Abstract
Heat stress limits growth and development of crops including grapevine which is a popular fruit in the world. Genetic variability in crops thermotolerance is not well understood. We identified and characterized heat stress transcription factor HSFA2 in heat sensitive Vitis vinifera ‘Jingxiu’ (named as VvHSFA2) and heat tolerant Vitis davidii ‘Tangwei’ (named as VdHSFA2). The transcriptional activation activities of VdHSFA2 are higher than VvHSFA2, the variation of single amino acid (Thr315Ile) in AHA1 motif leads to the difference of transcription activities between VdHSFA2 and VvHSFA2. Based on 41 Vitis germplasms, we found that HSFA2 is differentiated at coding region among heat sensitive V. vinifera, and heat tolerant Vitis davidii and Vitis quinquangularis. Genetic evidence demonstrates VdHSFA2 and VvHSFA2 are positive regulators in grape thermotolerance, and the former can confer higher thermotolerance than the latter. Moreover, VdHSFA2 can regulate more target genes than VvHSFA2. As a target gene of both VdHSFA2 and VvHSFA2, overexpression of MBF1c enhanced the grape thermotolerance whereas dysfunction of MBF1c resulted in thermosensitive phenotype. Together, our results revealed that VdHSFA2 confers higher thermotolerance than VvHSFA2, and MBF1c acts as their target gene to induce thermotolerance. The VdHSFA2 may be adopted for molecular breeding in grape thermotolerance.
Introduction
Heat stress (HS), as one of the main abiotic stresses, limits the yield of crops. With climate change, extreme high temperature becomes one of the major adverse environmental conditions that plants often encounter [1]. Heat stress generally impairs photosynthetic activity, reduces water content, and has negative effects on cell division and growth [2]. Heat stress can affect many physiological processes of plants, such as growth, development, and reproduction [3]. As sessile organisms, plants have to reprogram their transcripts, proteins, and metabolites to some extent in order to survive in heat stress conditions [3–6].
Heat stress response (HSR) is highly complicated in plants. Current knowledge of plant response to HS mainly includes heat stress transcription factors (HSFs), protein homeostasis, and reactive oxygen species (ROS) [1]. HSFs are the core regulatory factors for HSR and thermotolerance, and HSFs are required for the induction of major HS-induced genes such as HSPs [4, 7–9]. HSFs are not only involved in heat stress but also other abiotic stresses including drought, cold, salt, as well as plant growth and development [10]. The number of HSF members varied among plants, with 19 in grape (Vitis vinifera), 21 in Arabidopsis (Arabidopsis thaliana), and up to 56 in wheat (Triticum aestivum) [4, 10, 11]. The basic structure and promoter recognition of HSFs were highly conserved in eukaryotes although HSFs were different in size and sequence [4, 12]. A typical HSF structure generally contains DNA binding domain (DBD), oligomerization domain (OD) or HR-A/B, nuclear localization signal domain (NLS), C-terminal activation domain (CTAD), and nuclear export signal domain (NES) [4, 12, 13]. The DBD contains three α-helix bundle and an antiparallel four-stranded β-sheet, CTAD comprises some aromatic/hydrophobic/acidic (AHA) motifs [13, 14]. Different HSFs members had their specific roles in HSR despite the high similarity among HSF orthologs [15]. HSFA2 acts as key regulator involved in cellular response to various types of environmental stress. Heat stress can induce the expression of HSFA2, and HSFA2 promoted the expression of heat shock protein (HSP) genes and improved acquired thermotolerance of Arabidopsis [16]. The transcription level of HSFA2 was the highest among all 21 Arabidopsis HSFs after heat stress, and overexpression of the Arabidopsis HSFA2 gene improved plant abiotic stress such as heat resistance and salt/osmotic stress tolerance [17]. In tomato, loss function of HSFA2 reduced the viability and germination rate of pollen after heat stress during the stages of meiosis and microspore formation [15]. Overexpression of ZmHSFA2 in Arabidopsis improved the expression of Arabidopsis raffinose synthase gene, accompanied with increased raffinose content and plant thermotolerance [18]. These findings suggested an extensive role for HSFs in heat tolerance. In grapevine, VvHSFA2 may activate GOLS1 to induce thermotolerance, but there was no genetic evidence of HSFA2 in thermotolerance [19]. What is more, there are hardly any reports that allelic variations of HSFs result in thermotolerance difference in different plant species (or accessions), especially for HSFA2. In addition, exactly how HSFA2 regulates the downstream genes and contributes to heat tolerance in plants including grapevines remains to be explored.
MBF1, multiple bridge factor 1, is a highly conserved transcriptional coactivator that played a crucial role in development process and stress responses [20]. MBF1 contained two parts, N-terminal domain (named MBF1 domain) and C-terminal helix–turn–helix domain [20]. MBF1 was firstly isolated in silkworm, as a bridge between FTZ-F1 and TBP, mediating transactivation by FTZ-F1 [21]. Subsequent studies about MBF1 were performed in yeast [22]. Three MBF1 isoforms exist in the genome of Arabidopsis. MBF1a and MBF1b are involved in the regulation of various developmental processes, while MBF1c is a player of responses to different stress such as salinity and heat [23–27]. In tomato, the transcription level of MBF1-like protein ER24 is up-regulated upon ethylene treatment [28]. Recently, MBF1c were more reported in response to heat stress, such as in wheat, Chinese kale and lily [29–31]. In grape, there is some research about MBF1a, which is related to drought stress and high temperature [32, 33]. Rienth et al. reported that VvMBF1c showed an induction in grape response to high temperature [33, 34]. However, the function of MBF1c is not validated in grapevine, and which transcriptional factor directly modulates MBF1c remains unknown in plants.
Grape (V. vinifera L.) is a very popular fruit in the world and with important economic value. However, heat stress can have harmful effects on grape quality and production. With the publishing of the grape genome, genes related to many quality traits such as anthocyanin, resveratrol, sugar, cold, and dehydration have been identified [35–39]. These studies provided information for the study of grape quality formation. Information on grape heat stress is limited although there are some studies about morphological and physiological changes in response to heat stress. Nevertheless, a growing body of omics data provided novel insights for grape heat stress response [5, 40]. Our previous study pointed to the fact that the expression of HSFA2 was increased at transcription and protein level after heat stress, indicating that HSFA2 may play a potential role in grape thermotolerance [5]. However, we lacked genetic evidence of HSFA2 inducing thermotolerance in grapevines. V. vinifera accessions that people often consume are sensitive to high temperature, while Vitis davidii and Vitis quinquangularis appear more thermotolerant than V. vinifera [41, 42]. Therefore, it is also very much worth exploring the mechanism of HSFA2 among these grape accessions displaying different thermotolerant capacity.
In this study, we reported the identification and characterization of HSFA2 gene, which plays a key role in thermotolerance in grapevine based on genetic evidence. HSFA2 is differentiated at the coding region among heat sensitive V. vinifera, and heat tolerant Vitis davidii and Vitis quinquangularis. The difference in sequences between VvHSFA2 and VdHSFA2 may contribute to the different thermotolerance among grape accessions. Moreover, VdHSFA2 can regulate more target genes than VvHSFA2. MBF1c, as the target gene of HSFA2, plays a large role in thermotolerance in grapevines. Our results indicate that natural variations of HSFA2 enhance thermotolerance in Vitis.
Results
Expression of HSFA2 in V. vinifera and V. davidii grapevine leaves in response to high temperatures
Our previous study indicated that HSFA2 may play an important role in grape response to heat [5, 19]. Based on these results, we further explored the expression of HSFA2 in heat sensitive ‘Jingxiu’ (V. vinifera) and heat tolerant ‘Tangwei’ (V. davidii) grapevines in response to heat stress. After 40°C and 45°C treatments for 2 h, the expression level HSFA2 in leaves significantly (P < 0.05, the same as below) increased when compared to control condition (25°C). Additionally, HSFA2 showed higher expression in ‘Tangwei’ than in ‘Jingxiu’ upon heat stress (Fig. 1a).
Figure 1.

The expression and promoter activities of Vitis vinifera ‘Jingxiu’ and Vitis davidii ‘Tangwei’ grapevines HSFA2 under different temperatures. a Expression analysis of HSFA2 in grapevine leaves was conducted using RT-PCR. bHSFA2 promoter activities were examined by transient expression assay in Arabidopsis protoplasts. Relative Luc/Ren value was measured. cHSFA2 promoter activities were examined by transient expression assay in Nicotiana benthamiana under room temperature and heat stress. Agrobacterium containing the VvHSFA2 and VdHSFA2 promoter vectors were transformed into N. benthamiana leaves, respectively. Some transformed lines were treated at 37°C for 1 h or 2 h, and some allowed to still in growth chamber (25°C). A Tanon 5200 Multi luminometer (China) was used to observe fluorescence signal. Bar: 5 mm. d Relative Luc/Ren values were measured by transient expression assay in N. benthamiana under room temperature (25°C) and heat stress (37°C). pVvHSFA2 indicates the HSFA2 promoter from V. vinifera ‘Jingxiu’; pVdHSFA2 indicates the HSFA2 promoter from Vitis davidii ‘Tangwei’. Data are based on three independent biological replicates. Student’s t-test was used to determine significant differences (*P < 0.05; **P < 0.01).
Sequences and activities comparison of VvHSFA2 and VdHSFA2 promoters
The HSFA2 isoforms from ‘Jingxiu’ and ‘Tangwei’ were named as VvHSFA2 and VdHSFA2, respectively. Two kb of their corresponding promoter regions were cloned and sequenced. Alignment of VvHSFA2 and VdHSFA2 promoters revealed 44 single nucleotide polymorphisms (SNPs) and 5 Indels (Fig. S1, see online supplementary material). The Plant CARE database (http://bioinformatics.psb. ugent.be/webtools/plantcare/html/) was used to search conserved motifs. The Plant CARE search revealed that VvHSFA2 and VdHSFA2 promoters include 32 common motifs whereas five motifs were present only in the VvHSFA2 promoter (Fig. S2, see online supplementary material). We conducted transient expression assay in Arabidopsis protoplasts to evaluate VvHSFA2 and VdHSFA2 promoter activities. As shown in Fig. 1b, the relative activity of the VdHSFA2 promoter was higher than the one from the VvHSFA2 promoter. The promoter activity of VvHSFA2 and VdHSFA2 was further compared in the tobacco leaf assay under room temperature (25°C) and heat stress (37°C). As shown in Fig. 1c and d, the HSFA2 promoter activity increased after 37°C heat treatments. In addition, the activity of VdHSFA2 promoter was higher than VvHSFA2 promoter under room temperature (25°C) or heat stress (37°C).
Sequence analysis, subcellular localization, and transcriptional activity of VvHSFA2 and VdHSFA2
The CDS of HSFA2 were obtained from leaves of ‘Jingxiu’ and ‘Tangwei’, respectively. Sequence analysis revealed that both VvHSFA2 and VdHSFA2 transcripts are 1134 bp, which encode 377 amino acids containing a putative HSF domain. VvHSFA2 and VdHSFA2 have 11 SNPs (Fig. S3, see online supplementary material) leading to differences for seven amino acid residues distributed in DBD, HR-A/B, and AHA domains (Fig. 2a). Phylogenetic analysis of the VvHSFA2 and VdHSFA2 proteins and their orthologs in various plants was conducted. The phylogenetic analysis was constructed based on the amino acid sequence alignments from representative species. The results showed that VvHSFA2 and VdHSFA2 share the highest sequence identity, followed by MdHSFA2 (Malus domestica HSFA2) (Fig. S4, see online supplementary material).
Figure 2.

Conserved motif, subcellular localization, and transcriptional activities of HSFA2 from Vitis vinifera ‘Jingxiu’ (VvHSFA2) and Vitis davidii ‘Tangwei’ (VdHSFA2). a Conserved domain analysis of VvHSFA2 and VdHSFA2 amino acid sequences. Conserved domain is shown in the bottom of the sequence. DNA binding domain (DBD) contains three α-helix bundle and a small four-stranded antiparallel β-sheet; oligomerization domain (OD) contains HRA and HRB; NLS, nuclear localization signal; NES, nuclear export signal; C-terminal activation domains (CTAD) are rich in aromatic, hydrophobic, and acidic amino acid residues, so CTAD is also called AHA. bVvHSFA2-eGFP and VdHSFA2-eGFP were transiently transformed into epidermal cells of N. benthamiana leaves, empty vector (EV) as control. Images under bright field (left), fluorescence (middle), and the merged images are shown on the right. Bar: 20 μm. c Analysis of VvHSFA2 and VdHSFA2 transcriptional activity in yeast. The coding sequences of VvHSFA2 and VdHSFA2 were fused with pGBDK7 vector including Gal4 binding domain (BD), respectively. Constructed vector was transformed into yeast strain Y2HGold. The transformed yeast was grown on SD/−Trp, SD/−Trp/-His/−Ade (SDO) and SD/−Trp/-His/−Ade/X-α-gal (SDO/X). Combinations of AD-T with BD-p53 and BD-lam were used as positive and negative control, respectively. d Schematic diagrams indicate constructed plasmids in the transient expression assays in Arabidopsis protoplasts. The CDS of VvHSFA2, VdHSFA2, and VdHSFA2 mutation were cloned into CTB7-GAL4BD vector as the effector, respectively. Negative control was the empty vector. LUC was used as the reporter to detect the transcriptional activation of VvHSFA2, VdHSFA2, and VdHSFA2 mutation. Ren was as internal reference. e Transcriptional activity analysis of VvHSFA2 and VdHSFA2 in Arabidopsis protoplasts by dual luciferase assay. Constructed vector was co-transformed Arabidopsis protoplasts with firefly LUC reporter vector and the renilla LUC vector. GalBD was used as negative control. f Comparison of transcriptional activity of VvHSFA2, VdHSFA2, and VdHSFA2 mutation in Arabidopsis protoplasts by dual luciferase assay. g Partial diagrams of VvHSFA2, VdHSFA2, and VdHSFA2 mutation are used in f. Amino acid mutant was shown in red characters in VdHSFA2 mutation. VdHSFA2-mutant1 indicates that the 315 th amino acid ‘T(Thr)’ (distributing in the region of AHA1) was changed into ‘I (Ile)’ in VdHSFA2 protein through base mutant; VdHSFA2-mutant2 indicates that the 329 th amino acid ‘G (Gly)’ was changed into ‘E(Glu)’ in the VdHSFA2 protein; VdHSFA2-mutant3 indicates that the 336 th amino acid ‘P(Pro)’ was changed into ‘R(Arg)’ in the VdHSFA2 protein. Data are based on three independent biological replicates. Duncan’s test was used to determine significant differences (P < 0.05).
Next, we explored the subcellular localization of VvHSFA2 and VdHSFA2. VvHSFA2-GFP and VdHSFA2-GFP fusion constructs were transfected into N. benthamiana leaves, respectively. Fluorescence analysis of N. benthamiana overexpressing VvHSFA2-GFP and VdHSFA2-GFP indicated nuclear localization, while the GFP control fluorescence was evenly distributed throughout the cell (Fig. 2b).
To explore whether VvHSFA2 and VdHSFA2 possess transcriptional activity, VvHSFA2 and VdHSFA2 were constructed into the pGBKT7 vector with the Gal4 DNA binding domain (BD), respectively. The BD-VvHSFA2, BD-VdHSFA2 plasmids and empty vector BD were expressed individually into yeast strain Y2HGold. As shown in Fig. 2c, yeast cells carrying BD-VvHSFA2, BD-VdHSFA2 and the positive control exhibited alpha-galactosidase activity, indicating that VvHSFA2 and VdHSFA2 possess transcriptional activity in yeast. A similar assay was conducted using a dual-luciferase reporter assay in Arabidopsis protoplasts. The effector and reporter constructs are shown in Fig. 2d. As shown in Fig. 2e, both VvHSFA2 and VdHSFA2 triggered luciferase activity, in higher proportion for VdHSFA2 when compared to VvHSFA2. To further explore which SNP may explain the different transactivation properties observed between VvHSFA2 and VdHSFA2, we muted three different SNPs located in AHA1 and AHA1-adjacent domains of VdHSFA2 into VvHSFA2 in turn. The muted VdHSFA2 were named as VdHSFA2mut1, VdHSFA2mut2, VdHSFA2mut3 in order of the location of the three SNPs (Fig. 2g). The three muted VdHSFA2 with Gal4 DNA binding domain were co-transformed into Arabidopsis protoplasts with LUC and TRL plasmid, respectively. The results showed that only VdHSFA2mut1 (Thr315 → Ile315), resulting from the nucleotide polymorphism of C/T at the 944th base of HSFA2 coding region, caused significant lower transcriptional activity when compared to VdHSFA2 wild type (Fig. 2f).
To explore whether heat tolerance is correlated with the VdHSFA2mut1 in grape, we measured the heat tolerance of 41 grape accessions (Table S1, see online supplementary material), and combined cloning and sequencing of HSFA2 isoforms from each germplasm. As shown in Table 1, allele (Ile315) was possessed in 38 V. vinifera accessions which are heat sensitive (lower Fv/Fm values). However, allele (Thr315) was possessed in two V. davidii accessions and one V. quinquangularis accession which are heat tolerant (higher Fv/Fm values).
Effect of VvHSFA2 and VdHSFA2 on heat tolerance in grape
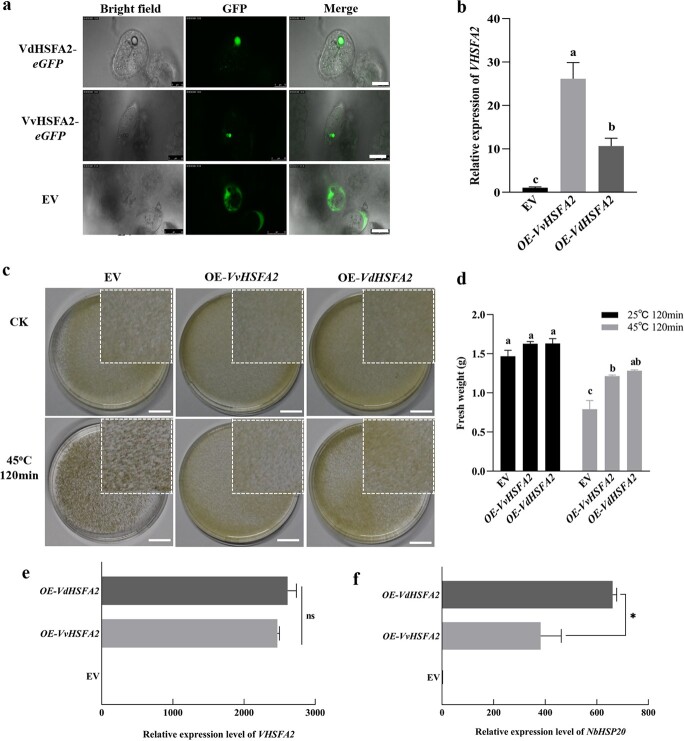
To investigate the function of HSFA2, transgenic grape suspension cells overexpressing either VvHSFA2 (OE-VvHSFA2) or VdHSFA2 (OE-VdHSFA2) were produced using 35S promoter-driven HSFA2 constructs. Successfully transformed cells were identified by eGFP fluorescence and RT-qPCR (Fig. 3a and b).
Figure 3.
Overexpression of VvHSFA2 and VdHSFA2 improved heat tolerance in grape. a Detection of transgenic grape suspension cells by GFP fluorescence. The cells transformed with empty vector (EV) was as control. Bar: 20 μm. b Expression analysis of HSFA2 in transgenic grape suspension cells of empty vector (EV), overexpressing VvHSFA2 (OE-VvHSFA2), and overexpressing VdHSFA2 (OE-VdHSFA2) using RT-PCR. c Phenotypes of grape suspension cells of EV, OE-VvHSFA2, and OE-VdHSFA2 after 45°C for 2 h and 25°C for 7 d. The grape suspension cells grown under 25°C were as control. The cells in white box lines indicate a zoom on the center sector of the petri dishes. Bar: 1.5 cm. d Fresh weight of transgenic grape suspension cells of EV, OE-VvHSFA2, and OE-VdHSFA2 was measured after 45°C for 2 h and 25°C for 7 d. e Transgenic lines of similar expression levels of VvHSFA2 and VdHSFA2 were acquired in N. benthamiana. fNbHSP22.0 expression levels in corresponding transgenic lines in e. Data are based on three independent biological replicates. Duncan test (P < 0.05) or Student’s t-test (*P < 0.05) were used to determine significant differences. ns: not significant.
Compared with controls (grape cells transformed with empty vector (EV)), HSFA2 expression was up-regulated ~26 fold and ~ 10 fold in VvHSFA2 and VdHSFA2 overexpressing lines, respectively (Fig. 3b). The time for heat stress was the 6th day after subculturing. Then, we evaluated the thermotolerance of control and transgenic grape suspensions by determining their phenotype and fresh weight [43]. The appearance of OE-VvHSFA2 and OE-VdHSFA2 grape suspension cells was similar with EV cells when they grew in control conditions at 25°C (Fig. 3c). However, control cells (EV) became black after incubation to 45°C for 2 h and recovery at 25°C for 7 d (Fig. 3c). As shown in Fig. 3d, the fresh weight of EV and transgenic suspension cells all declined significantly after heat stress and recovery, but in lower proportion for HSFA2 transgenic cells than EV cells. Therefore, the overexpression of VvHSFA2 and VdHSFA2 significantly improved heat tolerance of grape, with a better effect of VdHSFA2. In addition, we transiently overexpressed VvHSFA2 and VdHSFA2 in grape tissue culture plantlets. Similarly, the results showed that the overexpression of VvHSFA2 and VdHSFA2 significantly improved heat tolerance of grape plants (Fig. S6, see online supplementary material).
To further compare the regulation ability between VvHSFA2 and VdHSFA2, we overexpressed VvHSFA2 and VdHSFA2 in N. benthamiana transiently. We chose three transgenic N. benthamiana lines of OE-VvHSFA2 and OE-VdHSFA2, respectively, showing similar HSFA2 expression level (Fig. 3e). HSP22.0 gene was shown to be transactivated by HSFA2 [17, 44], accordingly, we measured the HSP22.0 expression level. The results showed that HSP22.0 expression levels were enhanced in both N. benthamiana OE-VdHSFA2 and OE-VvHSFA2 lines; however, the last one appeared to a lesser extent (Fig. 3f).
To further address the function of HSFA2 on thermotolerance in grape, we investigated the thermotolerance of grape suspension cells in which HSFA2 was interrupted by CRISPR-Cas9. sgRNA is shown in Fig. 4a. The mutant hsfa2 grape suspension cells were detected by sequencing analysis. As shown in Fig. 4b, we took 10 colonies in which eight colonies were edited and resulted in five type mutations. The mutated amino acid sequences were shown in Fig. 4c, respectively. As expected, the appearance of hsfa2 suspension cells was similar with empty vector grape suspension cells when they grew at 25°C (Fig. 4d). By contrast, hsfa2 suspension cells became black after 45°C for 80 min and recovery at 25°C for 7 d (Fig. 4d). As shown in Fig. 4e, the fresh weight of controls and hsfa2 cells declined significantly after heat treatment and recovery, but the fresh weight of hsfa2 cells was lower than control cells. Collectively, these results indicated that hsfa2 grape suspension cells were more sensitive to heat stress than control cells. In addition, we generated transiently transgenic V. quinquangularis plants by VdHSFA2 RNA interference (RNAi-HSFA2). The result showed that loss-function of HSFA2 was more sensitive to heat stress than controls (Fig. S6, see online supplementary material).
Figure 4.
Knockout of HSFA2 decreased heat tolerance in grape. a Diagram illustration of HSFA2 target sites designed using CRISPR/Cas9 technology. The sequences of sgRNA are shown in red characters, PAM are signed in black. b Identification of HSFA2 knockout mutant (hsfa2). Ten colonies for target were analysed. The number of mutant sequences is shown in red. Sequencing results on both sides of the target site were shown. The wild type (WT) and mutant (mut) are shown on the left. Mutant types are shown on the right. c Mutations of amino acids in corresponding mutant sequencing in b. d Phenotypes of grape suspension cells of EV and hsfa2 mutant after heat treatments (45°C for 80 min) and 25°C for 7 d. The grape suspension cells grown under 25°C were as control. Bar: 1.5 cm. e Fresh weight of transgenic grape suspension cells of EV and hsfa2 mutant after 45°C for 80 min and 25°C for 7 d. Student’s t-test (**P < 0.01) was used to determine significant differences. ns: not significant.
Potential genes targeted by VvHSFA2 and VdHSFA2
We performed a ChIP-Seq assay in order to investigate the target genes of VvHSFA2 and VdHSFA2. We pulled down the putative VvHSFA2-bound and VdHSFA2-bound DNA sequences using antibodies specific for the VvHSFA2-GFP and VdHSFA2-GFP tags based on three biological replicates. Model Based Analysis of ChIP-Seq (MACS) was used to predict VvHSFA2 and VdHSFA2 binding sites, setting a false discovery cut-off of 0.05. The total number of reads and mapped reads of ChIP-Seq are shown in Table S3 (see online supplementary material). The total number of reads were from 34 183 564 to 49 015 034, and mapped reads were from 18 383 583 to 32 683 141. Their mapping rates were up to 47.41%–68.81% according to default parameters by BWA software (version: 0.7.15-r1140) [45]. Annotation of the VdHSFA2 binding peak regions showed that 38.8% were mapped in promoter regions (Fig. 5a and b).
Figure 5.
Genome-wide analysis of VdHSFA2 and VvHSFA2 targeted genes by ChIP-Seq and RNA-Seq. a Distribution of VdHSFA2 binding regions in three biological replicates in grape genome. b Distribution of VdHSFA2 binding sites around the transcription start sites (TSS) of genes. a and b of VvHSFA2 is shown in the Fig. S7 (see online supplementary material). c Motif of the most highly enriched VdHSFA2 and VvHSFA2 binding sites identified by Homer prediction. d Venn diagram showing an integration of ChIP-Seq and RNA-Seq results of VdHSFA2. e Venn diagram showing an integration of ChIP-Seq and RNA-Seq results of VvHSFA2. f Distribution of the VdHSFA2 and VvHSFA2 binding sites for MBF1c (VIT_211s0016g04080) gene loci. The binding sites were obtained from three independent biological replicates. The translation start site (ATG) positions and gene transcription direction were indicated with black arrows.
For VvHSFA2 peaks, 23.51% were located at promoter regions (Fig. S7a and b, see online supplementary material). The DNA motif bound by HSFA2 within peak sequences were identified with the Homer software. Despite the most significantly enriched motif of both VdHSFA2 and VvHSFA2 being Motif 1 (core sequence TTCNNGAANNTTCNN) (Fig. 5c), the motif analysis reported 108 motifs for VdHSFA2 (Table S4, see online supplementary material), while only 20 motifs of VvHSFA2 (Table S5, see online supplementary material). The identified 2040 peaks corresponded to 1879 genes in VdHSFA2 ChIP-Seq assay, whereas 621 peaks fitted to 353 genes in VvHSFA2 ChIP-Seq assay (Fig. 5d and e; Tables S6 and S7, see online supplementary material).
To determine which were transcriptionally regulated by HSFA2 among the above bounding genes, we performed RNA-Seq analysis of EV, OE-VvHSFA2, and OE-VdHSFA2 grape suspension cells. The total number of reads and mapped reads of RNA-Seq are shown in Table S8 (see online supplementary material). The total number of reads were from 50 236 622 to 64 994 682, and mapped reads were from 46 101 972 to 58 898 181. Their mapping rates were up to 88.67%–91.77% based on default parameters by Hisat2 software (http://ccb.jhu.edu/software/hisat2/index.shtml) [46]. Different expressed genes (DEGs) in OE-VvHSFA2 and OE-VdHSFA2 grape suspension cells are shown in Tables S9 and S10 (see online supplementary material), respectively. The combination of ChIP-Seq and RNA-Seq data showed that VdHSFA2 may directly regulate 116 genes among which 96 were up-regulated and 20 were down-regulated (Fig. 5d; Table S11, see online supplementary material); VvHSFA2 may directly regulate 36 genes among which 35 were up-regulated and one was down-regulated (Fig. 5e; Table S12, see online supplementary material). A total of 116 VdHSFA2-regulated genes and 36 VvHSFA2-regulated genes were characterized based on the Gene Ontology (GO) database Goatools (https://github.com/tanghaibao/Goatools), respectively. Among the categories, the GO terms such as response to heat, response to hydrogen peroxide, response to temperature stimulus, cellular response to heat, and UDP-galactosyltransferase activity were both enriched in VdHSFA2-regulated genes and VvHSFA2-regulated genes, but the numbers in these GO terms were higher in VdHSFA2-regulated genes than in VvHSFA2-regulated genes (Fig. S7c and d; Tables S13 and S14, see online supplementary material).
Half of the genes directly regulated by VvHSFA2 are 18 HSPs, including many HSP20, but also some HSP40, HSP70, HSP90, and HSP100. Additional regulated genes related to heat stress responses include MBF1c, HSFB2a, and universal stress protein PHO34. Galactinol synthase1 (GOLS1), which is involved in the synthesis of compatible solutes from the raffinose family oligosaccharides (RFOs), appeared regulated by VvHSFA2, as well as a catalase, a GEM-like protein, a BAG domain protein, a E3 ubiquitin-protein ligase (Table S12, see online supplementary material).
Most of the genes suspected to be directly regulated by VvHSFA2 were also extracted from the data collected with VdHSFA2. The genes directly regulated by VdHSFA2 included 38 HSPs family members, VdHSFA2 itself, various enzymes from the RFOs synthetic pathway, different transcription factors from ERF, MYB, WRKY, and bZIP families, two UDP-glycosyltransferase, and a betaine aldehyde dehydrogenase 1 (Table S11, see online supplementary material). The common and specific genes directly regulated by VdHSFA2 and/or VvHSFA2 were listed in Tables S15, S16, and S17, respectively (see online supplementary material).
HSFA2 mediates thermotolerance in grapevine through regulating MBF1c expression
MBF1c is a transcriptional coactivator with high conservation and plays important roles in diverse processes including thermotolerance [25, 31]. In our study, MBF1c belongs to the list of VdHSFA2-regulated genes and VvHSFA2-regulated genes (Fig. 5f; Tables S11 and S12, see online supplementary material). To evaluate if HSFA2 can regulate MBF1c expression and to explore if there is any difference in regulation efficiency between VdHSFA2 and VvHSFA2 for MBF1c, yeast one-hybrid assay (Y1H), ChIP-qPCR and luciferase (LUC) reporter assay were performed. Firstly, we cloned and sequenced the promoter regions from ‘Jingxiu’ and ‘Tangwei’, respectively. We found that they have the same promoter sequences (Fig. S8, see online supplementary material). According to the ChIP peak of VvHSFA2 and VdHSFA2 binding MBF1c promoter (Fig. 5f), we chose to clone the core 574 bp fragment of MBF1c promoter into the pLacZ reporter vector. As shown in Fig. 6a, VvHSFA2 and VdHSFA2 can strongly activate the LacZ reporter gene expression, indicating that VvHSFA2 and VdHSFA2 can directly bind to MBF1c. This was confirmed by ChIP-qPCR, our results indicating that both VvHSFA2 and VdHSFA2 can directly bind to the MBF1c promoter (Fig. 6b and c). To detect if the VvHSFA2 and VdHSFA2 can induce the expression of MBF1c in planta, luciferase (LUC) reporter assay was conducted using N. benthamiana leaves transiently transformed with 35S::VvHSFA2 and 35S::VdHSFA2 plasmids used as effectors and proMBF1c::LUC plasmid as reporter. When empty vector was used, weak LUC activity was observed, whereas overexpression of VvHSFA2 or VdHSFA2 enhanced significantly the LUC activity driven by the MBF1c promoter. Again, VdHSFA2 can transactivate the proMBF1c-dependent LUC activity in a stronger manner when compared to the effect of VvHSFA2 (Fig. 6e and f). Taken together, VvHSFA2 and VdHSFA2 could directly bind to the promoter of MBF1c in vitro and in vivo, in addition, the transcriptional activation ability of VdHSFA2 to MBF1c was stronger than VvHSFA2.
Figure 6.
VvHSFA2 and VdHSFA2 positively regulate the expression of MBF1c. a Y1H assay of VvHSFA2 and VdHSFA2 binding to MBF1c promoter. The coding sequences of VdHSFA2 and VvHSFA2 were fused with GAD vector including Gal4 transcriptional activation domain (AD), respectively. Promoter of MBF1c was fused with LacZ reporter genes. Constructed vector were co-transformed into EGY48 yeast, the transformed yeast was grown on SD/−Trp/−Leu/–Ura/X-α-gal. The transformants with GAD and Reporter: proMBF1c::LacZ were used as negative control. b and c ChIP-qPCR verification of VvHSFA2 and VdHSFA2 binding to MBF1c promoter. A specific GFP antibody against VvHSFA2-GFP and VdHSFA2-GFP tag was used to precipitate chromatin. IgG was used as an antibody control. d Schematic representation of constructed plasmids used in the transient expression assays in N. benthamiana. A 574-bp promoter fragment of MBF1c that contains two HSE motifs was used to drive the expression of the firefly luciferase reporter gene. The internal control was Renilla luciferase gene driven by the 35S promoter. e and f Transactivation of the MBF1c promoter by VvHSFA2 and VdHSFA2 by luciferase activity assay. The representative images of an N. benthamiana leaves 48 h after infiltration are shown. The relative luciferase activity was measured. Data are based on three independent replicates. Duncan test (P < 0.05) or Student’s t-test (*P < 0.05) were used to determine significant difference.
In addition, we explored whether MBF1c can directly regulate HSFA2. As a result, MBF1c can’t bind to VvHSFA2 and VdHSFA2 promoters by Y1H (Fig. S10, see online supplementary material). At the same time, we found that MBF1c cannott interact physically with VvHSFA2 or VdHSFA2 (Fig. S11, see online supplementary material).
Identification of MBF1c characteristics and verification of MBF1c-induced heat tolerance in grape
Accumulation of MBF1c transcripts was determined in ‘Jingxiu’ and ‘Tangwei’ grapevines exposed to HS. The same leaf samples as the VvHSFA2 and VdHSFA2 analysis was used to analyse MBF1c expression by qRT-PCR. As shown in Fig. 7a, expression of MBF1c increased in ‘Jingxiu’ and ‘Tangwei’ leaves exposed to HS. The MBF1c CDS of ‘Jingxiu’ and ‘Tangwei’ were cloned and sequenced, revealing differences for two nucleotides without consequence for the coded amino acid sequence (Fig. S9, see online supplementary material). We further explored the subcellular localization of MBF1c using MBF1c-GFP recombinant protein transiently expressed into N. benthamiana leaves. Fluorescence analysis revealed that MBF1c-GFP was detected in both cytosol and nuclei (Fig. 7b). The transcriptional activity of MBF1c assay was demonstrated in Arabidopsis protoplasts, as described above when studying transcriptional activity of HSFA2. The effector and reporter constructs are shown in Fig. 7c. The results showed that MBF1c possesses transcriptional activity (Fig. 7d).
Figure 7.
The expression, subcellular localization, transcriptional activities and function of MBF1c. a Expression analysis of MBF1c was conducted using RT-PCR. bMBF1c-eGFP was transiently transformed into epidermal cells of N. benthamiana leaves, empty vector (EV) as control. Images under bright field (left), fluorescence (middle), and the merged images (right) are shown. Bar: 20 μm. c Schematic diagrams indicate constructed plasmids in the transient expression assays in Arabidopsis protoplasts. The CDS of MBF1c was cloned into CTB7-GAL4BD vector as the effector. Negative control was the empty vector. LUC was used as a reporter to detect the transcriptional activation of MBF1c. Ren was as internal reference. d Transcriptional activity analysis of MBF1c in Arabidopsis protoplasts by dual luciferase assay. Constructed vector was co-transformed Arabidopsis protoplasts with firefly LUC reporter vector and the renilla LUC vector. GalBD were used as negative control. e Expression analysis of MBF1c in transgenic grape suspension cells of empty vector (EV) and overexpressing MBF1c (OE-MBF1c) using RT-PCR. f Phenotypes of grape suspension cells of EV and OE-MBF1c after heat treatment and 25°C for 7 d. The suspension cells grown under 25°C were as control. Bar: 1.7 cm. g Fresh weight of transgenic grape suspension cells of EV and OE-MBF1c after heat treatment and 25°C for 7 d. Student’s t-test was used to determine significant difference (*P < 0.05; **P < 0.01). ns: not significant.
To explore the role of MBF1c in grapevine thermotolerance, firstly, the recombinant protein MBF1c-GFP was stably expressed in grape suspension cells (OE-MBF1c). Compared with control cells (EV), MBF1c expression was ~2 fold higher in OE-MBF1c cells (Fig. 7e). The appearance of OE-MBF1c suspension cells was similar with EV grape suspension cells when they grew at 25°C (Fig. 7f). However, more OE-MBF1c suspension cells were still well than EV suspension cells after heat treatment of 45°C for 90 min and recovery 7 d at 25°C (Fig. 7f). As shown in Fig. 7g, the fresh weight of controls and OE-MBF1c suspension cells declined significantly after heat treatment and recovery, but the fresh weight of OE-MBF1c suspension cells was higher than EV cells. Secondly, we generated MBF1c mutant cells (mbf1c) of grape suspension cells in which MBF1c was interrupted by CRISPR-Cas9 gene editing. sgRNA is shown in Fig. 8a.
Figure 8.
. Knockout of MBF1c decreased heat tolerance in grape suspension cells. a Diagram illustration of MBF1c target sites design using CRISPR/Cas9 technology. The sequences of sgRNA are shown in red, PAM are in black. b Identification of MBF1c knockout mutant (mbf1c). The total of 18 colonies for each target were analysed. Red represents the number of mutated sequences. Sequencing results on both sides of the target site are shown. The wild type (WT) or mutant (mut) lines are shown on the left. Mutant types are shown on the right. c Mutations of amino acids in corresponding mutant sequencing in b. d Phenotypes of grape suspension cells of EV and mbf1c mutant after heat treatments (45°C for 85 min) and 25°C for 7 d. The grape suspension cells grown under 25°C were as control. Bar: 1.5 cm. e Fresh weight of transgenic grape suspension cells of EV and mbf1c mutant after 45°C for 85 min and 25°C for 7 d. Student’s t-test was used to determine significant difference (**P < 0.01). ns: not significant.
The mutant MBF1c (mbf1c) of grape suspension cells were detected by sequencing analysis. As shown in Fig. 8b, we took 18 colonies in which 10 colonies were edited and resulted in six type mutations. The mutated amino acid sequences were shown in Fig. 8c, respectively. Sequencing analysis revealed that MBF1c was successfully edited (Fig. 8b and c). As expected, the appearance of mbf1c grape suspension cells was similar with EV cell when grown at 25°C (Fig. 8d). However, more mbf1c suspension cells became black than EV cells after heat treatment of 45°C for 85 min and recovery 7 d at 25°C (Fig. 8d). At the same time, the fresh weight of controls and mbf1c cells declined significantly, but the fresh weight of mbf1c cells was lower than EV cells (Fig. 8e). Taken together, the results suggests that MBF1c plays an important role in thermotolerance of grape.
Discussion
In many model plants or common crops, such as Arabidopsis, rice, maize, wheat, tomato, soybean, HSFs play an important role in plant thermotolerance [15, 17, 47–50], acting as the core regulatory factors for HSR [4, 8, 9]. HSFA2 in particular activates the expression of HS-regulated genes and is important for thermotolerance acquisition and the heat stress memory [16, 51]. In this context, grapevine responses to heat stress are particularly important but still far from being fully understood [52, 53]. A similar pivotal role was proposed for HSFA2 in grapevine [5, 10, 19] but its precise function remains to be determined. Most accessions of V. vinifera, among which are the main consumed grape, have relatively weaker heat tolerance than wild V. daviddi and V. quinquangularis originated from the heat regions of southern China [41]. Consequently, the identification of HSFA2 sequence variation between these grape accessions would be of particular interest to help future breeding strategies aiming at producing grapevine with high quality and yield in warmer climates. To the best of our knowledge, there is only a report that a variation in the intron of HSFA2 attributes to enhanced thermotolerance in tomato [9], and no reports linking different HSFA2 variation to grapevine or the other plants.
In our previous studies, the expression of HSFA2 in V. vinifera leaves and fruits gradually increased with elevating temperature [5, 19]. Therefore, we firstly investigated HSFA2 expression change in V. vinifera ‘Jingxiu’ and Vitis daviddi ‘Tangwei’ grapevines exposed to high temperatures. As shown in Fig. 1a, the transcription levels of HSFA2 were higher in leaves of ‘Tangwei’ than ‘Jingxiu’ after similar heat stress. Accordingly, we also observed that the promoter activity of VdHSFA2 from ‘Tangwei’ was higher than VvHSFA2 from ‘Jingxiu’ under 25°C or 37°C (Fig. 1b, c and d). This result may be related to the stronger thermotolerance of V. daviddi when compared to V. vinifera. However, importantly, we want to know if natural variations of HSFA2 coding region confer thermotolerance in grapevine. The present work indicated that VvHSFA2 and VdHSFA2 are both located into the nucleus, while VdHSFA2 has higher transcriptional activity than VvHSFA2 (Fig. 2). Interestingly, VvHSFA2 and VdHSFA2 have 11 single nucleotide polymorphisms in the coding region (Fig. S3, see online supplementary material) leading to differences for seven amino acid residues distributed in DBD, HR-A/B, and AHA domains (Fig. 2a). Among these, although three amino acid residues difference between VvHSFA2 and VdHSFA2 were located at their AHA1 and adjacent domain (Fig. 2a), only when Thr315 in the AHA1 domain for VdHSFA2 was change into Ile315, VdHSFA2 transcriptional activity was decreased (Fig. 2f and g). The results indicate that Thr315 may be essential functional amino acid for the higher transcriptional ability of HSFA2. For instance, Arabidopsis HSFA2 activity was regulated through its Thr249 phosphorylation upon HS [54]. As mentioned above, our knowledge about the relationship between natural variations in key genes and plant thermotolerance efficiency is still fragmentary, particularly in horticulture crops. Recently, Li et al. found that an amino acid change between African rice and Asian rice leads to proteasome activity difference of thermo-tolerance 1 (TT1) [55]. It was shown that natural variation of SLG1 (cytosolic tRNA 2-thiolation protein 2) enhances higher heat tolerance in Oryza sativa subsp. indica compared to japonica [56]. There was also a report about natural variation about HSFA2 in tomato, but this research showed the SNP in HSFA2 intron brought about the different HSFA2 transcription splicing efficiency causing changes in thermotolerance, rather than SNPs of HSFA2 promoter or coding sequences [9]. To gain insights into whether the variation of HSFA2 is associated with grape thermotolerance, we evaluated the heat tolerance of various Vitis. Thirty-eight V. vinifera accessions bearing the same HSFA2 coding region (VvHSFA2) were described as heat sensitive, while two V. davidii accessions and one V. quinquangularis accession with the same HSFA2 coding region (VdHSFA2) were better tolerant to heat (Table 1; Table S1, see online supplementary material). In addition, some Vitis amurensis was also reported as relative thermotolerant [42]. However, we found that VaHSFA2 from V. amurensis possesses Ile315 (data not shown), which is the same as VvHSFA2. V. daviddi and V. quinquangularis are wild species distributed in the south of China. However, V. amurensis distributes in the northeast of China. Therefore, based on their different geographical distribution, we speculate that their thermotolerance mechanism may also be different; the thermotolerance of V. amurensis may not be determined by HSFA2 but by other factors. For example, a few reports demonstrated that DREB/CBF-type transcription factors function in cold stress-response as well as heat and drought response in plants [57, 58]. Therefore, as a cold tolerant grape, V. amurensis maybe acquire higher thermotolerance by DREB/CBF-type transcription factors.
Table 1.
Association of SNPs in HSFA2 with heat tolerance in grapevines.
| Species | SNP1 +100 |
SNP2 +229 |
SNP3 +527 |
SNP4 +819 |
SNP5 +944 |
SNP6 +986 |
SNP7 +1007 |
No. of accessions | Average Fv/Fm |
|---|---|---|---|---|---|---|---|---|---|
| Vitis vinifera | T | C | G | G | T | A | G | 38 | 0.26 |
| Vitis davidii, Vitis quinquangularis | A | T | T | C | C | G | C | 3 | 0.60** |
Note: SNP1–7 indicates the variation of HSFA2 CDS, and their positions relative to ATG are shown. SNP5 located in AHA1 is highlighted. Accessions list of V. vinifera, V. davidii and V. quinquangularis are shown in Table S1 (see online supplementary material). Student’s t-test was used to determine significant differences (**P < 0.01). Fv/Fm indicates the thermotolerance of Vitis.
So far, no genetic evidence was reported about the effect of HSFA2 on grape thermotolerance. Our work revealed that overexpression and mutation of HSFA2 increased or decreased thermotolerance of grape suspension cells, respectively (Figs 3 and 4). Although VdHSFA2 showed lower expression level than VvHSFA2 in the corresponding overexpression lines of grape suspension cells, OE-VdHSFA2 cells still presented higher thermotolerance capacity than OE-VvHSFA2 cells (Fig. 3d). These results should be the consequence of a higher transcriptional activity of VdHSFA2 when compared to VvHSFA2. To discard the influence of different overexpression levels of VdHSFA2 and VvHSFA2 on target genes, we generated tobacco leaves with the same expression of VdHSFA2 and VvHSFA2, and found that expression levels of HSP22.0 (one HSFA2 target gene) in OE-VdHSFA2 cells were higher than OE-VvHSFA2 lines (Fig. 3e and f). These results confirmed that transcriptional activity of VdHSFA2 is higher than VvHSFA2.
Because VdHSFA2 was more efficient than VvHSFA2 in improving grape heat tolerance (Fig. 3), we looked for potential difference in targeted genes between VvHSFA2 and VdHSFA2. In recent years, integration of ChIP-Seq and RNA-Seq has been an effective strategy for exploring targeted genes of transcriptional factor. Li et al. reported transcriptional regulatory framework of Opaque2 in Zea mays by integration of ChIP-Seq and RNA-Seq [59]. Zhang et al. (2020) combined ChIP-Seq and RNA-Seq to point out IbBBX24 binding to IbJAZ10 and IbMYC2 [60]. Here, we reported that VdHSFA2 can directly regulated 116 genes while VvHSFA2 can directly regulated only 36 genes after data mining of our ChIP-Seq and RNA-Seq experiments (Fig. 5d and e). Among these target genes, we found various HSPs and GOLSs already reported to be transactivated by HSFA2 in both Arabidopsis and grape [17, 19]. Interestingly, we noticed that more heat stress related genes, mostly from the HSP family were regulated by VdHSFA2 than VvHSFA2 (Tables S11, S12, S13, and S14, see online supplementary material).
Rienth et al. reported that a very strong correlation remained in the expression of HSFA2 and MBF1c in V. vinifera berries, following 2 hours of a rather moderate thermal stress (37°C) at the whole plant level, at different developmental stages and times during a nychthemeral cycle [33]. The result indicated that there may be a regulation relationship between HSFA2 and MBF1c in grape. Based on our results of ChIP-Seq and RNA-Seq, MBF1c was found to be a putative target gene of HSFA2 (Fig. 5f; Tables S11 and S12, see online supplementary material). Combining yeast one hybrid, ChIP-qPCR and luciferase (LUC) reporter assay, our results showed that HSFA2 can bind to the MBF1c promoter and transactivate its expression (Fig. 6). However, MBF1c can’t bind to the HSFA2 promoter (Fig. S10, see online supplementary material). Previous study described MBF1c as a bridge factor [20]; however, our results indicated that MBF1c can’t interact physically with HSFA2 (Fig. S11, see online supplementary material). Although the roles of MBF1c on heat, drought and salt tolerance were reported in some species, such as Arabidopsis, tomato, tobacco, potato, pepper, and wheat [24, 29, 61–63], the function of MBF1c in grape has not been studied yet. MBF1c transcripts accumulated in grape leaves exposed to heat stress (Fig. 7a). In our research, overexpression and mutation of MBF1c in grape suspension cells resulted in higher and weaker thermotolerance, respectively (Figs 7 and 8). These data indicate that MBF1c induces grape thermotolerance.
In conclusion, our work revealed that grape thermotolerance was modulated by HSFA2 variations, with the VdHSFA2 conferring higher heat tolerance than the VvHSFA2. The transcriptional activation activities of VdHSFA2 are higher than the ones from VvHSFA2, and VdHSFA2 can regulate more target genes than VvHSFA2. Interestingly, we found that the variation of single amino acid (Thr315Ile) only in the AHA1 motif results in difference of transcriptional activities between VdHSFA2 and VvHSFA2. This variation discovery could be of potential significance for understanding plant thermotolerance and for supporting future molecular breeding of thermotolerant grapevine. Finally, MBF1c is a target gene of HSFA2 and plays a relevant role in the grape response to heat stress.
Materials and methods
Plant materials and heat treatments
One-year-old ‘Jingxiu’ (V. vinifera) and ‘Tangwei’ (V. davidii) grapevines were used in this study. The grapevine growth condition and heat treatments were carried out as described by Jiang et al. [5]. The grapevines were grown in greenhouse with the relative humidity 70%–80%, temperature 25°C/18°C day/night, and light 800 μmol m−2 s−1. When the sixth leaves (counting from up to down) were mature, grapevines were divided into three groups, and each group had three seedlings at least. Grapevines of each group were treated at 25°C, 40°C, and 45°C for 2 h (all conditions were the same as above their growth conditions except for temperature), respectively. A total of 41 grape accessions were used in this study (Table S1, see online supplementary material). The grape accessions were grown at the Germplasm Repository for Grapevines in the Institute of Botany of the Chinese Academy of Sciences, located in Beijing. Healthy 30-day leaves were used to evaluate the thermotolerance. The evaluation of these germplasms was conducted in May of 2019 according to Xu et al. [41]. Tissue culture grape plants ‘Jingxiu’ (V. vinifera) and ‘Summer Black’ (V. vinifera × Vitis labrusca) were used for transiently overexpression of VdHSFA2 and VvHSFA2, among which ‘Summer Black’ was heat sensitive and had the same HSFA2 sequence as ‘Jingxiu’ (V. vinifera). One-year-old V. quinquangularis plants were used for transiently RNA interference of VdHSFA2. A. thaliana ecotype Columbia-0 (Col-0) and Nicotiana benthamiana were grown in a growth chamber with their suitable growing conditions.
A. thaliana ecotype Columbia-0 (Col-0) and N. benthamiana were grown in the greenhouse with conditions of a 16 h/8 h, light/dark cycle, and the relative humidity and temperature was 70–75%, 23°C, respectively. These materials were used for the research of HSFA2 or MBF1c function.
VvHSFA2 and VdHSFA2 gene isolation and analysis
Total RNA was extracted from mature grape leaves using RNAprep Pure PlantKit (Tiangen, Beijing, China). cDNA syntheses were performed using total RNA and the HiScript® III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). Based on the gene sequence of Pinot Noir HSFA2 acquired from the grape Genome Browser (https://phytozome.jgi. doe.gov/pz/portal.html), the primer pair for HSFA2 was designed using DNAMAN. VvHSFA2 and VdHSFA2 were cloned from cDNA by PCR. The HSFA2 sequences were aligned using DNAMAN. The domain was analysed according to Arabidopsis and O. sativa HSFs family. The primers used in the present study are listed in Table S2 (see online supplementary material).
Gene expression analysis
AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China) was used to conduct qRT-PCR. VvActin was used as an internal control. The qPCR condition was carried out according to the manufacturer’s instructions. The gene relative expression level was calculated using the 2-ΔΔCT method.
Analysis of HSFA2 promoter activity
The isolated 2 kb fragments of the VvHSFA2 and VdHSFA2 promoters were fused to a pGreenII-0800-LUC vector, respectively. CaMV35S::Ren was used as an internal control. The constructed vector was transformed into Arabidopsis protoplast. The transformed protoplasts were put at 25°C for 16 h before harvest. In addition, the constructed vector was transformed into Agrobacterium GV3101 (pSoup) for transformation of N. benthamiana leaves. After 48 h, some transformed lines were treated at 37°C for 1 h or 2 h, and some allowed to remain in the growth chamber (25°C). The activity of firefly luciferase (LUC) and Renilla luciferase (REN) were measured by Dual-Luciferase Reporter Assay System kit (Promega, Madison, USA). The Tanon 5200 Multi luminometer (China) was used to detect the fluorescence signal.
Subcellular localization
The vector 2300-GFP was used to study the subcellular localization of VvHSFA2 and VdHSFA2. The coding sequence (CDS) of VvHSFA2 was fused with 2300-GFP vector and signed as VvHSFA2-eGFP. The VdHSFA2-eGFP was constructed in the same way. The empty GFP vector (EV) was used as control. VvHSFA2-eGFP, VdHSFA2-eGFP, and EV were transformed into Agrobacterium EHA105, respectively. N. benthamiana leaves were injected with transformed Agrobacterium for transient transformation. The Agrobacterium transformed H2B-mCherry was used as nuclei marker. Leica TCS SP5 Confocal Scanning Microscope was used to detect the transformed N. benthamiana fluorescence signal after 48–72 h incubation period.
Transcriptional activity assay
Transcriptional activity assay was firstly conducted in yeast. The CDS of VvHSFA2 and VdHSFA2 were fused to the GAL4 BD vector, and the constructed vector was named as BD-VvHSFA2, BD-VdHSFA2, respectively. The constructed plasmids were transformed to yeast strain Y2HGold. Positive and negative controls were the yeast clones containing combinations of AD-T with BD-p53 and BD-Lam, respectively. Transcriptional activation activity was evaluated by spot assay and alpha-galactosidase activity.
Transcriptional activity assay was also conducted in Arabidopsis protoplasts by dual luciferase reporter assay. The CDS of VvHSFA2, VdHSFA2, VdHSFA2 mutation, and MBF1c were cloned into CTB7-GAL4BD vector as the effector, signed as GalBD-VvHSFA2, GalBD-VdHSFA2, GalBD-VdHSFA2mut1/2/3, and GalBD-MBF1c, respectively. The empty vector was used as negative control. LUC was used as reporter to detect the transcriptional activation of VvHSFA2, VdHSFA2, VdHSFA2 mutation, and MBF1c. Ren was the internal reference. The reporter and effector plasmids were co-transformed into Arabidopsis protoplasts by PEG-mediated transformation. The transformed Arabidopsis protoplasts were cultured at 25°C in the dark overnight. Dual-luciferase activity was measured by the Dual-Luciferase Reporter Assay System kit (Promega, Madison, USA).
Stable transformation of VvHSFA2 and VdHSFA2 in grape and heat treatments
Grape transformations were made in ‘41B’ (V. vinifera × Vitis berlandieri) grape suspension cells as previous described [64]. Overexpressed VvHSFA2 (OE-VvHSFA2) and VdHSFA2 (OE-VdHSFA2) vectors were constructed, respectively. Empty vector (EV) was as control. CRISPR/Cas9-based gene editing in grape was conducted according to the method of Ma and Liu [65]. The verified sequence was fused into sgRNA according to the online tool CRISPR-GE (Genome Editing)-Liu YG Lab (scau.edu.cn) [65]. sgRNA intermediate vectors with Arabidopsis U3b promoter (PYLsgRNA-AtU3b) was used in this study. HSFA2 and MBF1c target were imported into PYLsgRNA-AtU3b, respectively. The designed sgRNAs were ligated with pYLCRISPR/Cas9P35S-N by homologous recombination. The constructed vectors were transformed into Agrobacterium EHA105, then the transformed Agrobacterium were co-cultured with grape suspension cells. The infected grape suspension cells were cultured in liquid GM medium supplemented with antibiotic. The successfully transformed overexpression lines were identified by qRT-PCR and fluorescence detection. The successfully transformed mutation lines were identified by sequencing.
Grape suspension cells were grown at 25°C for six days, then they were treated as the following treatments: (i) the samples of control group remained at the optimal conditions (120 rpm, 25°C); and (ii) the samples of the heat treatment groups were transferred into shaking incubator (120 rpm, 45°C). When the heat treatments were finished, grape suspension cells were recovered at 25°C for seven days. The heat treatments time was identified based on the survival ability (Fresh weight) at 7 d after recovery (120 rpm, 25°C) from different heat treatments, the data is shown in Fig. S5 (see online supplementary material). Grape suspension cells were taken out from medium, and dried with blotting paper. Then these cells were weighted by electronic scales (Mettler Toledo, Shanghai, China).
Transient transformation of VvHSFA2 and VdHSFA2 in grapevine and heat treatments
Agrobacterium EHA105 that transformed OE-VvHSFA2 or OE-VdHSFA2 vectors were used for overexpression experiments, respectively. Agrobacterium EHA105 transformed empty vector was used as control. The HSFA2-specific sequence and its specific reverse transcription sequence were cloned into pFGC5941 through homologous recombination to obtain a functionally deficient HSFA2 construct, generating small interfering RNA (siRNA). The constructed vectors were transformed into Agrobacterium EHA105.
Six-week-old tissue culture grape plantlets of ‘Jingxiu’ and ‘Summer Black’ were infiltrated by Agrobacterium EHA105 with the above overexpressing construct. After infiltration, the plantlets were transferred to tissue culture medium for 4 days. Then, the plants were treated by high temperature: (i) the control samples remained at the optimal conditions (25°C); and (ii) the samples of the heat treatment groups were treated at 45°C 3 h for ‘Jingxiu’ and 45°C 2 h for ‘Summer Black’. Four weeks after one-year-old tissue culture plants of V. quinquangularis germinated, these plants were infiltrated by Agrobacterium EHA105 with the above silencing construct. Then, the infiltrated plants were transferred to soil. After 4 days, the infiltrated plants were treated by high temperature: (i) the control plants remained at the optimal conditions (25°C); and (ii) the heat treatment group plants were treated for 150 min at 40°C. The relative electrolyte leakage was measured as follows: 10 leaves discs (0.5 cm in diameter) were incubated in 5 ml of distilled water, then conductivity before and after being boiled was measured using industrial conductivity [66].
ChIP-Seq analysis
Overexpressed VvHSFA2, VdHSFA2 and empty lines in grape suspension cells were sampled for the ChIP-Seq analysis. Every sample with three biological replicates. The ChIP assay was conducted according Landt et al. [67], with some modifications. In brief, 3–5 g samples were used to cross-link DNA and protein with 1% (v/v) formaldehyde, and these samples were ground into powder in liquid nitrogen. Purified DNA-protein complexes were sonicated and incubated with anti-GFP antibody (Abeam, ab290). The precipitated DNA was purified, dissolved in distilled water, and stored at −80°C to construct an Illumina sequencing library. ChIP-Seq was conducted by Igenebook (Wuhan, China). The reads from ChIP-Seq were aligned with the genome of V. vinifera from the grapevine database (http://genomes.cribi.unipd.it/DATA/V2/V2/). The mapped reads were applied to MACS software (version: 2.1.1.20160309) to identify regions potentially bound by VvHSFA2 and VdHSFA2. The DNA motif within peak sequences was identified with Homer software (version 3).
RNA-Seq analysis
For RNA-Seq, overexpressed VvHSFA2, VdHSFA2, and empty lines in grape suspension cells were sampled. RNAprep Pure PlantKit (Tiangen, Beijing, China) was used to extract total RNA. RNA quality was checked with the NanoDrop2000, agarose gel electrophoresis and Agilent 2100. According to the Illumina standard instructions, RNA-Seq libraries were prepared. RNA-Seq was implemented on the Illumina HiSeq 6000 platform by Shanghai Majorbio. DEGs were defined as those with P-value (P ≤ 0.05) and the fold change (|log2FC| ≥ 1).
ChIP-qPCR
The prepared DNA in ChIP was used for qPCR. The fold changes were calculated based on the relative enrichment in anti-GFP compared with anti-IgG immunoprecipitate. The expression levels were normalized to the input sample. Each sample was evaluated based on three replicates.
Yeast one-hybrid assay
Yeast one-hybrid assay was conducted as previously described Lin et al. [68]. The CDS of VvHSFA2 and VdHSFA2 were constructed into GAD vector (contain GAL4 transcriptional activation domain), respectively. The successfully constructed vector were signed as GAD-VvHSFA2 and GAD-VdHSFA2, respectively. The MBF1c promoter fragment contained HSE motif and was constructed into pLacZ vector, signed as proMBF1c::LacZ. Plasmids for AD fusions were each co-transformed with LacZ reporter constructs into yeast strain EGY48. Transformed yeast was grown on SD/−Trp/-Ura dropout plates containing X-gal for blue color reaction.
Dual-luciferase assay
To explore the effect of HSFA2 on MBF1c transcriptional regulation, we performed LUC reporter assays in N. benthamiana leaves. The MBF1c core promoter containing the HSFA2 binding motif was constructed into pGreen0800-LUC to generate the reporter vector, proMBF1c::LUC. The 35S::VvHSFA2 and 35S::VdHSFA2 vectors were used as effectors, and the 35S empty vector was used as a control. The constructed plasmids were transformed into Agrobacterium EHA105, and N. benthamiana leaves were co-infected by Agrobacterium infiltration as Wang et al. [64] described. LUC activity was detected using Dual-Luciferase Reporter Assay System kit (Promega, Madison, USA). The analysis was performed in at least three biological replicates.
Yeast two-hybrid assays
Yeast two-hybrid (Y2H) was performed using the Matchmaker Gold yeast two-hybrid system (Clontech, Palo Alto, USA). The CDS of HSFA2 and MBF1c were constructed into pGADT7 and pGBKT7 vectors, respectively. The yeast strain Y2HGold was co-transformed with different combinations of pGADT7 and pGBKT7 recombinant vectors and cultured on SD/−Leu/−Trp selection medium. Positive colonies were plated on SD/−Leu/−Trp/-His/−Ade selection medium, providing 40 mg L of 1x-α-Gal. The positive control was AD-T combined with BD-p53, and the negative control was AD-T combined BD-Lam.
Supplementary Material
Contributor Information
Xinna Liu, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Haiyang Chen, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Shenchang Li, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China; University of Chinese Academy of Sciences, Beijing 100049, China.
David Lecourieux, EGFV, Bordeaux Sciences Agro, INRAE, ISVV, Bordeaux University, Villenave d'Ornon F-33882, France.
Wei Duan, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China.
Peige Fan, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China.
Zhenchang Liang, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China.
Lijun Wang, Beijing Key Laboratory of Grape Science and Enology and Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; China National Botanical Garden, Beijing 100093, China.
Acknowledgement
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFD1000300), the National Natural Science Foundation of China (Grant No. 31872065 and U21A20227), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA23080602). We would like to thank Professors Xianjun Song and Jingbo Jin from the Institute of Botany, Chinese Academy of Sciences. This research was conducted as part of the LIA INNOGRAPE International Associated Laboratory.
Author contributions
X.L. performed the work and initiated the draft. H.C. measured heat tolerance of grapevine accessions. S.L. helped to clone promoters. D.L. critically reviewed the manuscript. W.D., P.F., and Z.L. helped to design the experiment. L.W. obtained funding, designed the experiment and revised the final version.
Data availability
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.
Conflict of interests
The authors declare no conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
References
- 1. Gong Z, Xiong L, Shi Het al. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63:635–74. [DOI] [PubMed] [Google Scholar]
- 2. Ohama N, Sato H, Shinozaki Ket al. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22:53–65. [DOI] [PubMed] [Google Scholar]
- 3. Katano K, Honda K, Suzuki N. Integration between ROS regulatory systems and other signals in the regulation of various types of heat responses in plants. Int J Mol Sci. 2018;19:3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scharf KD, Berberich T, Ebersberger Iet al. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta. 2012;1819:104–19. [DOI] [PubMed] [Google Scholar]
- 5. Jiang J, Liu X, Liu Cet al. Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiol. 2017;173:1502–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li B, Gao K, Ren Het al. Molecular mechanisms governing plant responses to high temperatures. J Integr Plant Biol. 2018;60:757–79. [DOI] [PubMed] [Google Scholar]
- 7. Liu HC, Liao HT, Charng YY. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34:738–51. [DOI] [PubMed] [Google Scholar]
- 8. Wu Z, Liang J, Wang Cet al. Alternative splicing provides a mechanism to regulate LlHSFA3 function in response to heat stress in lily. Plant Physiol. 2019;181:1651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu Y, Mesihovic A, Jiménez-Gómez JMet al. Natural variation in HsfA2 pre-mRNA splicing is associated with changes in thermotolerance during tomato domestication. New Phytol. 2020;225:1297–310. [DOI] [PubMed] [Google Scholar]
- 10. Liu GT, Chai F, Wang Yet al. Genome-wide identification and classification of HSF family in grape, and their transcriptional analysis under heat acclimation and heat stress. Hortic Plant J. 2018;4:133–43. [Google Scholar]
- 11. Xue GP, Sadat S, Drenth Jet al. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J Exp Bot. 2014;65:539–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C, Zhang Q, Shou HX. Identification and expression analysis of OsHsfs in rice. J Zhejiang Univ Sci B. 2009;10:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zang D, Wang J, Zhang Xet al. Arabidopsis heat shock transcription factor HSFA7b positively mediates salt stress tolerance by binding to an E-box-like motif to regulate gene expression. J Exp Bot. 2019;70:5355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nover L, Bharti K, Doring Pet al. Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones 2001;6:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fragkostefanakis S, Mesihovic A, Simm Set al. HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 2016;170:2461–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charng YY, Liu HC, Liu NYet al. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007;143:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogawa D, Yamaguchi K, Nishiuchi T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J Exp Bot. 2007;58:3373–83. [DOI] [PubMed] [Google Scholar]
- 18. Gu L, Jiang T, Zhang Cet al. Maize HSFA2 and HSBP2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J. 2019;100:128–42. [DOI] [PubMed] [Google Scholar]
- 19. Pillet J, Egert A, Pieri Pet al. VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries. Plant Cell Physiol. 2012;53:1776–92. [DOI] [PubMed] [Google Scholar]
- 20. Jaimes-Miranda F, Chávez Montes RA. The plant MBF1 protein family: a bridge between stress and transcription. J Exp Bot. 2020;71:1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li FQ, Ueda H, Hirose S. Mediators of activation of fushi tarazu gene transcription by BmFTZ-F1. Mol Cell Biol. 1994;14:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takemaru K, Harashima S, Ueda Het al. Yeast coactivator MBF1 mediates GCN4-dependent transcriptional activation. Mol Cell Biol. 1998;18:4971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsuda K, Tsuji T, Hirose Set al. Three Arabidopsis MBF1 homologs with distinct expression profiles play roles as transcriptional co-activators. Plant Cell Physiol. 2004;45:225–31. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki N, Rizhsky L, Liang Het al. Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiol. 2005;139:1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki N, Bajad S, Shuman Jet al. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem. 2008;283:9269–75. [DOI] [PubMed] [Google Scholar]
- 26. Arce DP, Godoy AV, Tsuda Ket al. The analysis of an Arabidopsis triple knock-down mutant reveals functions for MBF1 genes under oxidative stress conditions. J Plant Physiol. 2010;167:194–200. [DOI] [PubMed] [Google Scholar]
- 27. Zandalinas SI, Balfagón D, Arbona Vet al. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot. 2016;67:5381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zegzouti H, Jones B, Frasse Pet al. Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 1999;18:589–600. [DOI] [PubMed] [Google Scholar]
- 29. Qin D, Wang F, Geng Xet al. Overexpression of heat stress-responsive TaMBF1c, a wheat (Triticum aestivum L.) multiprotein bridging factor, confers heat tolerance in both yeast and rice. Plant Mol Biol. 2015;87:31–45. [DOI] [PubMed] [Google Scholar]
- 30. Zou L, Yu B, Ma XLet al. Cloning and expression analysis of the BocMBF1c gene involved in heat tolerance in Chinese kale. Int J Mol Sci. 2019;20:5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding L, Wu Z, Teng Ret al. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic Res. 2021;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan Q, Hou H, Singer SDet al. The grape VvMBF1 gene improves drought stress tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss Org. 2014;118:571–82. [Google Scholar]
- 33. Rienth M, Torregrosa L, Luchaire Net al. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (vitis vinifera) fruit. BMC Plant Biol. 2014;14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rienth M, Torregrosa L, Sarah Get al. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol 2016;16:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lecourieux F, Lecourieux D, Vignault Cet al. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in grapevine cells. Plant Physiol. 2010;152:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Movahed N, Pastore C, Cellini Aet al. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J Plant Res. 2016;129:513–26. [DOI] [PubMed] [Google Scholar]
- 37. Tu M, Wang X, Zhu Yet al. VlbZIP30 of grapevine functions in dehydration tolerance via the abscisic acid core signaling pathway. Hortic Res. 2018;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang J, Xi H, Dai Zet al. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J Exp Bot. 2019;70:715–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun X, Zhang L, Wong DCJet al. The ethylene response factor VaERF092 from Amur grape regulates the transcription factor VaWRKY33, improving cold tolerance. Plant J. 2019;99:988–1002. [DOI] [PubMed] [Google Scholar]
- 40. Lecourieux F, Kappel C, Pieri Pet al. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing cabernet sauvignon grape berries. Front Plant Sci. 2017;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu H, Liu G, Liu Get al. Comparison of investigation methods of heat injury in grapevine (Vitis) and assessment to heat tolerance in different cultivars and species. BMC Plant Biol. 2014;14:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Jiang J, Fan Xet al. Identification of heat tolerance in Chinese wildgrape germplasm resources. Horticulturae. 2020;6:68. [Google Scholar]
- 43. George IS, Pascovici D, Mirzaei Met al. Quantitative proteomic analysis of cabernet sauvignon grape cells exposed to thermal stresses reveals alterations in sugar and phenylpropanoid metabolism. Proteomics. 2015;15:3048–60. [DOI] [PubMed] [Google Scholar]
- 44. Nishizawa A, Yabuta Y, Yoshida Eet al. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006;48:535–47. [DOI] [PubMed] [Google Scholar]
- 45. Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yokotani N, Ichikawa T, Kondou Yet al. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta. 2008;227:957–67. [DOI] [PubMed] [Google Scholar]
- 48. Jiang Y, Zheng Q, Chen Let al. Ectopic overexpression of maize heat shock transcription factor gene ZmHsf04 confers increased thermo and salt-stress tolerance in transgenic Arabidopsis. Acta Physiol Plant. 2018;40:9. [Google Scholar]
- 49. Ding X, Guo Q, Li Qet al. Comparative transcriptomics analysis and functional study reveal important role of high-temperature stress response gene GmHSFA2 during flower bud development of CMS-based F1 in soybean. Front Plant Sci. 2020;11:600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Z, Li G, Zhang Het al. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: characterization and functional roles. J Plant Physiol. 2020;246–247:153135. [DOI] [PubMed] [Google Scholar]
- 51. Friedrich T, Oberkofler V, Trindade Iet al. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat Commun. 2021;12:3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Venios X, Korkas E, Nisiotou Aet al. Grapevine responses to heat stress and global warming. Plants (Basel). 2020;9:1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gomes E, Maillot P, Duchene E. Molecular tools for adapting viticulture to climate change. Front Plant Sci. 2021;12:633846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Evrard A, Kumar M, Lecourieux Det al. Regulation of the heat stress response in Arabidopsis by MPK6-targeted phosphorylation of the heat stress factor HsfA2. PeerJ. 2013;1:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li XM, Chao DY, Wu Yet al. Natural alleles of a proteasome alpha2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet. 2015;47:827–33. [DOI] [PubMed] [Google Scholar]
- 56. Xu Y, Zhang L, Ou Set al. Natural variations of SLG1 confer high-temperature tolerance in indica rice. Nat Commun. 2020;11:5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kidokoro S, Watanabe K, Ohori Tet al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015;81:505–18. [DOI] [PubMed] [Google Scholar]
- 58. Yun SD, Kim MH, Oh SAet al. Overexpression of C-repeat binding factor1 (CBF1) gene enhances heat stress tolerance in Arabidopsis. J Plant Biol. 2022;65:253–60. [Google Scholar]
- 59. Li C, Qiao Z, Qi Wet al. Genome-wide characterization of cis-acting DNA targets reveals the transcriptional regulatory framework of opaque2 in maize. Plant Cell. 2015;27:532–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang H, Zhang Q, Zhai Het al. IbBBX24 promotes the jasmonic acid pathway and enhances fusarium wilt resistance in sweet potato. Plant Cell. 2020;32:1102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matsushita Y, Miyakawa O, Deguchi Met al. Cloning of a tobacco cDNA coding for a putative transcriptional coactivator MBF1 that interacts with the tomato mosaic virus movement protein. J Exp Bot. 2002;53:1531–2. [PubMed] [Google Scholar]
- 62. Arce DP, Tonón C, Zanetti MEet al. The potato transcriptional co-activator StMBF1 is up-regulated in response to oxidative stress and interacts with the TATA-box binding protein. J Biochem Mol Biol. 2006;39:355–60. [DOI] [PubMed] [Google Scholar]
- 63. Guo WL, Chen RG, Du XHet al. Reduced tolerance to abiotic stress in transgenic Arabidopsis overexpressing a Capsicum annuum multiprotein bridging factor 1. BMC Plant Biol. 2014;14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Z, Wong DCJ, Wang Yet al. GRAS-domain transcription factor PAT1 regulates jasmonic acid biosynthesis in grape cold stress response. Plant Physiol. 2021;186:1660–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma X, Liu YG. CRISPR/Cas9-based multiplex genome editing in monocot and dicot plants. Curr Protoc Mol Biol. 2016;115:31.6.1–21. [DOI] [PubMed] [Google Scholar]
- 66. Xu M, Tong Q, Wang Yet al. Transcriptomic analysis of the grapevine LEA gene family in response to osmotic and cold stress reveals a key role for VamDHN3. Plant Cell Physiol. 2020;61:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Landt SG, Marinov GK, Kundaje Aet al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22:1813–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin R, Ding L, Casola Cet al. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 2007;318:1302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.