Abstract
Respiratory tract resident memory T cells (TRM), typically generated by local vaccination or infection, can accelerate control of pulmonary infections that evade neutralizing antibody. It is unknown whether mRNA vaccination establishes respiratory TRM. We generated a self-amplifying mRNA vaccine encoding the influenza A virus nucleoprotein that is encapsulated in modified dendron-based nanoparticles. Here we report how routes of immunization in mice, including contralateral versus ipsilateral intramuscular boosts, or intravenous and intranasal routes, influenced influenza-specific cell-mediated and humoral immunity. Parabiotic surgeries revealed that intramuscular immunization was sufficient to establish CD8 TRM in lung and draining lymph nodes. Contralateral, compared to ipsilateral, intramuscular boosting broadened the distribution of lymph node TRM and T follicular helper cells, but slightly diminished resulting levels of serum antibody. Intranasal mRNA delivery established modest circulating CD8 and CD4 T cell memory, but augmented distribution to the respiratory mucosa. Of note, combining intramuscular immunizations with an intranasal mRNA boost achieved high levels of both circulating T cell memory and lung TRM. Thus, routes of mRNA vaccination influence humoral and cell-mediated immunity, and intramuscular prime-boosting establishes lung TRM that can be further expanded by an additional intranasal immunization.
One Sentence summary:
Intramuscular mRNA vaccination induces pulmonary resident memory T cells that can be further expanded with intranasal boosting.
Introduction
Despite the successful control and in some cases eradication of numerous infectious diseases, classical vaccination approaches have failed to eliminate endemic pathogens such as HIV, malaria, tuberculosis, and influenza, highlighting a need for innovative vaccine design. To combat the emergent threat of SARS-CoV-2, novel mRNA vaccine technologies were rapidly developed, adopted, and shown to prevent severe disease outcomes (1-4).
Standard immunization with a SARS-CoV-2 mRNA vaccine includes two intramuscular (IM) immunizations at 21 (BNT162b2) or 28 day (mRNA-1273) intervals, followed by one or more additional boosts five months later. Advantages of mRNA vaccination include the relatively potent immunogenicity for CD8 T cells as well as CD4 T cells and antibodies, the absence of vector immunity which permits homologous boosting, and the rapidity of production at scale. mRNA vaccines are being considered for diverse disease conditions, including universal influenza vaccines and cancer, both of which likely depend on regional cell-mediated immunity (5, 6). This furthers the impetus to better characterize the differentiation and distribution of memory CD8 and CD4 T cells after mRNA vaccination.
mRNA vaccine-elicited T cells have been proposed to contribute to protection upon infection of SARS-CoV-2 variants of concern (7, 8). In contrast to neutralizing antibodies, T cells recognize partially conserved epitopes and hence may not be completely evaded by mutations in the spike protein of SARS-CoV-2. Similarly, T cells recognize epitopes in the nucleoprotein of influenza viruses that may be conserved despite antigenic drift and antigenic shift, unlike neutralizing antibody targets on hemagglutinin (HA) and neuraminidase (NA) (9, 10). However, mRNA vaccine-elicited T cells have been best characterized in blood, and remain underexplored in the lung, the local site of influenza and SARS-CoV-2 infections. Indeed, even in mouse studies, little work has been done on antigen-specific T cells in tissues, including their migration properties, following mRNA vaccination.
Unlike circulating memory T cell populations that patrol blood and lymph, resident memory T cells (TRM) are the predominant surveyors of nonlymphoid tissues and accelerate pathogen control in the event of local infection (11, 12). Upon reactivation, CD8+ TRM are poised for rapid cytotoxic and innate-like ‘sensing and alarm’ functions which establish an antiviral tissue microenvironment that is further supported by the IFN-γ production of CD4+ TH1 TRM (13-16). In addition, lung resident CD4+ T follicular helper cells (TFH) support local antibody production after heterosubtypic reinfection (15, 17). TRM have also been identified in lung-draining lymph nodes (LNs), and local LN memory T cells may make independent contributions to respiratory immunity (18, 19). Because of the known protective role of pulmonary TRM in mice and humans, whether mRNA prime-boost vaccination elicits bona fide TRM in the lung or regional LNs is clinically relevant (16, 19-35). Induction of pulmonary TRM might represent a supplementary strategy to bolster the efficacy of vaccines designed to elicit neutralizing antibodies or to induce protective immunity in immunocompromised individuals that have an impaired ability to generate antibodies (16, 20, 22, 24, 36). However, previous reports indicate that local antigen expression within the lung is required to establish pulmonary TRM (20, 23, 37-41). Therefore, IM mRNA vaccinations may not establish TRM in the respiratory mucosa.
Here, we characterized how antigen-specific CD8 and CD4 T cell and serum antibody responses related to immunization routes after vaccination with modified dendron-based nanoparticle (MDNP)-encapsulated self-amplifying mRNA encoding an influenza nucleoprotein (NP). RNA encapsulation in MDNPs is driven by charge-based interactions between the ionizable dendritic material and the RNA phosphate backbone, and hydrophobic interactions with additional lipid excipients. This is in contrast to ionizable lipids currently used in the clinic, which are monocationic. In comparison to previously-reported lipid nanoparticles (LNPs) used for self-amplifying RNA, the vaccine platform used in this study allows delivery of greater masses of self-amplifying mRNA per unit mass of delivery compounds with minimal acute reactogenicity and systemic toxicity (42).
Results
Impact of ipsilateral versus contralateral intramuscular mRNA-vaccination on CD8 T cell, CD4 T cell, and humoral immunity
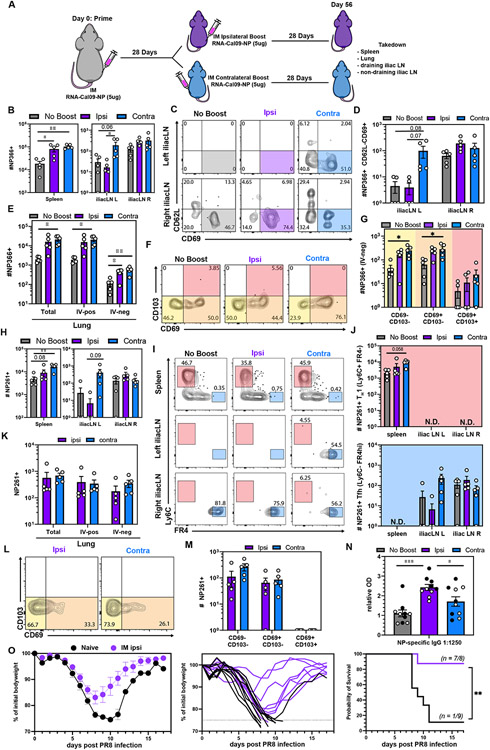
To assess memory T cell populations after mRNA vaccination, we primed C57BL/6J mice with 5 μg of mRNA encoding the Cal/09 influenza nucleoprotein (NP) in the right hamstring. ≥28 days later, we administered an additional 5 μg dose of mRNA. To examine whether the administration site of the booster impacted the regionalization of the memory T cell response, we boosted in either the right (ipsilateral) or the left (contralateral) hamstring (Fig. 1A). We used MHC I (H-2Kb NP366-375) and MHC II (I-Ab NP261-277) tetramers to track antigen-specific CD8 and CD4 T cell responses in various tissues ≥28 days after the last immunization (Fig S1 A-B) (43). Compared to a single immunization, both ipsilateral and contralateral boosting increased the number of H-2Kb NP366-375+ CD8 memory T cells in the spleen (Fig. 1B). Contralateral immunization was required for establishing memory CD8 T cells in the contralateral LN (Fig. 1C-D). This unexpected result might be explained by the lack of CD62L expression on most memory CD8 T cells (Fig. 1C). We also observed that IM immunization induced a trackable extravascular (IV-neg) CD69− and CD69+ H-2Kb NP366-375+ CD8 T cell population in the lung that was bolstered upon boosting (Fig 1E-G).
Fig 1. Impact of ipsilateral versus contralateral intramuscular mRNA-vaccination on CD8 T cell, CD4 T cell, and humoral immunity.
(A) Mice were primed in the right hamstring then boosted in either the right (ipsilateral) or left (contralateral) hamstring. ≥28 days post boost, spleen, iliac LN, medLN and lungs were examined. (B) Quantification of antigen-specific CD8+ T cells in the indicated SLOs. (C-D) Representative flow cytometry plot (C) and quantification (D) of NP366-specific CD8+ CD69+CD62L− SLO TRM. (E) Enumeration of antigen-specific CD8+ T cells in the indicated lung compartments. (F-G) Representative flow cytometry plot (F) and quantification (G) of NP366+ CD8 T cell subsets in the lung parenchyma (IV-neg). (H) Enumeration of antigen-specific CD4+ T cells in the indicated SLOs. (I-J) Representative flow cytometry plot (I) and quantification (J) of NP261-specific CD4+ TH1 and TFH in SLOs. (K) Quantification of antigen-specific CD4+T cells in the indicated lung compartments. (L-M) Representative flow cytometry plot (L) and quantification (M) of NP261-specific CD4+ T cell subsets in the lung parenchyma (IV-neg). (N) NP IgG specific serum antibody titers. Open and closed circles represent data from two independent experiments. (O) Ipsilaterally prime-boosted mice were challenged with Influenza A/PR8 IN. Weight relative to initial bodyweight over time (left, middle), and survival (right). Data represent N = 2 independent experiments with n = 4-5 mice per group except for (N-O) where data was pooled from N = 2 independent experiments with n = 8-10 mice. Data are shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as determined by one-way ANOVA and Tukey’s multiple comparisons test (for 3 groups) or unpaired two-tailed Student’s t test (2 groups) or with log-rank Mantel-Cox test for survival. N.D. = not determined.
mRNA prime-boost vaccination also induced regionalization of antigen-specific memory CD4 T cells (Fig 1H). We used the marker combinations Ly6C+FR4lo and Ly6C−FR4hi to define TH1 and TFH memory subsets, respectively (44-46). TH1 memory cells were mostly restricted to the spleen, whereas long-lived TFH were essentially exclusive to the draining LNs (Fig 1 I-J, Fig S1 C-D). Spleen contained a population of cells expressing intermediate levels of FR4 (FR4int). FR4 is also a known Treg marker, however FR4int I-Ab NP261-277 tetramer+ CD4 T splenocytes do not express Foxp3 (47) (Fig. S1E). IM prime-boost vaccination also induced I-Ab NP261-277 tetramer+ CD4 memory T cells in the lung parenchyma (Fig 1 K-M). We next investigated the humoral response and found that NP-specific IgG titers were higher in ipsilaterally boosted mice compared to both unboosted and contralateral boosted mice (Fig. 1N). These data indicate that the side of boosting impacts the quality of the immune response, where contralateral boosting established CD69+ CD8 and CD4 TFH memory T cells in the left iliac LN, but ipsilateral boosting generated slightly higher antibody levels.
We next tested whether ipsilateral prime-boost IM vaccination conferred protection. Mice were IM prime-boost vaccinated, rested for 38 or 39 additional days, depending on the experiment, then anesthetized with ketamine/xylazine. Mice were then challenged intranasally (IN) with influenza A/PR8. Weight loss and survival were compared alongside contemporaneously challenged age-matched naïve C57BL/6J mice. Cal/09-NP mRNA vaccinated mice were largely protected from lethal influenza A/PR8 challenge (Fig. 1O).
Comparing routes of immunization reveals that intranasal prime-boost vaccination induces more CD103+ CD8 T cells in the lung parenchyma
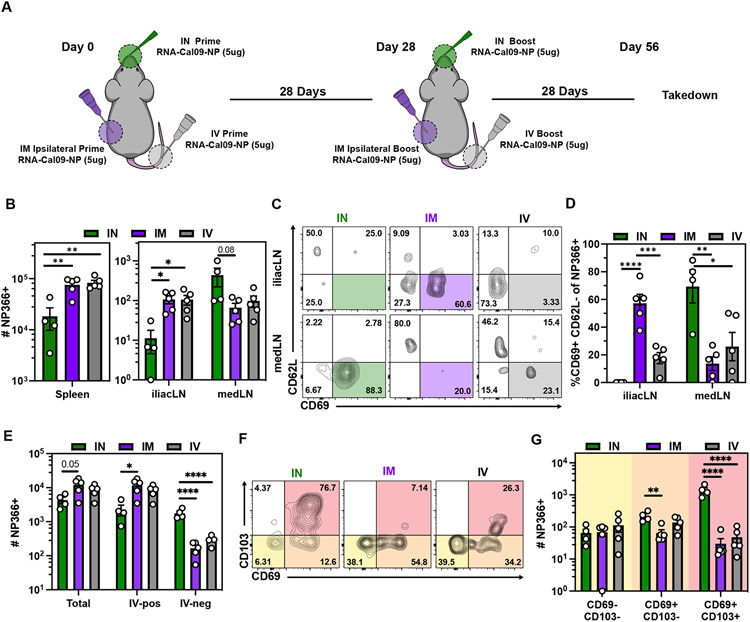
Human mRNA vaccination is administered by IM injection, though immunization via alternative routes may be an approach to induce specific immunological outcomes (48). Conventional RNA lipid nanoparticles (LNPs) cause material-induced inflammatory responses which can cause morbidity and mortality upon IN instillation in mice due to the sensitivity of the respiratory airway to acute inflammation (Fig. S2A-B, Table S1) (49). The MDNP formulation selected for this study resulted in complete survival following IN RNA vaccination. Moreover, compared to equivalent RNA doses of conventional LNP, this delivery formula induced negligible material-induced inflammation measured by the induction of IP-10, IL-6, MCP-1, CXCL1, and RANTES after IM immunization (Fig S2C). Liver toxicity studies of this MDNP formulation containing the self-amplifying Cal/09 influenza NP mRNA was assessed by serum alkaline phosphatase (ALP) and alanine transaminase (ALT) activity measurement at a high dose (10μg), and while a transient increase in ALT was observed, levels remained within acceptable ranges. (Fig. S2D-E). Given this opportunity, we tested how IM, IN, and intravenous (IV) routes of immunization would compare with respect to memory T cell differentiation and distribution to the respiratory mucosa and lung-draining mediastinal LN (medLN) (Fig. 2A). We observed that the magnitude of H-2Kb NP366-375 tetramer+ T cells following IM and IV vaccination were largely comparable in secondary lymphoid organs (Fig. 2B). In contrast to IM immunization, IV immunization did not robustly establish CD62L−CD69+ memory CD8 T cells in the muscle-draining iliac LN (Fig 2C-D). IN vaccination resulted in fewer memory T cells in the spleen and iliac LN but generated more in the medLN (Fig. 2B). After IN vaccination, medLN H-2Kb NP366-375 tetramer+ T cells phenocopied T cells in the iliac LN of IM immunized mice (CD62L−CD69+), which is consistent with a SLO-TRM phenotype (Fig. 2C-D)(50). These data demonstrate that vaccination route influences the regionalization and phenotype of mRNA vaccine-elicited T cells.
Fig 2. Comparing routes of immunization reveals that intranasal prime-boost vaccination induces more CD103+ CD8 T cells in the lung parenchyma.
(A) Mice were either IM, IV or IN prime-boosted and spleen, iliac LN, medLN and lungs were examined ≥28 days post boost. (B) Quantification of antigen-specific CD8+ T cells in the indicated SLOs. (C-D) Representative flow cytometry plot (C) and quantification (D) of NP366-specific CD8+ CD69+CD62L− SLO TRM. (E) Quantification of antigen-specific CD8+ T cells in the indicated lung compartments. (F-G) Representative flow cytometry plot (F) and quantification (G) of NP366+ CD8 T cell subsets in the lung parenchyma (IV-neg). Data represent N = 2 independent experiments with n = 4-5 mice per group. Data are shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as determined by one-way ANOVA and Tukey’s multiple comparisons test.
While IM and IV vaccinations induced demonstrable T cells within the extravascular compartments of the lung, most cells were labeled with iv antibody. In comparison, IN prime-boost vaccination established ~10x more extravascular NP366+ T cells within the lung (Fig. 2E). Unlike IM and IV elicited CD8 T cells, most influenza-specific memory CD8 T cells following IN mRNA administration were CD69+CD103+, a phenotype previously associated with localization in proximity to airways (Fig. 2F-G) (18, 20, 51-53). Independent of route, mRNA vaccination induced an NP-specific memory CD8 T cell population in the nasal-associated lymphoid tissues (NALT), trachea, and bronchoalveolar lavage fluid (BAL) (Fig. S3A-C). These populations persisted for at least 123 and 160 days after the last IN and IM immunization, respectively, in both the NALT and trachea. While CD8 T cells in the BAL following IN vaccination contracted over time, IM prime-boost was less effective at inducing memory T cells in the BAL fluid (Fig. S3A-C). Together, these data highlight that all routes of immunization established broadly distributed memory T cells, but that IN immunization increased the magnitude of CD69+CD103+ CD8 T cells in the lung and medLN.
mRNA vaccination generates long-lived TFH in the draining LN but not in the lung
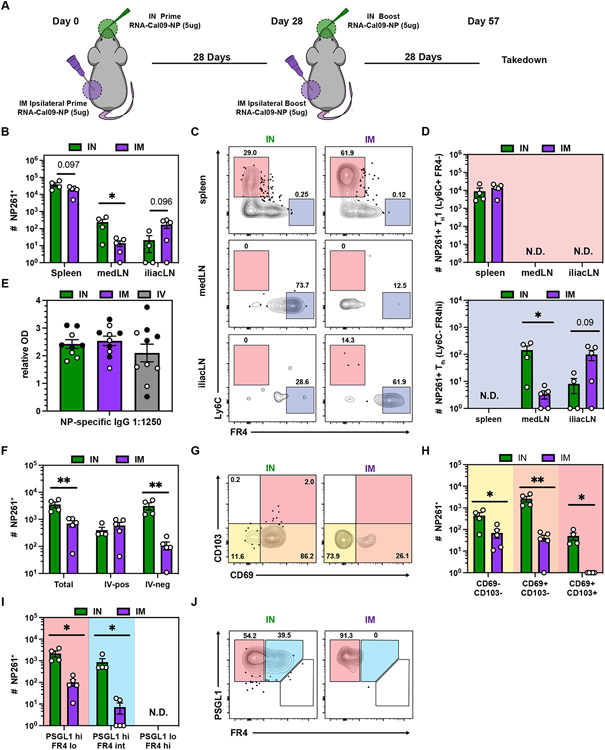
The impact of IN mRNA delivery on the CD8 memory compartment prompted us to investigate whether the route also impacted antigen-specific CD4 memory T cell magnitude and phenotype in the lung and draining LN (Fig. 3A). In contrast to CD8 T cells, the frequency of antigen-specific CD4 T cells in the spleen following IN or IM vaccination was comparable, whereas the abundance of I-Ab NP261-277+ T cells in the different LNs correlated with the vaccination route (Fig. 3B). Again, TH1 cells were enriched in spleen and TFH were restricted to the draining LN (Fig. 3 C-D, Fig. S3A-B). NP-specific IgG serum antibody titers were not affected by vaccination route (Fig. 3E). We observed a significant increase in I-Ab NP261-277+ CD4s in the lung after IN vaccination which was completely accounted for by an increase in extravascular cells (Fig. 3F), many of which were CD69+ (Fig. 3G-H). In contrast to NP366+ CD8 memory T cells, CD103 upregulation was minimal, consistent with previously reported phenotypes of CD4 T cells in non-lymphoid tissues (54). In addition to well-described pulmonary TH1 resident CD4 T cells (PSGL1hi FR4lo), a TFH-like memory population (PSGL1lo FR4hi) was recently identified in the lung following influenza infection that promoted local antibody production upon reactivation (14, 15). In contrast to influenza infection, mRNA vaccination did not induce I-Ab NP261-277+ PSGL1lo FR4hi cells in the lung (Fig. 3I-J, Fig. S4C). However, IN vaccination reproducibly induced an FR4 intermediate population that otherwise phenocopied TH1-like resident memory T cells (Fig. S4D) (14, 15). I-Ab NP261-277+ CD4 T cells in the lung and NP-specific IgG antibodies in BAL fluid persisted for at least 123 days post immunization after both IM and IN immunization (Fig. S4E). Taken together, IN vaccination induced long-lived TFH in the medLN and in contrast to IM vaccination generated a robust pulmonary CD4 T cell memory population.
Fig 3. mRNA vaccination generates long-lived TFH in the draining LN but not in the lung.
(A) Mice were either IM or IN prime-boosted and spleen, iliac LN, medLN and lungs were examined ≥28 days post boost. (B) Quantification of antigen-specific CD4+ T cells in the indicated SLOs. (C-D) Representative flow cytometry plot (C) and quantification (D) of NP261-specific CD4+ TH1 and TFH in SLOs. (E) NP IgG specific serum antibody titers. Open and closed circles represent data from two independent experiments. (F) Quantification of antigen-specific CD4+ T cells in the indicated lung compartments. (G-H) Representative flow cytometry plot (G) and quantification (H) of NP261-specific CD4+ T cell subsets in the lung parenchyma (IV-neg). (I-J) Quantification (I) and representative flow cytometry plot (J) of NP261-specific CD4+ T cell subsets in the lung parenchyma (IV-neg). Data represent N = 2 independent experiments with n = 4-5 mice per group. Data are shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as determined by one-way ANOVA and Tukey’s multiple comparisons test (for 3 groups) or unpaired two-tailed Student’s t test (2 groups). N.D. = not determined.
All mRNA immunization routes were sufficient to induce pulmonary resident memory CD8 T cells
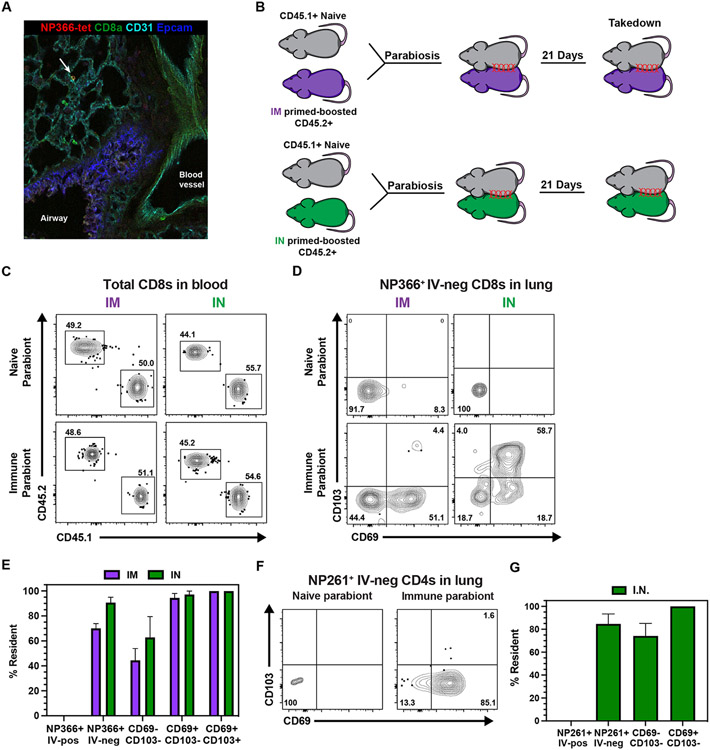
Upon IM mRNA prime-boost vaccination, we noted a population of CD8 and CD4 memory T cells in the lung, some of which expressed CD69. To further validate the presence of H-2Kb NP366-375 tetramer+ CD8 T cells in the lung after IM immunization, we performed in situ tetramer staining and found influenza-specific memory T cells in the lung parenchyma (Fig. 4A). Although CD69 is often used as a proxy to denote resident memory T cells, many TRM do not express CD69 and not all CD69+ cells are resident (11, 50). Moreover, the establishment of TRM in the lung often requires local antigen recognition (20, 23, 37-41). Therefore, we wondered whether or not IM vaccine-induced T cells were resident or recirculating. To assess migration of mRNA vaccine-elicited pulmonary T cells, we conjoined CD45.2+ mRNA primed-boosted mice to congenically mismatched naive CD45.1+ C57BL/6J mice via parabiosis surgery (Fig. 4B). While circulating bloodborne populations equilibrate between the two mice, the failure of cells to equilibrate in corresponding tissues is strong evidence for residency. Three weeks after surgery, the blood contained equal proportions of CD45.2+ and CD45.1+ CD8 T cells, demonstrating successful anastomosis between the immune and naive parabionts (Fig. 4C). To calculate the proportion of resident T cells, we compared the number of antigen-specific T cells in the immune versus naive parabiont using a previously reported equation (11). Unexpectedly, extravascular pulmonary NP366+ CD8 T cells were preferentially present in the immune parabiont – demonstrating that IM mRNA vaccination elicits bona fide resident memory T cells (Fig. 4D-E). The few NP366+ CD8 T cells found in the lung of the naive parabiont were >90% CD69− CD103−. The proportion of resident cells increased stepwise from 40% in the CD69−CD103− compartment to 95% and 100% residence in the CD69+CD103− and CD69+CD103+ subsets, respectively. To test whether local antigen deposition impacts the formation of pulmonary TRM, we repeated parabiosis with IN prime-boosted mice. Not only did IN vaccination boost the number of extravascular pulmonary T cells by ~10 fold, the fraction of resident T cells also increased from ~70% to ~90% (Fig. 2E, Fig. 4 D-E). We also assessed residence within the CD4 compartment. Our analysis was limited to the IN vaccination route due to insufficient recovery of antigen-specific CD4 T cells upon parabiosis after IM vaccination. Following IN mRNA vaccination, ~90% of I-Ab NP261-277+ CD4 T cells resided in the lung for at least three weeks and this proportion increased to 100% if the analysis was limited to CD69+ cells (Fig. 4 F-G). In conclusion, mRNA vaccination induces bona fide TRM independent of vaccination route.
Fig 4. All mRNA immunization routes were sufficient to induce pulmonary resident memory CD8 T cells.
(A) Representative image of in situ NP366-tetramer staining in the lung upon IM prime-boost. (B) CD45.2+ mice were either IM or IN prime-boosted and then conjoined to CD45.1+ naïve hosts. After 21 days, lungs were harvested to examine recirculating and resident populations antigen-specific CD8 and CD4 T cells. (C) Representative flow cytometry plots depicting CD45.1+ and CD45.2+ CD8 T cells to demonstrate equilibration in the blood. (D-E) Representative flow cytometry plot (D) and quantification (E) of NP366-specific CD8 T cell subsets in the lung parenchyma. (F-G) Representative flow cytometry plot (F) and quantification (G) of NP261-specific CD4 T cell subsets in the lung parenchyma. Data represent N = 2 independent experiments with n = 4-5 mice per group, except for (A) which represents N = 1 independent experiment with n = 3 mice. Data are shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001as determined by unpaired two-tailed Student’s t test.
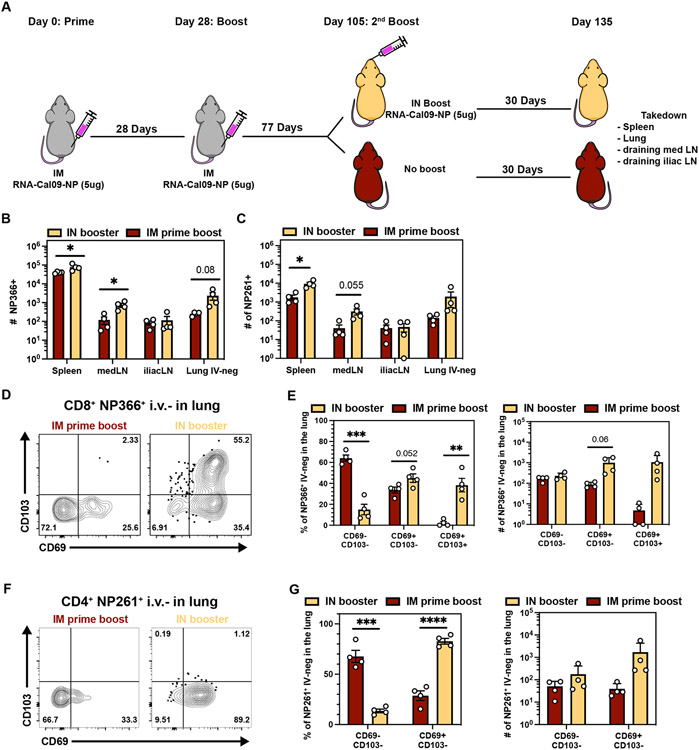
Combining intramuscular immunizations with an intranasal mRNA boost achieves high levels of both circulating memory and lung TRM
While IM vaccination resulted in a large population of NP366+ CD8 T cells in the circulation, IN immunization biased differentiation towards a higher number of pulmonary TRM. We next tested if 1) antigen-specific memory T cells retain the potential to generate abundant lung TRM populations and 2) whether one could generate a large pool of both circulating and pulmonary T cells by giving IM prime-boosted mice a secondary IN boost (Fig 5A). Compared to IM prime-boost alone, IN boosting increased the number of antigen-specific CD8 and CD4 T cells in the spleen, medLN, and lung (IV-neg), but not in the iliac LN (Fig. 5B-C). IN boosting expanded the proportion and number of CD69+CD103+ CD8 T cells and CD69+ CD4 T cells in the extravascular compartment of the lung, comparable to the IN prime-boost regimen (Fig 2F-G, 3G-H, Fig.5D-G). These data demonstrate that combining IM prime-boost immunizations with a third IN mRNA immunization achieved high levels of both circulating and lung memory T cells.
Fig 5. Combining intramuscular immunizations with an intranasal mRNA boost achieves high levels of both circulating memory and lung TRM.
(A) IM prime-boosted mice received a second booster via IN route and spleen, iliac LN, medLN and lungs were examined ≥28 days post secondary boost. (B-C) Quantification of antigen-specific CD8+ T cells (B) and CD4+ T cells (C) in the indicated compartments. (D-E) Representative flow cytometry plot (D) and quantification (E) of IV-neg NP366-specific CD8+ T cell subsets in the lung. (F-G) Representative flow cytometry plot (F) and quantification (G) of IV-neg NP261-specific CD4+ T cell subsets in the lung. Data represent N = 2 independent experiments with n = 4-5 mice per group. Data are shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as determined by unpaired two-tailed Student’s t test.
Discussion
In this study, we characterized the abundance, phenotype, and anatomic distribution of mRNA vaccine-elicited antigen-specific CD8 and CD4 memory T cells (Fig. S5). Independent of route of administration, mRNA vaccination induced TRM in the lung, where frontline cellular immunity is known to contribute to protection against certain respiratory infections (16, 19-35). Thus, mRNA vaccines may fulfill an important criterion in the pursuit of a universal influenza vaccine: establishing cellular immune memory at the primary site of infection. Importantly, we found that prime-boosted memory T cells elicited by IM vaccinations retain the potential to generate abundant pulmonary TRM upon an additional IN boost. These data could inform a strategy to leverage pre-existing memory T cells to bolster frontline immunity at the site of infection without compromising the circulating compartment. This could have implications for future iterations of SARS-CoV-2 vaccination, or vaccines for other respiratory pathogens. Including conserved T cell epitopes in vaccines that also establish humoral immunity may provide immunity in immunocompromised individuals that have impaired ability to efficiently generate antibodies or against pathogens that evolve to evade vaccine-elicited neutralizing antibodies. With that said, this study focused primarily on the differentiation and distribution of mRNA vaccine-elicited memory CD4 and CD8 T cells. However, we demonstrated that IM mRNA prime-boost vaccination solely encoding Cal/09 NP was still sufficient to protect mice against lethal influenza A virus, PR8 strain. NP is not thought to be a significant target of neutralizing antibodies, but contains conserved T cell epitopes that could elicit other mechanisms of immunity that could also cross-react with serological variants. Future studies will be needed to determine how to best integrate cell-mediated immunity with other vaccine-elicited mechanisms (which may include various classes of antibodies) to optimize long-term protective potential. This is a complex issue, and will depend on the route, dose, virulence, and antigenic profile of the pathogens in question, as well as availability of animal models that adequately predict human outcomes.
Lazcko and colleagues demonstrated the presence of activated CD69+ IV-neg T cells in the lung 10 days after IM mRNA-LNP vaccination and a recent study from Mao et al. showed persistence of antigen-specific CD8 T cells in the lung for at least 56 days, raising the question of whether TRM are established (55, 56). Here, we report the persistence of vaccine-elicited antigen-specific CD8 and CD4 T cells in the lung for at least 188 days after IM priming. Numerous studies have shown that establishment of pulmonary CD8 TRM requires local antigen recognition (20, 23, 37-41). To our surprise, based on migration assays in parabiotic mice most CD8 memory T cells, including CD69− cells, were TRM. Although we would not expect antigen to be present in the lung after IM immunization, we cannot exclude this possibility. Nevertheless, IN immunization was much more effective at establishing pulmonary TRM compared to IV and IM immunization. IN vaccination also induced persisting memory CD8 T cells in BAL fluid and in the upper respiratory tract. However, NP-specific serum IgG titers were similar in the BAL fluid between IM and IN vaccine delivery. Another group using an IM mRNA prime followed by a protein boost reported that IN protein delivery is particularly efficient in inducing IgA in BAL fluid (55). While we were not able to detect NP-specific IgA, that might be consistent with the absence of vaccine-induced pulmonary TFH or due to technical limitations.
We found it surprising that IN and IV immunizations were effective at establishing memory CD8 and CD4 T cells, as the vaccine is tailored for IM administration. A previous report of self-amplifying RNA delivery by the IN route using multiple delivery systems including LNPs showed poor immunogenicity relative to more typical IM or intradermal injections, and was associated with rapid clearance (57). For the most part, IN delivery of mRNA vaccines have been attempted using well-established polymer-based transfection reagents, though such polymer-based approaches have not historically reached clinical application successfully, possibly in part due to toxicity issues (58-61). Moreover, IN LNP can induce lethal inflammatory responses in mice, complicating studies of alternative administration routes (49). The MDNP formulation here did not have that effect and induced reduced levels of proinflammatory cytokines compared to commonly used LNPs. It is unclear whether this could be translated to humans. Of note however, we found that IN immunization of mice that had previously received two IM immunizations resulted in particularly robust memory CD8 T cell responses in both the pulmonary and circulating compartments. CD4 T cells were augmented in lung as well. These data add to the possibility that future mRNA vaccinations, perhaps combined with systemic immunizations, might be tailored to focus immunity at specific sites including the respiratory mucosa based on modulating the site of boosting.
A common question concerning mRNA vaccines is whether one should get a booster vaccine in the same (ipsilateral) or opposite (contralateral) arm. In the mouse model, we found moderate differences in the immunological parameters that we measured. Serum antibody titers were higher after ipsilateral versus contralateral boosting. A recent report indicated that affinity maturation was also enhanced by same-site boosting, suggesting that ipsilateral boosting may offer advantages for humoral immunity (62). Opposite-site boosting readily affected T cell responses. Like Lederer et al., we showed that IM mRNA elicits TFH memory cells in the vaccine-site draining LNs, however, we extended these data and showed that TFH memory cells were restricted to the immunization-site draining LNs (and absent from the contralateral LN, spleen, and lung) (63). Moreover, we demonstrated that IN vaccination was sufficient to establish TFH in the medLN and contralateral IM immunization broadened distribution of this population. This observation also extended to CD69+CD62L− memory CD8 T cells, a phenotype previously shown to correlate with LN residence (50). LN TRM are putatively derived from retrograde migration from upstream nonlymphoid tissues, so this population may reflect ex-TRM derived from muscle (18). However, preliminary attempts to examine muscle tissue did not yield viable lymphocyte populations that could be analyzed. We also observed that TFH and TRM-phenotype CD8 T cells were uniquely established in the lung-draining LN after IN immunization.
RNA-based vaccines are rapidly scalable against essentially any protein antigen and are not vulnerable to vector immunity that could otherwise limit opportunities for boosting or reusing the same vector, encoding different antigens, in future vaccines. The prospects are exciting for combatting emerging pathogens, antigenically variable pathogens that might be addressed by megavalent vaccination, and perhaps non-infection conditions such as personalized tumor vaccines. For these reasons, it is important to more fully characterize RNA vaccine immunogenicity. The establishment of TRM is of obvious importance, but difficult to assess in humans, despite COVID-19 providing intense interest and subject availability. Genetically defined mice and parabiosis surgery provided an opportunity to address this fundamental question and suggest that RNA vaccines may be effectively exploitable for establishing TRM.
Materials and Methods
Study design
The aim of this study was to characterize the self-amplifying mRNA-MDNP vaccine-elicited CD8 and CD4 T cell responses against the Cal/09 nucleoprotein of the Influenza virus in secondary lymphoid organs and the lung. We used MHC I and II tetramers to identify polyclonal antigen-specific CD8 and CD4 memory T cells. Analysis of experimental data was conducted unblinded. Detailed description of experimental replicates, sample sizes, and statistical analysis can be found in the figure captions or in the “Statistical analysis” section.
Mice
Female C57BL/6J (B6) and B6.SJL-PtprcaPepcb/BoyJ (B6 CD45.1), were purchased from Jackson Laboratory and maintained under specific pathogen-free conditions at the University of Minnesota according to the Institutional Animal Care and Use Committees guidelines. All B6 mice used in the experiments were female and 6-9 weeks of age at the timepoint of the first immunization. For inflammatory response testing, BALB/cJ mice were purchased from Jackson Laboratory and maintained according to Tiba Biotech’s Institutional Animal Care and Use Committee guidelines. All BALB/cJ mice were 6-10 weeks of age at time of experimentation.
MDNP vaccine production
The Cal/09 nucleoprotein coding sequence was cloned into a DNA plasmid encoding a self-amplifying mRNA template based on the genome of Venezuelan equine encephalitis virus, followed by RNA synthesis by run-off in vitro transcription from an upstream T7 promoter and subsequent enzymatic capping essentially as reported previously (64). Expression potency of each lot of self-amplifying mRNA produced for this work was validated by transfection of BHK cells and subsequent immunoblot using anti-influenza A virus nucleoprotein (NP) antibody (clone 1C5-1B7; NR-43899) obtained through BEI Resources Repository, NIAID, NIH. A representative blot is shown in Fig. S6. The capped self-amplifying RNA was dissolved in 10 mM citrate buffer and mixed using a NanoAssemblr Ignite microfluidic mixer (Precision NanoSystems) with a proprietary ethanolic solution of an ionizable modified dendron provided by Tiba Biotech (at an N:P ratio of 4), plus cholesterol, DOPE, and DMG-PEG2000 (Avanti Polar Lipids) in a 100:288:60:1 molar ratio respectively. Nanoparticles were dialyzed against sterile, endotoxin-free PBS using 20,000 Da molecular weight cutoff dialysis cassettes and sterile filtered using 0.2 micron polyethersulfone filters (CELLTREAT Scientific Products). Nanoparticle diameter and polydispersity index were assessed by dynamic light scattering and encapsulation efficiency measured by RiboGreen® (Thermo) dye-exclusion assay. Representative nanoparticle characteristics of the final Cal/09 nucleoprotein self-amplifying mRNA vaccine are provided in Table S2.
ALP and ALT measurement
ALP and ALT were measured in serum samples using the QuantiChrom™ Assay Kit (BioAssay Systems) or Colorimetric Activity Assay Kit (Cayman Chemical Company), respectively.
Immunizations
Mice were immunized with 5 μg (50 μl at 0.1 mg/ml) of mRNA encapsulated in MDNP encoding for the nucleoprotein of the Influenza A H1N1 Cal/09 strain per dose for all vaccine delivery routes. Vaccine was either delivered IM (right or left hamstring), IN, or IV. For injections, mice were either anaesthetized with vaporized isoflurane (IM) or ketamine and xylazine (IN).
Lymphocyte isolation from spleen, LNs, and lung
Mice were injected intravenously (tail vein) 15 minutes prior to sacrifice with NAD-induced cell death (NICD) protector (25 μg, BioLegend, #149802) to prevent NAD-induced cell death during tissue processing (46). Intravascular staining was used to discriminate cells present in the vasculature from cells present in the tissues parenchyma as previously described (65). Briefly, 3 minutes prior to euthanasia mice were injected retroorbitally with 3 μg of fluorescently conjugated αThy1.2 antibody (BV650, BD, #740443). Where indicated, bronchoalveolar lavage fluid was collected in three 1 ml lavages with cold PBS before the lung was excised. Lymphocyte isolation from spleen, LNs, trachea, nasal associated lymphoid tissues and lung was performed as described (11, 21). Spleen and LN single cell suspensions were generated via dissociation through 70μm filters (Falcon, #352350). For lung and trachea cell isolations, tissues were excised, minced, and digested in Collagenase I (Worthington, #LS004197) for 1 hour at 37°C shaking at 200 rpm. Tissue homogenates were then mechanically dissociated using a gentleMACS Dissociator (Miltenyi Biotec) and subsequently passed through a 70 μm filter. Finally, lung lymphocytes were enriched on a Percoll gradient. Cell enumerations were determined using PKH26 Reference Microbeads (Sigma-Aldrich, # P7458-100ML) as previously described (66).
Flow cytometry
To identify influenza-specific CD8+ T cells, isolated lymphocytes were surface-stained with homemade PE-conjugated H-2Kb NP366-375 tetramer for 1 hour at room temperature in the presence of viability dye (Ghost Dye Red 780, Tonbo Biosciences, #13-0865-T500), dasatinib (50 nM), Fc Shield (Tonbo, #70-0161-M001), and indicated antibodies. Splenic influenza-specific CD4+ T cells were isolated by staining single cell suspensions with homemade APC-conjugated I-Ab NP261-277 tetramer for 1 hour at room temperature in the presence of dasatinib (50 nM) followed by tetramer enrichment (66, 67). CXCR5 (BV421, BioLegend, #145512) was added at the time of tetramer staining. Further surface staining was performed for 30 minutes on ice in the presence of viability dye (Ghost Dye Red 780, Tonbo Biosciences, #13-0865-T500). Lung and LN-derived I-Ab NP261-277 tetramer+ CD4 T cells were identified by staining single cell suspensions with I-Ab NP261277 APC tetramer for 1 hour at room temperature in the presence of viability dye (Ghost Dye Red 780, Tonbo Biosciences, #13-0865-T500), Dasatinib (50nM), Fc Shield (Tonbo, #70-0161-M001), and indicated antibodies. All stained samples were acquired with LSR Fortessa flow cytometers (BD) and analyzed with FlowJo software (TreeStar).
Isolated H-2Kb NP366-375 tetramer+ lymphocytes were stained with the following antibodies: CD8a (BUV496, BD, #750024), CD45.2 (AF700, BioLegend, #109822), CD69 (BV421, BD, #562920), CD103 (BV510, BD, #563087), CD44 (BV785, BioLegend, #103041), CD62L (BUV737, BD, #612833), and were indicated CD45.1 (APC, BioLegend, # 110714)
Isolated I-Ab NP261-277 tetramer+ lymphocytes were stained with the following antibodies: CD4 (BUV496, BD, #612952), CD45.2 (AF700, BioLegend, #109822), CD103 (BV510, BD, #563087), CD44 (BV785, BioLegend, #103041), CD62L (BUV737, BD, #612833), Ly6C (FITC, BD, #553104), CD69 (PE-Dazzle, BioLegend, #104536), FR4 (PE-Cy7, eBioscience, #25-5445-82), PSGL1 (BV605, BD, #740384), CXCR6 (BV711, BioLegend , #151111), PD-1 (BUV395, BD, #744549), and were indicated CD45.1 (PE, BD, #553776).
Enzyme-linked immunosorbent assay (ELISA)
96-well plates (ThermoFisher, #44-2404-21) were coated with the Cal/09 influenza nucleoprotein (1 μg/ml, Sino Biological, #40205-V08B-100) and incubated overnight at 37°C. Using the Invitrogen ELISA Kit (#88-50400-88), plates were blocked for 2 hours at 37°C before diluted sera were added and incubated for 1 hour at 37°C. HRP-conjugated goat anti-mouse IgG detection antibody (Jackson ImmunoResearch, #115-035-062) was incubated for 1 hour at 37°C before plates were developed with 1x TMB for 15 min followed by addition of stop solution (Biofix, #NC9141468). Optical density was measured at 450 nm wavelength using a Tecan Infinite M Plex.
Immunohistochemistry
Lung tissues were manually cut into sections using surgical blades and incubated with PE-conjugated-H-2Kb NP366-375 overnight at 4°C. Sections were fixed in 2% paraformaldehyde in PBS for 2 hours at 4°C and incubated in 30% sucrose overnight at 4°C before freezing in Tissue-Tek O.C.T. compound. 20 μM thick sections were generated using the Leica Cryostat and subsequently stained with CD8 BV480 (BD, #566096), CD31 AF488 (BioLegend, #102514), EpCAM AF594 (BioLegend, #118222) and anti-PE (Novus Biologicals, #NB120-7011). Tetramer staining was further amplified using Cy3-donkey anti-rabbit (Jackson ImmunoResearch, #711-165-152). Images were acquired with a 20x immersion objective (set to glycerol NA 0.75) and voxel size of 0.568 μm on a Leica Stellaris 8 using laser scanning confocal microscopy with a 1 AU pinhole. Grouped sequential scans with excitation wavelengths of 405, 440, 498, 550, 594, and 647 nm were used with spectral HyD-S detectors with emission ranges set to 410-25, 445-510, 505-550, 555-585, 600-630, and 655-690 nm respectively.
Parabiosis surgery
Parabiosis surgeries were performed as previously described (13). In brief, mice were anaesthetized with Ketamine and Xylazine and fur was depilated on each mouse along the opposite lateral flank via electric clippers. The skin was wiped clean and sterilized with alcohol prep pads, Betadine solution, and 70% alcohol. Identical incisions were made on the lateral aspect of each mouse from the hip to shoulder and 9 mm wound clips (BD, #427631) were used to approximate and join the dorsal and ventral skins of adjacent mice. Conjoined mice were then allowed to rest for 21 days before sacrifice and analysis. Equilibration was confirmed in the peripheral blood prior to euthanasia.
Influenza virus infection
Vaccinated and age matched naïve C57BL/6 mice were anesthetized with ketamine and xylazine followed by IN infection with a lethal dose (30 PFU) of influenza A/PR8 virus. Mice were monitored by daily weighing. A euthanization endpoint was defined at a weight loss in excess of 25% of the initial body weight.
Statistical analysis
Data were analyzed and visualized using Prism 9 (GraphPad). Statistical significance was determined by one-way ANOVA and Tukey’s multiple comparisons test (more than two groups) or unpaired two-tailed Student’s t test (two groups), or with log-rank Mantel-Cox test for survival with P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are shown as mean ± SEM or SD as indicated in figure legends. Fitted linear regression models in Fig S3 A-C and S4E were computed in R (version 4.1.3) using the packages ggplot2 and ggpmisc. For visualization purposes only, values equal to zero were adjusted to 1 to appear on the axis of log-scaled data.
Supplementary Material
Data file S1: Raw data file (Excel spreadsheet)
Fig. S1: Impact of ipsilateral versus contralateral intramuscular mRNA-vaccination on CD8 T cell, CD4 T cell, and humoral immunity.
Fig. S2: MDNP-encapsulated mRNA induces less acute inflammation than LNPs composed of DLin-MC3-DMA or SM-102.
Fig. S3: Longevity of vaccine-elicited CD8 T cells in the respiratory tract.
Fig. S4: mRNA vaccination generates long-lived TFH in the draining LN but not in the lung.
Fig S5.: Anatomic distribution of mRNA vaccine elicited CD8 and CD4 memory T cells.
Fig. S6: Cal/09 nucleoprotein expression mediated by saRNA vectors in BHK cells.
Table S1: Clinical scoring rubric for inflammation and respiratory distress.
Table S2: Nanoparticle size, polydispersity, and encapsulation efficiency.
Acknowledgments
We thank the Masopust and Vezys group members for helpful discussions. We thank the Dr. Ryan Langlois for generously providing influenza virus. Funding: 75N93019C00051 Collaborative Influenza Vaccine Innovation Centers (CIVICs) (D.M., R.A.), Minnesota Partnership for Biotechnology and Medical Genomics (D.M., R.V.), National Institutes of Health grant 3R01AI084913 (D.M.), Swiss National Science Foundation (SNSF) grant P2BSP3_200187 (M.K.)
Footnotes
Competing interests: P.T, J.S.M., C.W.M., J.S.C. are employees of Tiba Biotech LLC and have equity interests. Tiba Biotech has filed a patent on the MDNP RNA delivery system (patent application PCT/US21/25542), naming P.T. and J.S.C. as inventors. J.S.C. is also an inventor on US Patent No. 10,548,959 entitled “Compositions and Methods for Modified Dendrimer Nanoparticle Delivery.” C.W.M. has worked as a freelance consultant advising commercial and non-profit organizations working in the fields of vaccines and viral vectors. The other authors declare no competing interests.
Data and Materials Availability:
The self-amplifying mRNA vaccine construct used in this study is available through a Material Transfer Agreement with Tiba Biotech. Requests should be directed to J.S.C. at Tiba Biotech.
References and Notes
- 1.Ssentongo P et al. , SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis 22, 439 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feikin DR et al. , Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 399, 924–944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR et al. , Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 384, 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N et al. , BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med 384, 1412–1423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardi N, Hogan MJ, Porter FW, Weissman D, mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary N, Weissman D, Whitehead KA, mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov 20, 817–838 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rydyznski Moderbacher C et al. , Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 183, 996–1012 e1019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noh JY, Jeong HW, Kim JH, Shin EC, T cell-oriented strategies for controlling the COVID-19 pandemic. Nat Rev Immunol 21, 687–688 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayward AC et al. , Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am J Respir Crit Care Med 191, 1422–1431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gras S et al. , Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci U S A 107, 12599–12604 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert EM et al. , Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust D, Soerens AG, Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37, 521–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenkel JM, Fraser KA, Vezys V, Masopust D, Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14, 509–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son YM et al. , Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci Immunol 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarnalekha N et al. , T resident helper cells promote humoral responses in the lung. Sci Immunol 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL, Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol 84, 9217–9226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son YM, Sun J, Co-Ordination of Mucosal B Cell and CD8 T Cell Memory by Tissue-Resident CD4 Helper T Cells. Cells 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolley JM et al. , Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J Exp Med 217, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paik DH, Farber DL, Influenza infection fortifies local lymph nodes to promote lung-resident heterosubtypic immunity. J Exp Med 218, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T et al. , Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95, 215–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzolla A et al. , Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2, (2017). [DOI] [PubMed] [Google Scholar]
- 22.Zens KD, Chen JK, Farber DL, Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng MZM, Wakim LM, Tissue resident memory T cells in the respiratory tract. Mucosal Immunol 15, 379–388 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teijaro JR et al. , Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187, 5510–5514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner DL et al. , Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7, 501–510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzolla A et al. , Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J Clin Invest 128, 721–733 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bree GJ et al. , Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med 202, 1433–1442 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piet B et al. , CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest 121, 2254–2263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jozwik A et al. , RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun 6, 10224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S, Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol 88, 11034–11044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J et al. , Airway Memory CD4(+) T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity 44, 1379–1391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo PA et al. , Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 54, 797–814 e796 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao M et al. , Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26, 842–844 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Kinnear E et al. , Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol 11, 290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luangrath MA, Schmidt ME, Hartwig SM, Varga SM, Tissue-Resident Memory T Cells in the Lungs Protect against Acute Respiratory Syncytial Virus Infection. Immunohorizons 5, 59–69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slutter B et al. , Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YT et al. , Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol 85, 4085–4094 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakim LM, Gupta N, Mintern JD, Villadangos JA, Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol 14, 238–245 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Takamura S et al. , Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 213, 3057–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMaster SR et al. , Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol 11, 1071–1078 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGill J, Van Rooijen N, Legge KL, Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med 205, 1635–1646 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geall AJ et al. , Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci U S A 109, 14604–14609 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson SA et al. , Intranasal Nanoparticle Vaccination Elicits a Persistent, Polyfunctional CD4 T Cell Response in the Murine Lung Specific for a Highly Conserved Influenza Virus Antigen That Is Sufficient To Mediate Protection from Influenza Virus Challenge. J Virol 95, e0084121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hale JS et al. , Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38, 805–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall HD et al. , Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity 35, 633–646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunzli M et al. , Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci Immunol 5, (2020). [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi T et al. , Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity 27, 145–159 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Zeng C, Zhang C, Walker PG, Dong Y, Formulation and Delivery Technologies for mRNA Vaccines. Curr Top Microbiol Immunol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndeupen S et al. , The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beura LK et al. , T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells. Immunity 48, 327–338 e325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laidlaw BJ et al. , CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41, 633–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rihs S et al. , Differential expression of alpha E beta 7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by alpha 4 beta 1-integrin crosslinking and TGF-beta. Am J Respir Cell Mol Biol 15, 600–610 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Erle DJ, Brown T, Christian D, Aris R, Lung epithelial lining fluid T cell subsets defined by distinct patterns of beta 7 and beta 1 integrin expression. Am J Respir Cell Mol Biol 10, 237–244 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Beura LK et al. , CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J Exp Med 216, 1214–1229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao T et al. , Unadjuvanted intranasal spike vaccine elicits robust protective mucosal immunity against sarbecoviruses. Science, 2022 Oct 27:eabo2523, (2022). doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laczko D et al. , A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 53, 724–732 e727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderluzzi G et al. , The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J Control Release 342, 388–399 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M et al. , Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater 64, 237–248 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Li M et al. , Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J Control Release 228, 9–19 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Srisomboon Y et al. , Airway Exposure to Polyethyleneimine Nanoparticles Induces Type 2 Immunity by a Mechanism Involving Oxidative Stress and ATP Release. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang J et al. , Nanotechnologies in Delivery of DNA and mRNA Vaccines to the Nasal and Pulmonary Mucosa. Nanomaterials (Basel) 12, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuraoka M et al. , Recall of B cell memory depends on relative locations of prime and boost immunization. Sci Immunol 7, eabn5311 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lederer K et al. , SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 53, 1281–1295 e1285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chahal JS et al. , Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci U S A 113, E4133–4142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson KG et al. , Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9, 209–222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moon JJ et al. , Tracking epitope-specific T cells. Nat Protoc 4, 565–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lissina A et al. , Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods 340, 11–24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data file S1: Raw data file (Excel spreadsheet)
Fig. S1: Impact of ipsilateral versus contralateral intramuscular mRNA-vaccination on CD8 T cell, CD4 T cell, and humoral immunity.
Fig. S2: MDNP-encapsulated mRNA induces less acute inflammation than LNPs composed of DLin-MC3-DMA or SM-102.
Fig. S3: Longevity of vaccine-elicited CD8 T cells in the respiratory tract.
Fig. S4: mRNA vaccination generates long-lived TFH in the draining LN but not in the lung.
Fig S5.: Anatomic distribution of mRNA vaccine elicited CD8 and CD4 memory T cells.
Fig. S6: Cal/09 nucleoprotein expression mediated by saRNA vectors in BHK cells.
Table S1: Clinical scoring rubric for inflammation and respiratory distress.
Table S2: Nanoparticle size, polydispersity, and encapsulation efficiency.