Abstract
We used Listeria monocytogenes, a gram-positive, facultative intracellular bacterium, to study the gut mucosal immune responses following oral infection. We employed a germfree (GF) mouse model to try to accentuate the development of a humoral mucosal immune response in the gut, and we used oral colonization with one of the mutants, actA-negative (ΔactA) L. monocytogenes, to restrict infection largely to the gut. The ΔactA mutant was able to colonize the intestinal mucosa of formerly GF mice for long periods of time without causing disease while eliciting secretory immunoglobulin A (IgA) responses, as evidenced by gut tissue fragment culture assays. Flow cytometric analyses and immunohistochemical methods showed the development of only minimal germinal center reactions (GCR) in Peyer's patches and more robust GCR in mesenteric lymph nodes. Pronounced increases in total (natural) IgA production occurred in gut tissues by day 7 and were maintained for up to 90 days. Levels of specific IgA were modest in gut tissues on day 14, increased until day 76, and stabilized at day 90. We also observed a significant rise in serum IgA and IgG1 levels following oral infection by listeriae. Upon colonization, the organisms mainly infected the intestines and intestinal lumen, and we only sporadically observed few colony-forming bacteria in the liver and spleen. We observed a marked rise in IgA-secreting cells, including listeria-specific IgA antibody-secreting cells, in the lamina propria of the small intestine by enzyme-linked immunospot assays. To ascertain whether some of the IgA was specific for listeriae, we performed Western blot analysis to test the reactivity of IgA from fragment cultures to antigens in sonicates of L. monocytogenes. We detected IgA binding to antigenic proteins with molecular masses of 96, 60, 40, and 14 kDa in the Listeria sonicates.
Listeria monocytogenes is a gram-positive, facultative, intracellular pathogen which is widely distributed in the environment in soil, water, vegetation, etc., and it can be prevalent in spoiled food, especially that derived from milk and meat (4, 8, 10, 11). It is both a human and an animal pathogen, and L. monocytogenes infection can lead to septicemia, possibly followed by meningitis and other related central nervous system disorders (1), especially in immunocompromised individuals (21, 22). A variety of experimental studies concerning pathogenesis and protective host responses have been carried out by parenteral infection with listeriae, and several excellent animal models have been established (2, 16, 20, 24, 37, 39). However, studies using oral infection are fewer (3, 19, 25, 29, 40, 50). Nevertheless, the most common route of listeria infection is through the gastrointestinal tract (23), as evidenced by the several outbreaks of listeriosis caused by ingestion of contaminated food materials (10, 11, 42). Some studies have shown that mice may be as susceptible to oral as to parenteral infection by listeriae (38), but it has been more commonly found that conventionally reared, immunocompetent mice are far more susceptible to systemic listeriosis and death if the inoculum is given parenterally rather than orally (50).
Most studies have indicated that serum antibody (Ab) responses to listeriae in systemically infected or parenterally injected animals are very limited and do not play a significant role in resolving the infection (9, 12, 15, 30). However, serum Ab responses to a variety of listerial antigens have been detected in convalescing humans, and Abs to the p60 protein of L. monocytogenes have been proposed to retrospectively diagnose systemic infection in humans (13, 14). We know of no substantial data concerning whether mammalian hosts express a gut humoral, mucosal immune response upon oral inoculation with listeriae. However, it seems possible that such a response might increase resistance to natural, oral infection, for instance, by blocking the uptake of listeriae by enterocytes via the internalin–E-cadherin pathway (35).
Since L. monocytogenes is a poor colonizer of the gut of conventional mice, we used germfree (GF) mice for most of our studies. It has been observed that oral infection with wild-type (WT) listeriae can easily be fatal to GF immunocompetent mice and to conventionally reared severe combined immunodeficient (SCID) mice (27, 50); therefore, we used an avirulent strain, the ΔactA mutant. The ActA protein of L. monocytogenes is involved in movement of bacteria in the cytoplasm after entrance into the host cell by contributing to the polymerization and linking of host F actin (48). The ΔactA mutant strain fails to display this function and thus exhibits neither intracellular movement nor cell-to-cell transmission. Previously, Harty and Bevan (17) have used this mutant as an intravenous vaccine for the protection of interferon gamma-negative mice against parenteral infection with WT listeriae. Our data show that oral colonization of formerly GF mice with the ΔactA mutant induces specific immunoglobulin A (IgA) Ab responses in gut-associated lymphoid tissues (GALT).
MATERIALS AND METHODS
Mice.
GF BALB/c mice were originally obtained from the University of Wisconsin, Madison. The mice were fed a sterile (autoclavable) mouse diet (PMI Feeds, Inc., St. Louis, Mo.). Separate GF isolators were dedicated for generation of pups, and the adult mice were maintained in a sterile environment within flexible film isolators in the gnotobiotic facility of the Biology Department, University of Pennsylvania, both before and after colonization with bacteria. Conventional mice were purchased from the Jackson Laboratory, Bar Harbor, Maine.
Bacteria and immunization.
L. monocytogenes strain 10403s (WT) and ΔactA mutant DP-L1942, which contains an in-frame deletion in actA (7), were gifts from D. Portnoy, University of Pennsylvania, and were grown as previously described (16). The 50% lethal dose of strain 10403s was 104 for BALB/c mice when introduced intravenously (5), and strain DP-L1942 was avirulent when introduced parenterally (17). Strains 10403s and DP-L1942 were grown in brain heart infusion (BHI; Difco) broth containing 50 μg of streptomycin per ml; aliquots were frozen and stored at −70°C. Freshly thawed bacterial stocks were grown at 37°C for 2 to 3 h in the presence of streptomycin, centrifuged, washed with phosphate-buffered saline (PBS), and diluted to the desired concentration. Mice were inoculated orally with a loop containing 0.2 ml of fluid/mouse and then housed in a formerly sterile isolator; noninoculated GF littermates were kept in a separate sterile isolator and used as controls. Typically, groups of three mice were used for each time point studied. For colonization, GF mice were orally inoculated with 5 × 108 CFU of ΔactA mutant DP-L1942.
Translocation of L. monocytogenes in the tissues of mice.
At different times following oral inoculation with L. monocytogenes, translocation of bacteria was detected as follows. Tissues were removed aseptically from the sacrificed mice and homogenized in sterile PBS. Each of the homogenates was serially diluted in sterile PBS, then plated on BHI plates containing streptomycin (50 μg/ml), and incubated at 37°C for 24 h; CFU were recorded. At random, colonies were picked for Gram staining and fluorescent Ab staining to confirm their identity.
Fragment culture of SI, PP, and MLN.
The general method for Peyer's patch (PP) organ culture was described previously (28). Briefly, PP were dissected with a sharp, sterile Beaver blade from the small intestines (SI) of mice and placed in ice-cold RPMI 1640 medium containing 10 mM HEPES, 0.01% gentamicin, and 10% fetal calf serum. The PP and mesenteric lymph nodes (MLN) were washed by five successive transfers through fresh medium, leaving tissues in each wash for 10 min before each transfer. In the case of SI, approximately 4- to 8-mm segments of duodenum, jejunum, and ileum were excised, opened longitudinally, and washed five times in Hanks' buffer containing 50 mM EDTA, then three times with Hanks' buffer alone, and once more with complete RPMI 1640. Two pieces of SI, one piece of PP, and one piece of MLN were placed separately in the wells of a sterile 24-well flat-bottom culture plate in 1.0 ml of conditioned Kennett's HY medium containing 10% fetal bovine serum, 1% l-glutamine, antibiotic-antimycotic solution (100 U penicillin/ml, 0.1 mg of streptomycin/ml), and 0.25 mg of amphotericin β (Fungizone; GIBCO, Grand Island, N.Y.) per ml and cultured for 7 days under 90% oxygen–10% CO2 at 37°C. The culture supernatants were then aspirated and frozen before use.
Estimation of Abs in serum.
Blood was collected at various times from the mice infected with the ΔactA mutant by cardiac puncture. Sera were separated and frozen at −20°C until used. Listeria-specific IgA and IgG1 were quantitated by radioimmunoassay (RIA).
RIA.
The RIA used in our laboratory has been described previously (26). Total IgA was determined using goat anti-mouse Fab (Southern Biotechnology Associates [SBA], Birmingham, Ala.)-coated RIA plates, and listeria-specific IgA was determined using plates coated with sonicates of the ΔactA mutant. The assays were linear for immunoglobulin concentrations of 0.5 to 10 ng/20 μl. Sonicates of listeriae were prepared in the following manner. An overnight culture from a single colony of the ΔactA mutant was prepared in 2.0 ml of BHI medium containing 50 μg of streptomycin per ml. This 2-ml culture was inoculated into 250 ml of BHI medium with streptomycin and grown to log phase at 37°C. Bacteria were harvested by centrifugation and washed twice with sterile cold PBS. The bacterial pellet was suspended in 16 ml of PBS, and 2-ml portions were each sonicated three times in a 5-ml glass tube for 20 s on ice, with 10-s cooling-off intervals. The sonicate was centrifuged to collect each supernatant, and the cell debris was subjected to sonication again. The combined supernatant obtained after sonication was filtered through a 0.45-μm-pore-size filter, and its protein concentration was estimated.
ELISPOT assay.
The enzyme-linked immunospot assay (ELISPOT) assay was performed as described elsewhere (6).
Fluorescence-activated cell sorting (FACS).
Cells harvested from experimental and control mice were incubated with an appropriate dilution of fluorochrome-coupled reagents in PBS containing 0.04% sodium azide (Sigma) for 30 min on ice. The cells were then washed three times prior to analysis on a FACS IV flow cytometer (Becton Dickinson, Sunnydale, Calif.). We used fluorescein isothiocyanate (FITC)-labeled peanut agglutinin (PNA) in conjunction with a phycoerythrin (PE)-conjugated anti-B-cell marker, goat PE anti-kappa (SBA), to stain germinal center (GC) B cells (41). We also used FITC-labeled goat anti-mouse IgA (SBA) together with PE anti-kappa.
Immunoperoxidase staining of frozen sections.
Immunohistochemistry was performed using 5-μm frozen tissue sections made after embedding in OCT compound (Miles, Inc., Elkhart, Ind.). Air-dried sections were rehydrated in PBS and then blocked with 1% bovine serum albumin in PBS for 1 h. Staining was carried out using biotinylated PNA or monoclonal Abs (MAbs) and developed with avidin-biotin-horseradish peroxidase (HRP) (ABC kit; Vector Laboratories, Burlingame, Calif.).
Western blot analysis.
An aliquot of the sonicated supernatant of the ΔactA mutant of L. monocytogenes was placed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (2% SDS, 5% 2-mercaptoethanol, 0.05% bromophenol blue, 0.062% Tris-HCl [pH 6.8]), and SDS-PAGE was performed in 7.5 and 12% separating gels with a 4% stacking gel. Proteins were electrophoretically transferred to an Immobilon-P transfer membrane (Millipore) overnight with 30-mA current. After 1 h of blocking with 10 mM Tris-buffered saline containing 1% dry milk, the membranes were incubated with fragment culture supernatant as the source for IgA Ab (1:50 dilution) for 6 h. After several washes with Tris-buffered saline containing 0.05% Tween, the membranes were incubated with alkaline phosphatase (AP)-linked goat anti-mouse IgA (1:2,500) for 1 h at room temperature. After several washes with 100 mM Tris-HCl (pH 9.5), the protein bands on the membrane were revealed by treatment with nitroblue tetrazolium and with a solution of the substrate for AP, 5-bromo-4-chloro-3-indolylphosphate, in the dark.
RESULTS
Total IgA and listeria-specific IgA responses occur within the GALT of immunocompetent GF mice following oral colonization with the ΔactA mutant of L. monocytogenes.
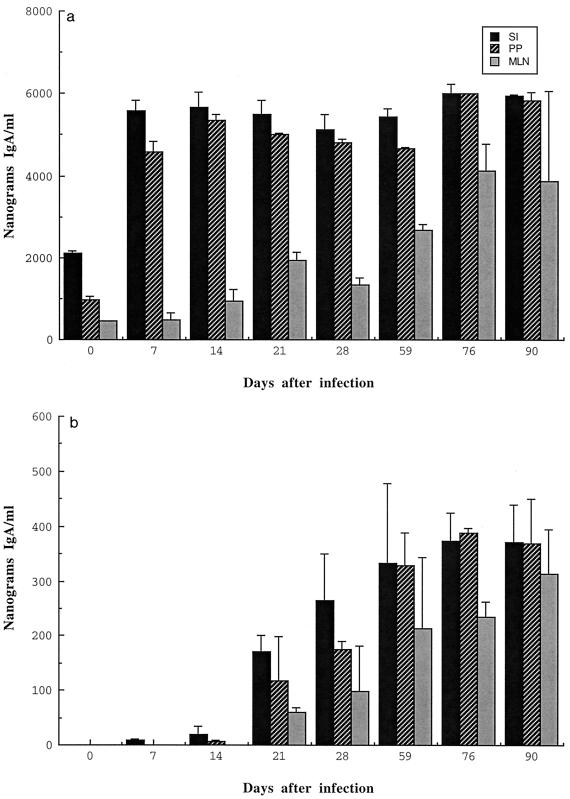
Organ cultures of PP, SI, and MLN from BALB/c mice monoassociated with ΔactA listeriae were performed at several times after intestinal colonization. We have previously shown that our organ cultures accurately reflect the immune status of the mucosal tissues at the times the tissues were analyzed (46, 47). These cultures were set up in triplicate. The supernatants from these cultures were analyzed for total natural IgA and listeria-specific IgA. Figure 1 shows a set of typical data from one of several sets of mice orally colonized with the ΔactA mutant. The total IgA expressed by GALT fragment cultures from BALB/c mice increased three- to fivefold within 7 days of colonization and remained at that high level for about 90 days, the last time point of the experiment. The listeria-specific IgA Ab expressed by these cultures showed a detectable rise from near baseline levels by 14 days after colonization to about 3 to 5% of the total IgA, by days 21 to 28, and it stabilized at approximately 7 to 8% at day 76. In MLN, the level of specific IgA increased slowly until day 90. These results showed that colonization with the ΔactA mutant of L. monocytogenes can induce a specific IgA response in GALT tissues.
FIG. 1.
Detection of total IgA and listeria-specific IgA in supernatants of fragment cultures of SI, PP, and MLN from BALB/c mice. At various times after colonization with the ΔactA mutant of L. monocytogenes, organs were collected from groups of three BALB/c mice at each time point, and organ culture assays were performed. Supernatants were used for estimating total IgA (a) and listeria-specific IgA (b) by RIA. Data represent means ± standard deviations.
Detection of listeria-specific IgA ASC in the gut lamina propria.
To confirm the occurrence of a specific IgA response after colonization, we determined whether ΔactA listeria-specific antibody-secreting cells (ASC) were present among intestinal lamina propria cells. We performed ELISPOT assays for estimating both total IgA and listeria-specific IgA-secreting cells. We used the samples from 21 and 28 days postinfection, when significant specific immune responses were first observed by fragment culture assays. As shown in Table 1, antigen-specific ASC were detected in both day 21 and day 28 samples; at day 28, the number of ASC was almost twofold higher than at day 21, but the ratio to total IgA-secreting cells remained the same. The presence of ASC in the lamina propria supports our findings for fragment cultures that the ΔactA mutant can induce both total and specific IgA responses in the gut.
TABLE 1.
Relative frequencies by ELISPOT analysis of total and ΔactA listeria-specific IgA ASC in gut lamina propria of GF and formerly GF BALB/c mice monoassociated with ΔactA L. monocytogenes for 21 and 28 days
| Days after infection | Frequencya
|
% of specific cells | |
|---|---|---|---|
| Total IgA | ΔactA-specific IgA | ||
| 0 | 1,066 ± 251 | ||
| 21 | 9,375 ± 3,346 | 47 ± 6 | 0.50 |
| 28 | 16,833 ± 2,608 | 82 ± 30 | 0.49 |
Mean frequency ± standard deviation of number of spots formed in three replicate wells with cell suspensions from three mice per group. Fivefold dilutions were assayed, and the dilution yielding between 5 and 50 spots per well was used to estimate the number of ASC per 106 mononuclear cells.
Western blot analysis.
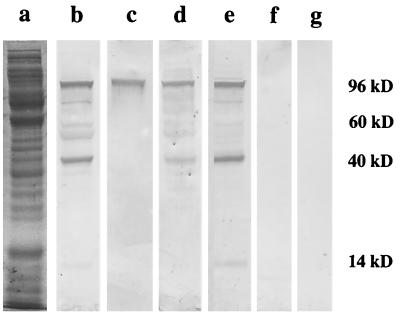
To further support our observation that there was a specific IgA response in gut tissues after oral infection with the ΔactA mutant, we tried to identify particular antigens reactive with IgA Abs secreted by single SI fragment cultures. To do this, we performed Western blot analysis using (i) ΔactA listeria sonicates as mixtures of antigens which were resolved in SDS-polyacrylamide gels and (ii) SI fragment culture supernatant as the developing IgA Ab. From many fragment culture supernatants, we arbitrarily chose as probing Abs samples which had given high scores by RIA. As shown in Fig. 2, we detected at least four antigens migrating with molecular masses of 96, 60, 40, and 14 kDa. As negative controls, we used fragment culture supernatants from mice monoassociated with segmented filamentous bacteria (SFB), which are gram-positive, spore-forming, anaerobic bacteria which can induce a potent gut mucosal immune response (44). SFB and the ΔactA mutant induce production of similar levels of total IgA in gut fragment cultures (1,500 to 2,000 ng/ml). When we used fragment culture supernatants from mice monoassociated with SFB, we detected no reactivity with listeria proteins.
FIG. 2.
Western blot analyses of antigens from sonicates of the L. monocytogenes ΔactA mutant. Antigens were separated on a 12% polyacrylamide gel, electrotransferred to a cellulose membrane, and probed with supernatants of four individual SI fragment cultures which were positive for Ab by RIA. Bound IgA was detected by AP-labeled goat anti-mouse IgA. Lanes: a, proteins of a supernatant of listeria sonicate visualized by Coomassie blue staining; b to e, samples probed with different supernatants of fragment cultures from listeria-monoassociated mice; f and g, samples probed with supernatants of fragment cultures from SFB-monoassociated mice.
Translocation of the ΔactA mutant of L. monocytogenes from the SI.
Previously, we had attempted to colonize conventionally reared SCID mice with 1 × 108 to 3 × 108 ΔactA listeriae given orally. Despite the mice being immunologically incompetent, we could detect no gut luminal listeriae beyond day 7 and no translocated bacteria in systemic tissues up to the time of the organisms' disappearance from the gut (unpublished data). We presumed that ΔactA listeriae are poor competitors with the already established gut flora. However, we expected that we might colonize the intestines of GF, immunocompetent mice with this mutant for extended periods, as we have done with certain commensal bacteria (43, 44), and that its chronic presence in the lumen of the gut might favor translocation to other organs. Thus, we orally inoculated GF BALB/c mice with 108 ΔactA listeriae and measured CFU in the intestinal content, liver, spleen, and brain at various times up to day 90. At all times we found large numbers of listeriae in luminal contents, but only occasionally did we find CFU in homogenates of liver or spleen, and then relatively few (Table 2). No CFU were ever detected in brain samples. Thus, as reasonably expected based on its defect, the growth of the orally administered ΔactA mutant is restricted largely to the gut.
TABLE 2.
Translocation of ΔactA L. monocytogenes from the lumen of the gut to the liver and spleen of formerly GF BALB/c micea
| Days after infection | CFU/organ
|
||
|---|---|---|---|
| SI | Liver | Spleen | |
| 7 | 7.78 × 104 | 0 | 0 |
| 2.86 × 106 | 3.00 × 102 | 1.14 × 103 | |
| 2.28 × 103 | 9.96 × 103 | 0 | |
| 14 | 1.24 × 106 | 1.27 × 106 | 0 |
| 3.20 × 104 | 0 | 8.80 × 104 | |
| 5.20 × 104 | 0 | 0 | |
| 21 | 2.40 × 106 | 7.52 × 102 | 0 |
| 8.60 × 105 | 1.45 × 103 | 0 | |
| 2.89 × 107 | 0 | 0 | |
| 28 | 5.6 × 104 | 0 | 0 |
| 7.20 × 107 | 3.00 × 105 | 0 | |
| 2.00 × 106 | 1.75 × 104 | 0 | |
| 59 | 1.45 × 107 | 5.90 × 103 | 2.00 × 103 |
| 4.90 × 103 | 0 | 0 | |
| 2.55 × 105 | 0 | 0 | |
| 76 | 1.17 × 108 | 5.35 × 105 | 0 |
| 5.60 × 107 | 0 | 0 | |
| 1.36 × 109 | 8.75 × 105 | 0 | |
| 90 | 6.80 × 108 | 1.05 × 105 | 2.60 × 104 |
| 2.98 × 109 | 2.55 × 103 | 1.10 × 102 | |
| 3.00 × 107 | 2.10 × 103 | 0 | |
No organisms were found in any of the brain samples.
Development of GCR in PP and MLN.
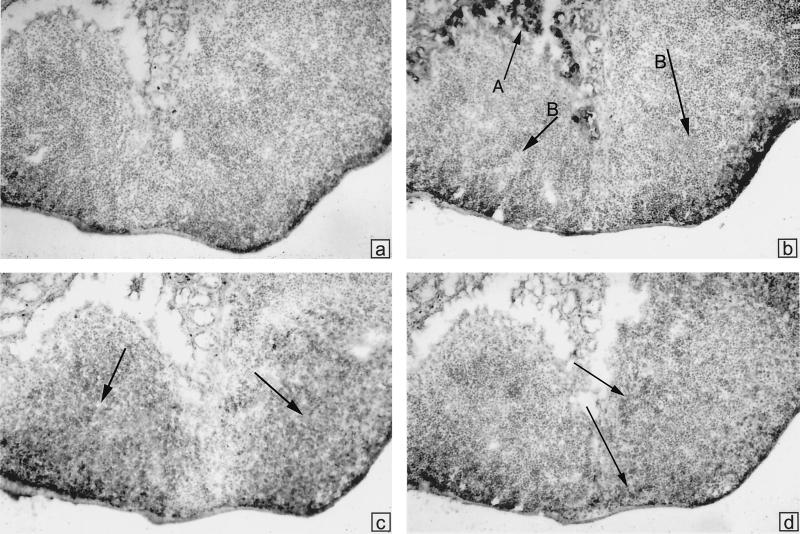
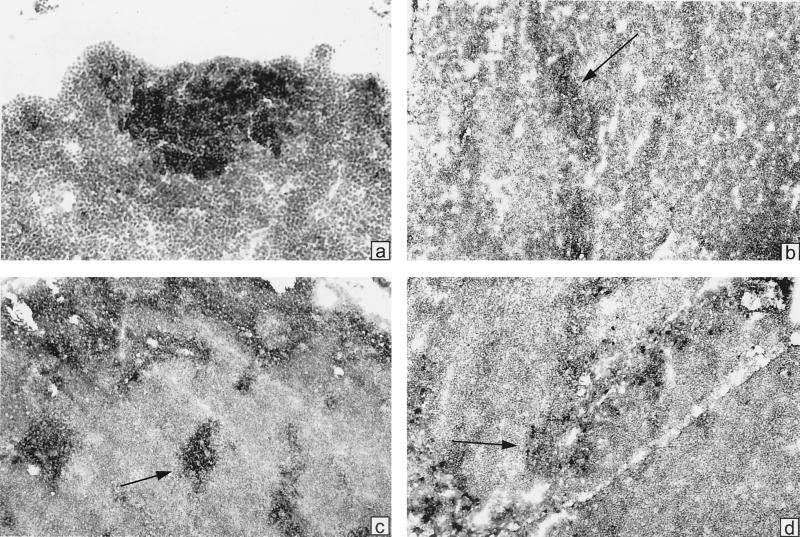
To investigate whether colonization of the gut by the ΔactA mutant of listeriae could induce GC reactions (GCR) in B-lymphoid follicles of PP and MLN, an indicator of the local induction of a humoral, mucosal immune response in GALT of formerly GF, orally infected mice (43, 47), we used immunohistochemical analyses of tissue sections. The PP and MLN were taken at day 21 after colonization, a time often found to coincide with near-maximal GCR. We generally stained with the lectin PNA, which selectively binds to B cells in GC, and with labeled anti-IgM, anti-IgA, or anti-CD4. Figure 3 shows a general observation: no indication of GCR in the PP. PNA stains mostly in the region of goblet cells, not in the region of B-cell follicles. The B-cell follicles are rather uniformly stained for IgM, and CD4+ cells are seen in the interfollicular zones. In some MLN, however, intensely positive PNA+ and IgM+ follicles were found; these contained some IgA+ cells, which are also scattered in surrounding cords (Fig. 4). Thus, it appears that heavy colonization of the gut with ΔactA listeriae does not significantly perturb the PP but rather stimulates GCR in some draining MLN.
FIG. 3.
Immunoperoxidase staining of cryostat sections from PP to assess GCR in mice colonized with L. monocytogenes, ΔactA for 21 days. (a) Hematoxylin stain plus HRP-labeled avidin. (b) Also stained with biotinylated PNA and HRP-labeled avidin. Arrow A points toward positively stained goblet cells, arrows B point to B-cell follicles which are unstained and show no GCR. (c) Biotinylated anti-IgM and HRP-avidin. Arrows point to centers of each of two B-cell follicles which are rather uniformly positive. (d) Biotinylated anti-CD4 and HRP-avidin. Positive cells are seen in the interfollicular regions.
FIG. 4.
Immunoperoxidase staining of cryostat sections from MLN to assess GCR in mice colonized with L. monocytogenes ΔactA for 21 days. (a) Stained with biotinylated PNA and HRP-labeled avidin; (b) stained with biotinylated anti-CD4 and HRP-avidin; (c) stained with biotinylated anti-IgM and HRP-avidin; (d) stained with biotinylated anti-IgA and HRP-avidin. All sections were counterstained with hematoxylin.
Previously, we correlated the transient appearance of GCR in GALT tissues by FACS analyses of cell suspensions (43, 44). Using FACS analyses, we were unable to detect meaningful changes in the content of PNA+ kappa chain+ or IgA+ kappa chain+ B cells in either PP or MLN over a 28-day period after gut colonization. These observations confirm the paucity of GCR observed in these tissues by histochemical analyses and attest to the limitations of the FACS method when only small and regionally limited GCR occur.
Serum Ab response to oral infection by the ΔactA mutant of L. monocytogenes.
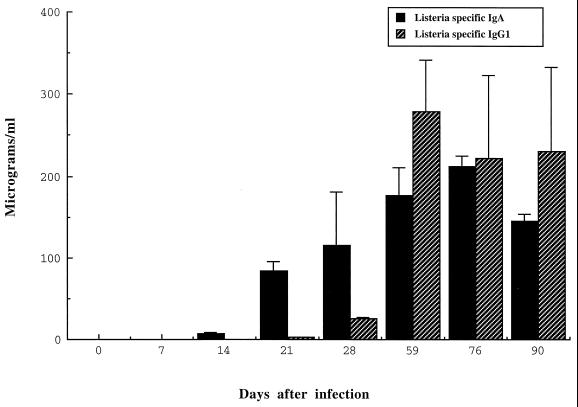
To determine the levels of serum IgA and IgG1 Abs, we collected blood at various time from mice infected with the ΔactA mutant, and estimated Ab concentrations by our standardized RIA. As shown in Fig. 5, listeria-specific serum IgA increased from day 21 after infection until day 76. Then the level stabilized until day 90, the last time point of the experiment. In the case of listeria-specific IgG1, the level was minimal until day 28 postinfection and increased until day 59.
FIG. 5.
Detection of listeria-specific IgA in the sera from BALB/c mice. At various times after colonization with the ΔactA mutant of L. monocytogenes, sera were collected and used for listeria-specific IgA by RIA. Data represent means ± standard deviations.
DISCUSSION
Using a mouse model, we sought to address whether oral inoculation of L. monocytogenes could elicit a gut mucosal immune response in mammals. Because virulent WT listeriae, given orally do not colonize the intestines of conventionally reared mice, large inocula (∼108) of bacteria must be given to effect at least a systemic immune response via translocation and dissemination (49). To favor chronic gut colonization and possible stimulation of the GALT, we decided to orally inoculate GF mice, which contain no potentially competitive enteric microorganisms. However, GF mice are exquisitely sensitive to orally introduced virulent listeria (50% lethal dose about 5 × 102 to 5 × 103) (27, 50). Thus, we chose to use an attenuated mutant, the ΔactA strain, which we have found to be innocuous when given orally to CNV SCID mice at a high dose (108) (27) and others have found to be a “safe” vaccine when given parenterally to immunocompromised gamma interferon-negative CNV mice (17). Another advantage of this mutant, suggested by our studies with SCID mice (31), is that its defect possibly could confine it largely to the intestines of the formerly GF mice, since the usual cell-to-cell transmission exhibited by listeriae should be minimized (45). Indeed, we found that ΔactA listeriae, given orally, extensively and chronically colonized the gut, but we could only sporadically detect relatively few CFU in the liver and spleen and none in the brain. We also found that this gut colonization was accompanied, within 21 to 28 days, by a marked increase in expression of total (nonspecific) IgA and the appearance of specific IgA Abs in organ fragment cultures of SI, MLN, and PP. The discernible specific IgA was about 7 to 8% of the total IgA. Supportive of listeria-specific IgA being produced in the gut were our findings of both specific and nonspecific ASC in cell suspensions of the gut lamina propria and the staining of particular but different antigens, separated via electrophoresis and analyzed by Western blotting with IgA from supernatants of fragment cultures. Also consistent with earlier, local gut stimulation was our finding of circulating IgA in the blood well before initiation of a serum IgG1 response (Fig. 5). This latter response likely was initiated peripherally by the occasional translocating and disseminating microbes.
Given the gut IgA responses elicited by intestinal colonization with the ΔactA mutant, we wondered whether it also produced GCR in the B-cell follicles of PP, as we found to occur transiently after gut colonization with commensal bacteria such as Morganella morganii or SFB (43, 44). However, analysis of the PP and MLN at 21 and 28 days following colonization, at times when specific IgA Abs were being expressed by gut tissues, showed no convincing GCR in PP (32). Instead, rather small and widely dispersed GCR were detected by immunohistochemistry in MLN. Our suggestion is that luminal listeriae mainly attach to the plasma membrane of enterocytes via the interaction of their internalin molecules with surface E-cadherin on host cells (35) and enter these or macrophages and dendritic cells, extended into intraepithelial spaces (33), rather than gain entry via M cells into lymphoid regions of PP. Thus, lymph draining the lamina propria may convey listerial antigens, via afferent lymphatics, into MLN, a conclusion which recent work of Havell et al. (18) also supports. Nevertheless, the result—generation of IgA plasma blasts, emigration, and accumulation in the gut lamina propria—may be similar regardless of the route of antigen entry and culminate in a gut mucosal IgA response.
A question left unresolved by these studies is whether specific IgA Abs, made in the gut lamina propria and released into the gut lumen, can be protective against gut infection by listeriae given orally. Although parenteral infection with WT listeriae does not effectively stimulate a systemic humoral response in laboratory animals, intraperitoneal (i.p.) vaccination of mice with recombinant, avirulent Salmonella enterica serovar Typhimurium, expressing the listeria p60 protein, did confer a measure of protection against i.p. challenge with WT listeria, based on translocation to the spleen, compared with the vector alone given i.p. (13). In support of the possible role of systemic IgG Abs in ameliorating parenteral listeria infections was the finding that an IgG1 MAb specific to listeriolysin O, given i.p., could also diminish translocates of WT listeria in the spleen following a subsequent i.p. challenge (9, 36). The motivation for our present work is supported by these findings. We have developed an admittedly contrived animal model to effect the clearly demonstrable expression of a mucosal IgA antibody response to listeriae in the gut. We are presently using this animal model to provide a library of IgG and IgA hybridomas against listerial antigens. Since we have found the CNV SCID mouse to be exceptionally vulnerable to orally initiated central nervous system listeriosis (27, 31), we plan to test these MAbs for protective effects against natural, oral infection in these mice. Recent mechanisms proposed for IgA antibodies to exclude viral pathogens from enterocytes, interfere with intracellular pathogenic processes, or even expel these pathogens from the gut lamina propria as IgA-antigen complexes (34) could all potentially be operative during gut listerial infections. We propose that any potentially protective antigen, identified using IgA MAbs, could be delivered to conventionally reared animals or humans by a mucosal vector in a fashion that would constitute an effective vaccine.
ACKNOWLEDGMENTS
This work was supported by grant AI-37108 from the National Institute of Allergy and Infectious Diseases. We thank the Lucille P. Markey Trust for funding of the Flow Cytometry Facility of the Cancer Center, University of Pennsylvania.
We thank Hank Pletcher for assistance with the FACS IV flow cytometer. We thank Alec McKay for the preparation of radiolabeled reagents and for assistance with the cell analyzer. We thank Al Chaney and Michelle Albright for generation and maintenance of GF mice, Judy Bun for immunohistochemical work, and Ethel Cebra for editorial assistance.
REFERENCES
- 1.Armstrong R W, Fung P C. Brainstem encephalitis due to Listeria monocytogenes: case report and review. Clin Infect Dis. 1993;16:689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft G J, Bosma M J, Bosma G C, Unanue E R. Regulation of macrophage Ia expression in mice with severe combined immune deficiency: induction of Ia expression by a T cell independent mechanism. J Immunol. 1986;137:4–9. [PubMed] [Google Scholar]
- 3.Barbour A H, Rampling A, Hormaeche C E. Comparison of the infectivity of isolates of Listeria monocytogenes following intragastric and intravenous inoculation in mice. Microb Pathog. 1996;20:247–253. doi: 10.1006/mpat.1996.0023. [DOI] [PubMed] [Google Scholar]
- 4.Ben Embareck P K. Presence, detection and growth of Listeria monocytogenes in sea food: a review. Int J Food Microbiol. 1991;23:17–34. doi: 10.1016/0168-1605(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 5.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 6.Bos N A, Meeuwsen C G, Wostmann B S, Pleasants J R, Benner R. The influence of exogenous antigenic stimulation on the specificity repertoire of background immunoglobulin-secreting cells of different isotypes. Cell Immunol. 1988;112:371–380. doi: 10.1016/0008-8749(88)90306-1. [DOI] [PubMed] [Google Scholar]
- 7.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton C B, Austin C C, Sobel J, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 9.Edelson B T, Cossart P, Unanue E R. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol. 1999;163:4087–4090. [PubMed] [Google Scholar]
- 10.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming D W, Cochi S L, MacDonald K L, Brondum J, Jayes P S, Plikaytis B D, Holmes M B, Audurier A, Broome C V, Reingold A L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985;312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel J K, Caldwell S A. Specific immunity and nonspecific resistance to infection: Listeria, protozoa, and viruses in mice and hamsters. J Infect Dis. 1975;131:201–209. doi: 10.1093/infdis/131.3.201. [DOI] [PubMed] [Google Scholar]
- 13.Gentschev I, Sokolovic Z, Kohler S, Krohne G F, Hof H, Wagner J, Goebel W. Identification of p60 antibodies in human sera and presentation of this listerial antigen on the surface of attenuated salmonellae by the HlyB-HlyD secretion system. Infect Immun. 1992;60:5091–5098. doi: 10.1128/iai.60.12.5091-5098.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grenningloh R, Darji A, Wehland J, Chakraborty T, Weiss S. Listeriolysin and IrpA are major protein targets of the human humoral response against Listeria monocytogenes. Infect Immun. 1997;65:3976–3980. doi: 10.1128/iai.65.9.3976-3980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hage-Chahine C M, Del Giudice G, Lambert P-H, Pechere J-C. Hemolysin-producing Listeria monocytogenes affects the immune response to T-cell-dependent and T-cell-independent antigens. Infect Immun. 1992;60:1415–1421. doi: 10.1128/iai.60.4.1415-1421.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harty J T, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFNγ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 18.Havell E A, Beretich G R, Carter P B. The mucosal phase of listeria infection. Immunobiology. 1999;201:164–177. doi: 10.1016/S0171-2985(99)80056-4. [DOI] [PubMed] [Google Scholar]
- 19.Hirose K, Suzuki H, Nishimura H, Mitani A, Washizu J, Matsuguchi T, Yoshikai Y. Interleukine-15 may be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats. Infect Immun. 1998;66:5677–5683. doi: 10.1128/iai.66.12.5677-5683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1-CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 21.Jones E M, MacGowan A P. Antimicrobial chemotherapy of human infection due to Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1995;14:165–175. doi: 10.1007/BF02310351. [DOI] [PubMed] [Google Scholar]
- 22.Jubb K V F, Kennedy P C, Palmer N. Pathology of domestic animals. 4th ed. San Diego, Calif: Academic Press, Inc.; 1993. pp. 1393–1397. [Google Scholar]
- 23.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann S H, Hug E, Vath U, Miller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listeria antigens depend on cooperation between specific L3T4+ and Lyt2+ T cells. Infect Immun. 1985;48:263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammerding A M, Glass K A, Gendron-Fitzpatrick A, Doyle M P. Determination of virulence of different strains of Listeria monocytogenes and listerial inocula by oral inoculation of pregnant mice. Appl Environ Microbiol. 1992;58:3991–4000. doi: 10.1128/aem.58.12.3991-4000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebman D A, Griffin P M, Cebra J J. Relationship between expression of IgA by Peyer's patch cells and functional IgA memory cells. J Exp Med. 1987;166:1405–1418. doi: 10.1084/jem.166.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee F. Oral listeriosis. Murine models for the study of pathogenesis. Ph. D. thesis. Philadelphia, Pa: University of Pennsylvania; 1995. [Google Scholar]
- 28.Logan A C, Chow K-P N, George A, Weinstein P D, Cebra J J. Use of Peyer's patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect Immun. 1991;59:1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald T T, Carter P B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980;28:516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackaness K B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 31.Manohar M, Portnoy D, Lee F, Cebra J J. Pathogenesis of CNS listeriosis and host mucosal immune responses vs. oral infection. Immunol Cell Biol. 1997;75(Suppl. 1):A22. [Google Scholar]
- 32.Manohar M, Jiang H Q, Bos N A, Cebra J J. A contrast between translocating Morganella morganii and Listeria monocytogenes, actA(−) colonizing the gut of formerly germ-free mice. Immunol Lett. 1999;69:68–12.20. [Google Scholar]
- 33.Maric I, Holt P G, Perdue M H, Bienenstock J. Class II MHC antigen (Ia) bearing dendritic cells in the epithelium of the rat intestine. J Immunol. 1996;156:1408–1414. [PubMed] [Google Scholar]
- 34.Mazanec M B, Nedrud J G, Kaetzel C S, Lamm M E. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- 35.Mengaud J, Ohayon H, Gounon P, Mage R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 36.Nato F, Reich K, Lopital S, Rouyre S, Geoffroy C, Mazie J C, Cossart P. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect Immun. 1991;59:4641–4646. doi: 10.1128/iai.59.12.4641-4646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pamer E G, Sijts A J, Villanueva M S, Busch D H, Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–136. doi: 10.1111/j.1600-065x.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 38.Pine L, Malcolm G B, Plikaytis B D. Listeria monocytogenes intragastric and intraperitoneal approximate 50% lethal doses for mice are comparable, but death occurs earlier by intragastric feeding. Infect Immun. 1990;58:2940–2945. doi: 10.1128/iai.58.9.2940-2945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roll J T, Czuprynski C J. Hemolysin is required for extraintestinal dissemination of Listeria monocytogenes in intragastrically inoculated mice. Infect Immun. 1990;58:3147–3150. doi: 10.1128/iai.58.9.3147-3150.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose M L, Birbeck M S C, Wallis V J, Forrester J A, Davies A J. Peanut lectin binding properties of germinal centers of mouse lymphoid tissue. Nature. 1980;284:364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- 42.Schuchat A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shroff K E, Meslin K, Cebra J J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talham G L, Jiang H-Q, Bos N A, Cebra J J. Segmented filamentous bacteria can make a major contribution to stimulating the physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, L. monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein P D, Cebra J J. The preference for switching to IgA expression by Peyer's patch germinal center B cells is likely due to intrinsic influence of their microenvironment. J Immunol. 1991;147:4126–4135. [PubMed] [Google Scholar]
- 47.Weinstein P D, Schweitzer P A, Cebra-Thomas J A, Cebra J J. Molecular genetic features reflecting the preference for isotype switching to IgA expression by Peyer's patch germinal center B cells. Int Immunol. 1991;3:1253–1263. doi: 10.1093/intimm/3.12.1253. [DOI] [PubMed] [Google Scholar]
- 48.Welch M D, Rosenblatt J, Skoble J, Portnoy D A, Mitchison T J. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto S, Russ F, Teixeira H C, Conradt P, Kaufmann S H E. Listeria monocytogenes-induced gamma interferon secretion by intestinal intraepithelial γ/δ T lymphocytes. Infect Immun. 1993;61:2154–2161. doi: 10.1128/iai.61.5.2154-2161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zachar Z, Savage D C. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect Immun. 1979;23:168–174. doi: 10.1128/iai.23.1.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]