Abstract
Introduction
Solitary keratoacanthoma (SKA) is generally considered as a well-differentiated form of squamous cell carcinoma, but it usually runs a benign course and a not aggressive behavior. Diagnostic criteria, prognosis, and treatment of SKA are not fully defined yet. Surgical treatment with fusiform excision represents the gold standard; nonoperative intralesional therapy of KA is uncommon but may provide a valid option in some categories of patients.
Case Series Presentation
We report our experience regarding the treatment of SKA with a hybrid treatment consisting of a minimally invasive technique such as curettage followed by intralesional corticosteroid administration in the same session. Six patients affected with KA were treated ending in a complete resolution, with good esthetic outcome, no relapse after 1 year, and satisfaction of the patients.
Discussion and Conclusion
The combined treatment allows us on the one hand to avoid radical surgery in selected patients and particular anatomic areas and on the other the side effects that the use of intralesional chemotherapy/immunosuppressive drugs can entail.
Keywords: Intralesional treatment, Intralesional corticosteroid, Noninvasive treatment, Solitary keratoacanthoma
Established Facts
Solitary keratoacanthoma (SKA) is generally considered as a well differentiated form of squamous cell carcinoma that usually runs a benign course with sometimes spontaneous involution. The clinical course is unpredictable, so treatment is indicated.
Guidelines for therapy of SKA are lacking. Treatment of choice when possible consists of surgical excision with no specific margins established. Other therapeutic approaches are elettrodessication, curettage, intralesional therapy, topical 5% imiquimod cream, and topical 5% 5-fluorouracil.
Rare experiences have been reported on the use of intralesional corticosteroids, and most of them are very dated.
Novel Insights
A new interesting approach could be a hybrid treatment consisting of a minimally invasive technique such as curettage followed by intralesional corticosteroid administration in the same session.
The excellent results obtained allow us to propose the two treatments in combination instead of separately.
Introduction/Literature Review
Solitary keratoacanthoma (SKA) is the most common subtype of KA, a skin tumor originating from a hyperproliferation of squamous epithelium of the hair follicle infundibulum [1]. Other subtypes include multiple KA Ferguson-Smith syndrome, generalized eruptive KA of Grzybowsky, KA centrifugum marginatum, giant KA, and mucosal and subungueal KA.
SKC generally manifests as a small papule that rapidly grows into a 1–2-cm nodule with a crateriform appearance. A maturation phase and a final involution into an atrophic scar follow; stages, maturation, stability, and regression are variable in length of time. Overall, lesion resolution rate is consistently high. Most KAs develop on sun-exposed hair-bearing areas or in areas of trauma [2].
Due to its histological organization, it is generally considered as a well differentiated form of squamous cell carcinoma, but according to what has been described in the literature and based on our experience, SKA usually runs a benign course and a not aggressive behavior. Diagnostic criteria, prognosis, and treatment of SKA are not fully defined yet [3, 4].
The standard therapeutic approach encompasses complete fusiform excision that represents the gold standard, elettrodessication, curettage, radiotherapy, intralesional therapy, topical 5% imiquimod cream, and topical 5% 5-fluorouracil (5-FU). Intralesional therapy of KA is uncommon but may provide a valid option in some categories of patients. The literature on this subject is mostly based on case reports and small case series.
The most used agents for intralesional treatment are methotrexate (MTX) and 5-FU followed by interferon alpha and bleomycin [5]. The use and efficacy of corticosteroids has been reported less frequently.
Based on the supposed proliferative nature of KA and the preference in avoiding complete excision or other longer treatments such as radiation therapy, in selected patients and/or particular anatomic areas, we decided to combine two therapeutic options: intralesional triamcinolone preceded by curettage of the KA. Six patients affected with KA were treated ending in a complete resolution, with good esthetic outcome, no relapse after 6 months, and satisfaction of the patients.
Case Series Presentation
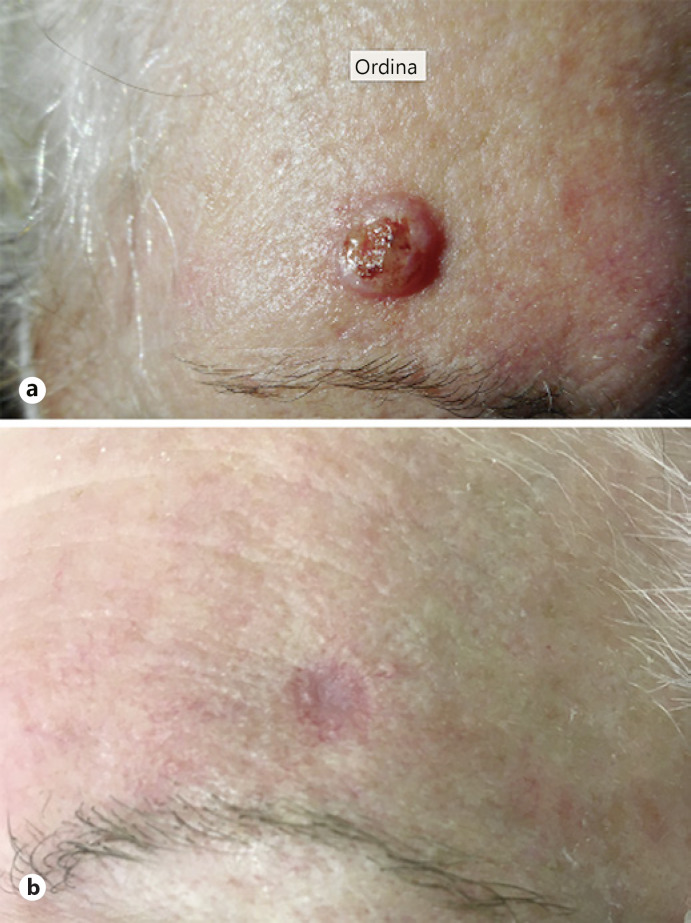
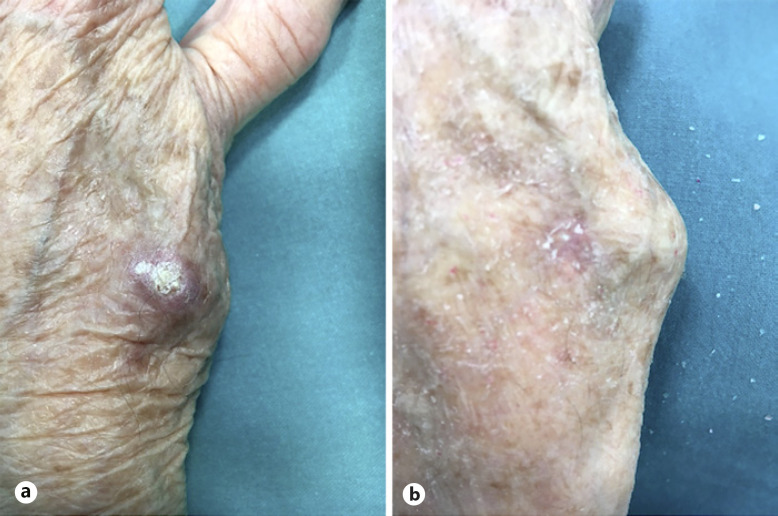
Six patients, affected with SKA, referred to the O.U. of Dermatology, University Hospital of Ferrara, Italy, from September to November 2020, were selected because of their advanced age, the presence of many comorbidities, and the need of a noninvasive treatment approach. KAs were located on the face (4/6) and extremities (2/6) (shown in Fig. 1a, 2a). The mean diameter of the lesions was 1.5 cm (range 1.1–2.3 cm).
Fig. 1.
a SKA of the forehead before treatment. b Complete healing of the lesion 6 weeks after first treatment.
Fig. 2.
a SKA of the hand before treatment. b Complete healing of the lesion 6 weeks after first treatment.
The procedure consisted of a local anesthesia by injection with mepivacaine 2%, followed by a medium/deep curettage up to the dermal plane and intralesional injection of a solution of triamcinolone acetonide 40 mg/mL diluted in physiological saline with a ratio of 1:5. It was prepared and aspirated into 0.5-mL syringes-8 mm-30 G. The solution was subsequently injected in the bottom and the edges of the area previously occupied by the SKA. The dosage of triamcinolone was adapted to the size of the KA and ranged between 8 and 12 mg. Following the curettage, the lesions were sent to the histological analysis, which confirmed the diagnosis of keratoacanthoma.
All patients were evaluated after 2, 6, 12, 24 weeks and 1 year. At the 2-week control, two cases showed only a thin crust, and in the remaining 4 cases, erythema and mild edema were still present. A second treatment was carried out with the same amount of triamcinolone previously injected. No further curettage was needed.
All patients showed complete recovery at the 6-week control, 1 month after the second treatment (shown in Fig. 1b, 2b). No signs of relapse, both clinical and dermatoscopic, were depicted at the 12 and 24 weeks and 1-year control. All patients underwent ultrasound of tributary lymph nodes, both in the staging phase and at the follow-up every 6 months. All ultrasound scans excluded secondary disorders of the lymph nodes and major salivary glands.
No relevant side effects were reported. All patients were happy about the procedure and the opportunity to avoid a surgical operation. All of them would advise other patients to use this procedure.
Discussion/Conclusion
KA can regress spontaneously, but final dimensions and persistence cannot be predicted. In consideration of their localization in visible locations and especially in the face, the wait-and-see strategy would not be acceptable due to its important functional and esthetic repercussions. Although very rarely described, the possibility of distant metastasis makes the need for treatment mandatory in our opinion [6].
Guidelines for therapy of SKA are lacking. Treatment of choice when possible consists of surgical excision with no specific margins established [7, 8, 9]. The patients we selected showed wide lesions located in different anatomical areas difficult to be approached with a surgical radical excision.
Most of the patients were under anticoagulants and antiplatelet agents that they could not suspend in relation to the comorbidities. They expressed the strong will to be treated in the least invasive way and with fewer complications. These patients expressly refused to undergo a complete radical excision with a wide cut and application of sutures and other treatments longer over time and with possible greater esthetic sequelae as radiotherapy.
Our choice was to try a hybrid approach that included a minimally invasive procedure and the use of an intralesional corticosteroid treatment. The decision of making 2 injections instead of one was empirically taken.
Following the curettage, we proposed histological confirmation to the patient despite the history and clinical appearance strongly suggested the diagnosis. With regard to intralesional techniques, there are two very recent reviews of the literature [3, 5].
There is no standardized guideline or treatment, and the evidence is based on case reports and case series. The most numerous case series concern the use of intralesional MTX (15 studies) and intralesional 5-FU (7 studies); only one study used intralesional bleomycin.
Rare experiences have been reported on the use of intralesional corticosteroids, and most of them are very dated. Recently, in the literature, we found only case reports on their use in multiple or eruptive forms and no experience on solitary and sporadic KA.
There are few data on their effectiveness, and various hypotheses have been made about their mechanism of action as a therapy of KAs. Although the immunosuppressive action of corticosteroids is known, some mechanisms seem to explain their efficacy in the treatment of keratoacanthomas. Keratoacanthomas originate from the hair follicle infundibulum; it is possible that corticosteroids inhibit the hair growth cycle at the initiation of anagen and the keratinocyte hyperplasia by inhibiting epidermal mitoses, DNA synthesis, and transcription of transforming growth factor beta, which is important for growth of keratoacanthomas [10, 11, 12, 13]. The hyper-proliferative nature of the lesion is the most plausible one. Although there are more studies regarding the use of other intralesional agents such as MTX or 5-FU, the choice to use corticosteroid was dictated by the lower risk of systemic effects. In fact, although rarely, some systemic side effects have been described following the use of the other intralesional chemotherapeutic agents [5]. This makes blood monitoring tests necessary during therapy with these agents which are not recommended with the use of intralesional corticosteroid. The combined technique of intralesional triamcinolone associated with curettage we applied in our 6 patients was easy to make and very effective, leading to complete remission of the lesions with no recurrence after 24 weeks of follow-up. This new hybrid technique could represent a valid less invasive treatment alternative for patients presenting difficult cases of SKA.
Statement of Ethics
The subjects (or their parents/legal guardians/next-of-kin) have given their written informed consent to publish their or publication of the details of their medical case and any accompanying image. Information revealing the subject's identity is avoided. All patients are identified by numbers and not by their real names. Ethics approval for this study was not required per local and national guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Vincenzo Bettoli: design of the study, interpretation of data, drafting of the manuscript, and critical revision of the manuscript. Pierantonia Zedde: acquisition of data, drafting of the manuscript, and critical revision of the manuscript. Natale Schettini, Pacetti, and Giulia Odorici: acquisition of data and critical revision of the manuscript. Monica Corazza: design of the study, interpretation of data, and critical revision of the manuscript. All the authors read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further Inquiries can be directed to the corresponding author.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Zito PM, Scharf R. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021. Nov 15, Keratoacanthoma. [Google Scholar]
- 2.Veerula VL, Ezra N, Aouthmany M, Graham TA, Wolverton SE, Somani AK. Multiple keratoacanthomas occurring in surgical margins and de novo treated with intralesional methotrexate. Cutis. 2016;98((6)):E12–5. [PubMed] [Google Scholar]
- 3.Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74((6)):1220–33. doi: 10.1016/j.jaad.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30((2 Pt 2)):326–333. doi: 10.1111/j.1524-4725.2004.30080.x. discussion 333. [DOI] [PubMed] [Google Scholar]
- 5.Kiss N, Avci P, Bánvölgyi A, Lőrincz K, Szakonyi J, Gyöngyösi N, et al. Intralesional therapy for the treatment of keratoacanthoma. Dermatol Ther. 2019;32((3)):e12872. doi: 10.1111/dth.12872. [DOI] [PubMed] [Google Scholar]
- 6.Hodak E, Jones RE, Ackerman AB. Solitary keratoacanthoma is a squamous-cell carcinoma: three examples with metastases. Am J Dermatopathol. 1993;15((4)):332–342. doi: 10.1097/00000372-199308000-00007. discussion 343–52. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein MC, Brodell RT, Bordeaux J, Honda K. The art and science of surgical margins for the dermatopathologist. Am J Dermatopathol. 2012;34((7)):737–745. doi: 10.1097/DAD.0b013e31823347cb. [DOI] [PubMed] [Google Scholar]
- 8.Rogers CR, Bentz ML. An evidence-based approach to the treatment of nonmelanoma facial skin malignancies. Plast Reconstr Surg. 2011;127((2)):940–948. doi: 10.1097/PRS.0b013e318204aeb2. [DOI] [PubMed] [Google Scholar]
- 9.Schell AE, Russell MA, Park SS. Suggested excisional margins for cutaneous malignant lesions based on Mohs micrographic surgery. JAMA Facial Plast Surg. 2013;15((5)):337–343. doi: 10.1001/jamafacial.2013.1011. [DOI] [PubMed] [Google Scholar]
- 10.Sanders S, Busam KJ, Halpern AC, Nehal KS. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28((10)):954–958. doi: 10.1046/j.1524-4725.2002.02069.x. [DOI] [PubMed] [Google Scholar]
- 11.Stenn KS, Paus R, Dutton T, Sarba B. Glucocorticoid effect on hair growth initiation: a reconsideration. Skin Pharmacol. 1993;6((2)):125–134. doi: 10.1159/000211097. [DOI] [PubMed] [Google Scholar]
- 12.Ghadially FN. The role of the hair follicle in the origin and evolution of some cutaneous neoplasms of man and experimental animals. Cancer. 1961;14:801–816. doi: 10.1002/1097-0142(199007/08)14:4<801::aid-cncr2820140417>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence N, Reed RJ. Actinic keratoacanthoma. Speculations on the nature of the lesion and the role of cellular immunity in its evolution. Am J Dermatopathol. 1990;12((5)):517–533. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further Inquiries can be directed to the corresponding author.