Abstract
Rhinella marina toad is abundant in Brazil. Its poison contains cardiac glycosides called bufadienolides, which are extensively investigated for their bioactivity. Our aim was to characterize the vasoactivity of Rhinella marina poison (RmP) on the aorta of male Wistar rats. For this, the RmP was first collected and processed to obtain an alcoholic extract. To determine cardiovascular effects of RmP, we performed in vivo tests by administering RmP intravenously in doses of 0.1–0.8 mg/kg. Vascular reactivity was also performed through concentration-response curves to RmP (10 ng/mL to 200 μg/mL) in aortic segments with and without endothelium. RmP induced a concentration-dependent contraction in rat aorta which was partly endothelium-mediated. Nitric oxide contributes with this response in view that incubation with L-NAME increased the contractile response. Additionally, treatment with indomethacin [cyclooxygenase, (COX) inhibitor], nifedipine (L-type voltage-gated calcium channels blocker), and BQ-123 (ETA receptors antagonist) decreased maximum response, and ketanserin (5-HT2 receptors antagonist) decreased pEC50, suggesting active participation of these pathways in the contractile response. On the other hand, apocynin (NADPH oxidase inhibitor) did not alter contractility. Incubation with prazosin (α1-adrenergic receptor antagonist) abolished the contractile response, suggesting that the RmP-induced contraction is dependent on the adrenergic pathway. In the Na+/K+ ATPase protocol, a higher Emax was observed in the RmP experimental group, suggesting that RmP potentiated Na+/K+ATPase hyperpolarizing response. When this extract was injected (i.v.) in vivo, increase in blood pressure and decrease in heart rate were observed. The results were immediate and transitory, and occurred in a dose-dependent manner. Overall, these data suggest that the poison extract of R. marina toad has an important vasoconstrictor action and subsequent vasopressor effects, and its use can be investigated to some cardiovascular disorders.
Keywords: Amphibian poisons, Aorta, Rhinella marina, Vascular reactivity, blood pressure
1. Introduction
The Bufonidae family, commonly called “true toads”, is composed of 634 species allocated in 52 genera with worldwide distribution (AmphibiaWeb. Bufonidae family, 2022). In Brazil, the Bufonidae family is characterized by eight genera with Rhinella being the most representative one. Popularly known as “sapo-cururu” or “cane toads”, Rhinella marina is native from the extreme south of Texas in North America to the center of South America, in the Brazilian Amazon (Segalla et al., 2014). This species can also be found in Hawaii and Australia where it was introduced in the early 1930s as an effort to control insect pests on sugar cane crops. However, its high adaptability and toxicity to predators enabled R. marina to spread rapidly, affecting the local fauna and ultimately ending up by being considered a pest in these territories (DeVore et al., 2021; Edwards et al., 2018; Kosmala et al., 2020).
Amphibians’ skin is characterized by the presence of mucous glands, which prevent skin dryness and maintain its homeostasis, and granular glands, that release poison as a form of chemical defense against predators and pathogens. RmP is produced and stored in parotoid macroglands, located along the toad’s neck and parascapular region (Johnnides et al., 2016; Mailho-Fontana et al., 2014). The secretion provides a passive protection against predators since the intoxication occurs when predators bite the toad, resulting in oral exposure to the poison (Sakate and Oliveira, 2000). RmP exposure leads to neurologic abnormalities, ptyalism, tachypnea and vomiting, and it can be fatal if not treated quickly (Roberts et al., 2000; Johnnides et al., 2016). Poisons of amphibians are a rich source of bioactive compounds with promising medicinal use (Kowalski et al., 2018). This particular poison may contain alkaloids, proteins, peptides, amines, and bufadienolides (Ferreira et al., 2013; Lebedev et al., 2017).
It was demonstrated that bufadienolides are the main active group of compounds of RmP. Marinobufagenin is the bufadienolide present in higher concentrations, followed by telocinobufagin, bufalin, and resibufogenin (Schmeda-Hirschmann et al., 2016). In addition, this poison has some arginyl-diacids (e.g. marinobufotoxin and bufalitoxin); alkaloids (e.g. bufotenidin and dehydrobufotenin); and biogenic amines (e.g. serotonin) (Kerkhoff et al., 2016; Pelissari et al., 2021).
Bufadienolides are part of an important group of steroid hormones that act as cardiotonic glycosides by inhibiting the Na+/K + ATPase pump. This enzyme blockade causes a sequence of events that leads to intracellular accumulation of Ca2+, resulting in a positive inotropic action on cardiac muscle (Morishita et al., 1991). Bufadienolides has also demonstrated antitumor (Gao et al., 2011), antiparasitic (Banfi et al., 2016), immunomodulatory (Manika et al., 1998), and antimicrobial (Cunha Filho et al., 2005) effects.
Vascular tone plays a fundamental role in the regulation of tissue perfusion, determining the supply of nutrients and oxygen (O2) to afford the demand (GOLAN, 2016; Touyz et al., 2018). The dysregulation in the release of contractile and relaxing factors in the blood vessel is a pre-requisite for the onset of several vascular diseases, such as hypertension, atherosclerosis, stroke, and obesity (Touyz et al., 2018). Pharmacological intervention strategies in these regulatory pathways have already resulted in numerous successful treatments. Therefore, the identification of new compounds that act in order to improve vascular function is essential.
Although preliminary evidence suggests that RmP has an important function on the cardiovascular system, these effects and the mechanisms by which this poison may affect the cardiovascular system have not been explored in depth. The present study aimed to investigate the vascular activity of RmP and the possible mechanisms involved in the responses observed and also examine the hemodynamic and cardiac alterations caused by RmP in conscious, free-moving rats. We hypothesized that RmP has a Na+/K+ ATPase-mediated vasoconstrictor action.
2. Material and methods
2.1. Poison collection
The toads were captured and identified by the team of biologists from the Federal University of Mato Grosso, under the coordination of Dr. Domingos de Jesus Rodrigues (IBAMA, SISBIO: 30,034–1). Subsequently, our team extracted the material secreted by the parotoid gland of the toads by manual compression. Then, the animals were returned to nature. Eleven animals from the same habitat in the amazon area were used and the extracts were prepared with the poison pool of all these toads. The toads poisons were collected in December, January and February, during the summer season in Brazil. Present work was registered at the Brazilian National System for the Management of Genetic Patrimony and Associated Traditional Knowledge (SisGen) under protocol n° A67857B.
2.2. Extract preparation
After collection, RmP was dried in a vacuum desiccator at room temperature for two days. Then, it was ground using a mortar and pistil and submerged in methanol 100% while submitted to an ultrasonic bath extraction for 10 min. The samples were filtered using a filter paper, and the solvent was removed in a vacuum pump, which was coupled to a rotary evaporator, and subsequently the samples were lyophilized to obtain the methanolic extract from R. marina poison (MERmP) collected from the parotoid gland. After total drying, the samples were stored at 4 °C (De Souza et al., 2020; Kerkhoff et al., 2016). For the in vitro experiments we used the dried MERmP dissolved in H2O and for the in vivo experiments it was dissolved in saline solution.
2.3. HPLC analysis
HPLC analysis was performed as described by Kerkhoff et al. (2016). Briefly, chromatography analysis was executed on a HPLC system LC Varian Pro Star 325 with ultraviolet detector Pro Star 325 with dual wavelength system, the equipment was operated by Galaxie Chromatogrphy Date System Software, provided by the equipment manufacturer. The separation was performed on Phenomenex Luna C18 (250.0 × 4.6 mm, 5 μm). Samples were eluted to mobile phase with water (eluent A) and acetonitrile (eluent B). Elution took place according to gradient and mobile phase flow as it follows: 0–45 min with 0–70% solvent B; flow: 0,80 mL/min. The injection volume was 20 μL and detection at 296 nm. The identification of the compounds was made by comparing the retention time (RT) of the peaks with the standards of the pure compounds (Resinobufagin, Marinobufagin and Bufalin), the standards were injected separately to obtain the RT of each compound. The other peaks were not identified by HPLC-UV due to lack of standards. However, in a previous work, the methanolic extract from RmP was analyzed by UHPLC-DAD-MicOTOF-MS-MS and twelve compounds were identified (Pelissari et al., 2021).
2.4. Animal experiments
All animal protocols were approved by the Ethics Committee on the Use of Animals of the University of Sao Paulo (CEUA, under protocol n° 089/2021) and followed the regulations of the National Council on Animal Experimental Control (CONCEA, Brazil).
Six to eight weeks old male Wistar rats (200–250 g) were obtained from the Central Animal Facility of the University of Sao Paulo (Ribeirao Preto campus). The animals were housed in individually ventilated cages (5 rats per cage) covered with wood shavings at controlled room temperature (22° - 24 °C) and humidity (40%–60%), under 12 h light-dark cycles with free access to standard chow diet (3.77 kcal g−1) – NUVILAB CR-1 (NuvitalVR, Colombo, Paraná, Brazil) and water.
2.5. Vascular reactivity
Rats were anesthetized with isoflurane (4%) and a thoracotomy was performed. The thoracic aorta artery was isolated, excessive fat and connective tissue were removed, and the vessels were cut into rings of 3 mm of length. In most cases the endothelium was left intact. However, in a small set of experiments, the endothelium was removed by gently rolling the vessel segment on a stainless-steel wire across a cotton soaked in Krebs-Henseleit solution. The absence of endothelium was confirmed by the inability of acetylcholine 1 μM to induce relaxation (less than 10%) after a precontraction with phenylephrine 1 μM. Each arterial segment was mounted in standard organ chambers for the recording of isometric tension by a PowerLab 8/SP data acquisition system (ADInstruments Pty Ltd., Australia). Vascular segments were immersed in Krebs-Henseleit solution (130 mM NaCl, 14.9 mM NaHCO3, 5.5 mM glucose, 4.7 mM KCl, 1.56 mM CaCl2.2H2O, 1.18 mM KH2PO4, 1.18 mM MgSO4.7H2O and 0.026 mM EDTA) at 37 °C, continuously bubbled with a mix of 95% O2 and 5% CO2 under a resting tension of 1.5 g.
After 60 min of stabilization, vessels were stimulated twice with KCl 75 mM, to assess integrity of the muscle tissue and maximal contractility. Subsequently, concentration-response curves for RmP (10 ng/mL a 200 μg/mL) were performed, and there was a 4-min interval between each concentration added. The effects of apocynin (30 μM), BQ-123 (1 μM), indomethacin (100 μM), ketanserin (30 nM), Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME – 100 μM), nifedipine (100 μM) and prazosin (1 μM) were investigated by their addition 30 min before RmP curves in vessels with intact endothelium. Only one concentration-response curve was performed in each aorta segment.
2.6. Na+/K+ ATPase activity
Functional activity of Na+/K+ ATPase was measured using the method described by Webb and Bohr (1978) and adapted by Rossoni et al. (2002). After 30 min of stabilization in standard Krebs-Henseleit solution, the arteries were incubated in a K+-free medium for 30 min. Then, vessels were contracted with phenylephrine (1 μM) or RmP (2 μg/mL), accordingly with the experimental group. Once a plateau was reached, KCl was cumulatively added (1–10 mM). Relaxation was calculated as a percentage, considering the contraction induced by either phenylephrine or RmP as 100%.
2.7. Hemodynamic measurements
The femoral artery and vein were canulated to perform hemodynamic measurements and to inject RmP intravascularly. Arterial and venous catheterization were performed as described by Jespersen et al. (2012). Briefly, rats were anesthetized with inhalator isoflurane (2%) and oxygen (10%) in facial mask. A small incision was placed in inguinal region to expose femoral vein and artery. To perform vascular catheterization, an incision was made on the vessel section and the catheter was pushed into the vessel. Then, the catheters were externalized on the animal’s back, channeled through a subcutaneous tract in order to prevent the animal’s access to the cannulas after anesthetic return.
Twenty-four hours after the cannulation surgery, blood pressure and heart rate measurements were recorded from the artery catheter by a pressure transducer coupled to a PowerLab 8/SP data acquisition system (ADInstruments Pty Ltd., Australia) in conscious rats. The catheter was connected to the equipment and the animal was allowed to acclimate for 60 min before the beginning of the experiments. After stabilization, saline solution or RmP were intravenous administered at the doses: 0.1 mg/kg, 0.2 mg/kg, 0.4 mg/kg and 0.8 mg/kg. The final vehicle or RmP volume was 0.2 mL in each injection. Each administration was performed in bolus for 30–60 s and each subsequent dose was injected after 30–40 min of the previous dose. During the interval of each dose, the cannula was washed using 0,2 mL of saline.
2.8. Statistical analysis
Data are presented as mean ± standard error of mean (SEM) and “n” represents the number of rats used in the experiments. Concentration-response curves were fitted with nonlinear regression using an interactive fitting program (GraphPad Prism 8.0; GraphPad Software Inc.), and two pharmacological parameters were obtained: the maximal effect generated by the agonist (Emax) and pEC50 (negative logarithm of the molar concentration producing 50% of the maximal response). Normality distribution of data was checked with D’Agostino Pearson test. Data were analyzed by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons as appropriate. The confidence level for the tests was 95%.
3. Results
3.1. Chromatography
Chromatogram shows a high concentration of bufadienolides in the RmP methanolic extract (Fig. 1). Marinobufagenin was the most abundant component (7.9% of total area), followed by bufalin (7.4% of total area), and resinobufagin (6.6% of total area). In addition to the identified compounds, two other compounds in the bufadienolides region were not identified in this work, occupying 5.0% and 22.6% of the total area. It is noteworthy that there is a considerable amount of peptides on the chromatogram, which elute between 10 and 15 min, taking up 50.4% of the total area. This definition was based in the analysis done by Pelissari S. et al., 2021.
Fig. 1.

HPLC-UV chromatogram of the RmP methanolic extract Analysis method: Separation was performed on Phenomenex Luna C18 Column (250.0 × 4.6 mm, 5 μm), samples were eluted using water (solution A) and acetonitrile (solution B) as mobile phase. Elution varied according to gradient and solution flow as follows: 0–45 min with 0–70% solvent B, flow 0.80 mL/min, injection volume was 20 μL and detection at 296 nm.
3.2. Vascular reactivity
In vascular reactivity experiments, RmP induced a substantial contractile response in intact aorta rings (~129% of 100 μM KCl-induced contraction) (Fig. 2A). Aortic rings with denuded endothelium (E−) presented an increase in the RmP-induced contraction evidenced by pEC50 when compared to E+ control group. There were no changes in Emax between these groups. To confirm endothelial contribution by NO production, we performed experiments with intact endothelium aortic rings (E+) in the presence of NOS inhibitor, L-NAME (100 μM). NOS blockade shifted the curve to the left as observed in the E–group (Fig. 2A; Table 1).
Fig. 2.

Effect of RmP on aorta segments with and without endothelium. A: Concentration-response curves to RmP in segments of aorta in the presence and absence of endothelium. Some rings were incubated with L-NAME (100 μM) prior to concentration-response curves. B: Emax and C: pEC50 from concentration-response curves. Each point represents mean ± SEM. *p < 0.05 versus E+.
Table 1.
Emax and pEC50 values from concentration-response curves to RmP in segments of aorta. Each point represents mean ± SEM.
| Emax | pEC50 | |
|---|---|---|
| E+ (n = 10) | 128.91 ± 2.30 | 2.72 ± 0.04 |
| E− (n = 6) | 128.91 ± 2.11 | 3.33 ± 0.06* |
| L-NAME - 100 μM (n = 4) | 128.15 ± 8.42 | 3.60 ± 0.19* |
| Apocynin - 30 μM (n = 4) | 113.74 ± 13.5 | 2.56 ± 0.27 |
| Indomethacin - 100 μM (n = 6) | 93.09 ± 8.09* | 2.71 ± 0.18 |
| Nifedipine - 100 μM (n = 6) | 58.24 ± 4.55* | 2.51 ± 0.06 |
| BQ-123 - 1 μM (n = 6) | 113.87 ± 5.61* | 2.79 ± 0.12 |
| Ketanserin - 30 nM (n = 6) | 124.51 ± 2.48 | 2.19 ± 0.08* |
| Prazosin - 1 μM (n = 6) | 45.13 ± 4.47* | 0.85 ± 0.01* |
p < 0.05 versus E+.
To understand the mechanisms associated with RmP induced-contraction, different pharmacological inhibitors were used. Accordingly, aortic segments were pre-incubated with apocynin (30 μM), an antioxidant and unspecific inhibitor of NADPH oxidase, or indomethacin (100 μM), a non-selective COX inhibitor. Nifedipine (100 μM) was also used to evaluate the role of L-type voltage-gated calcium channels in this response. It was observed that indomethacin and nifedipine decreased the contractile response, to ~27.7% and ~54.8% Emax, respectively. On the other hand, apocynin did not change the contractile response to RmP (Fig. 3; Table 1).
Fig. 3.

Contribution of COX, L-type calcium channel and ROS in the vasoconstriction effect of RmP on aorta segments. A: Concentration-response curves to RmP in segments of aorta in the presence and absence of indomethacin (100 μM), nifedipine (100 μM) or apocynin (30 μM). B: Emax and C: pEC50 from concentration-response curves. Each point represents mean ± SEM. *p < 0.05 versus E+.
Pre-incubation with ketanserin (30 nM), a selective 5-HT2 serotonin receptor antagonist, did not change the contractile response to RmP. On the other hand, pre-incubation with BQ-123 (1 μM), a selective ETA receptor antagonist attenuated the maximal response (Emax) in ~11.6%. To investigate the contribution of the α1-adrenergic receptor in the contractile response some rings were treated with prazosin (1 μM). Interestingly, pre-incubation of the vessels with prazosin significantly decreased the contractile response in ~64.9% of Emax (Fig. 4; Table 1).
Fig. 4.
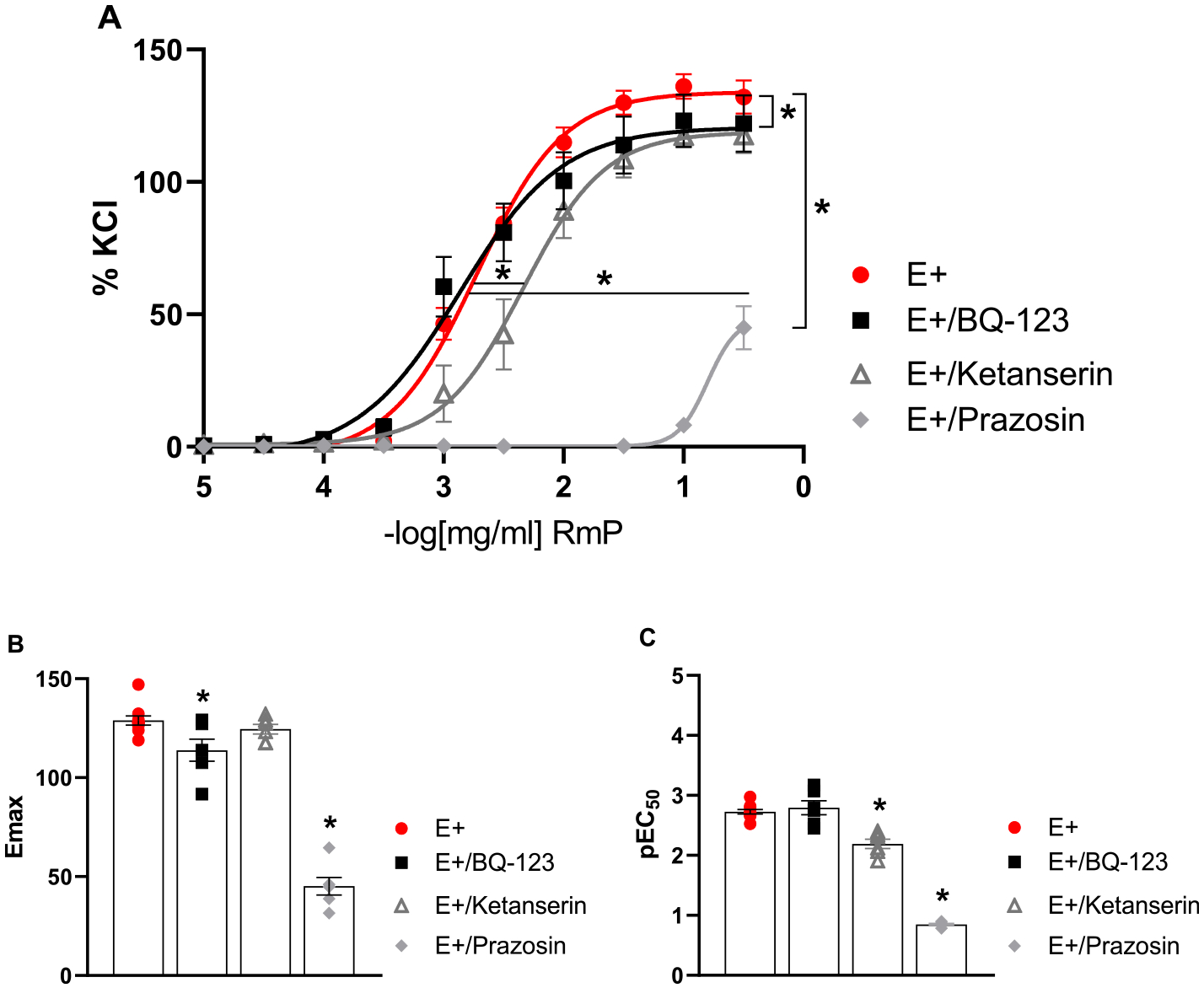
Contribution of endothelin, serotonin and α-adrenergic pathway in the effect of RmP on aorta segments. A: Concentration-response curves to RmP in segments of aorta in the presence and absence of BQ-123 (1 μM), ketanserin (30 nM) or prazosin (1 μM). B: Emax and C: pEC50 from concentration-response curves. Each point represents mean ± SEM. *p < 0.05 versus E+.
3.3. Na+/K+ ATPase activity
Na+/K+ ATPase activity was evaluated through the increase of K+ concentration in intact aortic rings previously contracted by phenylephrine or RmP. K+-induced relaxation was greater after pre-contraction with RmP when compared with the control group, which was pre-contracted with phenylephrine (Emax relaxation values: 78.74 ± 1.04% - control group; 92.94 ± 1.16% - RmP group). RmP also induced a leftward shift of the KCl concentration-response curve with significant alteration in EC50 values: 1.45 ± 0.09 – control group; and 0.38 ± 0.16 – RmP group (Fig. 5).
Fig. 5.

Indirect effect of RmP on Na+/K + -ATPase pump in aorta segments. A: Concentration-response curves to KCl (1–10 mM) in segments of aorta (n = 8) after pre-contraction with phenylephrine 1 μM (control) or RmP 2 μg/mL. B: Emax and C: pEC50 from concentration-response curves (A). Each point represents mean ± SEM. *p < 0.05 versus control.
3.4. In vivo tests
Intravenous administration of RmP increased mean arterial pressure (MAP) over time (seconds) in a dose dependent manner (Fig. 6A; Table 2). However, significant differences in blood pressure elevation with RmP compared with vehicle (Control) were observed with the higher RmP doses. Interestingly, changes in blood pressure return to baseline in less than 2 min. These data were confirmed by the area under the curve (AUC) (Fig. 6B).
Fig. 6.

In vivo effect of RmP injection in blood pressure. (A) Mean arterial pressure (MAP - mmHg) over time after intravenous injection of RmP (0.1 mg/kg – 0.8 mg/kg). (B) Area under the curve (AUC) of mean arterial pressure (MAP) curves after intravenous injection of RmP (0.1 mg/kg – 0.8 mg/kg). Each point represents mean ± SEM. *p < 0.05 versus control.
Table 2.
Mean arterial pressure (MAP – mmHg) values over time (s) after intravenous injection of RmP (0.1 mg/kg – 0.8 mg/kg) (n = 4). Each point represents mean ± SEM.
| Control | 0.1 mg/kg | 0.2 mg/kg | 0.4 mg/kg | 0.8 mg/kg | ||
|---|---|---|---|---|---|---|
| Time (s) | 0 | 105.1 ± 2.8 | 106.1 ± 4.5 | 104.7 ± 3.6 | 105.3 ± 3.2 | 105.9 ± 2.6 |
| 30 | 103.9 ± 6.9 | 127.7 ± 10.6 | 122.9 ± 15.6 | 125.5 ± 8.9* | 147.2 ± 8.7* | |
| 60 | 106.4 ± 5.7 | 121.8 ± 5.5 | 140.9 ± 9.0* | 150.0 ± 6.3* | 148.7 ± 8.7* | |
| 90 | 104.9 ± 7.3 | 104.4 ± 4.5 | 107.0 ± 3.5 | 115.9 ± 2.4 | 126.5 ± 6.3* | |
| 120 | 104.4 ± 6.2 | 104.3 ± 7.8 | 104.0 ± 4.2 | 109.6 ± 4.1 | 111.8 ± 4.1 |
p < 0.05 versus control.
Heart rate measurement was also performed, and it was observed that injection of the RmP at 0.8 mg/kg decreased this parameter. Interestingly, the lowest dose injected (0.1 mg/kg) produced a significant increase on heart rate at 90 s (Fig. 7; Table 3). Of note, there was no mortality of the animals after 24 h of RmP injection. These data suggested that RmP was not toxic at the tested doses.
Fig. 7.

In vivo effect of RmP injection in heart rate. Heart rate (beats per minute – BPM) over time (s) after intravenous injection of RmP (0.1 mg/kg – 0.8 mg/kg). Each point represents mean ± SEM. *p < 0.05 versus control.
Table 3.
Heart rate (beats per minute – BPM) values over time (s) after intravenous injection of RmP (0.1 mg/kg – 0.8 mg/kg) (n = 4). Each point represents mean ± SEM.
| Control | 0.1 mg/kg | 0.2 mg/kg | 0.4 mg/kg | 0.8 mg/kg | ||
|---|---|---|---|---|---|---|
| Time (s) | 0 | 350.6 ± 11.6 | 367.4 ± 21.5 | 370.4 ± 7.2 | 334.5 ± 6.3 | 341.4 ± 6.7 |
| 30 | 368.4 ± 16.7 | 376.6 ± 22.8 | 368.6 ± 22.4 | 356.0 ± 8.5 | 277.7 ± 25.0* | |
| 60 | 363.9 ± 10.1 | 393.8 ± 35.2 | 347.5 ± 14.0 | 339.1 ± 16.9 | 286.2 ± 20.0* | |
| 90 | 357.9 ± 10.4 | 405.6 ± 23.0* | 377.1 ± 9.6 | 347.5 ± 16.9 | 335.4 ± 9.8 | |
| 120 | 363.9 ± 17.8 | 389.7 ± 25.5 | 371.2 ± 11.4 | 355.3 ± 19.4 | 331.8 ± 12.5 |
p < 0.05 versus control.
4. Discussion
Rhinella marina toad is widely found in Brazilian Amazon. Its poison has been extensively studied due to its bioactivity, but much remains to be understood, particularly in the cardiovascular system. Thus, the aim of this study was to identify the mechanism through which the poison can cause cardiovascular alterations, using both in vivo and in vitro methods. The poison showed great contractile activity in vitro in aortic rings and the dose in vivo elevated the blood pressure.
Using quantitative HPLC-UV analyses, we observed that there was bufadienolides in the RmP, and that marinobufagenin was the main compound. Additionally, the chromatogram highlighted an accumulation of peptides and unidentified substances that may present pharmacological activity (Kerkhoff et al., 2016). In a previous work done in our laboratory by Pelissari et al. (2021), the methanolic extract analysis by liquid chromatography was performed using an ultra-high pressure liquid chromatography system with diode-array detection (DAD) and mass spectometry (MS) detection (UHPLC-DAD-microTOF), where 12 compounds present in the methanolic extract of RmP were identified, including 5 peptides and 7 bufadienolides.
It is important to mention that changes in location, pressure, and stress factors may affect various physiological aspects in toads, including the composition and potency of poisons. Alterations in morphological, behavioral, and physiological characteristics have been documented and reflect the toxicity risk of R. marina (Friesen and Shine, 2019; Gardner et al., 2020; Gardner et al., 2021).
Regarding vascular effects, we found that RmP induces vasoconstriction in aorta from Wistar rats. Interestingly, we observed that RmP is a potent vasoconstrictor, since this poison induced vascular contraction in a higher magnitude than KCl (75 mM). We suggest that the high contractile response observed in the experiments is due to the mixture of compounds present in the RmP, considering that concentration-response curves to 5H-T or marinobufagenin, which can be present in the poison, have smaller responses in rat aorta than those found with the crude poison used in this work (Bagrov et al., 1995). Given that α1-adrenergic receptor activation is an important mechanism to induce vasoconstriction in arteries (Pintérová et al., 2011), aortas were pre-incubated with prazosin (1 μM), an α1-adrenergic inhibitor. Blockade with prazosin significantly decreased contractile response in aorta. The influx of calcium via L-type voltage-gated calcium channels is an important downstream mechanism of α1-adrenergic receptor activation. Here, we observed that nifedipine (100 μM), decreased maximal response in ~54.8% when compared to control group. Overall, these data suggested that the contractile effect of RmP in aortas is mediated by the α1-adrenergic receptor and is partially calcium dependent. The effect of RmP on α1-adrenergic receptor can be direct or indirect, in view that it can bind directly and activate this receptor or it can stimulate the exocytosis of noradrenaline which can bind to the receptor.
The endothelium is responsible for synthesizing and releasing several substances that regulate vascular tone and consequently, blood pressure (Michelini and Rossoni, 2013). In this study, the removal of the endothelium or pre-treatment with L-NAME potentiated R. marina-induced contraction, suggesting an endothelial modulation, which is partially due to nitric oxide release.
In addition to nitric oxide, endothelial cells also synthetize cyclooxygenase-derived prostanoids and reactive oxygen species. These factors are important to maintain vascular homeostasis. Vascular dysfunction is correlated with development of cardiovascular diseases. In this scenario, low grade chronic inflammation induces the release of prostanoids, such as prostacyclin (PGI2) and thromboxane (TXA2) (Félétou et al., 2011), from endothelial cell through the cleavage of arachidonic acid by COX. An exacerbated ROS production promotes vasoconstriction mainly via reducing NO bioavailability resulting in oxidative stress (Mian and Martin, 1995). Previous studies have shown that RmP treatment produces ROS (Schmeda-Hirschmann et al., 2016), and induces leukocyte migration triggered by COX activation (De Medeiros et al., 2019). Based on this prior research, we performed some concentration-response curves to the RmP in the presence of indomethacin (100 μM) or apocynin (30 μM). Here we observed that the contraction response induced by RmP was partially mediated via activation of COX, but independent of NADPH oxidase activation.
Additionally, literature demonstrates the presence of serotonin, or 5-hydroxytryptamine (5-HT) in RmP (Mailho-Fontana, 2012). 5-HT acts as a hormone and neurotransmitter. Its actions are mainly due to the activation of 5-HT receptors. 5-HT2 receptor subtype is expressed in smooth muscle cells and its activation induces vasocontraction (Mohammad-Zadeh et al., 2008). Although our chromatogram did not identify 5-HT, we used ketanserin (30 nM) to investigate whether 5-HT indeed plays a role in RmP-induced contraction. Ketanserin caused a shift to the right in the concentration-response curve to RmP. These data suggest that RmP may have factors that share similarities with 5-HT. However, it is important to emphasize that several studies have shown that ketanserin may act on different receptors, such as α1-adrenergic receptors, in a concentration-dependent manner (Korstanje et al., 1986; Yoshio et al., 2001). Therefore, more specific experiments should be performed to confirm this inference.
RmP is rich in bufadienolides, such as marinobufagenin, which is described as a cardiac glycoside that acts by inhibiting the Na+/K+ ATPase (Bagrov et al., 1995; Strauss et al., 2019). Therefore, we performed an experiment to test the effects of RmP on the functional activity of Na+/K+ ATPase, as described by Rossoni et al. (2002). Our results indicate that the RmP intensified Na+/K+ ATPase-induced hyperpolarization. Although, this response is opposing contractile responses induced by RmP, it may be due to a modulatory mechanism. Puschett et al. (2012) suggests that resinobufagin, another bufadienolide of RmP, opposes marinobufagenin actions and induces vascular dilation. In RmP extract used in this work was found both, either resinobufagin or the marinobufagenin. And so, it is suggested that R. marina extract may potentiate the hyperpolarizing effect of Na+/K+ ATPase due to the action of unidentified compounds other than marinobufagenin or by a joint effect of different poison compounds.
RmP toxicosis is well known by veterinarians since young, smaller dogs are its main victims, and cases of intoxication require a quick treatment from an emergency care perspective (Roberts et al., 2000). Within 30–60 min after oral exposure to the poison, dogs present severe ptyalism, vomiting, and in the most severe cases, the intoxication can lead to seizures and cardiac dysrhythmias (Johnnides et al., 2016; Sakate and Oliveira, 2000). Regarding in vivo experiments, we have observed that R. marina induced an acute elevation of blood pressure. It is known that acute and transitory response in blood pressure is a typical response when a vasopressor drug is delivered via intravascular route. Subcutaneous or intraperitoneal administration provide a longer lasting effect, which tends to start about 45 min after administration (Dias et al., 2016; Romero-Imbachi et al., 2021). Since we have observed that R. marina binds α1-adrenoreceptor and it is a potent vasoconstrictor, it is possible to suggest that the elevation in the blood pressure was due to an increase in total peripheral resistance, rather than changes in cardiac output. It is well known that α–1 or α–2 adrenoceptors activation increases arterial pressure and total peripheral resistance but does not significantly change heart rate, left ventricular dP/dt, stroke volume, or cardiac output. Regarding the reduction in the heart rate, it is presumable that this change was due to the baroreceptors reflex feedback mechanism (Michelini and Rossoni, 2013) in response to the elevation in blood pressure.
5. Conclusion
In conclusion, we show that Rhinela marina poison induces an important vasoconstrictor effect via α1-adrenergic receptor activation and the release of endothelium-derived factors. In in vivo experiment, the administration of the poison increased the blood pressure. We propose that Rhinella marina poison extract could be used to identify new pharmacological tool for some cardiovascular disorders.
Acknowledgements
We are grateful to Brazilian agencies National Council for Scientific and Technological Development (CNPq 421162/2018–0 to G.F.B.) and Coordination of Superior Level Staff Improvement (CAPES). This work was also supported by National Institutes of Health (R01HL149762 and R00GM118885 to C.F.W.).
Footnotes
Credit authors statement
Cintia Vieira dos Santos: Conceptualization; Investigation; Writing – original draft; Caroline Aparecida Tomazelli: Resources. Jacqueline Kerkhoff: Methodology; Resources. Camilla Ferreira Wenceslau: Writing – Review and Editing. Adilson Paulo Sinhorin: Investigation; Resources. Domingos de Jesus Rodrigues: Resources. Fernando Silva Carneiro: Resources; Writing – Review and Editing. Gisele Facholi Bomfim: Writing – Review and Editing; Supervision; Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- AmphibiaWeb. Bufonidae family 2022. https://amphibiaweb.org/lists/Bufonidae.shtml. Accessed on January 25, 2022.
- Bagrov AY, Roukoyatkina NI, Pinaev AG, Dmitrieva RI, Fedorova OV, 1995. Effects of two endogenous Na+,K(+)-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur. J. Pharmacol 274 (1–3), 151–158. [DOI] [PubMed] [Google Scholar]
- Banfi FF, Guedes KDS, Andrighetti CR, Aguiar AC, Debiasi BW, Noronha J, da C, Rodrigues D. de J., Junior GMV, Sanchez BAM, 2016. Antiplasmodial and cytotoxic activities of toad venoms from southern amazon, Brazil. Kor. J. Parasitol 54 (4), 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Filho GA, Schwartz CA, Resck IS, Murta MM, Lemos SS, Castro MS, Kyaw C, Pires Júnior OR, Leite JRS, Bloch Júnior C, Schwartz EF, 2005. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon 45 (6), 777–782. [DOI] [PubMed] [Google Scholar]
- De Medeiros DSS, Rego TB, Dos Santos A.P. de A., Pontes AS, Moreira-Dill LS, Matos NB, Zuliani JP, Stábeli RG, Teles CBG, Soares AM, Sperotto A.R. de M., Moura DJ, Saffi J, Caldeira CA, da S, Pimenta DC, Calderon LA, 2019. Biochemical and Biological Profile of Parotoid Secretion of the Amazonian Rhinella Marina (Anura: Bufonidae), vol. 2019. BioMed research international [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EBR, De Sousa Júnior PT, De Vasconcelos LG, Rodrigues RDJ, Sinhorin VDG, Kerkhoff J, Pelissari S.R. do N., Sinhorin AP, 2020. Comparative study of the chemical profile of the parotoid gland secretions from Rhaebo guttatus from different regions of the Brazilian Amazon. Toxicon 179, 101–106. [DOI] [PubMed] [Google Scholar]
- DeVore JL, Shine R, Ducatez S, 2021. Spatial ecology of cane toads (Rhinella marina) in their native range: a radiotelemetric study from French Guiana. Sci. Rep 11 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias L, Rodrigues MAP, Rennó AL, Stroka A, Inoue BR, Panunto PC, Melgarejo AR, Hyslop S, 2016. Hemodynamic responses to Lachesis muta (South American bushmaster) snake venom in anesthetized rats. Toxicon 123, 1–14. [DOI] [PubMed] [Google Scholar]
- Edwards RJ, Enosi D, Amos TG, O’meally D, Richardson MF, Russel TL, Vallinoto M, Carneiro M, Ferrand N, Wilkins MR, Sequeira F, Rollins LA, Holmes EC, Shine R, White PA, 2018. Draft genome assembly of the invasive cane toad, Rhinella marina. Giga Sci 7 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Huang Y, Vanhoutte PM, 2011. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol 164 (3), 894–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira PMP, Lima DJB, Debiasi BW, Soares BM, Machado KC, Noronha JC, Rodrigues D. de J., Sinhorin AP, Pessoa C, Júnior GMV, 2013. Antiproliferative activity of Rhinella marina and Rhaebo guttatus venom extracts from Southern Amazon. Toxicon 72, 43–51. [DOI] [PubMed] [Google Scholar]
- Friesen CR, Shine R, 2019. At the invasion front, male cane toads (Rhinella marina) have smaller testes. Biol. Lett 15 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Popescu R, Kopp B, Wang Z, 2011. Bufadienolides and their antitumor activity. Nat. Prod. Rep 28 (5), 953–969. [DOI] [PubMed] [Google Scholar]
- Gardner ST, Assis VR, Smith KM, Appel AG, Mendonça MT, 2020. Innate immunity of Florida cane toads: how dispersal has affected physiological responses to LPS. J. Comp. Physiol. B 190 (3), 317–327. [DOI] [PubMed] [Google Scholar]
- Gardner ST, Kepas M, Simons CR, Horne LM, Savitzky AH, Mendonça MT, 2021. Differences in morphology and in composition and release of parotoid gland secretion in introduced cane toads (Rhinella marina) from established populations in Florida, USA. Ecol. Evol 11 (2), 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan DE, 2016. Principles of Pharmacology: the Pathophysiologic Basis of Drug Therapy Lippincott Williams & Wilkins. [Google Scholar]
- Jespersen B, Knupp L, Northcott CA, 2012. Femoral arterial and venous catheterization for blood sampling, drug administration and conscious blood pressure and heart rate measurements. JoVE: JoVE 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnnides S, Green T, Eubig P, 2016. Toad intoxication in the dog by Rhinella marina: the clinical syndrome and current treatment recommendations. J. Am. Anim. Hosp. Assoc 52 (4), 205–211. [DOI] [PubMed] [Google Scholar]
- Kerkhoff J, Noronha JAC, Bonfilio R, Sinhorin AP, Rodrigues D. de J., Chaves MH, Júnior GMV, 2016. Quantification of bufadienolides in the poisons of Rhinella marina and Rhaebo guttatus by HPLC-UV. Toxicon 119, 311–318. [DOI] [PubMed] [Google Scholar]
- Korstanje C, Sprenkels R, Doods HN, Hugtenburg JG, Boddeke E, Batink HD, Thoolen MJMC, Van Zwieten PA, 1986. Characterization of flufylline, fluprofylline, ritanserin, butanserin and R 56413 with respect to in-vivo alpha 1-, alpha 2- and 5-HT2-receptor antagonism and in-vitro affinity for alpha 1-, alpha 2 and 5-HT2-receptors: comparison with ketanserin. J. Pharm. Pharmacol 38 (5), 374–379. [DOI] [PubMed] [Google Scholar]
- Kosmala GK, Brown GP, Shine R, Christian K, 2020. Skin resistance to water gain and loss has changed in cane toads (Rhinella marina) during their Australian invasion. Ecol. Evol 10 (23), 13071–13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K, Marciniak P, Rosiński G, Rychlik L, 2018. Toxic activity and protein identification from the parotoid gland secretion of the common toad Bufo bufo. Comp. Biochem. Physiol. C Toxicol. Pharmacol 205, 43–52. [DOI] [PubMed] [Google Scholar]
- Lebedev DS, Ivanov IA, Kryukova EV, Starkov VG, Tsetlin VI, Utkin YN, 2017. Arginine derivatives of dicarboxylic acids from the parotid gland secretions of common toad Bufo bufo—new agonists of ionotropic γ-aminobutyric acid receptors. Dokl. Biochem. Biophys 474 (1), 178–182. [DOI] [PubMed] [Google Scholar]
- Mailho-Fontana PL, 2012. Comparative Study of the Cutaneous Chemical Defensive System in Two Species of Amazonian Toads (Rhinella Marina and Rhaebo Guttatus). Master’s Dissertation. Instituto Butantan, Sao Paulo, Brazil. [Google Scholar]
- Mailho-Fontana PL, Antoniazzi MM, Toledo LF, Verdade VK, Sciani JM, Barbaro KC, Pimenta DC, Rodrigues MT, Jared C, 2014. Passive and active defense in toads: the parotoid macroglands in Rhinella marina and Rhaebo guttatus. J. Exp. Zool. Part A, Ecological genetics and physiology 321 (2), 65–77. [DOI] [PubMed] [Google Scholar]
- Manika D, DasGupta SC, Gomes A, 1998. Immunomodulatory and antineoplastic activity of common Indian toad (Bufo melanostictus, Schneider) skin extract. Indian J. Pharmacol 30 (5), 311–317. [Google Scholar]
- Mian KB, Martin W, 1995. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric oxide to destruction by superoxide anion in rat aorta. Br. J. Pharmacol 115 (6), 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini LC, Rossoni LV, 2013. Vasomotricidade e regulação local de fluxo. In: AIRES MM (Ed.), Fisiologia, 4 ed. Guanabara Koogan, Rio de Janeiro, pp. 491–506. [Google Scholar]
- Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM, 2008. Serotonin: a review. J. Vet. Pharmacol. Therapeut 31 (3), 187–199. [DOI] [PubMed] [Google Scholar]
- Morishita S, Shoji M, Oguni Y, Ito C, Noguchi K, Sakanashi M, 1991. Congestive heart failure model in rabbits: effects of digoxin and a drug containing toad venom. Jpn. J. Pharmacol 56 (4), 427–432. [DOI] [PubMed] [Google Scholar]
- Pelissari SRN, Sinhorin VDG, Castoldi L, Vasconcelos LG, Rodrigues DJ, Ribeiro EBS, Kerkhoff J, Sinhorin AP, 2021. Methanolic extract of Rhinella marina poison: chemical composition, antioxidant and immunomodulatory activities. J. Braz. Chem. Soc 32, 1584–1597. [Google Scholar]
- Pintérová M, Kuneš J, Zicha J, 2011. Altered neural and vascular mechanisms in hypertension. Physiol. Res 60 (3), 381–402. [DOI] [PubMed] [Google Scholar]
- Puschett JB, 2012. Marinobufagenin predicts and resibufogenin prevents preeclampsia: a review of the evidence. Am. J. Perinatol 29 (10), 777–786. [DOI] [PubMed] [Google Scholar]
- Roberts BK, Aronsohn MG, Moses BL, Burk RL, Toll J, Weeren FR, 2000. Bufo marinus intoxication in dogs: 94 cases (1997–1998). J. Am. Vet. Med. Assoc 216 (12), 1941–1944. [DOI] [PubMed] [Google Scholar]
- Romero-Imbachi MR, Cupitra N, Ángel K, Gonźalez B, Estrada O, Calderón JC, Guerrero-Vargas J, Beltrán J, Narvaez-Sanchez R, 2021. Centruroides margaritatus scorpion complete venom exerts cardiovascular effects through alpha-1 adrenergic receptors. Comp. Biochem. Physiol. C Toxicol. Pharmacol 240. [DOI] [PubMed] [Google Scholar]
- Rossoni LV, Salaices M, Marín J, Vassallo DV, Alonso MJ, 2002. Alterations in phenylephrine-induced contractions and the vascular expression of Na+,K+-ATPase in ouabain-induced hypertension. Br. J. Pharmacol 135 (3), 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakate M, Oliveira PCLD, 2000. Toad envenoming in dogs: effects and treatment. J. Venom. Anim. Toxins 6, 52–62. [Google Scholar]
- Schmeda-Hirschmann G, Quispe C, Arana GV, Theoduloz C, Urra FA, Cárdenas C, 2016. Antiproliferative activity and chemical composition of the venom from the Amazonian toad Rhinella marina (Anura: Bufonidae). Toxicon 121, 119–129. [DOI] [PubMed] [Google Scholar]
- Segalla MV, Caramaschi U, Cruz CAG, Grant T, Haddad CFB, Langone JA, Garcia P.C. de A., 2014. Brazilian amphibians: list of species. Herpetol. Brasil 3 (2), 37–48. [Google Scholar]
- Strauss M, Smith W, Fedorova O, Schutte A, 2019. The Na+K+-ATPase inhibitor marinobufagenin and early cardiovascular risk in humans: a review of recent evidence. Curr. Hypertens. Rep 21 (5), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC, 2018. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res 114 (4), 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RC, Bohr DF, 1978. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. J. Vasc. Res 15 (1–3), 198–207. [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I, 2001. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn. J. Pharmacol 86 (2), 189–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


