ABSTRACT.
We report an autochthonous case of simple, localized cutaneous leishmaniasis in a healthy 18-month-old girl from southern Thailand. The patient presented with a solitary chronic cutaneous nodular lesion on her left cheek for approximately 1 year. Histopathological dissection of the cheek skin biopsy demonstrated remarkably nodular and interstitial infiltrates of lymphocytes and histiocytes full of intracellular oval-shaped amastigotes, consistent with cutaneous leishmaniasis. The Leishmania promastigotes were also cultured successfully from the lesion biopsy and were designated with the WHO code MHOM/TH/2021/CULE5. Using internal transcribed spacer 1-specific polymerase chain reaction, the parasite DNA was demonstrated in both saliva and lesion biopsy. Based on the BLASTn and phylogenetic analysis, the parasite was identified as Leishmania orientalis, clustered in the Mundinia subgenus. The patient responded well to a 6-week course of oral itraconazole, without recurrence. To our knowledge, this is the fourth case of autochthonous leishmaniasis resulting from L. orientalis and the youngest patient of leishmaniasis ever reported in Thailand. More importantly, we also demonstrate the clinical course of the lesion according to the timeline before and after treatment, which can help physicians better understand and provide an accurate diagnosis with appropriate treatment of this emerging parasitic disease.
INTRODUCTION
Human leishmaniasis is a neglected infectious disease that infects more than 1 million new cases worldwide annually.1 Approximately 53 Leishmania species have been recorded, 20 of which are known to be pathogenic to humans.2,3 Leishmania parasites have been known to be transmitted principally by phlebotomine sand flies.4 The spectrum of disease can be categorized broadly into three main forms: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis, and visceral leishmaniasis (VL).5 In Thailand, leishmaniasis is considered an emerging infectious disease because autochthonous leishmaniasis cases have increased continuously. The majority of cases are reported in the northern and southern provinces of the country, most frequently diagnosed with Leishmania martiniquensis and Leishmania orientalis.5,6 These two emerging Leishmania species have been classified recently in the same subgenus Mundinia with Leishmania sp. Ghana, Leishmania enriettii, and Leishmania macropodum.7–11 We describe the clinical presentation of autochthonous, localized CL caused by recently emerging L. orientalis in the youngest pediatric patient ever recorded in Thailand and compile all preceding information on indigenous leishmaniasis resulting from L. orientalis.
CASE DESCRIPTION
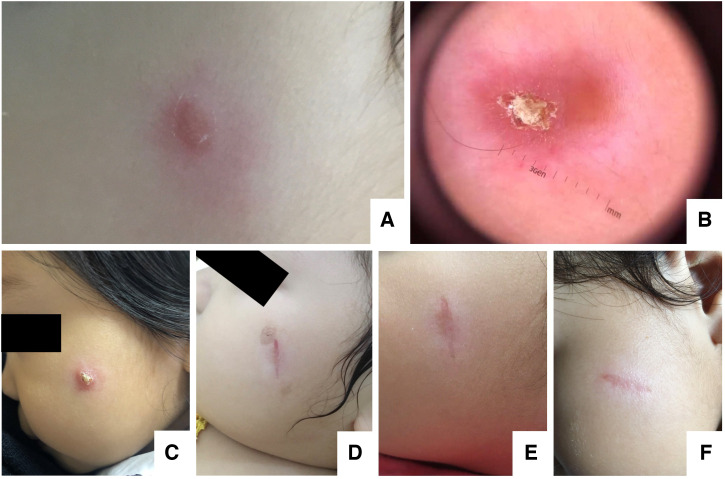
An 18-month-old Thai girl presented with a chronic, painless cutaneous nodule on her left cheek for ∼1 year. She was previously healthy with no known underlying disease. Initially, her mother noticed an erythematous papule on her left cheek when she was 6 months old. The papule gradually enlarged and transformed into a nodule. There was no history of travel outside Thailand before the appearance of the lesion. The patient was born and had lived in Phipun District, Nakorn Si Thammarat, southern Thailand until the age of 6 months, and then moved to Phuket. She had been evaluated several times at local private clinics and hospitals in Phuket; however, only a topical steroid cream, topical calcineurin inhibitors, and topical antibiotics were prescribed with no clinically significant improvement. On September 30, 2021, she visited Vachira Phuket Hospital with a 4 × 6 mm cutaneous erythematous nodule on her left cheek (Figure 1A). On October 15, 2021, a skin biopsy was performed and showed a dense, diffuse lymphohistiocytic infiltrate with numerous, small yeast-like intracellular organisms predominantly in the superficial dermis, and mild acanthosis with reactive changes at the overlying epidermis. CL and deep fungal infection, including penicilliosis, and histoplasmosis, were suspected. She was then referred to King Chulalongkorn Memorial Hospital in Bangkok for further investigations. Upon arrival at King Chulalongkorn Memorial Hospital November 23, 2021, a physical examination revealed the well-defined, solitary erythematous violaceous nodule and central overlying yellowish crust with a size of 5 × 7 mm on her left cheek (Figure 1B and C). No skin lesions in other sites were observed. Other examinations were within normal limits, including no fever, no lymphadenopathy, and no hepatosplenomegaly. Complete blood counts showed a hemoglobin level of 12.8 g/dL, a white blood cell count of 9,550 cells/mm3 (neutrophils, 33%; lymphocytes, 61%; monocytes, 4%; and atypical lymphocytes, 2%), and a platelet count of 414,000/mm3. Her HIV serology was negative. On December 7, 2021, an excisional biopsy of the lesion was performed. The histopathological examination was repeated (Figure 2) and showed the same findings as described previously by Vachira Phuket Hospital. In addition, the periodic acid–Schiff-, Gomori methenamine silver-, acid-fast bacilli-, and modified acid-fast bacilli-stained sections were negative for organisms. Cultures for Mycobacterium and fungus were also negative. Furthermore, the whole blood, saliva, and skin biopsy specimens were investigated for molecular diagnosis and Leishmania cultivation. Genomic DNA was extracted from the lesion biopsy, saliva, and whole blood specimens. Polymerase chain reaction (PCR) was conducted using specific primers (LeF: 5′-TCCGCCCGAAAGTTCACCGATA-3' and LeR: 5′-CCAAGTCATCCATCGCGACACG-3') with thermocycler condition reported previously12 to amplify a product of 371 bp spanning the internal transcribed spacer 1 (ITS1) with 18S and 5.8S ribosomal DNA flanking regions. Amplification was successful in biopsy and saliva specimens, but negative for whole blood (Figure 3). Direct sequencing and the BLASTn alignment were done, revealing that the obtained sequence was 100% identical to L. orientalis MHOM/TH/2010/PCM2 Trang (accession no. JX195640). The ITS1 sequence from this case was deposited in GenBank with accession no. ON303842. Leishmania orientalis promastigotes were cultured successfully from the lesion biopsy, exhibiting stage morphological variations (Figure 4). The culture isolate was assigned the WHO code MHOM/TH/2021/CULE5. The ITS1 phylogeny was also studied (Figure 5). Last, a definite diagnosis of localized CL resulting from L. orientalis was established. The patient was then treated with oral itraconazole 10 mg/kg/d for 6 weeks, with clinical improvement (Figure 1D–F). As of 4 months of follow-up, no relapse has been observed.
Figure 1.
(A) A cutaneous erythematous nodule on the left cheek in October 2021. (B, C) When referred to King Chulalongkorn Memorial Hospital, a well-defined, cutaneous, nodular and centrally crusted lesion with an irregular border was observed. Skin appearance 20 days postexcision (D), 48 days postexcision and 20 days after starting itraconazole (E), and 2 months postexcision and after completing a 6-week course of oral itraconazole (F).
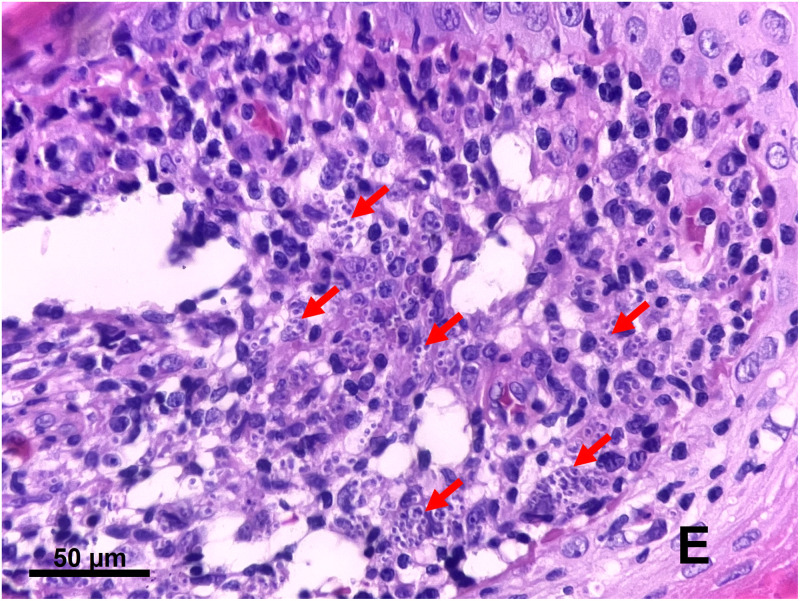
Figure 2.
Hematoxylin and eosin–stained section of the skin biopsy showing numerous intracellular amastigotes of Leishmania parasites in tissue macrophages (arrows), surrounded by several inflammatory cells in the upper dermis. E = epidermis.
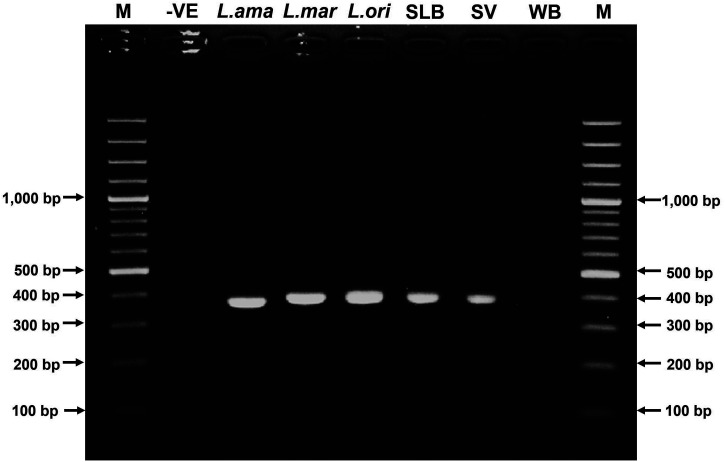
Figure 3.
Internal transcribed spacer 1 polymerase chain reaction products. Amplicon sizes are 362 to 379 bp. Lanes 1 through 9 are, from left to right, 100-bp ladder marker (M), negative control (-VE), Leishmania amazonensis MHOM/BR/1997/M2269 (L.ama, 362 bp), Leishmania martiniquensis MHOM/TH/2012/CULE1 (L.mar, 379 bp), Leishmania orientalis MHOM/TH/2014/LSCM4 (L.ori, 369 bp); skin lesion biopsy (SLB, 371 bp), saliva (SV, 371 bp), whole blood (WB, no band), and 100-bp ladder marker (M).
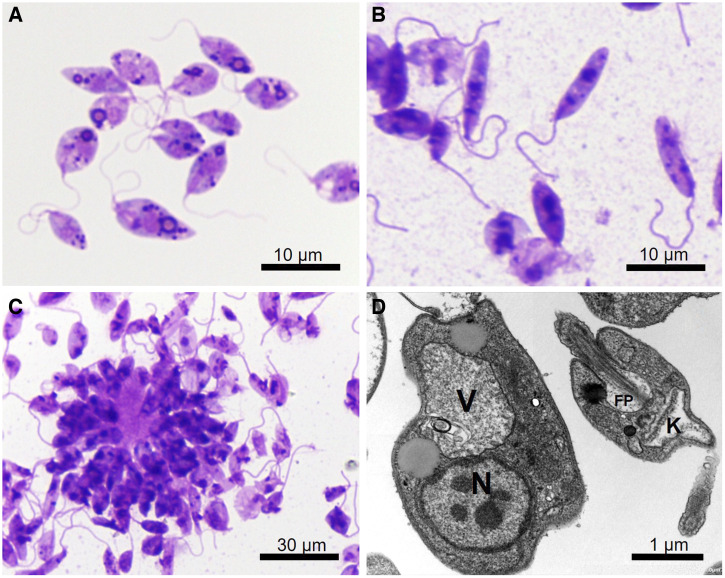
Figure 4.
Giemsa-stained Leishmania orientalis MHOM/TH/2021/CULE5 promastigotes from a culture of the patient’s biopsy tissue. (A) Procyclic form. (B) Leptomonad and nectomonad forms. (C) Rosette form. (D) Transmission electron micrograph showing the ultrastructure of the promastigotes. FP = flagellum within the flagellar pocket; K = kinetoplast; N = nucleus; V = large vacuole.
Figure 5.
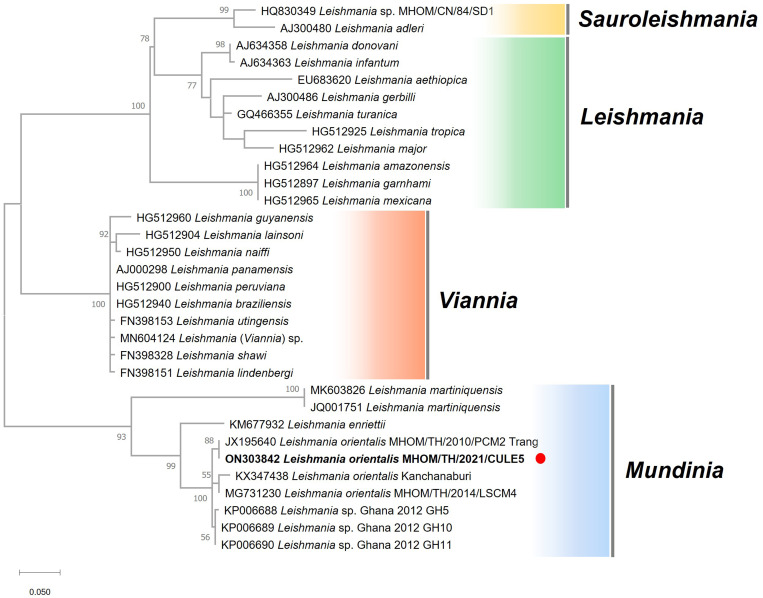
Molecular identification of the Leishmania clinical isolate as a member of the subgenus Mundinia. Maximum likelihood phylogeny of internal transcribed spacer 1 sequences from 32 Leishmania spp. isolates from four subgenera. Bootstrap branch support values greater than 50% are shown. The solid red circle indicates our patient.
DISCUSSION
Leishmania parasites are neglected pathogens that cause leishmaniasis in humans and animals worldwide. Since 1996, autochthonous leishmaniasis has been reported with increasing frequency. So far, more than 20 cases have been recorded, predominantly in the northern and southern provinces of Thailand. The two most prevalent Leishmania species in these indigenous cases were L. martiniquensis, and L. orientalis, respectively. Only one VL case resulting from L. infantum was reported in an HIV-seronegative male patient in 2008.13
We diagnosed an autochthonous case of localized CL in an 18-month-old Thai girl using Leishmania-specific PCR and in vitro parasite cultivation. Our study revealed that the multicopy ITS1 amplification and DNA sequencing analysis can greatly facilitate the definitive diagnosis of leishmaniasis. Based on the BLASTn and phylogenetic inference, the causative parasite was identified as L. orientalis. Interestingly, the histopathological examination in this case apparently exhibited a chronic, intense inflammatory infiltrate of lymphocytes and macrophages full of amastigote forms. Similar histopathological findings have also been reported in CL caused by other members of the Mundinia subgenus, including L. martiniquensis, and L. enriettii.9,14–18 Thus, it seems most likely that a remarkable feature of these Mundinia species is their ability to cause heavily parasitized macrophages, compared with Viannia and Leishmania subgenera.9,14–19 This patient was then considered the fourth leishmaniasis case caused by autochthonous L. orientalis, and the leishmaniasis patient with the youngest age reported thus far in Thailand.
In addition to the biopsy, we demonstrated, for the first time, the molecular detection of L. orientalis DNA using a saliva sample. As published previously,20 saliva has been reported as a promising clinical specimen for subclinical L. martiniquensis detection before the onset of symptoms. In addition, saliva can be used as an alternative source of parasite DNA for diagnosing molecularly malaria caused by Plasmodium falciparum and Plasmodium vivax.21,22 Therefore, saliva-based detection can be recommended as a rapid and noninvasive diagnostic test for disease surveillance and monitoring of emerging leishmaniasis in Thailand.
As reported previously6,23,24 in 2010, 2017, and 2018, the clinical presentations of leishmaniasis caused by L. orientalis could range from localized cutaneous to disseminated cutaneous, to visceral forms (Table 1). For the first two cases reported in 2010 and 2017, the parasites were formerly identified as Leishmania siamensis and designated as PCM2 Trang, and an anonymous isolate from Kanchanaburi, respectively. Although L. siamensis has been mentioned in several previous publications, this name was considered an invalid name because of the lack of formal parasite characterization.6,8 In 2018, Leishmania sp. strain LSCM4, isolated from a patient in Nan Province in 2014, was shown to be very similar genetically to the other two previous cases (PCM2 Trang and the anonymous isolate from Kanchanaburi), and thus was formally described with the taxonomic characterization as Leishmania (Mundinia) orientalis.6 Although its nomenclature refers to its eastern origin, L. orientalis has been reported exclusively in Thailand to date. Based on the ITS1 phylogeny, all four isolates of L. orientalis, including that in our patient, were identified as members of the same Mundinia subgenus as the other four Leishmania species (Figure 5).
Table 1.
Clinical information on autochthonous leishmaniasis cases resulting from Leishmania orientalis in Thailand from 2010 until 2021
| Patient no. | Year | Locality | Gender | Age, years | AIDS, CD4 count | Species | Manifestations | Treatment | Relapse | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | Trang | Female | 32 | Yes, CD4 = 107 cells/mm3 | L. orientalis MHOM/TH/2010/PCM2 Trang* | DCL (face, trunk, and legs), VL (severe anemia and generalized hepatomegaly) | There was no treatment because the patient died after 2 weeks from a seizure before amphotericin B administration | N/A | Bualert et al.23 |
| 2 | 2014 | Nan | Female | 57 | No | L. orientalis MHOM/TH/2014/LSCM4 | CL (left cheek and left mouth angle) | Oral amphotericin B (1 mg/kg/d for 1 day) and fluconazole (200 mg/kg/d for 45 days) | No | Jariyapan et al.6 |
| 3 | 2015 | Kanchanaburi | Female | 42 | Yes, CD4 = 89 cells/mm3 | L. orientalis anonymous isolate* (genetically identical to PCM2 Trang) | DCL (face, trunk, and both legs) | First intravenous amphotericin B (0.6 mg/kg/d for 4 weeks) and then additional intravenous amphotericin B (total dose, 1,260 mg), followed by itraconazole 400 mg/d until improved | No | Supsrisunjai et al.24 |
| 4 | 2021 | Nakhon Si Thammarat | Female | 1½ | No | L. orientalis MHOM/TH/2021/CULE5 | CL (left cheek) | Excisional biopsy and oral itraconazole (10 mg/kg/d for 6 weeks) | No | Our report |
CL = cutaneous leishmaniasis; DCL = disseminated cutaneous leishmaniasis; N/A = not available; VL = visceral leishmaniasis.
Species names identified previously as Leishmania siamensis and named retrospectively L. orientalis, with formal characterization in 2018.
Interestingly, all four L. orientalis isolates were also clustered in the phylogenetic tree closely with Leishmania sp. Ghana, which is responsible for localized CL in Ghana, West Africa. The existence of two closely related allopatric Leishmania species in different geographic localities strongly supports the hypothesis of supercontinent origins for the genus Leishmania before the breakup of Gondwana ∼200 million years ago.25,26 Therefore, phylogenomic analysis of the African and Asian Mundinia isolates will shed light on this speculation. In addition, most Ghanaian CL cases had a single lesion, and more than half the lesions were in the head region, similar to localized cutaneous presentation resulting from L. orientalis in our patient, in which the single lesion was located on the facial area. It has been noted8 that the head is typically an exposed area, especially at nighttime, and is more prone to a vector’s bite than other parts of the body.
From the clinical history known, our patient most likely acquired the infection before leaving Nakhon Si Thammarat, which is supported by the identical homology between the obtained ITS1 sequence with that of the PCM2 Trang isolate retrieved previously from the neighboring Trang Province. However, such two genetically similar isolates expressed different disease severity in patients with different immune statuses (Table 1). In addition, it is likely to have a greater degree of virulence in immunocompromised patients, with more severe manifestations, such as disseminated CL and VL. In contrast, mild CL was diagnosed in the non-HIV patients, with only a simple nodular lesion, as in our patient. Of note, CL should be added to the list of differential diagnoses for a chronic nodular lesion, especially in the leishmaniasis foci of the country.
The increasing incidence of autochthonous leishmaniasis cases in Thailand emphasizes the importance of identifying natural vectors and animal reservoirs of the parasites, which remain unclear. The DNA of L. orientalis (previously L. siamensis) was detected by heat shock protein 70-specific PCR in one female Sergentomyia iyengari sandfly collected around a VL patient’s house in Trang Province.27 In addition, the molecular detection of L. orientalis has been recently reported in Sergentomyia gemmea, collected from Chiang Rai Province, northern Thailand.28 However, a recent entomological investigation and phylogenetic analysis of the mitochondrial cytochrome b gene revealed that the morphological identification of Sergentomyia species is complicated, suggesting systematic revision for these potential vectors.29 More surprisingly, it was shown that L. orientalis promastigotes can develop in laboratory-reared biting midges (Culicoides sonorensis) and that C. sonorensis can transmit L. martiniquensis, L. orientalis, and Leishmania sp. Ghana to BALB/c mice in the laboratory.30,31 These findings implicate biting midges of the genus Culicoides as putative vectors in the transmission of human pathogenic Leishmania (Mundinia) species. Recently, L. martiniquensis DNA detection in biting midges Culicoides mahasarakhamense, collected from an affected area in Lamphun Province, northern Thailand, has been reported.32 Therefore, the species diversity of such hematophagous dipteran insects and the evidence of vector incrimination in different regions of Thailand, especially in the northern and southern provinces, need to be explored further. Essentially, the clinical isolates of autochthonous L. orientalis parasites need to be investigated to provide more biologic information about their differential virulence and to help us understand these emerging parasites more thoroughly.
ACKNOWLEDGMENTS
We acknowledge the staff members of the Center of Excellence in Vector Biology and Vector-Borne Disease, Department of Parasitology, Faculty of Medicine, Chulalongkorn University for their assistance in the laboratory investigations. We also give many thanks to our patient for her great contribution.
REFERENCES
- 1. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team , 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burza S, Croft SL, Boelaert M, 2018. Leishmaniasis. Lancet 392: 951–970. [DOI] [PubMed] [Google Scholar]
- 3. Herrera G. et al. , 2020. An interactive database of Leishmania species distribution in the Americas. Sci Data 7: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D, 2016. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis 10: e0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leelayoova S, Siripattanapipong S, Manomat J, Piyaraj P, Tan-Ariya P, Bualert L, Mungthin M, 2017. Leishmaniasis in Thailand: a review of causative agents and situations. Am J Trop Med Hyg 96: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jariyapan N, Daroontum T, Jaiwong K, Chanmol W, Intakhan N, Sor-Suwan S, Siriyasatien P, Somboon P, Bates MD, Bates PA, 2018. Leishmania (Mundinia) orientalis n. sp. (Trypanosomatidae), a parasite from Thailand responsible for localised cutaneous leishmaniasis. Parasit Vectors 11: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG, Shaw JJ, 2016. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 145: 430–442. [DOI] [PubMed] [Google Scholar]
- 8. Kwakye-Nuako G. et al. , 2015. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int J Parasitol 45: 679–684. [DOI] [PubMed] [Google Scholar]
- 9. Muniz J, Medina H, 1948. Cutaneous leishmaniasis of the guinea pig, Leishmania enriettii n. sp. Hosp (Lond) 33: 7–25. [PubMed] [Google Scholar]
- 10. Rose K, Curtis J, Baldwin T, Mathis A, Kumar B, Sakthianandeswaren A, Spurck T, Low Choy J, Handman E, 2004. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int J Parasitol 34: 655–664. [DOI] [PubMed] [Google Scholar]
- 11. Dougall AM, Alexander B, Holt DC, Harris T, Sultan AH, Bates PA, Rose K, Walton SF, 2011. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int J Parasitol 41: 571–579. [DOI] [PubMed] [Google Scholar]
- 12. Spanakos G, Piperaki ET, Menounos PG, Tegoa N, Flemetakis A, Vakalis NC, 2008. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg 102: 46–53. [DOI] [PubMed] [Google Scholar]
- 13. Maharom P, Siripattanapipong S, Mungthin M, Naaglor T, Sukkawee R, Pudkorn R, Wattana W, Wanachiwanawin D, Areechokchai D, Leelayoova S, 2008. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health 39: 988–990. [PubMed] [Google Scholar]
- 14. Phumee A, Chusri S, Kraivichian K, Wititsuwannakul J, Hortiwakul T, Thavara U, Silpapojakul K, Siriyasatien P, 2014. Multiple cutaneous nodules in an HIV-infected patient. PLoS Negl Trop Dis 8: e3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noppakun N, Kraivichian K, Siriyasatien P, 2014. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg 91: 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paraense WL, 1953. The spread of Leishmania enriettii through the body of the guineapig. Trans R Soc Trop Med Hyg 47: 556–560. [DOI] [PubMed] [Google Scholar]
- 17. Bryceson AD, Bray RS, Dumonde DC, 1974. Experimental cutaneous leishmaniasis: IV. Selective suppression of cell-mediated immunity during the response of guinea-pigs to infection with Leishmania enriettii. Clin Exp Immunol 16: 189–202. [PMC free article] [PubMed] [Google Scholar]
- 18. Pinheiro LJ, Paranaíba LF, Alves AF, Parreiras PM, Gontijo NF, Soares RP, Tafuri WL, 2018. Salivary gland extract modulates the infection of two Leishmania enriettii strains by interfering with macrophage differentiation in the model of Cavia porcellus. Front Microbiol 9: 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. González K, Diaz R, Ferreira AF, García V, Paz H, Calzada JE, Ruíz M, Laurenti M, Saldaña A, 2018. Histopathological characteristics of cutaneous lesions caused by Leishmania Viannia panamensis in Panama. Rev Inst Med Trop São Paulo 60: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siriyasatien P, Chusri S, Kraivichian K, Jariyapan N, Hortiwakul T, Silpapojakul K, Pym AM, Phumee A, 2016. Early detection of novel Leishmania species DNA in the saliva of two HIV-infected patients. BMC Infect Dis 16: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mharakurwa S, Simoloka C, Thuma PE, Shiff CJ, Sullivan DJ, 2006. PCR detection of Plasmodium falciparum in human urine and saliva samples. Malar J 5: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buppan P, Putaporntip C, Pattanawong U, Seethamchai S, Jongwutiwes S, 2010. Comparative detection of Plasmodium vivax and Plasmodium falciparum DNA in saliva and urine samples from symptomatic malaria patients in a low endemic area. Malar J 9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, Naaglor T, Ravel CEI, Baidouri F, Leelayoova S, 2012. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg 86: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Supsrisunjai C, Kootiratrakarn T, Puangpet P, Bunnag T, Chaowalit P, Wessagowit V, 2017. Disseminated autochthonous dermal leishmaniasis caused by Leishmania siamensis (PCM2 Trang) in a patient from central Thailand infected with human immunodeficiency virus. Am J Trop Med Hyg 96: 1160–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harkins KM, Schwartz RS, Cartwright RA, Stone AC, 2016. Phylogenomic reconstruction supports supercontinent origins for Leishmania. Infect Genet Evol 38: 101–109. [DOI] [PubMed] [Google Scholar]
- 26. Barratt J, Kaufer A, Peters B, Craig D, Lawrence A, Roberts T, Lee R, McAuliffe G, Stark D, Ellis J, 2017. Isolation of novel trypanosomatid, Zelonia australiensis sp. nov. (Kinetoplastida: Trypanosomatidae) provides support for a Gondwanan origin of dixenous parasitism in the Leishmaniinae. PLoS Negl Trop Dis 11: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siripattanapipong S, Leelayoova S, Ninsaeng U, Mungthin M, 2018. Detection of DNA of Leishmania siamensis in Sergentomyia (Neophlebotomus) iyengari (Diptera: Psychodidae) and molecular identification of blood meals of sand flies in an affected area, southern Thailand. J Med Entomol 55: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 28. Sriwongpan P. et al. , 2021. Prevalence and associated risk factors of Leishmania infection among immunocompetent hosts, a community-based study in Chiang Rai, Thailand. PLoS Negl Trop Dis 15: e0009545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Depaquit J. et al. , 2019. On the true identity of Sergentomyia gemmea and description of a closely related species: Se. raynali n. sp. Med Vet Entomol 33: 521–529. [DOI] [PubMed] [Google Scholar]
- 30. Chanmol W, Jariyapan N, Somboon P, Bates MD, Bates PA, 2019. Development of Leishmania orientalis in the sand fly Lutzomyia longipalpis (Diptera: Psychodidae) and the biting midge Culicoides sonorensis (Diptera: Ceratopogonidae). Acta Trop 199: 105157. [DOI] [PubMed] [Google Scholar]
- 31. Becvar T, Vojtkova B, Siriyasatien P, Votypka J, Modry D, Jahn P, Bates P, Carpenter S, Volf P, Sadlova J, 2021. Experimental transmission of Leishmania (Mundinia) parasites by biting midges (Diptera: Ceratopogonidae). PLoS Pathog 17: e1009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sunantaraporn S, Thepparat A, Phumee A, Sor-Suwan S, Boonserm R, Bellis G, Siriyasatien P, 2021. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Negl Trop Dis 15: e0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]