Abstract
Objective
To assess the effect of a novel naturally cultured rice with high resistant starch (RS) on postprandial glycemia in patients with type 2 diabetes compared to ordinary rice.
Design
This study is a randomized, double-blinded controlled trial.
Methods
Patients with type 2 diabetes were recruited, and postprandial glucose levels were measured at 5-time points after the ingestion of one of two types of cooked rice in random order. Paired t-tests were used to compare postprandial blood glucose changes and increment areas under the blood glucose curve between high-RS rice and ordinary rice.
Results
The increments of the postprandial blood glucose levels for high-RS rice were significantly lower than that for ordinary rice, i.e., 2.80 ± 1.38 mmol/L vs. 3.04 ± 1.50 mmol/L (P = 0.043) and 3.94 ± 2.25 mmol/L vs. 4.25 ± 2.29 mmol/L (P = 0.036) at 30 min and 60 min, respectively. The incremental areas under the blood glucose curve for high-RS rice were also significantly lower than that for ordinary rice, i.e., 42.04 ± 20.65 [mmol/(L·min)] vs. 45.53 ± 22.45 [mmol/(L·min)] (P = 0.043), 143.54 ±69.63 [mmol/(L·min)] vs. 155.15 ± 73.53 [mmol/(L·min)] (P = 0.026), and 354.61 ± 191.96 [mmol/(L·min)] vs. 379.78 ± 195.30 [mmol/(L·min)] (P = 0.042) at 30, 60, and 120 min, respectively. Repeated-measures ANOVA showed that postprandial glucose levels were not affected by the test order.
Conclusion
The novel high-RS rice as a staple food when substituting for widely consumed ordinary rice may provide potential health benefits by lowering blood glucose in patients with type 2 diabetes.
Keywords: resistant starch (RS), rice, diabetes, postprandial glucose level, randomized controlled trial (RCT)
Introduction
Diabetes is one of the most common chronic non-communicable diseases in China and worldwide and has become a great public concern. According to the data released by the International Diabetes Federation in 2019, the global prevalence of diabetes was 9.3% (1). While the number of people with diabetes in China has exceeded 100 million, it is ranking first in the world (2). Most cases of diabetes worldwide are type 2 diabetes (3). The epidemic of type 2 diabetes affects patients' health to varying degrees and may even threaten their lives in serious cases (4). In addition, the high cost of treatment imposes a huge economic burden on individuals and countries (2). Currently, lifestyle interventions are the major preventive and basic therapeutic measures for patients with diabetes (5, 6). The main strategy for reducing the risk of complications and improving the quality of life of patients with diabetes is intensive glycemic control (7, 8). Therefore, dietary interventions as a part of lifestyle change play an important role in controlling blood glucose levels (9).
Starch is one of the main forms of carbohydrates in the diet and is classified into three categories according to its digestibility: rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) (10). RS cannot be digested and absorbed in the small intestine but can be fermented by the microflora in the colon and subsequently exerts beneficial physiological effects. Therefore, RS is regarded as one type of dietary fiber (11). RS can be found in cereal products, seeds, beans, bananas, and potatoes, and in a commercially purified form as an additive to foods (12). The initial development of RS products was mainly based on corn with a high amylose (AM) content, more RS based on cassava, wheat, potato, and barley has also been made with great success (13). RS is classified into five forms, from RS1- to RS5, according to its origin and digestibility, i.e., physically inaccessible starch (RS1), high starch (RS2), modified starch (RS3), chemically modified starch (RS4), and starch-lipid complexes (RS5) (14). The unique properties of RS, such as its natural origin, mild flavor, white color, and low water retention, have made it a prominent topic for research on a variety of functional foods (15).
Based on accumulating evidence, RS is beneficial for human health. Animal studies have shown that RS intake ameliorates intestinal dysbiosis and chronic inflammation (11). Similar results have been obtained from population trials: wheat high in RS modulated fecal metabolites and microorganisms which are associated with gastrointestinal health in healthy adults (16). A meta-analysis showed that RS improved total cholesterol, low-density lipoprotein (LDL) cholesterol, and tumor necrosis factor-α (TNF-α) levels (17). The most well-studied function of RS might be the ability to improve glucose homeostasis. RS intervention has consistently been shown to help control postprandial glucose levels (18, 19). However, most of the published studies used RS as supplement in processed products [such as bagels (20) and cereal bars (21)] which are often consumed as snacks. Rice, on the contrary, as a main staple food for more than half of the global population (22) would be an ideal carrier for RS. Since refined ordinary rice usually has low fiber and high glycemic index (GI), it is not recommended as a daily staple food for patients with diabetes. So, cultivating high-RS rice with hypoglycemic properties may be a good option for diabetes control (23, 24). Although evidence for RS in reducing postprandial glycemic response is well-documented, most studies on diabetes used RS as supplement. Therefore, this study is about to confirm the effect of newly cultured high-RS rice on postprandial blood glucose levels in patients with type 2 diabetes, which may provide scientific evidence for the promotion of high-RS rice consumption to improve the health of diabetes.
Materials and methods
Study design
This study was a randomized, double-blinded controlled trial. The study was approved by the Research Ethics Committee of Chongqing Medical University (2021089). Subjects signed informed consent to take part in.
The trial site was in the Health Management Center of the Second Hospital of Chongqing Medical University, Chongqing, China.
Subjects
Subjects were recruited through the advertisement posted on the official social media (WeChat) account of the Health Management Center of the Second Hospital of Chongqing Medical University between November 2021 and February 2022. People who were interested in this study contacted the researcher for details. Inclusion criteria included: (1) age is between 40–70 years old; (2) diagnosed type 2 diabetes has medical records; (3) the patient's condition is stable without acute complications; (4) having the ability to do social communication. Exclusion criteria included patients with serious diseases or conditions, such as malignant tumors, severe heart disease, stroke, liver and kidney insufficiency, acute respiratory infections, fever, and those who have limited mobility. Subjects were interviewed at the first time of testing for basic information. A total of 106 patients with diagnosed type 2 diabetes finally were recruited.
Random grouping
Subjects were randomly assigned into two groups according to the order of testing. Group A: RS rice then ordinary rice; Group B: ordinary rice then RS rice.
Cards labeled with group assignments (Group A; Group B) were placed into sealed envelopes. The first time when subjects arrived at the test site, they were given an envelope randomly to decide the test order of the two rices. The rices of testing were blinded (cooked rices were indistinguishable by texture and taste in our pretest) to study subjects and the blood glucose sample testers.
Preparing testing meals
The Chongqing Academy of Agricultural Sciences cultured and provided both types of refined rice, i.e., high-RS rice and ordinary rice. High-RS rice has 8.44% of resistant starch, while it is only 0.46% in ordinary rice. The nutrition facts were shown in Table 1.
Table 1.
Nutrition facts of test rices* (per 100 g refined rice).
| Item | Unit | High-RS rice | Ordinary rice |
|---|---|---|---|
| Energy | KJ | 1,485 | 1,462 |
| Protein | G | 7.7 | 6.6 |
| Fat | G | 0.9 | 0.6 |
| Carbohydrates | G | 77.1 | 77.7 |
| Sodium | Mg | 0 | 0 |
| Dietary fiber | G | 1.2 | 0.8 |
| Resistant starch content | G | 8.44 | 0.46 |
Data were reported by an independent qualified laboratory in Beijing, China.
Rice was weighed, washed once with water, and cooked by using an electric rice cooker. The final rice-to-water ratio for cooking was 1:1.8 (decided by the taste and texture of cooked rice in pretest). The cooked rice/raw rice ratio was 2.39 and 2.46 for high-RS rice and ordinary rice, respectively (measured in pretest).
Three sizes of cooked rice (large portion: 200 g cooked rice; medium portion: 150 g cooked rice; small portion: 100 g cooked rice) were provided for subjects to choose according to their appetite.
To simulate the real diet, side dishes were also added, including a portion of boiled bok choy (100 g raw vegetable, 5 ml of soy sauce, and 5 ml of sesame oil), one cup (200 ml) of soup (5 grams nori and 1 stirred egg boiled in 2 liters of water), 1 boiled egg, and a small pinch of pickled vegetable.
Meals were freshly prepared on the testing day morning by the cafeteria staff in the hospital.
Test procedure
One week before testing day, subjects were required to maintain their usual lifestyle. On the day before the testing, subjects were told to avoid foods high in dietary fiber and sugar at dinner, to fast after 10:00 pm, and to keep fasting until the testing in the next morning. Subjects were also required to bring any medications they were prescribed to take in the morning, including hypoglycemic drugs.
Before taking the testing meal, 1 ml of fasting venous blood was collected to measure the fasting glucose. At their first bite of the meal, it was defined as the zero-time of trial. The whole meal was eaten up in 10 min. After the meal, subjects were reminded to take their medicine if prescribed.
Each time at 30, 60, 120, and 180 min after the meal, 1 ml of venous blood was drawn. Blood samples were collected from the subjects by nurses and sent to the hospital laboratory to do blood glucose tests.
After a 7-days washout period, the second round trial was conducted with the other rice according to the test order assigned at the first trial. In the second round, all except the rice type remained the same as in the first round for each subject, including meal size and medications.
Outcome indicators
Outcome indicators included the change of postprandial glucose level [ΔCt (mmol/L)] and the incremental area under the glucose curve (IAUC [mmol/ (L·min)]).
ΔCt is blood glucose level at a time point minus fasting blood glucose level.
IAUC is calculated using the trapezoidal rule, with the fasting blood glucose level as the baseline and ignoring the area below the baseline.
Statistical analysis
All continuous values were expressed as the mean ± standard deviation (SD). To control the confounding factors, studends t-tests and chi-square tests were used to analyze the baseline situation of the two AB groups. Paired t-test was used to compare the difference between groups at each time point. Repeated-measures ANOVA was used to detect the effect of test order (25). Statistical analysis was performed using SPSS 25.0 software. P < 0.05 was considered statistically significant.
Results
Overview of subjects
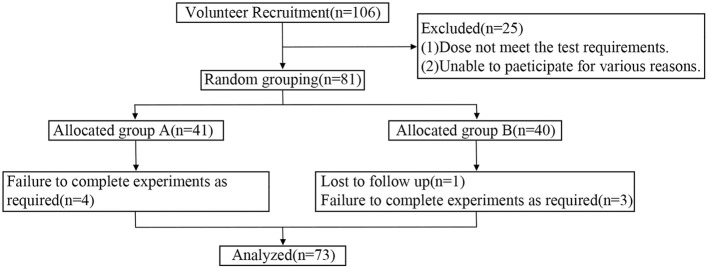
Eighty one out of 107 recruited patients with type 2 diabetes eventually were eligible for this study. During testing, 1 subject who failed two rounds of trial and 7 subjects who failed to fully follow the instructions were excluded from the data analysis. Ultimately, 73 subjects were included in this study. The process of subject screening was shown in Figure 1.
Figure 1.
Flow diagram of participant recruitment and included.
For those who were included, the age was 59.74 ± 8.89 years, the body mass index (BMI) was 23.22 ± 2.66 kg/m2, and the duration of diabetes was 9.49 ± 7.02 years. There were no significant differences between the two groups (two testing orders) in terms of baseline conditions such as mean age, sex, height, weight, and BMI (P > 0.05). The profile of the subjects was shown in Table 2.
Table 2.
General condition of the subjects (n = 73).
| Item |
Total
(n = 73) |
Group A
(n = 37) |
Group B
(n = 36) |
P |
|---|---|---|---|---|
| Age (years) | 59.74 ± 8.89 | 58.14 ± 8.60 | 61.39 ± 8.99 | 0.118 |
| Sex (male/female) | 46 / 27 | 25 / 12 | 21 / 15 | 0.414 |
| Height (cm) | 163.42 ± 7.77 | 163.35 ± 8.81 | 163.50 ± 6.65 | 0.936 |
| Body weight (kg) | 62.04 ± 8.27 | 62.60 ± 8.71 | 61.46 ± 7.87 | 0.559 |
| BMI (kg/m2) | 23.22 ± 2.66 | 23.45 ± 2.63 | 22.99 ± 2.71 | 0.462 |
| Hypertension (with/without) | 19 / 54 | 10 / 27 | 9 / 27 | 0.844 |
| Duration of diabetes | 9.49 ± 7.02 | 9.89 ± 6.67 | 9.08 ± 7.44 | 0.626 |
| Anti-diabetic drugs (yes/no) | 61 / 12 | 31 / 6 | 30 / 6 | 0.959 |
| Other prescribed drug (yes/no) | 9 / 64 | 5 / 32 | 4 / 32 | 0.755 |
| Rice portion (small, medium, and large) | 0 / 8 / 65 | 0 / 1 / 36 | 0 / 7 / 29 | 0.056 |
Each value is expressed as the mean ± SD. Group A, RS rice then ordinary rice; Group B, ordinary rice then RS rice.
The postprandial blood glucose level
The paired t-test showed that the subjects when taking the cooked high-RS rice had significantly lower changes in postprandial blood glucose than the time when taking cooked ordinary rice at 30 and 60 min (P < 0.05, Table 3).
Table 3.
Comparisons of postprandial blood glucose levels (mean ± SD) between high-RS rice meal and ordinary rice meal (n = 73).
| Item | High-RS rice | Ordinary rice | t | P |
|---|---|---|---|---|
| ΔC30min (mmol/L) | 2.80 ± 1.38* | 3.04 ± 1.50 | −2.057 | 0.043 |
| ΔC60min (mmol/L) | 3.94 ± 2.25* | 4.25 ± 2.29 | −2.131 | 0.036 |
| ΔC120min (mmol/L) | 3.00 ± 2.56 | 3.14 ± 2.54 | −0.698 | 0.488 |
| ΔC180min (mmol/L) | 1.13 ± 2.58 | 1.16 ± 2.17 | −0.158 | 0.875 |
ΔCt is the change between postprandial blood sugar and fasting blood glucose.
P < 0.05 (paired t-test).
Incremental area of the blood glucose curve
The incremental area under the blood glucose curve in patients with diabetes when taking cooked high-RS rice was smaller than that when taking cooked ordinary rice. The paired t-test revealed statistically significant differences between the two kinds of rice at 30, 60, and 120 min (P < 0.05, Table 4).
Table 4.
Comparison of IAUC (mean ± SD) between high-RS rice meal and ordinary rice meal (n = 73).
| Item | High-RS rice | Ordinary rice | t | P |
|---|---|---|---|---|
| IAUC30min [mmol/(L·min)] | 42.04 ± 20.65* | 45.53 ± 22.45 | −2.057 | 0.043 |
| IAUC60min [mmol/(L·min)] | 143.54 ± 69.63* | 155.15 ± 73.53 | −2.277 | 0.026 |
| IAUC120min [mmol/(L·min)] | 354.61 ± 191.96* | 379.78 ± 195.30 | −2.066 | 0.042 |
| IAUC180min [mmol/(L·min)] | 493.33 ± 308.03 | 519.78 ± 304.21 | −1.381 | 0.171 |
IAUC, incremental area under the postprandial glucose curve.
P < 0.05 (paired t-test).
The impact of testing order
Repeated-measures ANOVA showed that the treatment factors (types of rice) but not the test order caused the differences in postprandial blood glucose in patients with diabetes at 30 and 60 min (P < 0.05, Table 5).
Table 5.
Repeated ANOVA of postprandial glucose by test order.
| Item | Group A | Group B | Repeated-measures ANOVA( P) | |
|---|---|---|---|---|
| (n = 37) | (n = 36) | Treat | Test order | |
| ΔC30min (mmol/L) | 2.86 ± 1.50 | 2.98 ± 1.38 | 0.045* | 0.297 |
| ΔC60min (mmol/L) | 3.97 ± 2.32 | 4.22 ± 2.22 | 0.036* | 0.085 |
| ΔC120min (mmol/L) | 3.04 ± 2.59 | 3.09 ± 2.50 | 0.493 | 0.797 |
| ΔC180min (mmol/L) | 1.18 ± 2.33 | 1.10 ± 2.42 | 0.871 | 0.676 |
Group A, RS rice then ordinary rice; Group B, ordinary rice then RS rice.
P < 0.05.
Discussion
As a metabolic disease, diabetes will gradually progress with a variety of acute and chronic complications of varying severities, which is the main cause of disability and death of patients with diabetes (26). The development of diabetic complications is not only associated with persistent chronic hyperglycemia but also closely related to fluctuations in blood glucose levels (27, 28).
We have found in this study that patients with type 2 diabetes when consuming high-RS rice had smaller fluctuations in postprandial blood glucose levels than when consuming ordinary rice in the real world with antidiabetic drugs and simulated ordinary diet, and their glycemic changes were significantly lower at 30 min and 60 min after meals when consuming high-RS rice. Moreover, the incremental area under the blood glucose curve of patients with diabetes when consuming high-RS rice was also smaller than that of patients when consuming ordinary rice.
Other studies have also reported similar results. For example, Yuhi Saito (29) conducted two randomized, single-blinded, crossover trials to study the effects of a single intake of high-RS crackers and cooked high-RS rice on postprandial glycemic and insulin responses in healthy adults and compared them with rice crackers and cooked rice prepared from ordinary varieties, and the results indicated that both high-RS rice crackers and high-RS rice had lower postprandial glycemic and insulin responses. Takahashi (30) conducted a non-randomized crossover design trial in which five healthy men consumed two types of cooked rice, i.e. control (low-RS) and test (high-RS) rice, and found that high-RS rice improved postprandial hyperglycemia and hyperinsulinemia. But unlike our study, those studies investigated postprandial blood glucose in healthy people. The present study showed that naturally cultured high-RS rice has the same effects on postprandial blood glucose in type 2 diabetes. Although the benefits of resistant starch on diabetes have been reported in some studies, nearly all studies used RS supplements as intervention and postprandial blood glucose was not measured (31). Until now, the effects of naturally cultured high-RS rice on diabetes were rarely reported.
RS, as a special dietary carbohydrate, is considered one of the most important factors in the study of the relationship between carbohydrates and health (31). In our study, the refined high-RS rice has 8.44% of resistant starch, obviously high than ordinary refined rice which has <1% of RS. Not surprisingly, studies have shown that high-RS rice has a lower GI value than ordinary rice (32). Numerous studies have shown that the consumption of low-GI food has improved the drastic changes in postprandial blood glucose levels (33, 34). Moreover, a significant negative correlation was also observed between GI and RS (35). The novel high-RS rice in this study have a GI value of 65 when cooked, which is lower than 80 of ordinary cooked rice (result from our preliminary study, data have not been reported). The low GI value may explain the reduced postprandial glycemic response of high RS rice in diabetes compared with ordinary rice.
There are some strengths in this study. Firstly, randomized controlled trial (RCT) design was used in this study which can effectively control kinds of confounders. Secondly, a double-blinding method was used to avoid possible bias related to the researchers and subjects. Thirdly, postprandial glucose was measured at multiple time points, which provided an overall view of high-RS rice on postprandial glucose. Fourthly, this study tested high-RS rice in real-life conditions by simulating the real diet and allowing the medicine prescribed (31). Therefore, the results are reliable and can be generalized to the real world.
Some limitations of our study also must be mentioned. Since this study only measured postprandial glucose, the long term effects of high-RS rice on blood sugar and other health effects are still undecided. Therefore, studies about high-RS rice on diabetes or other metabolic diseases in the long term with more biomarkers are warranted.
Conclusion
The novel high-RS rice as a staple food when substituting for widely consumed ordinary rice may provide potential health benefits by lowering blood glucose in patients with type 2 diabetes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study approval number: 2021089.
Author contributions
YZ and L-LT reviewed the literature and completed the protocol design. L-LT, YM, X-YQ, W-QD, and M-XC conducted the clinical trial and were responsible for data collection. L-LT performed the statistical analysis and data interpretation under the direction of YZ, wrote the manuscript, and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to all staff at the Health Management Center and the Department of Laboratory Medicine of the Second Hospital of Chongqing Medical University for their assistance during the trial. We thank the Chongqing Academy of Agricultural Sciences for providing study materials and funds. We also thank the participants for participating in the study.
Funding
The Chongqing Academy of Agricultural Sciences sponsored this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (2019) 157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørensen HT. Prevention of diabetes mortality at ages younger than 25 years: access to medications and high-quality health care. Lancet Diabetes Endocrinol. (2022) 10:151–2. 10.1016/S2213-8587(22)00009-2 [DOI] [PubMed] [Google Scholar]
- 4.Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon Besançon S et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (2020) 162:108072. 10.1016/j.diabres.2020.108072 [DOI] [PubMed] [Google Scholar]
- 5.Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol. (2019) 7:452–61. 10.1016/S2213-8587(19)30093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsoutsouki J, Wunna W, Chowdhury A, Chowdhury TA. Advances in the management of diabetes: therapies for type 2 diabetes. Postgrad Med J. (2020) 96:610–8. 10.1136/postgradmedj-2019-137404 [DOI] [PubMed] [Google Scholar]
- 7.Asmelash D, Abdu N, Tefera S, Baynes HW, Derbew C. Knowledge, attitude, and practice towards glycemic control and its associated factors among diabetes mellitus patients. J Diabetes Res. (2019) 2019:2593684. 10.1155/2019/2593684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaribeygi H, Atkin SL, Katsiki N, Sahebkar A. Narrative review of the effects of antidiabetic drugs on albuminuria. J Cellular Physiol. (2019) 234:5786–97. 10.1002/jcp.27503 [DOI] [PubMed] [Google Scholar]
- 9.Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina. (2019) 55:546. 10.3390/medicina55090546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuis JH, Liu Q, Yada RY. Methodologies for increasing the resistant starch content of food starches: a review. Compr Rev Food Sci Food Saf. (2014) 13:1219–34. 10.1111/1541-4337.12104 [DOI] [Google Scholar]
- 11.Yuan H, Wang W, Chen D, Zhu X, Meng L. Effects of a treatment with Se-rich rice flour high in resistant starch on enteric dysbiosis and chronic inflammation in diabetic ICR mice. J Sci Food Agri. (2017) 97:2068–74. 10.1002/jsfa.8011 [DOI] [PubMed] [Google Scholar]
- 12.Lockyer S, Nugent A. Health effects of resistant starch. Nutr Bull. (2017) 42:10–41. 10.1111/nbu.12244 [DOI] [Google Scholar]
- 13.Thompson DB. Strategies for the manufacture of resistant starch. Trends Food Sci Technol. (2000) 11:245–53. 10.1016/S0924-2244(01)00005-X [DOI] [Google Scholar]
- 14.Si X, Zhou Z, Strappe P, Blanchard C. A comparison of RS4-type resistant starch to RS2-type resistant starch in suppressing oxidative stress in high-fat-diet-induced obese rats. Food Funct. (2017) 8:232–40. 10.1039/C6FO01225F [DOI] [PubMed] [Google Scholar]
- 15.Homayouni A, Amini A, Keshtiban AK, Mortazavian AM, Esazadeh K, Pourmoradian S. Resistant starch in food industry: a changing outlook for consumer and producer. Starch-Stärke. (2014) 66:102–14. 10.1002/star.201300110 [DOI] [Google Scholar]
- 16.Gondalia SV, Wymond B, Benassi-Evans B, Berbezy P, Bird AR, Belobrajdic DP. Substitution of refined conventional wheat flour with wheat high in resistant starch modulates the intestinal microbiota and fecal metabolites in healthy adults: a randomized, controlled trial. J Nutr. (2022) 152:1426–37. 10.1093/jn/nxac021 [DOI] [PubMed] [Google Scholar]
- 17.Halajzadeh J, Milajerdi A, Reiner Ž, Amirani E, Kolahdooz F, Barekat M, et al. Effects of resistant starch on glycemic control, serum lipoproteins and systemic inflammation in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled clinical trials. Crit Rev Food Sci Nutr. (2020) 60:3172–84. 10.1080/10408398.2019.1680950 [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Piao Z, Wan C, Lee G, Ruan X, Bai J. Breeding for three-line japonica hybrid rice combinations with high resistant starch content using molecular marker-assisted selection. Breed Sci. (2020) 70:409–14. 10.1270/jsbbs.20005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh Y, Crofts N, Abe M, Hosaka Y, Fujita N. Characterization of the endosperm starch and the pleiotropic effects of biosynthetic enzymes on their properties in novel mutant rice lines with high resistant starch and amylose content. Plant Sci. (2017) 258:52–60. 10.1016/j.plantsci.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Dainty SA, Klingel SL, Pilkey SE, McDonald E, McKeown B, Emes MJ, et al. Resistant starch bagels reduce fasting and postprandial insulin in adults at risk of type 2 diabetes. J Nutr. (2016) 146:2252–9. 10.3945/jn.116.239418 [DOI] [PubMed] [Google Scholar]
- 21.Wolever TM, van Klinken BJ-W, Bordenave N, Kaczmarczyk M, Jenkins AL, Chu Y, et al. Reformulating cereal bars: high resistant starch reduces in vitro digestibility but not in vivo glucose or insulin response; whey protein reduces glucose but disproportionately increases insulin. Am J Clin Nutr. (2016) 104:995–1003. 10.3945/ajcn.116.132431 [DOI] [PubMed] [Google Scholar]
- 22.Fukagawa NK, Ziska LH. Rice: importance for global nutrition. J Nutr Sci Vitaminol. (2019) 65(Suppl):S2–3. 10.3177/jnsv.65.S2 [DOI] [PubMed] [Google Scholar]
- 23.Yi D, Maike W, Yi S, Xiaoli S, Dianxing W, Wenjian S. Physiochemical properties of resistant starch and its enhancement approaches in rice. Rice Sci. (2021) 28:31–42. 10.1016/j.rsci.2020.11.005 [DOI] [Google Scholar]
- 24.Kwak JH, Paik JK, Kim HI, Kim OY, Shin DY, Kim H-J, et al. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis. (2012) 224:457–64. 10.1016/j.atherosclerosis.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 25.Park E, Cho M, Ki C-S. Correct use of repeated measures analysis of variance. Korean J Lab Med. (2009) 29:1–9. 10.3343/kjlm.2009.29.1.1 [DOI] [PubMed] [Google Scholar]
- 26.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. (2020) 16:377–90. 10.1038/s41581-020-0278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying C, Zhou X, Chang Z, Ling H, Cheng X, Li W. Blood glucose fluctuation accelerates renal injury involved to inhibit the AKT signaling pathway in diabetic rats. Endocrine. (2016) 53:81–96. 10.1007/s12020-016-0867-z [DOI] [PubMed] [Google Scholar]
- 28.Catargi B, Gerbaud E. editors. Glucose fluctuations and hypoglycemia as new cardiovascular risk factors in diabetes. Ann Endocrinol (Paris). (2021) 82:144–5. 10.1016/j.ando.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Watanabe T, Sasaki T, Watanabe K, Hirayama M, Fujita N. Effects of single ingestion of rice cracker and cooked rice with high resistant starch on postprandial glucose and insulin responses in healthy adults: two randomized, single-blind, cross-over trials. Biosci, Biotechnol, Biochem. (2020) 84:365–71. 10.1080/09168451.2019.1687282 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Fujita H, Fujita N, Takahashi Y, Kato S, Shimizu T, et al. A pilot study to assess glucose, insulin, and incretin responses following novel high resistant starch rice ingestion in healthy men. Diab Ther. (2022) 13:1383–93. 10.1007/s13300-022-01283-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HO, Qiu B, Xu TC, Zong AZ, Liu LN, Xiao JX. Effects of resistant starch on the indicators of glucose regulation in persons diagnosed with type 2 diabetes and those at risk: a meta-analysis. J Food Process Preserv. (2020) 44:18. 10.1111/jfpp.14594 [DOI] [Google Scholar]
- 32.Kumar A, Panda PA, Lal MK, Ngangkham U, Sahu C, Soren KR, et al. Addition of pulses, cooking oils, and vegetables enhances resistant starch and lowers the glycemic index of rice (Oryza sativa L). Starch-Starke. (2020) 72:7. 10.1002/star.201900081 [DOI] [Google Scholar]
- 33.Kaur J, Kaur K, Singh B, Singh A, Sharma S. Insights into the latest advances in low glycemic foods, their mechanism of action and health benefits. J Food Meas Charact. (2021) 16:533–46. 10.1007/s11694-021-01179-z [DOI] [Google Scholar]
- 34.Vlachos D, Malisova S, Lindberg FA, Karaniki G. Glycemic index (GI) or glycemic load (GL) and dietary interventions for optimizing postprandial hyperglycemia in patients with T2 diabetes: a review. Nutrients. (2020) 12:1561. 10.3390/nu12061561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Sahoo U, Baisakha B, Okpani OA, Ngangkham U, Parameswaran C, et al. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J Cereal Sci. (2018) 79:348–53. 10.1016/j.jcs.2017.11.013 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.