Abstract
BACKGROUND
Data are lacking on the comparative effectiveness of commonly used glucose-lowering medications, when added to metformin, with respect to microvascular and cardiovascular disease outcomes in persons with type 2 diabetes.
METHODS
We assessed the comparative effectiveness of four commonly used glucose-lowering medications, added to metformin, in achieving and maintaining a glycated hemoglobin level of less than 7.0% in participants with type 2 diabetes. The randomly assigned therapies were insulin glargine U-100 (hereafter, glargine), glimepiride, liraglutide, and sitagliptin. Prespecified secondary outcomes with respect to microvascular and cardiovascular disease included hypertension and dyslipidemia, confirmed moderately or severely increased albuminuria or an estimated glomerular filtration rate of less than 60 ml per minute per 1.73 m2 of body-surface area, diabetic peripheral neuropathy assessed with the Michigan Neuropathy Screening Instrument, cardiovascular events (major adverse cardiovascular events [MACE], hospitalization for heart failure, or an aggregate outcome of any cardiovascular event), and death. Hazard ratios are presented with 95% confidence limits that are not adjusted for multiple comparisons.
RESULTS
During a mean 5.0 years of follow-up in 5047 participants, there were no material differences among the interventions with respect to the development of hypertension or dyslipidemia or with respect to microvascular outcomes; the mean overall rate (i.e., events per 100 participant-years) of moderately increased albuminuria levels was 2.6, of severely increased albuminuria levels 1.1, of renal impairment 2.9, and of diabetic peripheral neuropathy 16.7. The treatment groups did not differ with respect to MACE (overall rate, 1.0), hospitalization for heart failure (0.4), death from cardiovascular causes (0.3), or all deaths (0.6). There were small differences with respect to rates of any cardiovascular disease, with 1.9, 1.9, 1.4, and 2.0 in the glargine, glimepiride, liraglutide, and sitagliptin groups, respectively. When one treatment was compared with the combined results of the other three treatments, the hazard ratios for any cardiovascular disease were 1.1 (95% confidence interval [CI], 0.9 to 1.3) in the glargine group, 1.1 (95% CI, 0.9 to 1.4) in the glimepiride group, 0.7 (95% CI, 0.6 to 0.9) in the liraglutide group, and 1.2 (95% CI, 1.0 to 1.5) in the sitagliptin group.
CONCLUSIONS
In participants with type 2 diabetes, the incidences of microvascular complications and death were not materially different among the four treatment groups. The findings indicated possible differences among the groups in the incidence of any cardiovascular disease. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; GRADE ClinicalTrials.gov number, NCT01794143.)
LONG-TERM COMPLICATIONS OF TYPE 2 diabetes mellitus, including microvascular and cardiovascular disease, account for most illness, deaths, and costs associated with this condition.1 Clinical trials have shown a benefit of decreased chronic hyperglycemia on diabetes-specific microvascular complications.2,3 In addition, trials have shown that some new classes of glucose-lowering medication have beneficial effects with respect to cardiovascular disease and kidney disease, largely in participants with preexisting disease,4–6 whereas other medications have been shown to have neutral effects7 or potentially harmful side effects.6,8,9
The primary aim of the randomized Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness (GRADE) Study was to compare the effectiveness of agents from four commonly used classes of glucose-lowering medications, when added to metformin, in achieving and maintaining glycemic targets in participants with type 2 diabetes mellitus of recent onset.10 These medications were insulin glargine U-100 (hereafter, glargine), the sulfonylurea glimepiride, the glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide, and the dipeptidyl peptidase 4 inhibitor sitagliptin.
The metabolic results of this trial are presented in our accompanying article in this issue of the Journal.11 In brief, glargine and liraglutide were more effective than glimepiride and sitagliptin in maintaining glycemic targets, albeit with small differences in the glycated hemoglobin level over time. The relative effects of the randomized therapies on prespecified secondary outcomes (microvascular complications and cardiovascular events and their risk factors) and heterogeneity among subgroups are presented here.
METHODS
TRIAL DESIGN AND OVERSIGHT
The general description of this trial and its methods is provided in the companion article.11 Here, we present a summary of the overall methods and additional methods relevant to the secondary outcomes. A full list of the inclusion and exclusion criteria is provided in the protocol, available with the full text of this article at NEJM.org. All the data were collected and analyzed by the research group. The authors vouch for the accuracy and completeness of the data and the fidelity of the trial to the protocol. The authors wrote the manuscript and made the decision to submit it for publication. No confidentiality restrictions were imposed by the funding agencies (including the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] of the National Institutes of Health) or by the companies that donated materials for the trial.
This trial was conducted at 36 clinical centers (Section S1 in the Supplementary Appendix, available at NEJM.org) and was designed by a subgroup of the investigators with NIDDK participation. In this parallel-group, comparative-effectiveness clinical trial, glucose-lowering medications approved by the Food and Drug Administration (FDA) were administered in accordance with their labeling, in combination with metformin.10 Randomization was conducted with the use of a centralized Web-based system and stratified according to trial site. The participants and clinic staff were aware of the treatment assignments; however, investigators at the laboratories and reading centers and the event adjudicators were unaware of the treatment assignments. The manufacturers contributed trial medications under clinical-trial agreements with the NIDDK but had no role in the design, conduct, or analysis of the trial. An NIDDK-appointed data and safety monitoring board oversaw the conduct of the trial, and all participating centers received approval from local institutional review boards.
PARTICIPANTS
The eligibility criteria and baseline characteristics of the participants have been published previously.12 In brief, we enrolled 5047 patients who had received a diagnosis of type 2 diabetes mellitus at 30 years of age or older, with the exception of American Indians and Alaska Natives, in whom the age at diagnosis was 20 years or older. Eligibility criteria included the following: diabetes that had been diagnosed within the previous 10 years and treated with at least 500 mg of metformin per day, but no other glucose-lowering medications, and a glycated hemoglobin level of 6.8 to 8.5% (50.8 to 69.4 mmol per mole) at the time of randomization. The criteria for exclusion included a history of a major cardiovascular event in the year before randomization, a New York Heart Association functional classification of III or higher, and an estimated glomerular filtration rate (eGFR) of less than 30 ml per minute per 1.73 m2 of body-surface area.
TREATMENTS
Participants were randomly assigned to receive one of the four treatments (glargine, glimepiride, liraglutide, or sitagliptin) in addition to metformin. The medications selected had to be FDA-approved for use in combination with metformin and in common clinical use at the time of the trial launch. During the run-in period before randomization, the metformin dose was increased to at least 1000 mg per day, with a target maximal dose (one that could be taken without unacceptable side effects) of 2000 mg per day. Immediate-release or extended-release formulations of metformin (Bristol Myers Squibb) were supplied to all the participants. The doses of the randomly assigned treatments were adjusted on the basis of their labeling. Glargine (Sanofi) was administered daily at an initial dose of up to 20 U and was adjusted on the basis of glucose levels monitored by the participants and to avoid hypoglycemia. The dose of glimepiride (Sanofi) was increased from 1 to 2 mg to a maximum of 8 mg per day, administered in divided doses, on the basis of glucose levels monitored by the participants and to avoid hypoglycemia. The dose of liraglutide (Novo Nordisk) was escalated to a maximum dose of 1.8 mg daily, depending on gastrointestinal side effects, and sitagliptin (Merck) at a dose of 100 mg was administered and adjusted depending on the participant’s kidney function.
During the trial, updated consensus recommendations on the choice of glucose-lowering medications in participants with prevalent cardiovascular or kidney disease were issued by the American Diabetes Association and the European Association for the Study of Diabetes.13,14 These recommendations were communicated to participants who were potentially eligible for these interventions and to their health care providers. The participants’ own health care providers were responsible for all medications other than the glucose-lowering medications specified in the trial protocol.
OUTCOMES AND ASSESSMENTS
The participants were evaluated every 3 months. The metabolic outcomes for the current trial are described in detail in the accompanying article.11 Data on the risk factors, microvascular complications, and cardiovascular outcomes that are the focus of this article were obtained with the use of standardized questionnaires, physical examinations, and laboratory analyses. Details regarding laboratory tests and other methods are provided in Sections S3 and S4 in the Supplementary Appendix. Risk factors related to the microvascular and cardiovascular outcomes included hypertension, defined as previously diagnosed hypertension, measured blood pressure of at least 140/90 mm Hg, or treatment with blood pressure–lowering agents, and dyslipidemia. Dyslipidemia was defined as fasting low-density lipoprotein cholesterol levels of at least 100 mg per deciliter (≥2.6 mmol per liter), triglyceride levels of at least 150 mg per deciliter (≥1.7 mmol per liter), high-density lipoprotein levels of less than 40 mg per deciliter (<1.0 mmol per liter) in men and less than 50 mg per deciliter (<1.3 mmol per liter) in women, or the use of lipid-lowering medications.
Kidney complications were assessed on the basis of the urinary albumin:creatinine ratio, which was measured every 6 months. Moderately increased albuminuria was defined as an albumin:creatinine ratio of 30 or greater, with albumin measured in milligrams and creatinine in grams, and confirmed at a subsequent visit. Severely increased albuminuria was defined as an albumin:creatinine ratio of 300 or higher. The eGFR was calculated from annual serum creatinine measurements, with renal impairment defined15 as an eGFR of less than 60 ml per minute per 1.73 m2. Participants in whom incident end-stage kidney disease (as defined by dialysis, transplantation, or death from kidney disease) developed during the trial were considered to have had an outcome event in the categories of albuminuria (moderately increased albuminuria and severely increased albuminuria) and renal impairment. Diabetic peripheral neuropathy was assessed annually with the modified Michigan Neuropathy Screening Instrument (MNSI), which included a 15-item interviewer-administered symptom questionnaire and a bilateral lower-extremity clinical examination assessing ankle reflexes and vibration sensation at the great toes.16 Scores on the MNSI range from 0 to 8, with higher scores indicating more severe symptoms of neuropathy. Diabetic peripheral neuropathy was defined as an MNSI score of 7 or higher or an examination score of 2.5 or higher, as previously described.16
Cardiovascular outcomes were classified and adjudicated by a committee whose members were unaware of the treatment assignments, according to the 2017 Cardiovascular and Stroke End-point Definitions for Clinical Trials.17 Two committee members independently reviewed each event and, if consensus could not be reached, a third member provided the tie-breaking assessment. A major adverse cardiovascular event (MACE) was defined as the time to the first nonfatal myocardial infarction or stroke or death from cardiovascular causes. The outcome “any cardiovascular disease” included the first incidence of any of the following: MACE, unstable angina or heart failure warranting hospitalization, or revascularization in any arterial bed.
STATISTICAL ANALYSIS
The statistical methods used herein are the same as those described in the companion article.11 With the exception of sensitivity analyses conducted in accordance with the protocol, all analyses were conducted in accordance with the intention-to-treat principle. Briefly, analyses of the nine outcomes were conducted with the use of standard methods for the analysis of event–time (survival) data, including hazard ratios and confidence limits from the Cox proportional-hazards model, and the comparison of each group with the other three groups combined. For an outcome requiring confirmation, time to the initial event was used. Short-term (1 year) and longer-term (4 years) changes in risk factors were assessed in longitudinal models that compared the differences among treatment groups in the average (least-squares mean) over 1 and 4 years, the latter time when 85.8% of the cohort was under follow-up owing to staggered entry. No treatment-by-visit interaction was observed. Analyses of the cumulative incidence of hypertension, dyslipidemia, and microvascular outcomes excluded participants with those conditions at baseline, whereas analyses of incident cardiovascular disease included all the participants, regardless of history of cardiovascular disease before the trial.
Estimates of pairwise treatment effects from Cox models are presented as hazard ratios with 95% confidence intervals. The widths of the confidence intervals have not been adjusted for multiple testing, and therefore any inferences drawn from these intervals may not be reproducible. No P values are reported.
Per-protocol sensitivity analyses were used to assess the effect of the trial medications while the participants were receiving their assigned medication. This was accomplished by including results only for participants who continued to take their assigned glucose-lowering medications and who did not take glucose-lowering medications other than those that were part of the trial regimen. For each of the microvascular and cardiovascular outcomes, subgroup analyses were conducted according to prespecified factors, including race, ethnic group, sex, baseline glycated hemoglobin level (by strata in thirds), age, duration of diabetes, and body-mass index (Section S5 in the Supplementary Appendix).
With a projected hazard rate of 0.04 events per year for moderately increased albuminuria, we estimated that 5000 participants would provide the trial with 88% power to detect a 33% relative difference in risk among the groups. During the trial, the observed rate was less at 0.0275 per year, resulting in 71% power for this outcome. For any cardiovascular disease, we estimated that a rate of 0.01 per year would provide 72% power to detect a 50% difference among the groups. The observed rate was higher (0.018 per year), resulting in 99% power to detect a 50% difference.
RESULTS
BASELINE CHARACTERISTICS OF THE PARTICIPANTS
The first of 5047 participants underwent randomization in July 2013, and enrollment concluded in August 2017. The baseline characteristics of the trial cohort have been reported previously,12 including those relevant to the microvascular and cardiovascular outcomes18 (Table S1). At baseline, the mean (±SD) age of the trial cohort was 57.2±10.0 years, and 41.5% of the participants were 60 years of age or older. A total of 63.6% of the participants were men, which reflected the inclusion of 10 Veterans Affairs medical centers as trial recruitment sites.12 A total of 65.7% of the participants identified as White, 19.8% as Black, and 3.6% as Asian; 0.6% of the participants identified as Hawaiian Islander or Pacific Islander, 2.7% as American Indian or Alaska Native, and 18.6% as Hispanic or Latinx. The mean duration of diabetes as reported by the participants was 4.2±2.7 years, and the daily metformin dose was 1994±205 mg. The mean body-mass index (the weight in kilograms divided by the square of the height in meters) was 34.3±6.8, and the mean glycated hemoglobin level was 7.5±0.5% (58.3±5.3 mmol per mole).
At baseline, the prevalence of hypertension and dyslipidemia, largely indicated by the use of medications, was 77% and 96%, respectively. The prevalence of diabetic peripheral neuropathy at baseline was 42%, and 6.4% of the participants reported having had a heart attack or stroke. The baseline characteristics of the recruited cohort resembled those of the U.S. population who had metformin-treated type 2 diabetes mellitus and who were of a similar age and had a similar duration of diabetes and range of glycated hemoglobin levels (Table S2). Randomization was effective, with similar baseline demographic and clinical characteristics among the treatment groups.
The trial cohort was followed until April 2021, with a mean (and median) follow-up of 5.0 years (interquartile range, 4.1 to 6.0; range, 0 to 7.6). Data on recruitment and enrollment are provided in Figure S1 in the Supplementary Appendix.
METABOLIC OUTCOMES, HYPERTENSION, AND DYSLIPIDEMIA
Glargine and liraglutide were more effective than glimepiride and sitagliptin in the maintenance of glycemic targets (the primary metabolic outcome). The glycated hemoglobin level at 4 years was 7.1% in both the glargine and liraglutide groups, as compared with 7.2% in the sitagliptin group and 7.3% in the glimepiride group.11
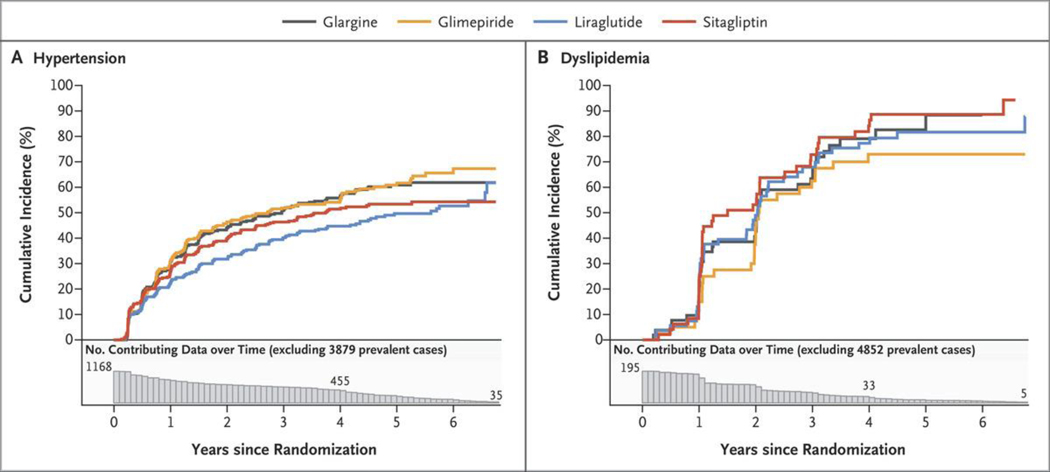
Figure 1 shows the cumulative incidence of hypertension among the 1168 of 5047 participants (23%) who did not have hypertension at baseline and the cumulative incidence of dyslipidemia among the 195 participants (4%) who did not have dyslipidemia at baseline. More than 60% of the participants who did not have hypertension at baseline and more than 90% of those who did not have dyslipidemia at baseline were later classified as having these conditions, largely because blood-pressure medications or lipid-lowering medications had been initiated. During the first year of follow-up, hypertension developed in approximately 25% of the participants who had not had hypertension previously, and this incidence reached approximately 60% by 6 years. Across the treatment groups, the curves separated beyond 1 year, with the glimepiride and glargine groups having the highest cumulative incidence of hypertension and the liraglutide group the lowest.
Figure 1. Cumulative Incidences of Hypertension and Dyslipidemia in the Intention-to-Treat Analyses.
Shown are the cumulative incidences of hypertension (Panel A) and dyslipidemia (Panel B) over 6.5 years of follow-up among participants who did not have each condition at baseline. The numbers plotted below the x axis of each panel are the numbers of participants at risk for the outcome at each follow-up time point (i.e., the number of participants in whom a specified outcome event had not developed by that time). Participants who had the condition at baseline were excluded, leaving 1168 of 5047 participants (23%) in the analysis of hypertension and 195 of 5047 participants (4%) in the analysis of dyslipidemia. There was no substantial difference among the groups with respect to the cumulative incidences of hypertension or dyslipidemia.
The average (least-squares mean) systolic blood pressure in all the participants over years 1 and 4 was highest in the glargine and glimepiride groups (129.1 mm Hg and 128.7 mm Hg, respectively), lowest in the liraglutide group (126.9 mm Hg), and intermediate in the sitagliptin group (128.1 mm Hg). Diastolic blood pressure did not differ according to treatment group. We also assessed differences among the treatment groups with respect to other risk factors over the short term (1 year) as compared with those over the long term (4 years). In the liraglutide group, the use of blood pressure–lowering medications increased from 80% in year 1 to 83% in year 4; in the other groups, the use of these medications ranged from 81 to 84% at year 1 and increased to 90 to 91% by year 4. Only approximately 10% of the participants without dyslipidemia at baseline had dyslipidemia in the first year, and this percentage increased to approximately 80% by year 4, with little subsequent increase.
MICROVASCULAR OUTCOMES
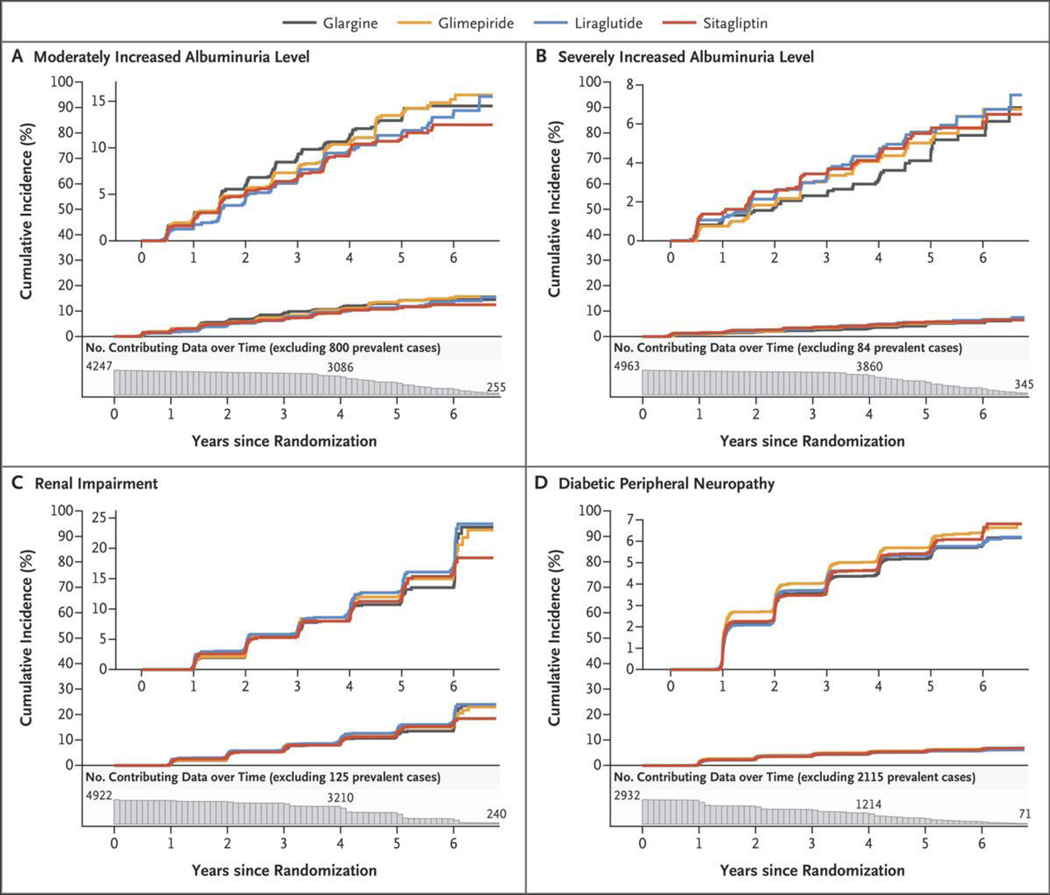
Figure 2 shows the cumulative incidences of confirmed moderately increased albuminuria levels (albumin:creatinine ratio ≥30 [as measured in milligrams of albumin to grams of creatinine] on two consecutive visits), severely increased albuminuria levels (albumin:creatinine ratio ≥300, an eGFR of less than 60 ml per minute per 1.73 m2, and diabetic peripheral neuropathy (assessed by the MNSI). For each outcome, Table 1 shows the rates, pairwise hazard ratios between the treatment groups, and hazard ratios for each agent as compared with the others combined. There were no major differences among the treatment groups in the cumulative incidence of a confirmed moderately increased or severely increased albuminuria level or renal impairment (eGFR, <60 ml per minute per 1.73 m2 of body-surface area), with overall incidence rates of 2.57, 1.08, and 2.91 events per 100 participant-years, respectively; the cumulative incidences were approximately 15%, 8%, and 20%, respectively, by the end of the trial. Likewise, there were no major differences among the groups in the incidence of diabetic peripheral neuropathy. The overall linearized hazard rate was 16.7 events per 100 participant-years, with diabetic peripheral neuropathy developing in approximately 20% of the participants over the first year of follow-up and reaching approximately 70% by the end of the trial.
Figure 2. Cumulative Incidences of Microvascular Outcomes in the Intention-to-Treat Analyses.
Shown are the cumulative incidences of the following conditions over 6.5 years of follow-up among participants who did not have one of these conditions at baseline: confirmed moderately increased albuminuria level (≥30 mg of albumin per gram of creatinine) or dialysis, transplantation, or death due to end-stage kidney disease (Panel A); severely increased albuminuria level (≥300 mg of albumin per gram of creatinine) or dialysis, transplantation, or death due to end-stage kidney disease (Panel B); renal impairment (estimated glomerular filtration rate of <60 ml per minute per 1.73 m2 of body-surface area) (Panel C); and diabetic peripheral neuropathy (Panel D). The numbers plotted below the x axis of each panel are the participants at risk for the outcome at each follow-up time point (i.e., the number of participants in whom a specified outcome event had not developed by that time). The insets show the same data on an enlarged y axis.
Table 1.
Microvascular Outcomes in the Intention-to-Treat Analysis.*
| Outcome | Glargine (N =1263) | Glimepiride (N = 1254) | Liraglutide (N = 1262) | Sitagliptin (N = 1268) | Total (N = 5047) |
|---|---|---|---|---|---|
| Moderately increased albuminuria level † | |||||
| No. of participants/no. at risk (%) | 136/1066(12.8) | 135/1046 (12.9) | 121/1040(11.6) | 115/1070(10.7) | 507/4222 (12.0) |
| Rate (95% Cl) — events/100 participant-yr | 2.76 (2.32–3.26) | 2.78 (2.33–3.29) | 2.46 (2.05–2.95) | 2.30 (1.90–2.76) | 2.57 (2.35–2.81) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 1.00 (0.78–1.26) | 1.12 (0.88–1.43) | 1.20 (0.94–1.54) | ||
| Glimepiride | 1.12 (0.88–1.44) | 1.21 (0.94–1.55) | |||
| Liraglutide | 1.07 (0.83–1.39) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 1.10 (0.91–1.34) | 1.11 (0.91–1.35) | 0.95 (0.77–1.16) | 0.86 (0.70–1.06) | |
| Severely increased albuminuria level ‡ | |||||
| No. of participants/no. at risk (%) | 59/1240(4.8) | 64/1220 (5.2) | 70/1229 (5.7) | 66/1246 (5.3) | 259/4935 (5.2) |
| Rate (95% Cl) — events/100 participant-yr | 0.97 (0.74–1.26) | 1.08 (0.83–1.38) | 1.17 (0.91–1.48) | 1.09 (0.85–1.39) | 1.08 (0.95–1.22) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 0.90 (0.63–1.28) | 0.83 (0.59–1.18) | 0.89 (0.63–1.26) | ||
| Glimepiride | 0.92 (0.66–1.29) | 0.99 (0.70–1.39) | |||
| Liraglutide | 1.07 (0.76–1.50) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 0.87 (0.65–1.17) | 1.00 (0.76–1.33) | 1.12 (0.85–1.47) | 1.02 (0.77–1.35) | |
| Renal impairment § | |||||
| No. of participants/no. at risk (%) | 144/1174 (12.3) | 151/1198 (12.6) | 170/1184 (14.4) | 145/1208 (12.0) | 610/4764 (12.8) |
| Rate (95% Cl) — events/100 participant-yr | 2.78 (2.35–3.28) | 2.88 (2.44–3.38) | 3.26 (2.79–3.78) | 2.73 (2.31–3.22) | 2.91 (2.69–3.15) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 0.96 (0.76–1.20) | 0.85 (0.69–1.07) | 1.02 (0.81–1.28) | ||
| Glimepiride | 0.89 (0.72–1.11) | 1.07 (0.85–1.34) | |||
| Liraglutide | 1.19(0.95–1.49) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 0.94 (0.78–1.13) | 1.00 (0.83–1.20) | 1.16 (0.97–1.38) | 0.92 (0.76–1.11) | |
| Diabetic peripheral neuropathy | |||||
| No. of participants/no. at risk (%) | 393/751 (52.3) | 427/728 (58.7) | 382/704 (54.3) | 405/723 (56.0) | 1607/2906 (55.3) |
| Rate (95% Cl) — events/100 participant-yr | 15.57 (14.07–17.19) | 18.22 (16.53–20.04) | 16.06 (14.49–17.75) | 16.87 (15.27–18.60) | 16.66 (15.85–17.49) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 0.85 (0.74–0.97) | 0.96 (0.84–1.11) | 0.92 (0.80–1.06) | ||
| Glimepiride | 1.14 (0.99–1.31) | 1.08 (0.95–1.24) | |||
| Liraglutide | 0.95 (0.83–1.10) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 0.91 (0.81–1.02) | 1.13 (1.01–1.27) | 0.96 (0.85–1.07) | 1.02 (0.91–1.14) |
The number of participants at risk excludes the prevalent cases at baseline that were not counted in either the numerator or denominator of the calculation of the rate. Pairwise hazard ratios were calculated from an analysis of the differences in the hazards among any of the four treatment groups, on the basis of a Cox proportional-hazards model, with treatment group as the only predictor variable. The 95% confidence intervals (CIs) were not corrected for multiple comparisons.
A moderately increased albuminuria level was defined as a confirmed urinary albumin:creatinine ratio of at least 30, as measured in milligrams of albumin to grams of creatinine.
A severely increased albuminuria level was defined as a urinary albumin:creatinine ratio of at least 300, as measured in milligrams of albumin to grams of creatinine.
Impaired renal function was defined as an estimated glomerular filtration rate of less than 60 ml per minute per 1.73 m2. Participants in whom incident end-stage kidney disease (as defined by dialysis, transplantation, or death from kidney disease) developed during the trial were considered to have had an outcome event in the categories of albuminuria (moderately increased albuminuria and severely increased albuminuria) and renal impairment.
CARDIOVASCULAR OUTCOMES
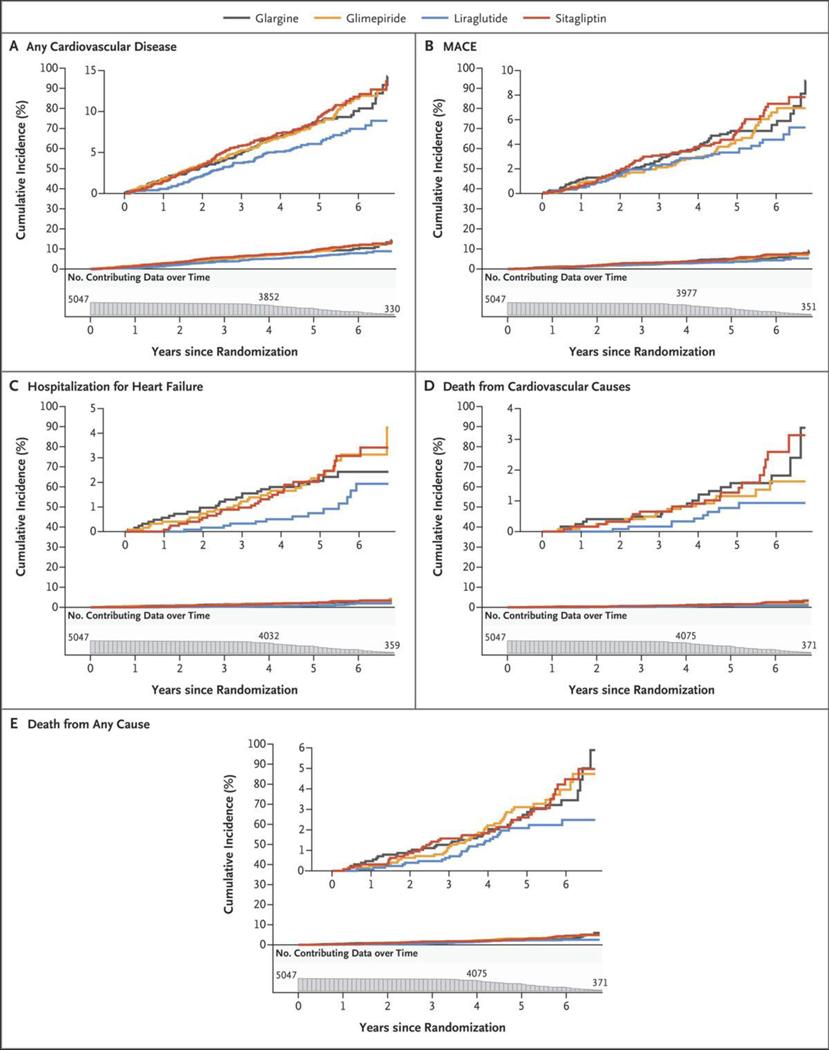
The incidences of cardiovascular events and death are shown in Figure 3A and Table 2. The trialwide rate of the aggregate of any cardiovascular event was 1.79 events per 100 participant-years, with the incidence reaching 10 to 15% among the treatment groups by the end of the trial. As shown in Figure 3A, the liraglutide group had few cases of any cardiovascular event over the first year, and the cumulative incidence increased linearly thereafter, whereas the other treatment groups appeared to have a linear increase starting from baseline and reaching approximately 14% at trial end, as compared with approximately 10% with liraglutide. In pairwise analyses, the hazard ratio for any cardiovascular disease in the liraglutide group as compared with the sitagliptin group was 0.68 (95% confidence interval [CI], 0.51 to 0.90), and the hazard ratio in the liraglutide group as compared with the glimepiride group was 0.71 (95% CI, 0.53 to 0.93), which was obtained by inverting the hazard ratio in the glimepiride group as compared with the liraglutide group (1.41) (Table 2). The incidence of any cardiovascular disease was similar in the liraglutide and glargine groups. A comparison of the liraglutide group with the other three groups combined revealed a hazard ratio of 0.71 (95% CI, 0.56 to 0.90).
Figure 3. Cumulative Incidences of Cardiovascular Outcomes and Mortality in the Intention-to-Treat Analyses.
Shown are the cumulative incidences of any cardiovascular disease (Panel A), a major adverse cardiovascular event (MACE) (Panel B), hospitalization for heart failure (Panel C), death from cardiovascular causes (Panel D), and death from any cause (Panel E) over 6.5 years of follow-up. The numbers plotted below the x axis of each panel are the participants at risk for the outcome at each follow-up time point (i.e., the number of participants in whom a specified outcome event had not developed by that time).
Table 2.
Cardiovascular and Mortality Outcomes in the Intention-to-Treat Analysis.*
| Outcome | Glargine (N = 263) | Glimepiride (N = 1254) | Liraglutide (N = 1262) | Sitagliptin (N = 1268) | Total (N = 5047) |
|---|---|---|---|---|---|
| Any cardiovascular disease † | |||||
| No. of participants/no. at risk (%) | 113/1257 (9.0) | 115/1247 (9.2) | 83/1251 (6.6) | 121/1264 (9.6) | 432/5019 (8.6) |
| Rate (95% Cl) | 1.87 (1.54–2.25) | 1.92 (1.59–2.31) | 1.36 (1.08–1.69) | 2.00 (1.66–2.39) | 1.79 (1.62–1.96) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 0.97 (0.75–1.26) | 1.37 (1.03–1.82) | 0.93 (0.72–1.21) | ||
| Glimepiride | 1.41 (1.07–1.87) | 0.96 (0.74–1.24) | |||
| Liraglutide | 0.68 (0.51–0.90) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 1.07 (0.87–1.33) | 1.12 (0.90–1.39) | 0.71 (0.56–0.90) | 1.18 (0.96–1.46) | |
| MACE | |||||
| No. of participants/no. at risk (%) | 65/1257 (5.2) | 59/1247 (4.7) | 48/1251 (3.8) | 69/1264 (5.5) | 241/5019 (4.8) |
| Rate (95% Cl) | 1.05 (0.81–1.34) | 0.96 (0.73–1.24) | 0.78 (0.57–1.03) | 1.12 (0.87–1.41) | 0.98 (0.86–1.11) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 1.09 (0.77–1.55) | 1.35 (0.93–1.96) | 0.94 (0.67–1.32) | ||
| Glimepiride | 1.24 (0.85–1.81) | 0.86 (0.61–1.22) | |||
| Liraglutide | 0.70 (0.48–1.01) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 1.12 (0.84–1.49) | 0.99 (0.74–1.33) | 0.75 (0.54–1.03) | 1.21 (0.91–1.60) | |
| Hospitalization for heart failure | |||||
| No. of participants/no. at risk (%) | 26/1257 (2.1) | 30/1247 (2.4) | 14/1251 (1.1) | 30/1264 (2.4) | 100/5019 (2.0) |
| Rate (95% Cl) | 0.42 (0.27–0.61) | 0.48 (0.33–0.69) | 0.22 (0.12–0.38) | 0.48 (0.32–0.68) | 0.40 (0.33–0.49) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 0.86 (0.51–1.45) | 1.85 (0.96–3.55) | 0.87 (0.51–1.47) | ||
| Glimepiride | 2.16 (1.14–4.06) | 1.01 (0.61–1.67) | |||
| Liraglutide | 0.47 (0.25–0.88) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 1.11 (0.70–1.76) | 1.36 (0.88–2.11) | 0.49 (0.28–0.86) | 1.35 (0.87–2.08) | |
| Death from cardiovascular causes | |||||
| No. of participants/no. at risk (%) | 21/1257 (1.7) | 16/1247 (1.3) | 9/1251 (0.7) | 21/1264 (1.7) | 67/5019 (1.3) |
| Rate (95% Cl) | 0.33 (0.21–0.51) | 0.26 (0.15–0.42) | 0.14 (0.07–0.27) | 0.33 (0.21–0.51) | 0.27 (0.21–0.34) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 1.29 (0.67–2.47) | 2.30 (1.05–5.01) | 1.00 (0.55–1.82) | ||
| Glimepiride | 1.78 (0.79–4.04) | 0.77 (0.40–1.48) | |||
| Liraglutide | 0.43 (0.20–0.95) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 1.43 (0.85–2.43) | 1.02 (0.58–1.82) | 0.47 (0.23–0.95) | 1.44 (0.85–2.44) | |
| Death from any cause | |||||
| No. of participants/no. at risk (%) | 42/1263 (3.3) | 43/1254 (3.4) | 27/1262 (2.1) | 41/1267 (3.2) | 153/5046 (3.0) |
| Rate (95% Cl) | 0.65 (0.47–0.87) | 0.67 (0.48–0.90) | 0.42 (0.27–0.60) | 0.63 (0.45–0.86) | 0.59 (0.50–0.69) |
| Pairwise hazard ratio (95% Cl) | |||||
| Glargine | 0.96 (0.63–1.46) | 1.54 (0.95–2.50) | 1.02 (0.66–1.57) | ||
| Glimepiride | 1.61 (1.00–2.61) | 1.07 (0.69–1.63) | |||
| Liraglutide | 0.66 (0.41–1.07) | ||||
| Sitagliptin | |||||
| Hazard ratio (95% Cl) in one agent as compared with the others combined | 1.15 (0.80–1.64) | 1.22 (0.85–1.73) | 0.64 (0.42–0.97) | 1.12 (0.78–1.60) |
The number of participants at risk excludes the prevalent cases at baseline that were not counted in either the numerator or the denominator of the calculation of the rate. Pairwise hazard ratios were calculated from an analysis of the differences in the hazards among any of the four treatment groups, on the basis of a Cox proportional-hazards model, with treatment group as the only predictor variable. The 95% confidence intervals were not corrected for multiple comparisons.
Any cardiovascular disease was defined as the first of any major adverse cardiovascular event (MACE; defined as death from cardiovascular disease or nonfatal myocardial infarction or stroke), unstable angina warranting hospitalization or revascularization, heart failure warranting hospitalization, or any revascularization event.
The rate of MACE was approximately 0.98 events per 100 participant-years, with the cumulative incidence increasing steadily to approximately 6 to 8% across the treatment groups by the end of the trial, with no material differences among the groups. The rate of hospitalization for heart failure was low overall (0.4 per 100 participant-years), and although there were nominal differences in the hazard rates among the four treatment groups, the low number of events precluded a definitive assessment. A total of 67 participants died from cardiovascular disease and 153 participants died from any cause, with corresponding rates of 0.27 and 0.59 per 100 participant-years, respectively (Fig. 3 and Table 2). The incidences of death from cardiovascular causes and all deaths were similar among the groups.
PER-PROTOCOL AND INTENTION-TO-TREAT ANALYSES
Per-protocol sensitivity analyses were performed and compared with the intention-to-treat analyses (Figs. S2 and S3 and Tables S3 and S4). There was no material difference among the treatment groups in the intention-to-treat analysis with respect to confirmed moderately increased albuminuria; however, the per-protocol analysis showed small differences favoring the liraglutide and sitagliptin groups over the glargine and glimepiride groups. The hazard ratios, which were were derived by inverting the ratios shown in Table S3, are as follows: hazard ratio in the liraglutide group as compared with the glargine group, 0.73; hazard ratio in the sitagliptin group as compared with the glargine group, 0.71; hazard ratio in the liraglutide group as compared with the glimepiride group, 0.73; and hazard ratio in the sitagliptin group as compared with the glimepiride group, 0.71.
There were no differences between the intention-to-treat and per-protocol analyses with respect to severely increased albuminuria level, renal impairment, or diabetic peripheral neuropathy. In the analyses of cardiovascular outcomes and death, in no instance did the per-protocol analysis yield materially larger differences among groups than did the intention-to-treat analysis. The differences among treatment groups with respect to any cardiovascular disease in the intention-to-treat analysis were unchanged in the per-protocol analysis.
SUBGROUP ANALYSES
Assessments of the homogeneity of treatment-group differences among predefined subgroup strata showed that the pattern of risks across treatment groups did not differ materially across any subgroups (Table S5). A post hoc analysis showed a substantially higher incidence of any cardiovascular disease among the participants with a history of stroke or myocardial infarction at baseline than among those without this history; the rates of any cardiovascular disease were nominally lower in both categories in the liraglutide group than in the other three treatment groups.
DISCUSSION
The current trial showed significant differences among the four randomized treatments, when added to metformin, in the ability to reach and maintain targeted glycated hemoglobin levels.11 Here, we evaluated secondary outcomes, including differential effects of these agents with respect to microvascular and cardiovascular disease and their risk factors. Most participants had hypertension or dyslipidemia at baseline, which is typical for a population with type 2 diabetes mellitus. More than 90% of the participants who did not have hypertension or dyslipidemia at baseline were later classified as having these conditions, largely because medications had been initiated by their own care providers. The only difference in the development of these conditions at 1 and 4 years of follow-up was that liraglutide may have had a relative benefit with respect to measured blood pressure. The glargine group may have had more incident hypertension because of the effect of insulin on sodium reabsorption in the kidney.19
Despite the differences among the treatment groups in glycemia and hypertension, both of which are long-recognized risk factors for microvascular complications,2,20 there were no material differences in any of the microvascular complications that were evaluated. The absence of the expected effect of lower glycemia on microvascular complications has been noted in some trials, including studies of diabetes prevention,21 and this absence has been ascribed to inadequate separation of glycemic levels over time, insufficient trial duration, threshold effects,22 or inadequate power. Any or all of these factors, including the small separation in glycemia,11 might have been operative in our trial.
The trial cohort was at relatively low risk for MACE or other cardiovascular events; only 6% of the participants had a history of myocardial infarction or stroke before the trial began, and none of the participants had had an event within 1 year before randomization. This trial was not designed or powered to detect differences among the treatment groups with respect to cardiovascular events or death from cardiovascular disease. Cardiovascular risk factors were generally well managed, so the observed differences in the incidence of any cardiovascular disease among the treatment groups are especially notable. Trials showing a beneficial effect of a number of GLP-1 receptor agonists with respect to cardiovascular disease have included populations with a higher cardiovascular risk at baseline than the population in the current trial.4,9,23 Nevertheless, in our trial, there was a difference in the incidence of any cardiovascular disease across the four treatment groups and in pairwise comparisons between the liraglutide and sitagliptin groups, between the liraglutide and glimepiride groups, and between the liraglutide group and the other three groups combined. These results should not be viewed as definitive proof that GLP-1 receptor agonists reduce the incidence of cardiovascular disease in low-risk populations. However, our results parallel the benefits with respect to cardiovascular disease that have been reported in populations with type 2 diabetes mellitus and higher cardiovascular risk at baseline than the population in the current trial.4,9,23
We observed differences among the groups in adherence to and discontinuation of the assigned medications such that there were some differences in the sensitivity analyses. In the per-protocol analysis, but not in the intention-to-treat analyses, the liraglutide and sitagliptin groups had a lower risk of moderate albuminuria than the glimepiride and glargine groups. However, liraglutide did not appear to mitigate decreases in renal function. In the comparison of liraglutide with the other three medications, the hazard ratio for an eGFR of less than 60 was 1.16 (95% CI, 0.97 to 1.38). In the per-protocol analyses, as in the intention-to-treat analyses, the risk of any cardiovascular disease was lower with liraglutide than with either glimepiride or sitagliptin.
The current trial used a comparative-effectiveness approach to examine four different glucose-lowering medications. The different effects of these agents on microvascular complications, cardiovascular risk factors, and cardiovascular outcomes should be considered along with their glycemic effects when choosing therapies for type 2 diabetes. In this trial involving participants with type 2 diabetes of generally brief duration, the incidences of microvascular complications and death were not materially different among the four treatment groups. The findings did provide support for possible differences among the treatment groups in the incidence of any cardiovascular disease.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by a grant (U01DK098246) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH); a U34 planning grant (U34-DK-088043) from the NIDDK; funding for the initial planning meeting regarding the U34 proposal from the American Diabetes Association; the National Heart, Lung, and Blood Institute; the Centers for Disease Control and Prevention; resources and facilities from the Department of Veterans Affairs; grants (P30 DK017047, P30 DK020541-44, P30 DK020572, P30 DK072476, P30 DK079626, P30 DK092926, U54 GM104940, UL1 TR000170, UL1 TR000439, UL1 TR000445, UL1 TR001102, UL1 TR001108, UL1 TR001409, 2UL1TR001425, UL1 TR001449, UL1 TR002243, UL1 TR002345, UL1 TR002378, UL1 TR002489, UL1 TR002529, UL1 TR002535, UL1 TR002537, UL1 TR002541, and UL1 TR002548) from the NIH; educational materials from the National Diabetes Education Program; and donated medications and supplies from Becton Dickinson, Bristol Myers Squibb, Merck, Novo Nordisk, Roche Diagnostics, and Sanofi.
We thank our participants, whose loyal dedication made this trial possible.
Footnotes
Contributor Information
The GRADE Study Research Group:
David M. Nathan, John M. Lachin, Ionut Bebu, Henry B. Burch, John B. Buse, Andrea L. Cherrington, Stephen P. Fortmann, Jennifer B. Green, Steven E. Kahn, M. Sue Kirkman, Heidi Krause-Steinrauf, Mary E. Larkin, Lawrence S. Phillips, Rodica Pop-Busui, Michael Steffes, Margaret Tiktin, Mark Tripputi, Deborah J. Wexler, and Naji Younes
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 3.Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013;11: CD008143. [DOI] [PubMed] [Google Scholar]
- 4.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 7.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42. [DOI] [PubMed] [Google Scholar]
- 8.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–89. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GRADE Study Research Group. Glycemia reduction in type 2 diabetes — glycemic outcomes. N Engl J Med 2022;387: 1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wexler DJ, Krause-Steinrauf H, Crandall JP, et al. Baseline characteristics of randomized participants in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2019;42:2098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17: 1281–9. [DOI] [PubMed] [Google Scholar]
- 17.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and stroke end-point definitions for clinical trials. Circulation 2018;137:961–72. [DOI] [PubMed] [Google Scholar]
- 18.Mather KJ, Bebu I, Baker C, et al. Prevalence of microvascular and macrovascular disease in the Glycemia Reduction Approaches in Diabetes — A Comparative Effectiveness (GRADE) study cohort. Diabetes Res Clin Pract 2020;165:108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarafidis PA, Bakris GL. The antinatriuretic effect of insulin: an unappreciated mechanism for hypertension associated with insulin resistance? Am J Nephrol 2007;27:44–54. [DOI] [PubMed] [Google Scholar]
- 20.UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317:713–20. [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan DM, Bennett PH, Crandall JP, et al. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia 2019;62: 1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012; 55:636–43. [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.