ABSTRACT.
To describe the serostatus of measles IgG antibodies in pregnant women and newborns, placental transfer, and factors that determine being below the threshold of 150 mIU/mL, a cross-sectional study was conducted. Blood samples of 790 pregnant women at the time of delivery and 734 umbilical cord samples were analyzed from eight hospitals in the Aburrá Valley of Antioquia, Colombia. Measles IgG antibody measurement was performed with ELISA. The proportion of individuals with antibodies < 150 mIU/mL was 13.9% (95% CI: 12.2–15.8) in pregnant women and 11.1% (95% CI: 9.2–13.4) in newborns. The geometric mean of the antibody level of the pregnant women was 552 mIU/mL (95% CI: 504–605) and in the umbilical cord 662 mIU/mL (95% CI: 604–727). A positive correlation between pregnant woman and umbilical cord antibodies was found. The median ratio of measles IgG antibodies in umbilical cord/pregnant woman was 1.22 for all participants. A seroprevalence below the threshold of 150 mIU/mL was found in newborns whose mothers were born between 1983 and 1994, compared with those born before that period, when exposure to the wildtype virus was common (adjusted prevalence ratio: 3.6, 95% CI: 1.3–9.6). These findings suggest that there are gaps in measles immunity among women of childbearing age, before pregnancy. To close this immune gap and support efforts to maintain measles control, serological screening for measles antibodies should be routinely included in reproductive health and antenatal care programs to identify women without immunity who should be vaccinated before pregnancy or after delivery.
INTRODUCTION
Measles is a highly contagious viral disease that can cause serious health problems and even death.1 It is among the main causes of death in infants despite the existence of a safe and cost-effective vaccine.2 Measles can go from being a mild disease to presenting complications such as otitis, diarrhea, pneumonia, and encephalitis, events that require hospitalization and, in a quarter of cases, can lead to disability due to brain damage, blindness, or hearing loss.3 Children under 12 months, pregnant women, and immunocompromised people are at high risk of complications.4 More than 2 million deaths occurred in the world per year before the introduction of the measles vaccine in the 1960s.5 Currently, measles remains a public health problem, causing more than 100,000 deaths annually.3
Despite the incompleteness of case reporting and registration of deaths due to measles in most Latin American countries6 before the introduction of the vaccine in this region in the 1970s, approximately 600,000 cases were reported annually. This represented an incidence > 150 cases per 100,000 population with high mortality rates in children: 14 to 55 deaths per 100,000 infants and 8 to 54 deaths in children 1 to 4 years of age.7 In Colombia, the change in the incidence rate before and after measles vaccination was 313 cases per 100,000 population in 19598 and 34.1 per 100,000 population in 1982,9 respectively.
Since 2008, none of the WHO regions has maintained a vaccination coverage equal or greater than the 95% required for herd immunity,4 the most critical situation after the COVID-19 pandemic.10,11 This can hamper successful elimination in many countries despite the use of two doses and additional strategies in regular vaccination programs.
Measles elimination may be affected by the resurgence of cases registered in the past decade. Worldwide, reported cases decreased between 2000 and 2016 (853,479 cases in 2000 to 132,490 cases in 2016), but in 2019, it increased by 556% (869,770 cases).12 Outbreaks have been reported in the United States, Africa, and Europe; in countries from the Region of the Americas, an increase in reported cases was more noticeable, rising from 92 cases in 2016 to 19,244 cases in 2019 (0.1–28 per million inhabitants)12 with the reestablishment of endemic transmission in Venezuela in 2018 and in Brazil in 2019.12,13 In Colombia, as in other countries in the region, there is concern about the accumulation of susceptible, which may influence the recent resurgence of the disease. In 2002, an outbreak was reported (139 cases), and in 2018 and 2019, 208 and 244 cases were confirmed, respectively.14
The resurgence of the disease may be due to the immune gap generated by the accumulation of susceptible individuals. This is because of lack of access to vaccination of socioeconomically disadvantaged population groups, failures of the health system, vaccine hesitancy in a globalized world, high rates of migration,15 and the weakening of public health in the face of war, social conflict, and political instability.13 The accumulation of susceptible individuals may also be due to loss of vaccine effectiveness, non-seroconversion, interference of vaccine antibodies with those maternally transferred by the placenta, or decreased immunity over time after elimination of the disease.16
The WHO has emphasized the need for good quality data at the subnational level to detect the accumulation of susceptible individuals and thus prevent the occurrence of outbreaks and resurgence of the disease.17 Vaccine coverage data often do not represent the population immunity profile or provide data on individual vaccination rates; the population census may be inaccurate, vaccine effectiveness may not be systematically analyzed, and there may be an absence of alerts for vaccinated individuals with incomplete schedules.18
Detection of susceptibility is possible through serosurveillance of the immune profiles of subgroups or the entire population. The periodic determination of the susceptibility of pregnant women through hospital serosurveillance can be a novel means of documenting progress in the elimination of measles as an alternative to probabilistic household surveys that require considerable time, resources, and logistical capacity.19,20 In addition to determining the magnitude and trend of seroprevalence in pregnant women, the loss of passive immunity in newborns can be anticipated to guide their protection during the first months of life.21 It has been recommended that women of childbearing age be identified and vaccinated before pregnancy because, as a live virus, the measles vaccine is contraindicated during pregnancy.22,23 This strategy can be complemented with postpartum vaccination in hospitals.23
This study used a hospital serosurveillance approach to detect measles serostatus in a cohort of pregnant women and their newborns residing in a subregion of Antioquia, Colombia, in an attempt to identify new measures to close the immune gap and sustain the elimination of measles.
MATERIALS AND METHODS
Study population.
The prevalence of measles IgG antibodies was determined through a cross-sectional study in paired samples of pregnant women at the time of delivery and umbilical cord, from a representative random sample obtained in a previous study.24 These samples were kept in the serum bank of the serosurveillance program of the Departmental Laboratory of Public Health of the Sectional Secretariat of Health and Social Protection of Antioquia,19,24 where they were also processed.
The study was conducted in a cohort of healthy pregnant women captured at the time of delivery between December 2015 and April 2016. Mothers with 37 or more weeks of gestation were included in the study. Those with multiple gestation, fever in the 72 hours before delivery (chorioamnionitis, sepsis), those seen in the intensive care unit, and those in an advanced stage of labor were excluded.
The population was pregnant women residing in the Aburrá Valley subregion of Antioquia, Colombia, whose delivery could be attended in one of 17 urban hospitals. A sample size of 1,000 pregnant women was estimated, and 790 met the inclusion criteria and agreed to participate. In 734, a sample was obtained from the mother and the umbilical cord. With prior informed consent, a survey was applied to record demographic and clinical data and information on pregnancy and delivery.
The study was approved by the ethics committee of the Héctor Abad Gómez National Faculty of Public Health at Universidad de Antioquia (session 129-15 October 2015 and 204-01 February. 2019) and by the ethics committees of each hospital.
Laboratory tests.
Measles IgG antibodies were measured with Enzygnost® Anti-measles virus/IgG (Siemens, Marburg, Germany), sensitivity 99.6% and specificity 100%,25 following the manufacturer’s recommendations. A quantitative result calculated using the alpha method expressed in international milliunits per milliliter (mIU/mL) was obtained. Participants were considered below the threshold of 150 mIU/mL (or nonimmune) if the antibody concentration was < 150 mIU/mL.21 Above the antibody threshold of 150 mIU/mL, individuals were classified as equivocal (150–350 mIU/mL) and positive (> 350 mIU/mL), according to the cutoff points established by the manufacturer.
Statistical analysis.
The demographic characteristics of the pregnant women and newborns were analyzed through descriptive measures such as arithmetic mean (± SD), median, minimum and maximum in the case of continuous variables. The categorical variables and seroprevalence were shown with absolute and relative frequencies. The prevalence estimate was made with the calculation of weighted proportions from the design effect of the multistage sampling.
Logarithmic transformation and calculation of the geometric mean (GM) of the measles IgG antibody concentration were performed. Using the Spearman correlation coefficient, the relationship between measles IgG antibodies from the mother and the umbilical cord was analyzed. Antibody transfer from pregnant to newborn was measured as the ratio of measles IgG in umbilical cord divided measles IgG in the mother.
The pregnant women participating in the study had access to measles vaccination in childhood and adulthood, with differential exposure to wild-type virus or to different vaccination strategies by birth cohort and maternal age:
Cohort I: pregnant women born between1972 and 1982. In 1973, when measles vaccination was officially introduced in Colombia, it was applied as a monovalent vaccine.19,26 This cohort was more likely to be exposed to wild-type virus or to receive at least one dose of measles-containing vaccine (MCV) at the start of massive immunization.
Cohort II: pregnant women born between 1983 and 1994. Starting in 1984, the regular vaccination strategy was complemented with updating campaigns.26
Cohort III: born between 1995 and 2002: These pregnant women had access to two-dose of MMR vaccine. Since 1995, the first dose of the triple viral vaccine (MMR against measles, mumps, and rubella) has been applied at 12 months of age. In 1997, the application of an MMR booster began at 10 years, which was changed to 5 years from 2004 onward.27 Between 1995 and 1997, vaccination campaigns applied MMR to populations born since 1984.27–29 During 2005–2006, a national vaccination campaign with measles and rubella-MR double viral vaccine was carried out, aimed at men and women between the ages of 14 and 39.28
To explore factors associated with being below the threshold of 150 mIU/mL, crude prevalence ratio (PR) and adjusted PR (aPR) with 95% CIs were calculated by multivariate analysis (binomial regression). Socioeconomic covariates were included in the regression analysis. Household overcrowding was defined when the average number of people per room in the home was greater than three. The social security system in health classified as “contributory,” individuals who had a formal employment relationship or financial resources to pay their contribution independently; “subsidized,” individuals who did not have these resources; “special or exception,” such as military and employees of state companies; and “uninsured poor population,” who were eligible for the subsidized regimen.30
The data were analyzed in SPSS® version 28 (IBM SPSS Statistics for Windows, Armonk, NY) and Stata14 (StataCorp, College Station, TX).
RESULTS
Participant characteristics.
The pregnant women were born primarily between 1983 and 1994 (cohort II). The average age of the pregnant women was 24.9 ± 6.3 years of age (minimum 13, maximum 43). They lived in urban areas, had studied for up to 11 years, had health insurance, had a partner, and their homes were not overcrowded. More than half had had up to two pregnancies; 1.6% had received steroids in the previous 6 months (Table 1).
Table 1.
Characteristics of mothers and newborns, Aburrá Valley, Colombia, 2016
| Characteristic | n | % |
|---|---|---|
| Mothers (N = 790) | ||
| Birth cohort by age and vaccination* | ||
| Cohort I (1972–1982) | 94 | 11.9 |
| Cohort II (1983–1994) | 442 | 55.9 |
| Cohort III (1995–2002) | 254 | 32.2 |
| Residential area | ||
| Urban | 751 | 95.1 |
| Rural | 39 | 4.9 |
| Maternal education (years) | ||
| ≤ 11 | 606 | 76.7 |
| > 11 | 182 | 23.0 |
| Unknown | 2 | 0.3 |
| Social security† | ||
| Contributory/special | 385 | 48.7 |
| Subsidized | 362 | 45.8 |
| Uninsured | 43 | 5.4 |
| Marital status | ||
| Married | 575 | 72.8 |
| Single/divorced | 215 | 27.2 |
| Overcrowding | ||
| Yes | 26 | 3.3 |
| No | 764 | 96.7 |
| Parity | ||
| 1 | 378 | 47.8 |
| 2 | 215 | 27.2 |
| 3 | 116 | 14.7 |
| ≥ 4 | 81 | 10.3 |
| Use of immunosuppressant steroids | ||
| Yes | 13 | 1.6 |
| No | 777 | 98.4 |
| Newborns (N = 734) | ||
| Type of delivery | ||
| Caesarean | 529 | 72.6 |
| Vaginal | 200 | 27.4 |
| Sex | ||
| Boys | 385 | 52.5 |
| Girls | 349 | 47.5 |
| Gestational age (weeks) | ||
| 37 | 140 | 19.1 |
| 38 | 166 | 22.6 |
| 39 | 202 | 27.5 |
| 40 | 191 | 26 |
| 41 | 35 | 4.8 |
| Birth weight (g) | ||
| < 2,500 | 42 | 5.7 |
| ≥ 2,500 | 690 | 94.3 |
Cohort I: born between1972 and 1982, start of massive immunization, natural exposure to the virus, or mothers who received at least one dose of measles-containing vaccine (MCV); cohort II: born between 1983 and 1994, MCV in regular program plus “catch-up” campaigns (1984), Measles Mumps and Rubella (MMR; 1995–1997) and MCV (2005); cohort III: born between 1995 and 2002: two-dose MMR.
Contributory/special: individuals had a formal employment relationship or had financial resources to pay their contribution independently/military and employees of state companies.
Most of the deliveries were caesarean, mainly between 37 and 39 weeks of gestation (69.2%). Of the newborns, 52.5% were boys; 5.7% had a birth weight below 2,500 g (Table 1).
Serological results of pregnant women and newborns.
The proportion of pregnant women who were below the threshold of 150 mIU/mL was 13.9% (95% CI: 12.2–15.8%), with a GM of antibodies of 552 mIU/mL (95% CI: 504–605). In the umbilical cord, the proportion of antibodies below the threshold of 150 mIU/mL was 11.1% (95% CI: 9.2–13.4%), with a GM of 662 mIU/mL (95% CI: 604–727) (Table 2).
Table 2.
Proportion of seroprevalence, geometric mean concentration and ratio of measles IgG antibodies in umbilical cord/pregnant woman, Aburrá Valley, Colombia, 2016
| Value | Measles IgG antibody level, mIU/mL | Total | |
|---|---|---|---|
| < 150 | ≥ 150 | ||
| Maternal serum | |||
| n | 115 | 675 | 790 |
| Weighted proportion (95% CI) | 13.9% (12.2–15.8) | 86.1% (84.2–87.8) | 100.0% |
| GMC (95% CI) | 71 (60–85) | 782 (725–845) | 552 (504–605) |
| Cord blood serum | |||
| n | 84 | 650 | 734 |
| Weighted proportion (95% CI) | 11.1% (9.2–13.4) | 88.9% (86.6–90.8) | 100.0% |
| GMC (95% CI) | 78 (67–91) | 873 (804–948) | 662 (604–727) |
| Cord serum/maternal serum ratio | |||
| n | 84 | 649 | 733 |
| Median (min–max) | 0.93 | 1.26 | 1.22 |
GMC = geometric mean concentration.
There were 171 (21.3%) pregnant women and 148 (19.2%) umbilical cords classified as equivocal, based on their serostatus. 504 (64.8%) pregnant women and 502 (69.7%) umbilical cords were classified as positive.
Transfer of antibodies from the pregnant woman to the umbilical cord.
The ratio of measles IgG antibodies in umbilical cord/pregnant woman was 1.22 for all participants. In pregnant–cord pairs below the threshold of 150 mIU/mL, this ratio was lower than 1 (Table 2).
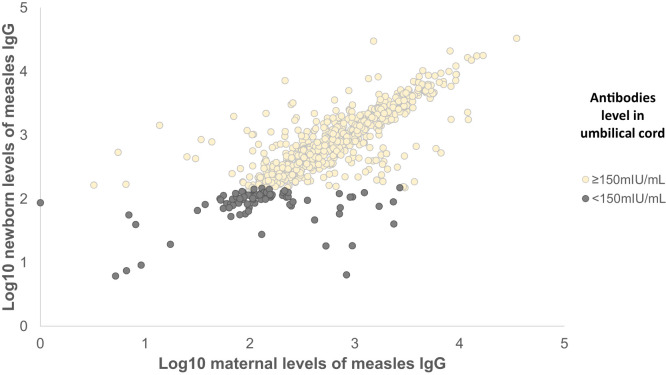
In Figure 1, a positive correlation was observed between the antibody titers of the pregnant women and those of the umbilical cord (Spearman correlation, 95% CI: 0.77, 0.74–0.80), even in those below the antibody threshold.
Figure 1.
Measles IgG antibody correlation in umbilical cord/pregnant woman, Aburrá Valley, Colombia, 2016.
Of the 733 samples analyzed in pairs of mother and umbilical cord, there were 81 (11.1%) with discordant results. The level of umbilical cord antibodies was below the threshold of 150 mIU/mL at 4.6% (29/626) when the mother was above the threshold of 150 mIU/mL and 48.6% (52/107) in mothers with antibodies below the threshold of 150 mIU/mL but the umbilical cord was above the threshold.
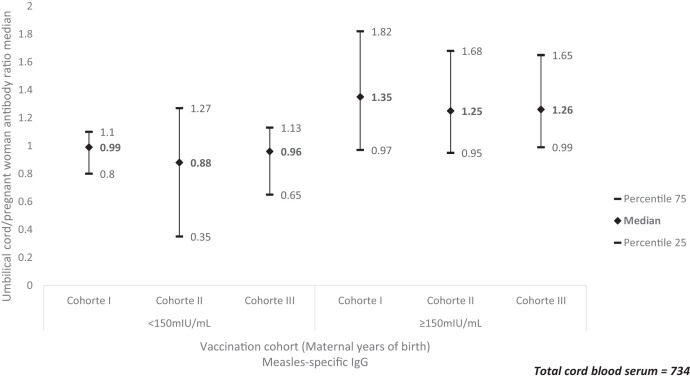
The umbilical cord/pregnant woman antibody ratio presented the same pattern between those above and below the threshold of 150 mIU/mL, with the highest value in cohort I, followed by cohort III. This ratio was lower in cohort II (Figure 2).
Figure 2.
Ratio of antibody transfer by birth cohort by maternal age and vaccination and level of protection, Aburrá Valley, Colombia, 2016. aCohort I: born between1972 and 1982: start of massive immunization, natural exposure to the virus, or mothers who received at least one dose of measles-containing vaccine (MCV); cohort II: born between 1983 and 1994: MCV in regular program plus “catch-up” campaigns (1984), Measles Mumps and Rubella (MMR; 1995–1997) and MCV (2005); cohort III: born between 1995 and 2002: two-dose MMR.
Newborns classified below the threshold of 150 mIU/mL.
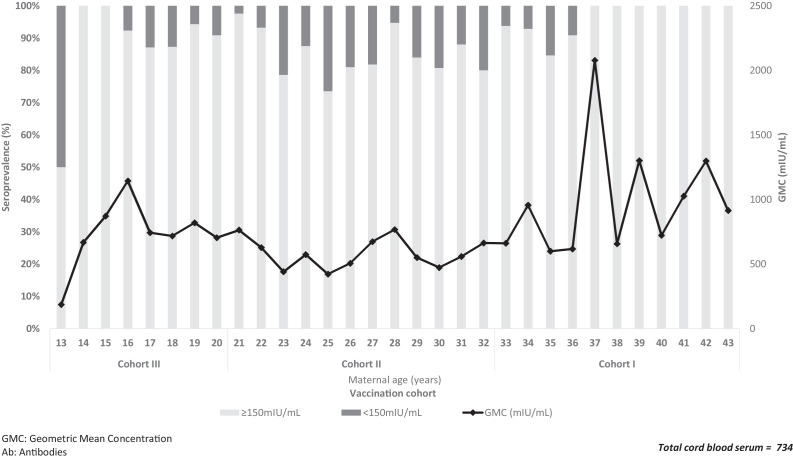
The seroprevalence below the threshold of 150 mIU/mL was observed primarily in newborns of mothers from the cohort II (born between 1983 and 1994, aged between 21 and 32 years). In cohort I, from 37 years of age onward, no antibodies below 150 mIU/mL was recorded, and the concentration of antibodies increased (Figure 3).
Figure 3.
Age-specific distribution of seroprevalence and geometric mean concentration of measles IgG antibodies in newborns in each birth cohort by maternal age and vaccination, Aburrá Valley, Colombia, 2016. GMC = geometric mean concentration. Ab = antibodies. Cohort I: born between 1972 and 1982: start of massive immunization, natural exposure to the virus, or mothers who received at least one dose of measles-containing vaccine (MCV); cohort II: born between 1983 and 1994: MCV in regular program plus “catch-up” campaigns (1984), Measles Mumps and Rubella (MMR; 1995–1997) and MCV (2005); cohort III: born between 1995 and 2002: two-dose MMR.
In both the univariate and multivariate analysis, a significant statistical association of being below the threshold of 150 mIU/mL in the newborn was observed when the pregnant woman belonged to cohort II (born from 1983 to 1994) compared with cohort I (born until 1982): aPR 3.6 (95% CI: 1.3–9.6) (Table 3).
Table 3.
Factors associated with newborns being measles antibodies below the threshold 150 mIU/mL (N = 734 umbilical cord samples), Aburrá Valley, Colombia, 2016
| Factors | Cord blood serum IgG (< 150 mIU/mL), n (%) | Univariate analysis PR (95% CI) | Multivariate analysis aPR (95% CI) |
|---|---|---|---|
| Mothers | |||
| Birth cohort by age and vaccination* | |||
| Cohort I (1972–1982) | 4 (4.7) | Ref | Ref |
| Cohort II (1983–1994) | 59 (14.4) | 3.1 (1.2–8.3)†‡ | 3.6 (1.2–11.4)‡ |
| Cohort III (1995–2002) | 21 (8.9) | 1.9 (0.7–5.4)† | 2.5 (0.7–8.7) |
| Maternal education (years) | |||
| ≤ 11 | 58 (10.3) | 0.7 (0.4–1.0)† | 0.7(0.4–1.3) |
| > 11 | 26 (15.4) | Ref | Ref |
| Social security§ | |||
| Contributory/Special | 47 (13.4) | Ref | Ref |
| Subsidized/Uninsured | 37 (9.7) | 0.7 (0.5–1.1)† | 0.8 (0.5–1.5) |
| Marital status | |||
| Married | 23 (11.6) | 1.0 (0.7–1.6) | |
| Single/divorced | 61 (11.4) | Ref | |
| Parity | |||
| 1 | 40 (11.3) | Ref | Ref |
| 2 | 19 (9.9) | 0.9 (0.5–1.5) | 0.7 (0.4–1.3) |
| 3 | 18 (16.2) | 1.4 (0.9–2.4)† | 1.4 (0.8–2.6) |
| ≥ 4 | 7 (9.1) | 0.8 (0.4–1.7) | 0.9 (0.4–2.0) |
| Newborns | |||
| Type of delivery | |||
| Caesarean | 61 (11.5) | 1.0 (0.6–1.6) | |
| Vaginal | 23 (11.5) | Ref | |
| Sex | |||
| Boys | 46 (11.9) | 1.1 (0.7–1.6) | |
| Girls | 38 (10.9) | Ref | |
| Gestational age (week) | |||
| 37 | 18 (12.9) | 1.3 (0.7–2.4) | |
| 38 | 20 (12.0) | 1.2 (0.7–2.2) | |
| 39 | 23 (11.4) | 1.1 (0.6–2.0) | |
| 40–41 | 23 (10.2) | Ref | |
| Birth weight (g) | |||
| < 2,500 | 4 (9.5) | 0.8 (0.3–2.1) | |
| ≥ 2,500 | 80 (11.6) | Ref | |
aPR = adjusted prevalence ratio; PR = prevalence ratio; Ref = reference.
Cohort I: born between1972 and 1982, start of massive immunization, natural exposure to the virus, or mothers who received at least one dose of measles-containing vaccine (MCV); cohort II: born between 1983 and 1994, MCV in regular program plus “catch-up” campaigns (1984), Measles Mumps and Rubella (MMR; 1995–1997), and MCV (2005); cohort III: born between 1995 and 2002: two-dose MMR.
Factors with P value < 0.25 (chi’s square or Fisher analysis was used for univariate analysis).
Items with P value < 0.05 with statistical significance were underlined (chi-square or Fisher analysis was used for univariate analysis; binomial regression was used for multivariate analysis).
Contributory/special: individuals who had a formal employment relationship or had financial resources to pay their contribution independently/military and employees of state companies.
DISCUSSION
Measles prevention continues to be a public health challenge, increasingly so with the SARS-CoV-2 pandemic, which has affected access to childhood vaccination programs around the world.10
With our study, potential obstacles to measles elimination were identified given the magnitude of the number of pregnant women and newborns below the threshold of 150 mIU/mL. Additionally, scientific evidence is provided for the development of new strategies that reduce the susceptibility of women of childbearing age and the mother–child group and that contribute to the maintenance of elimination goals. Given the limited number of local studies that address this issue,28,31–33 the findings are important due to the resurgence of the disease in Colombia after prolonged periods of absence.34
Despite not having a clearly established correlate of protection for measles,35,36 the observed 13.9% in pregnant women whose antibodies are below the threshold of 150 mIU/mL indicates the possible accumulation of susceptible individuals and the potential occurrence of cases in women of childbearing age. In Colombia, despite vaccination coverage close to 95%,37 measles cases were confirmed in women of childbearing age aged 10 to 54 years in 13% (19 cases) of the total registered in 2018 and 16% (29 cases) in 2019.34
The 11.1% in newborns whose antibodies are below the threshold of 150 mIU/mL shows the potential susceptibility of infants. In 2019, of the confirmed measles cases in Colombia, 18% (32 cases) were under 12 months.34 This susceptibility could be increased in infants due to the decrease in protective maternal antibodies before the first year of life.2,21
The frequency of participants below the threshold of 150 mIU/mL in our study is comparable to that reported in other studies. In Turkey, between April 2016 and April 2017, Cetin Coban et al. recorded a nonprotective prevalence of 20% in mothers and 15% in newborns,38 captured in hospitals in four provinces with the highest number of deliveries. They used the same test from our study and analyzed samples from 1,547 mothers and 1,529 newborns.
In our results, as in those of Cetin Coban et al., a positive correlation was obtained between the umbilical cord antibodies and those of the mother.38 These authors found a higher geometric mean of antibodies in newborns, whereas in our study, no differences were observed in the CIs for the ratio of umbilical cord and pregnant women, but an increase in the transfer of antibodies with gestational age was observed (Table 3). We found a positive correlation between maternal and newborn antibodies. The R2 value obtained from the Spearman correlation in this study indicates that approximately 40% of the changes in the level of antibodies in the umbilical cord can be explained by other variables, including a possible waning immunity over time due to reduced exposure to wild-type virus after advances in mass vaccination and measles elimination.38
In our study, the only factor that was associated with the measles serostatus of newborn (below the threshold of 150 mIU/mL) was the birth cohort by maternal age and the vaccination schedule. Mothers who were probably exposed to wild-type measles virus before the start of vaccination had higher antibody levels than those vaccinated afterward,21 which is consistent with what other authors have found. In 1996, authors carried out a serological survey of measles in Medellin, Colombia, in a random population sample aged 1 to 14 years, where it was found that 26% did not have serological protection. The authors postulated that the higher level of antibodies in children born before mass vaccination with MMR in 1995 was possibly due to exposure to the wild-type virus31 and the high measles incidence rate in the prevaccination era in Colombia.8
In South Africa, Gieles et al. carried out a study with the objective of determining specific IgG antibodies against measles (using the Indirect ELISA test, Euroimmun, Lübeck, Germany), they observed higher titers and prevalence of positivity in pregnant women born before the introduction of vaccination on a regular basis in the country, suggesting a waning of the protection induced by the vaccine, faster than that acquired by natural exposure.39
Likewise, Lam et al. found that the susceptibility was higher among the youngest pregnant women (born since 1989), measuring measles seroprevalence in 1,366 pregnant women using a chemiluminescence assay in New York City in 2019. After finding a serologically nonimmune to measles rate of 23% even in women with a vaccination history, authors postulated that despite vaccination strategies, the potential for susceptibility persists for several reasons, such as primary vaccination failures, waning immunity, or the limitations of commercial tests to determine immunity.40
This waning of measles immunity in adolescents and young adults, those mainly affected in outbreaks of the disease even in populations with high vaccination coverage, was also raised by Lee et al. in a study carried out in Taiwan in 1,096 participants using Enzygnost® enzyme immunoassay, VIDAS® enzyme-linked fluorescent assay, and LIAISON® XL chemiluminescent immunoassay tests.41
Unlike our findings, other authors identified the number of pregnancies38,40 and the time elapsed since the second dose of the vaccine as factors related to nonimmunity.42 However, they recognized the presence of collinearity of these factors with age.
A strength of our work is the multistage random sampling of hospital institutions designed and conducted in eight delivery centers from various municipalities throughout the subregion. Another strength was the use of a commercial ELISA test for the determination of antibodies, with a sensitivity of 99.6% and a specificity of 100%. The use of the manufacturer’s recommended cutoff point of 150 mIU/mL facilitated comparability with other studies.42–44
Our work also had several limitations. Individual data on immunization and disease history were not available. To counteract this, the birth cohorts by maternal age were grouped according to the vaccination strategy of the Expanded Program on Immunizations.
Since a correlate of protection for measles is still being discussed, it is not conclusive that having IgG antibodies < 150 mIU/mL correlates with susceptibility.35,36 Further studies are needed to assess cellular immunity to confirm whether pregnant women with antibodies below the threshold of 150 mIU/mL may have some protection against the disease.36 Studies are also needed on the transfer of antibodies through breastfeeding, as are those that provide more information on the strategy of advancing the first dose of the vaccine to 6 months of age without interfering with the antibodies transferred by the mother and those generated in the regular vaccination scheme of children.21
In conclusion, this study determined that more than 10% of newborns had antibodies below the threshold of 150 mIU/mL, although apparently there is transfer of antibodies from the mother to the newborn. The possibility of a newborn having antibodies < 150 mIU/mL was influenced by the pregnant women’s previous exposure according to the birth cohort by age, with a higher prevalence of antibodies in those born before the start of vaccination compared with those born in the post-vaccination period. Thus, women from cohort I had a greater chance of being exposed to the wild-type virus than did women from the other two cohorts because, at that time, there was no massive immunization or vaccination only consisted of receiving one dose of MCV. Due to “catch-up” campaigns, those in “cohort II” had a higher possibility of being vaccinated but less so than those in than “cohort III,” who received the regular program with two-dose MMR.
Vaccination of childbearing-age women is recommended to increase antibodies due to the possibility of waning of vaccine immunity as a complementary measure to support the elimination of measles. In addition, it is important to identify pregnant women with incomplete vaccination schedules and carry out postpartum vaccination.23,45 The application of an additional dose of vaccine for women of childbearing age in the preconception consultation could increase the passively transferred antibodies to the newborn and potentially prolong its duration.45
Hospital serosurveillance of antibodies against measles in pregnant women and the umbilical cord, as reported in this work, can identify new strategies for vaccination and measles elimination by periodically monitoring herd immunity and the eventual waning in vaccine immunity over time.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1. Strebel P, Orenstein W, 2019. Measles. N Engl J Med 381: 349–357. [DOI] [PubMed] [Google Scholar]
- 2. Guerra FM, Crowcroft NS, Friedman L, Deeks SL, Halperin SA, Severini A, Hatchette TF, Bolotin S, 2018. Waning of measles maternal antibody in infants in measles elimination settings – a systematic literature review. Vaccine 36: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 3. Murray AF, Englund JA, Tielsch JM, Katz J, Shrestha L, Khatry SK, Carlin K, Leclerq SC, Steinhoff MC, Chu HY, 2018. Measles and rubella seroprevalence in mother-infant pairs in rural Nepal and the United States: pre- and post-elimination populations. Am J Trop Med Hyg 99: 1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson KM, Odahowski CL, 2016. Systematic review of measles and rubella serology studies. Risk Anal Off Publ Soc Risk Anal 36: 1459–1486. [DOI] [PubMed] [Google Scholar]
- 5. Strebel PM, Papania MJ, Gastanaduy PA, Goodson JL, Orenstein WA, Offit PA, Edwards KM, Plotkin SA. Plotkin’s Vaccines. 7th ed. Philadelphia, PA: Elsevier, 579–618. [Google Scholar]
- 6. José Manuel Borgoño Z, 1983. Current impact of measles in Latin America. Rev Infect Dis 5: 417–421. [PubMed] [Google Scholar]
- 7. Castillo-Solorzano CC, Matus CR, Flannery B, Marsigli C, Tambini G, Andrus JK, 2011. The Americas: paving the road toward global measles eradication. J Infect Dis 204: S270–S278. [DOI] [PubMed] [Google Scholar]
- 8. PAHO , 1962. Health Conditions in the Americas, 1957–1960. Washington, DC: Panamerican Health Organization. [Google Scholar]
- 9. PAHO , 1986. Health Conditions in the Americas, 1981–1984 v1. Washington, DC: Panamerican Health Organization. [Google Scholar]
- 10. Kraus N, Condon SB, 2021. Measles (Rubeola): a case of vaccine hesitancy and pregnancy. J Midwifery Womens Health 66: 391–396. [DOI] [PubMed] [Google Scholar]
- 11. McNally VV, Bernstein HH, 2020. The effect of the COVID-19 pandemic on childhood immunizations: ways to strengthen routine vaccination. Pediatr Ann 49: e516–e522. [DOI] [PubMed] [Google Scholar]
- 12. Patel MK. et al. , 2020. Progress toward regional measles elimination—worldwide, 2000–2019. MMWR Morb Mortal Wkly Rep 69: 1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hotez PJ, Nuzhath T, Colwell B, 2020. Combating vaccine hesitancy and other 21st century social determinants in the global fight against measles. Curr Opin Virol 41: 1–7. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization , 2020. Immunization, vaccines and biologicals. vaccine preventable diseases vaccines monitoring system 2020 global summary reference time series: MEASLES. Available at: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencemeasles.html. Accessed May 21, 2021.
- 15. Feemster KA, Szipszky C, 2020. Resurgence of measles in the United States: how did we get here? Curr Opin Pediatr 32: 139–144. [DOI] [PubMed] [Google Scholar]
- 16. Hayman DT, 2019. Measles vaccination in an increasingly immunized and developed world. Hum Vaccin Immunother 15: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization , 2019. Measles vaccines: WHO position paper, April 2017—Recommendations. Vaccine 37: 219–222. [DOI] [PubMed] [Google Scholar]
- 18. Winter AK, Martinez ME, Cutts FT, Moss WJ, Ferrari MJ, McKee A, Lessler J, Hayford K, Wallinga J, Metcalf CJE, 2018. Benefits and challenges in using seroprevalence data to inform models for measles and rubella elimination. J Infect Dis 218: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hincapié-Palacio D. et al. , 2019. Serosurveillance for vaccine-preventable diseases: a look inside the pertussis experience. Biomed Rev Inst Nac Salud 39: 130–143. [DOI] [PubMed] [Google Scholar]
- 20. Durrheim D, 2018. Measles elimination, immunity, serosurveys, and other immunity gap diagnostic tools. J Infect Dis 218: 341–343. [DOI] [PubMed] [Google Scholar]
- 21. Gonçalves G, Nunes C, Mesquita J, Nascimento MSJ, Frade J, 2016. Measles antibodies in cord blood in Portugal: possible consequences for the recommended age of vaccination. Vaccine 34: 2750–2757. [DOI] [PubMed] [Google Scholar]
- 22. Congera P, Maraolo AE, Parente S, Schiano Moriello N, Bianco V, Tosone G, 2020. Measles in pregnant women: a systematic review of clinical outcomes and a meta-analysis of antibodies seroprevalence. J Infect 80: 152–160. [DOI] [PubMed] [Google Scholar]
- 23. Leuridan E, Nunes M, Jones C, 2019. Maternal Immunization. San Diego, CA: Academic Press. [Google Scholar]
- 24. Hincapié-Palacio D, Hoyos MC, Ochoa J, Montoya N, García D, Osorio E, 2018. Effect of maternal immunization against pertussis in Medellin and the metropolitan area, Colombia, 2016–2017. Vaccine 36: 3984–3991. [DOI] [PubMed] [Google Scholar]
- 25. Siemens , 2015. Enzygnost® Anti-Measles-Virus IgG.
- 26. Mayor Mora A, Menjura F, Arias A, 2010. Cruzada interminable por la niñez colombiana: historia del Programa Ampliado de Inmunizaciones (PAI) en Colombia 1979–2009. Bogotá Minist Protección Soc Organ Panam Salud 2: 1979–2009. [Google Scholar]
- 27. Morón-Duarte LS, Castillo-Pabón JO, 2012. La vigilancia epidemiológica de sarampión y rubéola en el marco del plan de eliminación: Colombia 1995–2009. Rev Salud Publica (Bogota) 14: 1–14. [DOI] [PubMed] [Google Scholar]
- 28. Hincapie-Palacio D, Lenis Ballesteros V, Ospina MC, Toro OLP, Díaz FJ, 2013. Seroprevalence of rubella in Colombia: a birth-year cohort analysis. Rev Saude Publica 47: 1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Velandia M, Castillo O, Higuera AB, Pastor D, 2004. Eliminación de la rubéola y el síndrome de rubéola congénita, plan de acción 2004–2010. Inf Quinc Epidemiol Nac 9: 321–336. [Google Scholar]
- 30. Pinto DM, Muñoz AL, 2010. Colombia: Sistema general de seguridad social en salud. Estrategia del BID 2011–2014. Washington, DC: División de Protección Social y Salud, Inter-American Development Bank. [Google Scholar]
- 31. De los Angeles Rodríguez M, Restrepo C, Uribe G, Díaz FJ, Jaramillo N, Melguizo M, 1999. Estado serológico para sarampión en población de uno a 14 años. Medellín 1996. Colomb Med (Cali) 30: 8–82. [Google Scholar]
- 32. Bermúdez-Forero A, Mercado-Reyes M, Tavera P, Rey-Benito G, 2007. Determinación de la potencia del componente “sarampión” de la vacuna de virus vivo de sarampión y rubéola USP utilizada en la jornada de vacunacion 2005. Univ Sci (Bogota) 12: 87–94. [Google Scholar]
- 33. Mercado MM, Rey G, Gutiérrez MF, Ruíz JG, Trespalacios AA, Bermudez A del P, 2009. Estimación de la proporción de seropositividad contra el sarampión en madres y presencia de anticuerpos antes y después de la vacuna en sus hijos de 6 y 12 meses de edad. Pediatria (Napoli) 42: 25–37. [Google Scholar]
- 34. National Institute of Health, Republic of Colombia , 2019. PortalSivigila 2019 Buscador. Available at: http://portalsivigila.ins.gov.co/Paginas/Buscador.aspx#. Accessed November 25, 2020.
- 35. Plotkin SA, 2020. Is there a correlate of protection for measles vaccine? J Infect Dis 221: 1571–1572. [DOI] [PubMed] [Google Scholar]
- 36. Bolotin S. et al. , 2020. What Is the evidence to support a correlate of protection for measles? A systematic review. J Infect Dis 221: 1576–1583. [DOI] [PubMed] [Google Scholar]
- 37. Ministerio de Salud y Protección Social, Republic of Colombia Información vacunas y programas de vacunación Ministerio de Salud Colombia. Available at: https://www.minsalud.gov.co/salud/publica/Vacunacion/Paginas/pai.aspx. Accessed July 10, 2021.
- 38. Cetin Coban S. et al. , 2019. Prevalence of protective measles virus antibody levels in umbilical cord blood samples and sera of mothers and transplacental transport ratio in Turkey. Jpn J Infect Dis 72: 185–192. [DOI] [PubMed] [Google Scholar]
- 39. Gieles NC, Mutsaerts E, Kwatra G, Bont L, Cutland CL, Jones S, Moultrie A, Madhi SA, Nunes MC, 2020. Measles seroprevalence in pregnant women in Soweto, South Africa: a nested cohort study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 26: 515.e1–515.e4. [DOI] [PubMed] [Google Scholar]
- 40. Lam MTC, Schmidt-Beuchat E, Geduldig E, Brustman LE, Choi KH, Overbey JR, Woods KL, Al-Ibraheemi Z, 2021. What is the prevalence of measles immunity among pregnant women? Am J Perinatol 38: 16–22. [DOI] [PubMed] [Google Scholar]
- 41. Lee Y-C, Lee Y-H, Lu C-W, Cheng S-Y, Yang K-C, Huang K-C, 2020. Measles immunity gaps in an era of high vaccination coverage: a serology study from Taiwan. Travel Med Infect Dis 36: 101804. [DOI] [PubMed] [Google Scholar]
- 42. Gonçalves G, Frade J, Nunes C, Mesquita JR, Nascimento MSJ, 2015. Persistence of measles antibodies, following changes in the recommended age for the second dose of MMR-vaccine in Portugal. Vaccine 33: 5057–5063. [DOI] [PubMed] [Google Scholar]
- 43. Poethko-Müller C, Mankertz A, 2012. Seroprevalence of measles-, mumps- and rubella-specific IgG antibodies in German children and adolescents and predictors for seronegativity. PLoS One 7: e42867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vandermeulen C, Mathieu R, Geert L-R, Pierre VD, Karel H, 2007. Long-term persistence of antibodies after one or two doses of MMR-vaccine. Vaccine 25: 6672–6676. [DOI] [PubMed] [Google Scholar]
- 45. Arora M, Lakshmi R, 2021. Maternal vaccines—safety in pregnancy. Best Pract Res Clin Obstet Gynaecol 76: 23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]