ABSTRACT.
Timely treatment-seeking behavior can reduce morbidity and mortality due to infectious diseases. Patterns of treatment-seeking behavior can differ by access to health care, and perceptions of disease severity and symptoms. We evaluated the association between self-reported symptoms at last illness and the level of treatment-seeking behaviors. We analyzed cross-sectional data from 1,037 participants from the lowlands and highlands of Western Kenya from 2015 using logistic regression models. There was considerable heterogeneity in the symptoms and treatment-seeking behaviors reported among individuals who were febrile at their last illness. A greater number of self-reported categories of symptoms tended to be associated with a higher likelihood of treatment-seeking in both sites. Participants were significantly more likely to seek treatment if they reported fever, aches, and digestive symptoms at last illness than just fever and aches or fever alone, but the frequency of treatment-seeking for fever in combination with aches and respiratory symptoms did not follow a consistent pattern. Among those who sought treatment, most used a formal source, but the patterns were inconsistent across sites and by the number of symptoms categories. Understanding the drivers of treatment-seeking behavior after febrile illness is important to control and treat infectious diseases in Kenya.
INTRODUCTION
Prompt diagnosis and early treatment are key to manage malaria and other infections effectively in endemic areas and are key pillars for the WHO’s global strategy for the elimination of malaria.1–10 Seeking and receiving immediate treatment after fever onset both prevents illness progression and limits infectiousness and transmission contribution. However, challenges persist, as the decision to seek treatment of an illness can be influenced by symptom severity and recognition, physical/geographic accessibility to sources of care, perceived disease severity, and quality of services, among other factors.2,3,9,11–15
In Kenya, where malaria transmission and burden greatly vary across the country, about 70% of the population is at risk of malaria infection, with approximately 13 million people at risk in endemic areas and 19 million in the epidemic and seasonal areas.16–18 The Kenyan Ministry of Health has a multipronged control strategy that involves using artemisinin-based combination therapy (ACT) as the primary line of treatment and also recommends confirmatory diagnosis of malaria among people of all ages and from all transmission settings, among other interventions.17,19 The healthcare service provision of Kenya classifies the services in the following levels: 1) community facilities (e.g., community health workers), 2) health dispensaries/clinics, 3) health centers, 4) county/district hospitals, 5) county/district referral hospitals, and 6) national referral hospitals.20,21 Each level have different responsibilities and resources, with levels 4 through 6 focusing on high-end curative and rehabilitating treatments.20 However, Kenyans may seek treatment of febrile illness from different sources, including shops, chemists, traditional healers, and private health facilities, among others,1,19,22 not all of which have the capacity to provide recommended care consistently.
Febrile illnesses are often associated with pathogens that cause high morbidity and mortality burdens in low-income countries. Fever, a general marker of illness, is thought to be an important symptom prompt to seek care for individuals and their children.12,23–25 In malaria-endemic areas, self-reported fever is often used as a proxy for malaria infection, and it is commonly used as a screening criterion among children under 5 years of age.8,12,26 However, fever is neither unique nor universal to Plasmodium infections. Malaria has been the leading cause of febrile illness for decades in several countries in sub-Saharan Africa, but its incidence has been declining in recent years due to increased coverage of control methods (e.g., insecticide-treated bed nets and indoor residual spraying) and improved access to ACT to treat clinical malarial cases.25,27,28 Despite significantly improved access to diagnostics, a significant number of health centers still rely on clinical diagnosis to make treatment and referral decisions.17,29,30 In older individuals, febrile illness is less common with Plasmodium infection, leading to underdiagnosis.26 Conversely, in younger children, acute febrile illnesses were commonly attributed to malaria and treated presumptively without diagnostic confirmation.31–33 However, the guidelines released by the WHO in 2010, recommend parasitological confirmation for all individuals showing fever and suspected of having malaria before providing treatment.34 A study conducted in two highlands sites of Kenya showed that the majority of cases of malaria occur among people older than 5 years of age and that reporting symptoms other than fever could lead to delayed detection and treatment.26 Many previous studies have focused on just fever or a specific set of malaria-associated symptoms, especially among children under 5 years of age, but the experience of other symptoms likely influences treatment-seeking behaviors. The decision to seek treatment is based on an individual’s experience of symptoms or observation of symptoms in their children or family members and not underlying causes.
Residents of western Kenya experience high risks of infection from endemic or epidemic malaria, among other febrile infectious diseases, including acute respiratory infections (i.e., pneumonia and influenza).4,32,35 However, little is known about symptoms at last illness, including fever, as a predictor of treatment-seeking in the lowlands and highlands of Western Kenya. This study used self-reported fever and other symptom combinations to assess symptoms experienced at last illness as a driver of treatment-seeking behaviors. The study was intended to evaluate the association between self-reported symptoms at last illness and treatment-seeking behaviors. Many studies have identified age, wealth quartile, severity perception of malaria, and education level as predictors of treatment-seeking.2,36,37 Therefore, we aimed to determine whether these factors are associated with treatment-seeking and source of treatment. We hypothesized that participants reporting fever in conjunction with other symptoms at the last time of illness would have been more likely to seek treatment than those reporting only fever. We further hypothesized that participants who sought treatment would have been more likely to use formal sources of treatment if they reported multiple categories of symptoms in addition to fever at their last febrile illness. This work classifies hospitals and clinics as formal sources because it was more likely that participants who reported going to these facilities received malaria diagnostic testing to guide treatment. Chemists, herbalists, spiritual healers, and “other” were classified as informal treatment sources based on the assumption that treatment would be unlikely to depend on diagnostic test results.
MATERIALS AND METHODS
Study site description and design.
Cross-sectional data were collected from households in western Kenya after the rainy season onset between June and August 2015.38,39 Selected villages were in the highlands (Kapkangani) and the lowlands (Miwani). Kapkangani, located in Nandi district in the western Kenyan highlands, has a population primarily comprised of Kalenjin and Luhya ethnic groups and mostly used in small-scale agriculture. The highlands are located at 1,500 meters above the sea level and have a cool climate (10–26°C).18,40,41 Miwani is located on the Kano Plain, Kisumu district, and encompasses the lowlands of western Kenya. People are primarily of the Luo tribe and primarily work on farming with some occasionally employed by the sugar cane and rice farms. The lowlands are located close to Lake Victoria and have a dry and hot climate (18–34°C) between January and March.18,41,42 In general, Kenya experiences hot temperatures from December through March and cool temperatures from June through August, with variations across the country.18 Kenya has a two rainfall patterns, taking place between March and May (characterized by long rains) and October and December (characterized by short rains).18 The study sites were chosen for their differences in malaria transmission and prevalence. Malaria transmission in the highlands is low and intermittent or epidemic, showing considerably yearly variation but predictable as it follows the seasonality of the rainfall patterns.4,41,43 Moreover, malaria is generally more severe and causes higher mortality in the highlands due to its long-term fluctuations of transmission.40,41 The incidence of malaria is high in the lowlands, with transmission occurring year-round (holo-endemic).4,41,43
Household sampling and participants recruitment.
Before household selection, the study site was mapped using ArcGIS and enumerated using updated data from the 2014 updated census from five sublocations (two in the lowlands and three in the highlands).38,39 All household members were listed using demographic information, including age, gender, and relationship with household head.38 This study involved sampling 50 households from each of the five sublocations from a randomly enumerated list, yielding a total of 250 households. Additional households were sampled proportional to size for each village using a list created of all households that were located withing a 1-km buffer around any previously selected household using ArcGIS.38 The sampling strategy involved a 20% oversample of households to supplement the sample sizes of the villages and to allow for replacement in case the household members of selected households were absent or declined participation from this study.38 Households were selected and visited in random order until the required sample size for each village was obtained.
For this survey, a household was defined as individuals who regularly ate meals together.38 Households were included if participants had resided in the study area for a period of at least 1 month. Household of all sizes and configurations were included. Participants were recruited in person by trained field personnel.38
Data collection and management.
Female household heads or primary female caretakers were interviewed to list all household members and complete household-level and individual-level surveys. Male household heads were surveyed when there was no female household head or she was absent. The head of household provided the information for all the children living in their household. The surveys collected information about demographics, treatment-seeking and symptoms at last illness, and risk perception and prevention of malaria.
A team leader was assigned for each field team and was responsible for reviewing and initiating all the data collected.38 After approval from the team leader, the forms were placed inside envelopes sorted by households and returned to the research office.38 Here, the site leader reviewed the forms, and if any discrepancies were noted, the forms were returned to the field teams for correction the next day.38
Measures and variables.
The primary outcomes of interest were any self- or caregiver-reported treatment-seeking at last illness and source of treatment sought. Treatment-seeking was dichotomized into sought treatment or did not seek treatment. Participants were asked to indicate all locations where they sought medical care for themselves or their family members. Sources of treatment were dichotomized into formal and informal, with subjects identified as seeking treatment at formal sources if they selected at least one formal source. Hospitals and clinics were classified as formal sources, and chemists, herbalists, spiritual healers and “other” were classified as informal treatment sources.
The primary explanatory variable was self-reported symptoms, measured by asking household respondents to select all symptoms they had experienced the last time they were ill within the past 12 months from a detailed list. Self-reported symptoms at last illness were then classified into the following mutually exclusive categories: 1) fever only; 2) fever and any ache(s) (headaches or body aches); 3) fever, any ache(s), and any digestive symptom(s) (inability to feed, diarrhea, or vomiting); 4) fever, any ache(s), and any respiratory symptom(s) (cough, difficulty breathing, or fast breathing); and 4) fever and any other symptom(s) or combination of symptoms (aches, digestive, respiratory, convulsions/loss of consciousness, and rash).
The present analyses were restricted to the subset of individuals that reported having a fever at last illness within the previous month. Participants were excluded if 1) they experienced fever more than 1 month before the time of the survey, 2) their treatment-seeking status or the source of treatment was unknown or missing; 3) the research team (Kenyan Medical Research Institute [KEMRI]) provided on-site treatment at the time of the survey; or 4) they declined answers or had missing values for the questions about the symptoms at last illness.
The covariates considered for univariate comparisons included sex, age, household wealth quartile (based on an inverse frequency-weighted asset index,38 ranging from lowest to highest), education level of female household head, and perceived malaria severity. Perceived malaria severity was measured by asking household respondents to rate the seriousness of malaria as a problem for their families and their communities, each on a scale of 1 to 5, with 5 being the most severe. The responses for the family and community questions were summed, and the perceived severity could therefore range from 2 to 10, which we then categorized as “low” (2–4), “moderate” (5–7), or “high” (8–10).
Data analysis.
Descriptive statistics were used to present the estimated proportions for both the outcomes of any treatment-seeking among those with a fever at last illness and of seeking-treatment at a formal source among those who chose to seek any treatment. Univariate comparisons between self-reported symptoms and other covariates, and treatment-seeking and source of treatment-seeking were tested using Pearson’s χ2 tests. We used unadjusted logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of 1) treatment-seeking and 2) source of treatment among those who reported treatment-seeking at last febrile illness, stratified by site. Multilevel models were used to account for clustering among observations at the household level. “Fever and any aches” was used as the reference category for the logistic regression models, as the number of individuals in the highlands who experienced fever alone was small and led to imprecise estimates. Because the malaria epidemiology differs dramatically between the highlands and lowlands, models were run separately for each site. All tests were two-sided, P values < 0.05 were considered significant, and all analyses were performed using STATA 14 (StataCorp, College Station, TX).44
Ethics statement.
The current study involved secondary analyses of deidentified data from the primary study. Ethical approval for the primary study was granted by the KEMRI as minimal risk (SSC #2810). The Institutional Review Board from the University of Arizona deferred review for this study to KEMRI. All residents aged 18 years and older of selected households provided informed consent for the study. Parental consent and assent were obtained for the household members aged 7 to 17 years.
RESULTS
Population characteristics.
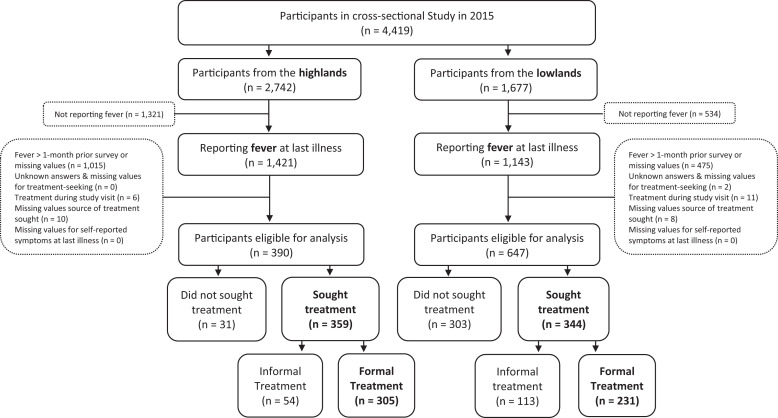
Of the 4,419 individuals, 390 (37.6%) of 2,742 from the highlands and 647 (62.4%) of 1,677 from the lowlands reported having a fever at the time of survey or within a month before and provided complete information on symptoms and treatment-seeking (Figure 1). More participants were female than male from both sites, but no significant difference in sex was observed between the highlands and lowlands (P = 0.39). In general, participants reporting fever in the lowlands tended to be in the highest wealth quartiles (P < 0.001), to have primary or some primary education (P < 0.001), and to have higher perceived malaria severity (< 0.001) than participants reporting fever in the highlands (Table 1). Symptom categories also differed significantly between highland and lowland participants (P < 0.001). In all, 54% and 78% of participants from the highlands and lowlands, respectively, reported fever at last illness. Moreover, participants from the lowlands reported more symptoms (N = 647), than participants from the lowlands (N = 390). Fever was most frequently accompanied by aches alone in the lowlands (N = 208, 32.2%) and by aches and digestive symptoms in the highlands (N = 230, 59.0%). In all, 359 (92.1%) people in the highland villages sought some form of treatment of their last illness compared with only 344 (53.2%) in the lowland villages (P < 0.001) (Table 1). Participants who sought treatment at last illness in the highlands and lowlands were more likely to have sought some formal treatment (85.0% and 67.2%, respectively) than solely informal treatment, and those in the highlands were significantly more likely to seek formal treatment than those in the lowlands (P < 0.001). A flow diagram of the overall classification of self-reported symptoms is included in Supplemental Figure 1.
Figure 1.
Flow diagram of participants from the cross-sectional study in Western Kenya in 2015 included in the analysis. Outcomes of interest are indicated in bold.
Table 1.
Characteristics of participants reporting fever in the previous month in lowlands and highlands of Western Kenya, 2015*
| Overall (N = 1,037) | Highlands (N = 390) | Lowlands (N = 647) | P value† | |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Self-reported symptoms‡ | ||||
| Fever alone | 178 (17.2) | 11 (2.8) | 167 (25.8) | < 0.001 |
| Fever + aches | 245 (23.6) | 37 (9.5) | 208 (32.2) | |
| Fever + aches + digestive symptoms | 293 (28.3) | 230 (59.0) | 63 (9.7) | |
| Fever + aches + respiratory symptoms | 99 (9.6) | 31 (8.0) | 68 (10.5) | |
| Fever + other symptoms | 222 (21.4) | 81 (20.8) | 141 (21.8) | |
| Treatment-seeking | ||||
| Sought treatment | 703 (67.8) | 359 (92.1) | 344 (53.2) | < 0.001 |
| Did not seek treatment | 334 (32.2) | 31 (8.0) | 303 (46.8) | |
| Source of treatment§ | ||||
| At least one formal source | 536 (76.2) | 305 (85.0) | 231 (67.2) | < 0.001 |
| Only informal sources | 167 (23.8) | 54 (15.0) | 113 (32.9) | |
| Perceived malaria severity‖ | ||||
| Low | 111 (10.8) | 47 (12.2) | 64 (9.9) | < 0.001 |
| Moderate | 533 (51.7) | 291 (75.6) | 242 (37.5) | |
| High | 387 (37.5) | 47 (12.2) | 340 (52.6) | |
| Gender | ||||
| Female | 603 (58.2) | 233 (59.9) | 370 (57.2) | 0.39 |
| Male | 433 (41.8) | 156 (40.1) | 277 (42.8) | |
| Household wealth quartile | ||||
| Lowest | 205 (19.8) | 96 (24.6) | 109 (16.9) | < 0.001 |
| Second | 289 (27.9) | 171 (43.9) | 118 (18.2) | |
| Third | 243 (23.4) | 53 (13.6) | 190 (29.4) | |
| Highest | 300 (28.9) | 70 (18.0) | 230 (35.6) | |
| Education of household head¶ | ||||
| No education | 35 (3.4) | 19 (4.9) | 16 (2.5) | < 0.001 |
| Primary or some primary | 749 (72.7) | 269 (69.3) | 480 (74.7) | |
| Secondary or some secondary | 179 (17.4) | 57 (14.7) | 122 (19.0) | |
| Other | 68 (6.6) | 43 (11.1) | 25 (3.9) | |
| Age category# | ||||
| Young children | 242 (23.3) | 86 (22.1) | 156 (24.1) | 0.04 |
| School children | 302 (29.1) | 121 (31.0) | 181 (28.0) | |
| Young adults | 206 (19.9) | 61 (15.6) | 145 (22.4) | |
| Adults | 215 (20.7) | 91 (23.3) | 124 (19.2) | |
| Elderly | 72 (6.9) | 31 (8.0) | 41 (6.3) | |
Missing data for specific characteristics in this table ranged from 0% to 4%.
P values are for the test of highlands versus lowlands.
Aches includes body aches and headaches; digestive symptoms include inability to feed, diarrhea, and vomiting; respiratory symptoms includes cough, difficulty breathing, and fast breathing; other symptoms includes convulsions/loss of consciousness, rash, and symptom combinations not otherwise classified.
Formal treatment includes hospital and clinics; informal treatment includes chemist, herbalist, spiritual healer, and other sources of treatment.
Perceived malaria severity was defined as the sum of two household-level questions about perceived malaria severity that were each ranked on a scale of 1 (least severe) to 5 (most severe), and each household was then categorized as low = 2–4, moderate = 5–7, or high = 8–10 perceived severity.
Based on the education level of the female household head; “other” educational level includes tertiary education, other, and no female household head.
Young children includes ≤ 5 years; school children includes > 5 to ≤ 15 years; young adults includes > 15 to ≤ 30 years; adults includes > 30 to ≤ 60 years; elderly includes > 60 years.
Factors associated with treatment-seeking at last febrile illness.
Odds ratios for predictors of treatment-seeking at last illness were obtained using multilevel unadjusted logistic regression models accounting for household-level clustering (Table 2). Participants who reported fever without other symptoms were much less likely to seek treatment than people who reported other symptom categories in conjunction with fever at their last illness in both sites but not significant for the highlands. Reporting symptoms in a greater number of categories tended to be associated with a higher likelihood of treatment-seeking in both sites. In the highlands, a large proportion of participants reported experiencing fever, aches, and digestive symptoms at their last illness (59.0%), for which they were highly likely to report seeking treatment (61.3%). In the highlands, those with fever, aches, and digestive symptoms were significantly more likely to seek treatment than those with just fever and aches (OR = 5.13, 95% CI: 1.87–14.09).
Table 2.
Predictors of any treatment-seeking among those experiencing fever in the previous month in Western Kenya, 2015
| Predictors | Highlands | Lowlands | ||||
|---|---|---|---|---|---|---|
| Sought treatment | Sought treatment | |||||
| Total N | n (%) | Crude OR (95% CI) | Total N | n (%) | Crude OR (95% CI) | |
| Self-reported symptoms* | ||||||
| Fever alone | 11 | 8 (2.2) | 0.62 (0.12–3.32) | 167 | 28 (8.1) | 0.19 (0.11–0.33) |
| Fever + aches | 37 | 30 (8.4) | 1.00 [reference] | 208 | 107 (31.1) | 1.00 [reference] |
| Fever + aches + digestive | 230 | 220 (61.3) | 5.13 (1.87–14.09) | 63 | 53 (15.4) | 5.00 (2.35–10.70) |
| Fever + aches + respiratory | 31 | 29 (8.1) | 3.38 (0.64–18.01) | 68 | 55 (16.0) | 3.99 (1.99–8.02) |
| Fever + other symptoms | 81 | 72 (20.1) | 1.87 (0.60–5.78) | 141 | 101 (29.4) | 2.38 (1.47–3.87) |
| Perceived malaria severity† | ||||||
| Low | 47 | 42 (11.9) | 1.00 [reference] | 64 | 57 (16.6) | 1.00 [reference] |
| Moderate | 291 | 273 (77.1) | 1.81 (0.64–5.09) | 242 | 133 (38.8) | 0.15 (0.06–0.35) |
| High | 47 | 39 (11.0) | 0.58 (0.14–2.49) | 340 | 153 (44.6) | 0.10 (0.04–0.22) |
| Gender | ||||||
| Female | 233 | 215 (60.1) | 1.00 [reference] | 370 | 214 (62.2) | 1.00 [reference] |
| Male | 156 | 143 (39.9) | 0.92 (0.44–1.94) | 277 | 130 (37.8) | 0.64 (0.47–0.88) |
| Household wealth quartile | ||||||
| Lowest | 96 | 93 (25.9) | 1.00 [reference] | 109 | 63 (18.3) | 1.00 [reference] |
| Second | 171 | 158 (44.0) | 0.39 (0.10–1.55) | 118 | 69 (20.1) | 1.03 (0.55–1.93) |
| Third | 53 | 47 (13.1) | 0.25 (0.06–1.04) | 190 | 115 (33.4) | 1.12 (0.62–2.01) |
| Highest | 70 | 61 (17.0) | 0.22 (0.06–0.84) | 230 | 97 (28.2) | 0.53 (0.30–0.94) |
| Education of household head‡ | ||||||
| No education | 19 | 17 (4.8) | 1.00 [reference] | 16 | 10 (2.9) | 1.00 [reference] |
| Primary or some primary | 269 | 254 (71.2) | 1.99 (0.41–9.79) | 480 | 249 (72.8) | 0.65 (0.19–2.18) |
| Secondary or some secondary | 57 | 47 (13.2) | 0.55 (0.11–2.78) | 122 | 71 (20.8) | 0.84 (0.23–3.01) |
| Other | 43 | 39 (10.9) | 1.15 (0.20–6.54) | 25 | 12 (3.5) | 0.55 (0.13–2.32) |
| Age category§ | ||||||
| Young children | 86 | 81 (22.6) | 1.00 [reference] | 156 | 96 (27.9) | 1.00 [reference] |
| School children | 121 | 110 (30.6) | 0.62 (0.19–2.02) | 181 | 106 (30.8) | 0.88 (0.56–1.38) |
| Young adults | 61 | 55 (15.3) | 0.57 (0.16–1.99) | 145 | 61 (17.7) | 0.45 (0.28–0.73) |
| Adults | 91 | 83 (23.1) | 0.64 (0.22–1.86) | 124 | 61 (17.7) | 0.61 (0.38–0.96) |
| Elderly | 31 | 30 (8.4) | 1.85 (0.21–16.6) | 41 | 20 (5.8) | 0.60 (0.29–1.21) |
CI = confidence interval; OR = odds ratio.
Aches includes body aches and headaches; digestive symptoms include inability to feed, diarrhea, and vomiting; respiratory symptoms includes cough, difficulty breathing, and fast breathing; other symptoms includes convulsions/loss of consciousness, rash, and symptom combinations not otherwise classified.
Perceived malaria severity was defined as the sum of two household-level questions about perceived malaria severity that were each ranked on a scale of 1 (least severe) to 5 (most severe), and each household was then categorized as low = 2–4, moderate = 5–7, or high = 8–10 perceived severity.
Based on the education level of the female household head; “Other” educational level includes tertiary education, other and no female household head.
Young children includes ≤ 5 years; school children include > 5 – ≤ 15 years; young adults include > 15 – ≤ 30 years; adults include > 30 – ≤ 60 years; elderly include > 60 years.
Participants from the lowlands self-reporting fever, aches, and digestive symptoms had significantly higher odds of treatment-seeking (OR = 5.00; 95% CI: 2.35–10.70) compared with participants self-reporting just fever and aches, with even fewer seeking treatment of fever alone (OR = 0.19, 95% CI: 0.11–0.33). There was considerable variability in the predictors of treatment-seeking when comparing the lowlands and highlands, with perceived malaria severity, gender, house wealth quartile, and age reaching statistical significance in the lowlands. For example, those participants with a high perceived malaria severity in the lowlands were significantly less likely to seek treatment than those with a low perceived malaria severity (OR = 0.10, 95% CI: 0.04–0.22). There were no statistically significant associations between the education of the female household head in either site.
Interestingly, among those who sought some form of treatment, the percentage of people using at least one formal treatment source did not consistently increase with the number of symptom categories reported in either the highlands or lowlands (Table 3). However, these relationships were based on small sample sizes, which limited the power of the analyses. In the highlands, those with fever, aches, and digestive symptoms were more likely to seek formal treatment than those with just fever and aches (OR = 1.95, 95% CI: 0.18–17.11), but these differences did not reach statistical significance. In the lowlands, people reporting fever, aches, and respiratory symptoms (OR = 1.85, 95% CI: 0.85–4.05) and those only reporting fever (OR = 1.72, 95% CI: 0.57–5.21) had higher odds of formal treatment-seeking compared with those self-reporting fever and aches, but again, the differences did not reach statistical significance. Factors associated with the source of treatment at last illness were inconsistent across study sites. In the highlands, the ORs for seeking formal treatment decreased with increasing perception of malaria severity; in the lowlands, the ORs for seeking formal treatment increased as the perceived malaria severity increased (ORHigh = 3.61, 95% CI: 1.73–7.53). The household wealth quartile was not a significant predictor of using formal treatment sources in the highlands but increasing household wealth quartile strongly predicted the odds of seeking formal treatment in the lowlands.
Table 3.
Predictors for using formal treatment sources when seeking treatment among those experiencing fever in the previous month in Western Kenya, 2015
| Predictors | Highlands | Lowlands | ||||
|---|---|---|---|---|---|---|
| Formal treatment* | Formal treatment* | |||||
| Total N | n (%) | Crude OR (95% CI) | Total N | n (%) | Crude OR (95% CI) | |
| Self-reported symptoms† | ||||||
| Fever alone | 11 | 7 (2.3) | 1.75 (0.18–17.11) | 167 | 21 (9.1) | 1.72 (0.57–5.21) |
| Fever + aches | 37 | 24 (7.9) | 1.00 [reference] | 208 | 68 (29.4) | 1.00 [reference] |
| Fever + aches + digestive | 230 | 195 (63.9) | 1.95 (0.79–4.84) | 63 | 32 (13.9) | 0.87 (0.46–1.67) |
| Fever + aches + respiratory | 31 | 23 (7.5) | 0.96 (0.25–3.62) | 68 | 42 (18.2) | 1.85 (0.85–4.05) |
| Fever + other symptoms | 81 | 56 (18.4) | 0.88 (0.32–2.41) | 141 | 68 (29.4) | 1.18 (0.65–2.14) |
| Perceived malaria severity‡ | ||||||
| Low | 47 | 36 (12.0) | 1.00 [reference] | 64 | 27 (11.7) | 1.00 [reference] |
| Moderate | 291 | 233 (77.7) | 0.97 (0.37–2.54) | 242 | 86 (37.4) | 2.03 (0.97–4.27) |
| High | 47 | 31 (10.3) | 0.65 (0.18–2.28) | 340 | 117 (50.9) | 3.61 (1.73–7.53) |
| Gender | ||||||
| Female | 233 | 185 (60.9) | 1.00 [reference] | 370 | 148 (64.1) | 1.00 [reference] |
| Male | 156 | 119 (39.1) | 0.80 (0.42–1.53) | 277 | 83 (35.9) | 0.79 (0.49–1.27) |
| Household wealth quartile | ||||||
| Lowest | 96 | 75 (24.6) | 1.00 [reference] | 109 | 36 (15.6) | 1.00 [reference] |
| Second | 171 | 137 (44.9) | 1.57 (0.75–3.27) | 118 | 48 (20.8) | 1.71 (0.81–3.65) |
| Third | 53 | 39 (12.8) | 1.17 (0.47–2.89) | 190 | 76 (32.9) | 1.46 (0.74–2.90) |
| Highest | 70 | 54 (17.7) | 1.85 (0.71–4.84) | 230 | 71 (30.7) | 2.05 (1.00–4.18) |
| Education of household head§ | ||||||
| No education | 19 | 14 (4.6) | 1.00 [reference] | 16 | 4 (1.8) | 1.00 [reference] |
| Primary or some primary | 269 | 217 (71.6) | 1.26 (0.38–4.18) | 480 | 165 (72.1) | 2.95 (0.70–12.41) |
| Secondary or some secondary | 57 | 42 (13.9) | 1.80 (0.42–7.64) | 122 | 53 (23.1) | 4.42 (0.95–20.64) |
| Other | 43 | 30 (9.9) | 0.71 (0.17–2.96) | 25 | 7 (3.1) | 2.10 (0.34–12.94) |
| Age category‖ | ||||||
| Young children | 86 | 71 (23.3) | 1.00 [reference] | 156 | 68 (29.4) | 1.00 [reference] |
| School children | 121 | 94 (30.8) | 0.83 (0.34–2.04) | 181 | 74 (32.0) | 0.95 (0.52–1.73) |
| Young adults | 61 | 46 (15.1) | 0.72 (0.29–1.81) | 145 | 40 (17.3) | 0.78 (0.38–1.60) |
| Adults | 91 | 68 (22.3) | 0.64 (0.28–1.46) | 124 | 34 (14.7) | 0.52 (0.26–1.04) |
| Elderly | 31 | 26 (8.5) | 0.92 (0.26–3.25) | 41 | 15 (6.5) | 1.24 (0.41–3.70) |
CI = confidence interval; OR = odds ratio.
Formal treatment includes hospital and clinics; informal treatment includes chemist, herbalist, spiritual healer, and other sources of treatment.
Aches includes body aches and headaches; digestive symptoms include inability to feed, diarrhea, and vomiting; respiratory symptoms includes cough, difficulty breathing, and rapid breathing; other symptoms includes convulsions/loss of consciousness, rash, and symptom combinations not otherwise classified.
Perceived malaria severity was defined as the sum of two household-level questions about perceived malaria severity that were each ranked on a scale of 1 (least severe) to 5 (most severe), and each household was then categorized as low = 2–4, moderate = 5–7, or high = 8–10 perceived severity.
Based on the education level of the female household head; “other” educational level includes tertiary education, other, and no female household head.
Young children includes ≤ 5 years; school children include > 5 to ≤ 15 years; young adults include > 15 to ≤ 30 years; adults include > 30 to ≤ 60 years; elderly include > 60 years.
DISCUSSION
This study demonstrated that there is an association between self-reported symptoms at last illness and treatment-seeking behaviors. People with fever at last illness are a complex group because they may be experiencing additional symptoms that can influence their decision to seek treatment. Our results show that relatively few people sought treatment of fever alone in either site. We found that self-reporting multiple categories of symptoms was significantly associated with increased treatment-seeking in the lowlands and highlands of Western Kenya. Another interesting finding was the considerable heterogeneity in self-reported symptom distribution and treatment-seeking behaviors even in relatively close geographic areas. The heterogeneity of treatment-seeking behaviors can be masked if having fever is the only potential predictor measured in a study. Taken together, these results suggest limited generalizability of findings on treatment-seeking behaviors for fever. Our results showed that most people sought treatment from formal sources at their last illness, which is comparable to a study in southwest Ethiopia.2 Seeking treatment of febrile illness can help prevent illness progression and can aid in the control and elimination of malaria in endemic countries by decreasing its transmission.
Although we found that few people sought treatment of fever alone, we were not able to explore if this was a result of using over-the-counter pain killers to treat fever at home. Our results showed differences in reported symptom categories between the lowlands and the highlands. In the case of malaria, this was expected because its prevalence varies in both sites. The Malaria Indicator Survey conducted in 2015, estimated that the prevalence of malaria (confirmed with microscopy) was 3.1% in the highlands and 26.7% in the lowlands. Also, it is known that people living in the highland endemic areas are more likely to manifest more severe and intense symptoms than people living in the lowland epidemic areas due to the absence of partial immunity from repeated infections.45 However, the burden of malaria has declined in Kenya in the past 2 decades.18,32 In a study conducted among febrile children in Kenya, 41% of children were diagnosed with acute respiratory infections (with influenza being the most detected) and only 5.2% of febrile children were confirmed to have malaria.32 Therefore, the etiologies of nonmalaria febrile illnesses and their burden in the highlands and lowlands of western Kenya are unclear.18,32
In general, participants from the highlands sought more treatment of different combinations of symptoms than those from the lowlands. This difference in treatment-seeking for fever between lowlands and highlands could have been a result of the access to healthcare services. The residents of the highlands have access to more sophisticated and staffed health care facilities, including health centers and county hospitals, which are higher tiers of care in Kenya. In contrast, access to healthcare is limited in the lowlands, due to its distance and levels of care, with community facilities being more predominant. However, information about measuring access to healthcare services was not collected in this study.
Factors associated with treatment-seeking and source of treatment at last illness appear to be inconsistent across our two major study sites and when compared with the literature.37,46–48 For instance, in our study, elderly participants were most likely to report seeking treatment at last illness in the highlands. Studies from southeast Ethiopia and Malawi reported higher odds of treatment-seeking among children under 5 years compared with other ages.2,37 An unexpected finding of our study was that higher perceived malaria severity was associated with lower odds of treatment-seeking and use of formal treatment sources in the highlands. Treatment-seeking behaviors have been studied using other metrics for the perception of symptoms and severity of malaria.2,12 For example, a study in southwest Ethiopia found that participants would delay seeking treatment of febrile illness because malaria was perceived as less severe illness, based solely on qualitative data.2 The findings of our study emphasize that further research is needed to understand treatment-seeking behaviors and identify potential drivers due to the high degree of heterogeneity and lack of generalizability of our findings.
Our results demonstrated that self-reporting a greater number of symptoms was associated with a higher likelihood of treatment-seeking in the lowlands and highlands. These results have implications on how healthcare services delivery and case management can be improved to ensure prompt diagnosis and treatment, which are crucial to reduce the morbidity and mortality caused by febrile illness, especially malaria. Currently, community health workers (CHWs) are a component of the integrated community case management (iCCM) model designed to improve diagnosis and treatment of pneumonia, diarrhea, and uncomplicated malaria that are affecting the population, particularly children under 5 years, in resource-limited countries, including Kenya.35,49 In Kenya, CHWs are classified as level 1 within the healthcare service provision classification and approximately 10 to 12 CHWs are assigned to community units or sublocations to provide primary healthcare delivery to up to 100 households.49 However, anecdotal data from collaborators showed that the current work of CHWs in our study sites mostly consists of referring sick people to the nearest health facility for confirmatory diagnosis and proper treatment. Therefore, the results of this study can be used to push forward improvements in the diagnostic capacity among CHWs. More specifically, in this study we observed how those participants with fever, aches, and respiratory symptoms were not seeking as much care as people presenting digestive symptoms. This raises a concern as respiratory symptoms, such as difficulty breathing, are more difficult to address and treat at home. Pneumonia is a leading cause of death in Kenyan children and adults.50,51 A simple improvement of diagnostic capacity could be to provide CHWs with pulse oximeters to measure oxygen levels in patients presenting respiratory symptoms.
We further recommend that public health messaging among the selected sites at the time of the study increase awareness about nonmalaria febrile illnesses. Messaging should address respiratory infections, such as influenza and COVID-19, and the importance of getting the appropriate treatments. We found that 11.5% (n = 45) and 49.1 (n = 316) participants from the highlands and lowlands, respectively, reported sharing antimalarial drugs between household members. This raises a concern about participants using antimalarials to treat nonmalarial infections when antibiotics or other treatments may be more appropriate. Improper use of malarial medication can further increase drug resistance.52
The present study had multiple strengths. First, this is the first study we are aware that reports the association between self-reported symptoms and treatment-seeking behaviors in this part of western Kenya. Second, the study included participants of all ages. Many previous studies have focused on treatment-seeking behaviors for children aged under 5 or under 15 years,2,12,53–57 but it is becoming increasingly apparent that older adults may play an important role in perpetuating malaria transmission in communities. Understanding the choices that might lead them to obtain malaria treatment has important implications for opportunities to reduce the number of community parasite reservoirs in endemic areas. Third, we explored the heterogeneity of self-reported symptoms and treatment-seeking behaviors at several locations in close geographic proximity. It has been documented that national-level estimates of care-seeking are likely to mask the geographic, economic, and cultural heterogeneity in the use and access of healthcare services.58 Our results support this notion, with major differences in symptom patterns and treatment behaviors between the highland and lowland sites. Fourth, we created categories based on a combination of self-reported symptoms, which have not been considered in previous studies in western Kenya. Fifth, we use a combination of potential treatment categories (formal and informal), which was similar to that used in a study of fever management in Kenya.23 However, many studies have previously reported self-treatment, traditional healers, drug sellers, and other diverse sources of treatment individually.2,3,8,11,59
One of the limitations of this study was that the survey did not capture information on issues of quality of care and cost of treatment and transportation, which have been reported to be associated with choices about treatment-seeking for febrile illness.1,15,53,60 Because we conducted a cross-sectional study, the data collected does not capture the temporality of febrile illness. Another limitation is that treatment-seeking outcomes were based on self-report without validation using healthcare facility registries and may therefore be subject to error. If any social desirability bias was introduced when asking participants about the type of treatment sought (formal versus informal) at last illness, we could not address it due to the absence of validation of reported treatment-seeking. Even though participants were allowed to choose multiple sources of treatment at last illness, we did not measure whether treatment from different sources was a result of the failure of self-treatment at home.8,56 More specifically, there is the possibility that those participants reporting fever only could have used over-the-counter pain killers to treat their illness. Another limitation of this study is that even though treatment-seeking at last illness was commonly reported among the surveyed participants, mild symptoms may not have been considered when self-reporting the symptoms experienced during their last illness. Moreover, the absence of information about the severity of illness could have introduced recall bias into our study because we can assume that participants will better recall a severe illness over mild illnesses.
Future research is needed to understand the influence of perception of vulnerability and severity of illness by age groups on treatment-seeking behaviors in the study area. On the other hand, measuring cost of treatment and proximity to healthcare facilities may be of interest in the future because this information can shed light on the influence of external factors in the decision-making process of seeking treatment of febrile illness. Future research is needed to understand whether treatment-seeking at healthcare facilities comes after failing to self-treat at home. Furthermore, it is important to measure and compare the impact of CHWs on care-seeking behaviors for febrile illness in the highlands and lowlands of western Kenya and determine whether there have been any shifts in treatment seeking from high-level sources to community-based care. Studies should be conducted to estimate the prevalence of malaria and nonmalarial febrile illnesses among the residents of the highlands and lowlands of western Kenya to help inform resource allocation and improvement of diagnosis and treatment of infectious diseases. Lastly, there remains a need to improve the algorithms used to differentiate the clinical manifestations of malaria from nonmalaria febrile illness to ensure proper diagnosis and timely treatment in combination with improving access to RDT and microscopy for diagnosis.
The results of this study showed an association between the number of self-reported symptoms and treatment-seeking behaviors at last febrile illness. Also, we found that a high degree of heterogeneity exists even when comparing close geographic areas. Understanding the underlying drivers of treatment-seeking behavior is important to control and treat infectious diseases such as malaria in the communities in Kenya and other at-risk areas. In particular, addressing gaps in treatment seeking for respiratory symptoms, when pneumonia is the second leading cause of death in Kenya, will be a critical component of response to influenza and COVID-19.
Supplemental files
ACKNOWLEDGMENTS
We thank the field assistants for collecting the data for the project and the community members of Kapkangani and Miwani for their participation.
Note: Supplemental figure at www.ajtmh.org.
REFERENCES
- 1. Chuma J Okungu V Molyneux C , 2010. Barriers to prompt and effective malaria treatment among the poorest population in Kenya. Malar J 9: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birhanu Z Abebe L Sudhakar M Dissanayake G Yihdego YY Alemayehu G Yewhalaw D , 2016. Malaria related perceptions, care seeking after onset of fever and anti-malarial drug use in malaria endemic settings of southwest Ethiopia. PLoS One 11: e0160234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okeke TA Okafor HU , 2008. Perception and treatment seeking behavior for malaria in rural Nigeria: implications for Control. J Hum Ecol 24: 215–222. [Google Scholar]
- 4. Omondi CJ Onguru D Kamau L Nanyingi M Ong’amo G Estambale B , 2017. Perennial transmission of malaria in the low altitude areas of Baringo County, Kenya. Malar J 16: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battle KE et al. 2016. Treatment-seeking rates in malaria endemic countries. Malar J 15: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith LA Bruce J Gueye L Helou A Diallo R Gueye B Jones C Webster J , 2010. From fever to anti-malarial: the treatment-seeking process in rural Senegal. Malar J 9: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yasuoka J Kikuchi K Nanishi K Ly P Thavrin B Omatsu T Mizutani T , 2018. Malaria knowledge, preventive actions, and treatment-seeking behavior among ethnic minorities in Ratanakiri Province, Cambodia: a community-based cross-sectional survey. BMC Public Health 18: 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deressa W , 2007. Treatment-seeking behaviour for febrile illness in an area of seasonal malaria transmission in rural Ethiopia. Malar J 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitiku I Assefa A , 2017. Caregivers’ perception of malaria and treatment-seeking behaviour for under five children in Mandura District, West Ethiopia: a cross-sectional study. Malar J 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization , 2021. Global Technical Strategy for Malaria 2016–2030 Update. Available at: https://www.who.int/publications/i/item/9789240031357. Accessed June 1, 2022.
- 11. Ndyomugyenyi R Magnussen P Clarke S , 2007. Malaria treatment-seeking behaviour and drug prescription practices in an area of low transmission in Uganda: implications for prevention and control. Trans R Soc Trop Med Hyg 101: 209–215. [DOI] [PubMed] [Google Scholar]
- 12. Kassile T Lokina R Mujinja P Mmbando BP , 2014. Determinants of delay in care seeking among children under five with fever in Dodoma region, central Tanzania: a cross-sectional study. Malar J 13: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCombie SC , 1996. Treatment seeking for malaria: a review of recent research. Soc Sci Med 43: 933–945. [DOI] [PubMed] [Google Scholar]
- 14. Uzochukwu BSC Onwujekwe OE , 2004. Socio-economic differences and health seeking behaviour for the diagnosis and treatment of malaria: a case study of four local government areas operating the Bamako initiative programme in south-east Nigeria. Int J Equity Health 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maslove DM Mnyusiwalla A Mills EJ McGowan J Attaran A Wilson K , 2009. Barriers to the effective treatment and prevention of malaria in Africa: a systematic review of qualitative studies. BMC Int Health Hum Rights 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenya Ministry of Health , 2016. The Kenya Malaria Communication Strategy 2016–2021—Kenya Ministry of Health. Available at: https://www.thecompassforsbc.org/sites/default/files/strengthening_tools/Kenya%20SBCC%202016-2020.pdf. Accessed September 2, 2020.
- 17. Musuva A Ejersa W Kiptui R Memusi D Abwao E , 2017. The malaria testing and treatment landscape in Kenya: results from a nationally representative survey among the public and private sector in 2016. Malar J 16: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Division of National Malaria Programme and ICF , 2021. Kenya Malaria Indicator Survey 2020. Available at: https://dhsprogram.com/methodology/survey/survey-display-579.cfm. Accessed 2021.
- 19. Chuma J Abuya T Memusi D Juma E Akhwale W Ntwiga J Nyandigisi A Tetteh G Shretta R Amin A , 2009. Reviewing the literature on access to prompt and effective malaria treatment in Kenya: implications for meeting the Abuja targets. Malar J 8: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Coordinating Agency for Population and Development (NCAPD) [Kenya] , Ministry of Medical Services (MOMS) [Kenya], Ministry of Public Health and Sanitation (MOPHS) [Kenya], Kenya National Bureau of Statistics (KNBS) [Kenya], Macro I 2011. Kenya Service Provision Assessment Survey 2010. Available at: https://esaro.unfpa.org/en/publications/kenya-service-provision-assessment-survey-2010-kspa. Accessed April 15, 2022.
- 21. East L Arudo J Loefler M Evans C , 2014. Exploring the potential for advanced nursing practice role development in Kenya: a qualitative study. BMC Nurs 13: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amin AA Marsh V Noor AM Ochola SA Snow RW , 2003. The use of formal and informal curative services in the management of paediatric fevers in four districts in Kenya. Trop Med Int Health 8: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 23. Guyatt HL Snow RW , 2004. The management of fevers in Kenyan children and adults in an area of seasonal malaria transmission. Trans R Soc Trop Med Hyg 98: 111–115. [DOI] [PubMed] [Google Scholar]
- 24. Adinan J Damian DJ Mosha NR Mboya IB Mamseri R Msuya SE , 2017. Individual and contextual factors associated with appropriate healthcare seeking behavior among febrile children in Tanzania. PLoS One 12: e0175446–e0175446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyaoke BA Mureithi MW Beynon C , 2019. Factors associated with treatment type of non-malarial febrile illnesses in under-fives at Kenyatta National Hospital in Nairobi, Kenya. PLoS One 14: e0217980–e0217980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mutanda AL Cheruiyot P Hodges JS Ayodo G Odero W John CC , 2014. Sensitivity of fever for diagnosis of clinical malaria in a Kenyan area of unstable, low malaria transmission. Malar J 13: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chipwaza B Mugasa JP Mayumana I Amuri M Makungu C Gwakisa PS , 2014. Community knowledge and attitudes and health workers’ practices regarding non-malaria febrile illnesses in eastern Tanzania. PLoS Negl Trop Dis 8: e2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wainaina M Vey da Silva DA Dohoo I Mayer-Scholl A Roesel K Hofreuter D Roesler U Lindahl J Bett B Al Dahouk S , 2022. A systematic review and meta-analysis of the aetiological agents of non-malarial febrile illnesses in Africa. PLoS Negl Trop Dis 16: e0010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riley C Dellicour S Ouma P Kioko U Omar A Kariuki S Ng’ang’a Z Desai M Buff AM Gutman JR , 2018. Knowledge and adherence to the national guidelines for malaria diagnosis in pregnancy among health-care providers and drug-outlet dispensers in rural western Kenya. Am J Trop Med Hyg 98: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akulayi L et al. 2017. Testing times: trends in availability, price, and market share of malaria diagnostics in the public and private healthcare sector across eight sub-Saharan African countries from 2009 to 2015. Malar J 16: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamooya BM Chongwe G Dambe R Halwiindi H , 2016. Treatment-seeking behaviour for childhood fever among caretakers of Chivuna and Magoye rural communities of Mazabuka District, Zambia: a longitudinal study. BMC Public Health 16: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Meara WP et al. 2015. Etiology of pediatric fever in western Kenya: a case-control study of falciparum malaria, respiratory viruses, and streptococcal pharyngitis. Am J Trop Med Hyg 92: 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elven J et al. 2020. Non-malarial febrile illness: a systematic review of published aetiological studies and case reports from Africa, 1980–2015. BMC Med 18: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization , 2022. WHO Guidelines for Malaria. Available at: https://www.who.int/publications/i/item/guidelines-for-malaria. Accessed May 30, 2022.
- 35. Bedford KJA Sharkey AB , 2014. Local barriers and solutions to improve care-seeking for childhood pneumonia, diarrhoea and malaria in Kenya, Nigeria and Niger: a qualitative study. PLoS One 9: e100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fenny AP Asante FA Enemark U Hansen KS , 2015. Malaria care seeking behavior of individuals in Ghana under the NHIS: are we back to the use of informal care? BMC Public Health 15: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coalson JE Cohee LM Walldorf JA Bauleni A Mathanga DP Taylor TE Wilson ML Laufer MK , 2019. Challenges in treatment for fever among school-age children and adults in Malawi. Am J Trop Med Hyg 100: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coalson JE Santos EM Little AC Anderson EJ Stroupe N Agawo M Hayden M Munga S Ernst KC , 2020. Insufficient ratio of long-lasting insecticidal nets to household members limited universal usage in western Kenya: a 2015 cross-sectional study. Am J Trop Med Hyg 102: 1328–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santos EM Coalson JE Jacobs ET Klimentidis YC Munga S Agawo M Anderson E Stroupe N Ernst KC , 2019. Bed net care practices and associated factors in western Kenya. Malar J 18: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Githeko AK Ototo EN Guiyun Y , 2012. Progress towards understanding the ecology and epidemiology of malaria in the western Kenya highlands: opportunities and challenges for control under climate change risk. Acta Trop 121: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsushita N Kim Y Ng C F S Moriyama M Igarashi T Yamamoto K Otieno W Minakawa N Hashizume M , 2019. Differences of rainfall–malaria associations in lowland and highland in western Kenya. Int J Environ Res Public Health 16: 3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mutuku FM Bayoh MN Hightower AW Vulule JM Gimnig JE Mueke JM Amimo FA Walker ED , 2009. A supervised land cover classification of a western Kenya lowland endemic for human malaria: associations of land cover with larval Anopheles habitats. Int J Health Geogr 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Omukunda E Githeko A Ndong’a MF Mushinzimana E Atieli H Wamae P , 2013. Malaria vector population dynamics in highland and lowland regions of western Kenya. J Vector Borne Dis 50: 85–92. [PubMed] [Google Scholar]
- 44. StataCorp , 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 45. Taylor SM Molyneux ME Simel DL Meshnick SR Juliano JJ , 2010. Does this patient have malaria? JAMA 304: 2048–2056. [DOI] [PubMed] [Google Scholar]
- 46. Sumba PO Wong SL Kanzaria HK Johnson KA John CC , 2008. Malaria treatment-seeking behaviour and recovery from malaria in a highland area of Kenya. Malar J 7: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klein EY Lewis IA Jung C Llinás M Levin SA , 2012. Relationship between treatment-seeking behaviour and artemisinin drug quality in Ghana. Malar J 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dzator J Asafu-Adjaye J , 2004. A study of malaria care provider choice in Ghana. Health Policy 69: 389–401. [DOI] [PubMed] [Google Scholar]
- 49. Owek CJ Oluoch E Wachira J Estambale B Afrane YA , 2017. Community perceptions and attitudes on malaria case management and the role of community health workers. Malar J 16: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osano BO Were F Mathews S , 2017. Mortality among 5–17 year old children in Kenya. Pan Afr Med J 27: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Institute of Health Metrics and Evaluation , 2019. Kenya. Available at: https://www.healthdata.org/kenya. Accessed June 2, 2022.
- 52. Hyde JE , 2007. Drug-resistant malaria—an insight. FEBS J 274: 4688–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Getahun A Deribe K Deribew A , 2010. Determinants of delay in malaria treatment-seeking behaviour for under-five children in south-west Ethiopia: a case control study. Malar J 9: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Singh R Musa J Singh S Ebere UV , 2014. Knowledge, attitude and practices on malaria among the rural communities in Aliero, northern Nigeria. J Family Med Prim Care 3: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nabyonga Orem J Mugisha F Okui AP Musango L Kirigia JM , 2013. Health care seeking patterns and determinants of out-of-pocket expenditure for malaria for the children under-five in Uganda. Malar J 12: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Romay-Barja M Jarrin I Ncogo P Nseng G Sagrado MJ Santana-Morales MA Aparcio P Valladares B Riloha M Benito A , 2015. Rural-urban differences in household treatment-seeking behaviour for suspected malaria in children at Bata District, Equatorial Guinea. PLoS One 10: e0135887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Franckel A Lalou R , 2009. Health-seeking behaviour for childhood malaria: household dynamics in rural Senegal. J Biosoc Sci 41: 1–19. [DOI] [PubMed] [Google Scholar]
- 58. O’Meara WP Karuru S Fazen LE Koech J Kizito B Tarus C Menya D , 2014. Heterogeneity in health seeking behaviour for treatment, prevention and urgent care in four districts in western Kenya. Public Health 128: 993–1008. [DOI] [PubMed] [Google Scholar]
- 59. Burton DC Flannery B Onyango B Larson C Alaii J Zhang X Hamel MJ Breiman RF Feikin DR , 2011. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr 29: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tolhurst R Nyonator FK , 2006. Looking within the household: gender roles and responses to malaria in Ghana. Trans R Soc Trop Med Hyg 100: 321–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.