North America (35%).34 Mutational status also guides appropriate therapeutic strategies for different biological mCRC subtypes.5,24 Thus, improving testing rates further would greatly benefit optimization of treatment decisions.
In contrast to BRAF and RAS, MSI status was assessed much less frequently, in only 60.0% of patients overall. Variability of testing practices was high across Europe, including regions without routine MSI testing during the study inclusion period (2016-2018). However, MSI screening is now recommended in the ESMO guidelines (2020) in both curative and palliative treatment settings.24,35
Surprisingly, 6.8% of BRAFV600E-mutant patients in CAPSTAN CRC had a concomitant RAS mutation, although this is rare and these mutations are generally considered mutually exclusive.36,37 The reasons are unclear, but may include the high heterogeneity of the BRAFV600E-mutant population or reporting errors due to heterogeneous testing procedures across countries.37
There are some limitations to CAPSTAN CRC. First is the inherent issue of real-world evidence studies regarding low internal validity, human error, and quality control during data reporting. Despite this, real-world evidence can complement data from RCTs by providing valuable insights into routine clinical practices that guide treatment decisions.38 Second, population size is relatively small compared with some cohort studies, and particularly small for the ‘by country’ analysis [UK (n = 17) and Austria (n = 4)]. However, CAPSTAN CRC provides further information regarding treatment practices, effectiveness, and safety in this often-overlooked subpopulation. Similarly, the study includes patients from only seven European countries. Surveys revealed, for example, that mCRC BRAF testing is far less commonly carried out in Eastern European than Northern and Western European countries.34 Therefore, caution is required when extrapolating conclusions on treatment practices to the entirety of Europe. Finally, the treatment landscape for these patients is diverse and still evolving; this study is a snapshot of mCRC treatment practices and outcomes. The time frame in which the study was conducted should, therefore, be considered when evaluating the results.
A promising therapeutic avenue in BRAFV600E-mutant mCRC lies in the development of targeted agents, and their combined use to inhibit multiple points in the mitogen-activated protein kinase signaling pathway and overcome mechanisms of treatment resistance.39,40 BEACON CRC demonstrated the efficacy of combining BRAF and EGFR inhibition, using encorafenib and cetuximab, versus standard chemotherapy after prior systemic treatment.27 ANCHOR CRC, which assessed the efficacy of encorafenib, binimetinib, and cetuximab as first-line treatment for patients with BRAFV600E-mutant mCRC, reported an encouraging median PFS of 5.8 months and median OS of 17.2 months.32 Further ongoing clinical trials in BRAFV600E-mutant CRC evaluate different treatment combinations.41,42 For example, the phase III BREAKWATER trial (NCT04607421) is assessing encorafenib in combination with cetuximab in BRAFV600E-mutant, treatment-naive patients with or without chemotherapy.16,43
Immunotherapy is another promising treatment strategy in BRAFV600E-mutant mCRC. In the phase III KEYNOTE-177 trial, first-line pembrolizumab in patients with mismatch repair deficient/MSI tumors significantly prolonged PFS versus chemotherapy, with a similar benefit observed in patients with BRAFV600E-mutant and BRAF wild-type mCRC.44 The phase II CheckMate-142 study found that the combination of nivolumab plus ipilimumab provided a robust and durable clinical benefit, including long-term survival, for patients with MSI-high mCRC, including those with BRAF mutations.45, 46, 47
Conclusions
The BRAFV600E mutation is a biomarker for prognosis as well as treatment response, and screening is now recommended in combination with RAS and mismatch repair/MSI status determination. The CAPSTAN CRC study helps to elucidate the real-world European management of BRAFV600E-mutant mCRC, demonstrating the aggressiveness of this disease where the median OS was 12.9 months and only 52.5% of patients were able to receive a potentially effective second-line therapy. The study’s findings highlight the importance of testing for BRAFV600E mutations to personalize and optimize treatment, and the close monitoring necessary for patients with this mutational status. There remains a clear unmet need to establish the best treatment strategies for this population in the first-line setting.
Acknowledgments
Funding
This work was supported by Pierre Fabre (no grant number). The authors were responsible for all content and editorial decisions and received no honoraria for the development of this manuscript. Editorial support was provided by Anna Monk of Meditech Media, funded by Pierre Fabre.
Disclosure
EM has Personal fees from AstraZeneca, Amgen, Bayer, Merck Serono, Roche, Servier, and Pierre Fabre. CCr reports receiving personal fees from Amgen, Bayer, F. Hoffman–La Roche, and Sirtex. TM reports receiving personal fees from Servier and Pierre Fabre; and honoraria from Sandoz, Pierre Fabre, Sanofi, AAA, Merck Serono, and Servier. JV reports receiving honoraria from Amgen, Hoffman La-Roche, Merck-Serono, Sanofi, and Sysmex Inostics. IV reports receiving honoraria from Bristol Myers Squibb. DT reports financial interests in Merk Serono, Novartis, and BMS; and receiving honoraria from Amgen, BMS, Servier, Roche, Ipsen, Sanofi, Astra Zeneca, Novartis, and Merk Serono. PJC has a consulting/advisory role with Lilly, Novartis, Amgen, Roche, MSD, and Pierre Fabre; and reports receiving fees for travel expenses from Novartis, Ipsen, Roche, and Lilly. BC reports receiving personal fees from Roche, Sanofi, and Amgen; and honoraria from Sanofi. SKi reports receiving honoraria from Bayer, Boehringer Ingelheim, Ipsen, MSD, Sanofi, and Servier; and research funding from Boehringer Ingelheim, Pfizer, Roche, and Sanofi. IG reports receiving honoraria from Amgen, Merck Serono, Pierre Fabre, Roche, and Servier; and research funding from Roche. BA reports receiving personal fees from AstraZeneca, Bristol Myers Squibb, Pierre Fabre, Roche, and Servier. CCa, AZ, and SKh are all employees of Pierre Fabre. DA reports financial and personal interests in Bayer, Bristol Myers Squibb, Lilly, Merck (EMD), Mologen, Sanofi, Servier, Sirtex, Symphogen, Terumo, and Roche. IV has declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Xu W., Wang Y., Li X., et al. Risk factors and risk prediction models for colorectal cancer metastasis and recurrence: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:172. doi: 10.1186/s12916-020-01618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araghi M., Arnold M., Rutherford M.J., et al. Colon and rectal cancer survival in seven high-income countries 2010-2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project) Gut. 2021;70:114–126. doi: 10.1136/gutjnl-2020-320625. [DOI] [PubMed] [Google Scholar]
- 4.Stintzing S., Miller-Phillips L., Modest D.P., et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50–60. doi: 10.1016/j.ejca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E., Cervantes A., Adam R., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 6.Taieb J., Lapeyre-Prost A., Laurent Puig P., et al. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br J Cancer. 2019;121:434–442. doi: 10.1038/s41416-019-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran R.B., André T., Atreya C.E., et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen R., Liu H., Fiskum J., et al. BRAF V600E mutation in first-line metastatic colorectal cancer: an analysis of individual patient data from the ARCAD database. J Natl Cancer Inst. 2021;113:1386–1395. doi: 10.1093/jnci/djab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCN NCCN Clinical Practice Guidelines. Colon Cancer version v.3.2021 2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Available at.
- 10.Cremolini C., Antoniotti C., Stein A., et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020:JCO2001225. doi: 10.1200/JCO.20.01225. [DOI] [PubMed] [Google Scholar]
- 11.Wirapati P., Pomella V., Vandenbosch B., et al. Velour trial biomarkers update: impact of RAS, BRAF, and sidedness on aflibercept activity. J Clin Oncol. 2017;35:3538. [Google Scholar]
- 12.Yoshino T., Portnoy D.C., Obermannova R., et al. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE - a global phase III study. Ann Oncol. 2019;30:124–131. doi: 10.1093/annonc/mdy461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venderbosch S., Nagtegaal I.D., Maughan T.S., et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cutsem E., Lenz H.-J., Köhne C.-H., et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 15.Di Nicolantonio F., Martini M., Molinari F., et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 16.Grothey A., Fakih M., Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959–967. doi: 10.1016/j.annonc.2021.03.206. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E., Cervantes A., Nordlinger B., et al. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 18.Kopetz S., Grothey A., Yaeger R., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 19.Kopetz S., Grothey A., Cutsem E.V., et al. Encorafenib plus cetuximab with or without binimetinib for BRAF V600E metastatic colorectal cancer: updated survival results from a randomized, three-arm, phase III study versus choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC) J Clin Oncol. 2020;38:4001. [Google Scholar]
- 20.Stintzing S., Heinrich K., Tougeron D., et al. Randomized study to investigate FOLFOXIRI plus either bevacizumab or cetuximab as first-line treatment of BRAF V600E-mutant mCRC: the phase-II FIRE-4.5 study (AIO KRK-0116) J Clin Oncol. 2021;39:3502. [Google Scholar]
- 21.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 23.Cox D.R. Regression models and life-tables. J Royal Stat Soc Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 24.ESMO ESMO Pocket Guidelines: Lower Gastrointestinal Cancer. ESMO2020. http://interactiveguidelines.esmo.org/esmo-web-app/home/gl_home.php?GL=45 Available at.
- 25.Loupakis F., Cremolini C., Salvatore L., et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Cremolini C., Loupakis F., Antoniotti C., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 27.Tabernero J., Grothey A., Van Cutsem E., et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cremolini C., Antoniotti C., Rossini D., et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 29.Ince W.L., Jubb A.M., Holden S.N., et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 30.Price T.J., Hardingham J.E., Lee C.K., et al. Impact of KRAS and BRAF gene mutation status on outcomes from the Phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2011;29:2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 31.Van Cutsem E., Kohne C.H., Lang I., et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 32.Van Cutsem E., Taieb J., Yaeger R., et al. O-10 ANCHOR CRC: results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol. 2021;32:S222. doi: 10.1200/JCO.22.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayhanian H., Goode E., Sclafani F., et al. Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: a retrospective matched case-control study. Clin Colorectal Cancer. 2018;17:e69–e76. doi: 10.1016/j.clcc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Desai J., Kopetz S., Grothey A., et al. Global BRAF testing practices in metastatic colorectal cancer (mCRC) J Clin Oncol. 2021;39 [Google Scholar]
- 35.Luchini C., Bibeau F., Ligtenberg M.J.L., et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30:1232–1243. doi: 10.1093/annonc/mdz116. [DOI] [PubMed] [Google Scholar]
- 36.Sahin I.H., Kazmi S.M., Yorio J.T., et al. Rare though not mutually exclusive: a report of three cases of concomitant KRAS and BRAF mutation and a review of the literature. J Cancer. 2013;4:320–322. doi: 10.7150/jca.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanelli G.N., Dal Pozzo C.A., Depetris I. The heterogeneous clinical and pathological landscapes of metastatic Braf-mutated colorectal cancer. Cancer Cell Int. 2020;20:30. doi: 10.1186/s12935-020-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichler H.G., Pignatti F., Schwarzer-Daum B., et al. Randomized controlled trials versus real world evidence: neither magic nor myth. Clin Pharmacol Ther. 2020;109:1212–1218. doi: 10.1002/cpt.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopetz S., Desai J., Chan E. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33:4032–4038. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao M., Tian F., Mariadason J.M. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013;19:657–667. doi: 10.1158/1078-0432.CCR-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauri G., Bonazzina E., Amatu A., et al. The evolutionary landscape of treatment for brafv600e mutant metastatic colorectal cancer. Cancers (Basel) 2021;13:137. doi: 10.3390/cancers13010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kam A.E., Eng C. BRAF V600E mutated metastatic colorectal cancer: current progress and future directions. Expert Opin Biol Ther. 2021;21:1311–1313. doi: 10.1080/14712598.2021.1908994. [DOI] [PubMed] [Google Scholar]
- 43.Kopetz S., Grothey A., Yaeger R., et al. BREAKWATER: randomized phase 3 study of encorafenib (enco) + cetuximab (cetux) ± chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC) J Clin Oncol. 2021;39:TPS3619. [Google Scholar]
- 44.Andre T., Shiu K.K., Kim T.W., et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 45.Lenz H.J., Lonardi S., Zagonel V., et al. Nivolumab plus low-dose ipilimumab as first-line therapy in microsatellite instability-high/DNA mismatch repair deficient metastatic colorectal cancer: clinical update. J Clin Oncol. 2020;38:11. [Google Scholar]
- 46.Overman M.J., McDermott R., Leach J.L. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diab M. Shedding light on BRAF: management of colorectal cancer in the era of personalized medicine. JCO Oncol Pract. 2021;17:731–733. doi: 10.1200/OP.21.00467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.