Abstract
The occurrence of peritoneal adhesions in surgical patients is positively correlated with tumor necrosis factor (TNF) levels. In a model of septic peritonitis—cecal ligation and puncture—TNF neutralization prevented formation of peritoneal adhesions and increased mortality, most likely because localization of the septic focus was prevented. To discriminate between the coagulation-independent protective TNF effect and a potential protective procoagulant TNF effect, formation of peritoneal adhesions after CLP was inhibited with heparin, hirudin, or urokinase. Each treatment increased mortality and increased the number of bacteria in the peritoneal lavage fluid, kidney, and liver to various degrees. Under these experimental conditions, antibiotics prevented death. In coagulation-compromised mice, lethality was further enhanced by additional TNF neutralization. These findings demonstrate that peritoneal adhesions early in septic peritonitis are an important mechanism of innate immunity that prevents increased spread of bacteria and reduces mortality.
Although tumor necrosis factor (TNF) was regarded for many years as the major cytokine causing morbidity and mortality in sepsis and septic shock (13), clinical studies testing TNF inhibitors in septic shock did not yield encouraging results (11, 30). Besides the many sepsis/septic shock models demonstrating that TNF inhibitors protect animals from bolus injections with lipopolysaccharide (LPS) or bacteria, there are also reports concerning the use of TNF inhibitors in models of bacterial peritonitis where either no effect on survival (1, 10, 23) or even deleterious effects (7) were observed. The observation that mice treated with an anti-TNF monoclonal antibody after cecal ligation and puncture (CLP) show both increased mortality and reduced peritoneal adhesions raised the question of whether survival after CLP depends on adhesions (8). Peritoneal adhesions induced by intestinal bacteria can be inhibited by TNF neutralization (12). In addition to experimental data, after surgical intra-abdominal manipulations in patients, higher grades of adhesions correlated with higher levels of TNF both in serum and peritoneal exudate (15).
TNF has procoagulant and antifibrinolytic effects both in blood and on mesothelium (6, 26, 27). At higher TNF concentrations these properties might lead to disseminated intravascular coagulation (DIC), which is uniformly regarded as harmful during sepsis. Therefore, prevention of coagulation might protect against sepsis (3). Antithrombin III or hirudin, indeed, ameliorated DIC and protected rats against sequelae of intravenous administration of LPS alone or injection of bacteria together with an antibiotic, whereas heparin plus antibiotic had no effect on survival in the latter model. This might be due to the fact that the anticoagulant function of heparin is indirect by accelerating antithrombin III binding to thrombin (4, 5, 19).
The situation in bacterial peritonitis, however, is different since anticoagulant treatment may enhance the spread of bacteria by preventing localization of the septic focus. Therefore, anticoagulant treatment in bacterial infections in the peritoneal cavity may lead to an exacerbation of the disease despite prevention of DIC. There are controversial opinions as to whether abscesses are favorable or detrimental in bacterial peritonitis (14). At least initially, localization and containment of the bacterial insult within the peritoneal cavity, even with abscess formation as its consequence, might be beneficial to the host (16, 29). Besides TNF, interleukin-12 (IL-12) seems to be another cytokine important for formation of protective abscesses, because increased lethality of CLP after IL-12 neutralization was correlated with irregular organization of cecal abscesses (24).
Results of our experiments indicate that part of the protective effect of TNF after CLP is most probably its procoagulant effect. This conclusion is based on the findings that treatment with anti-TNF antibodies, inactivation of the TNF gene, and direct use of drugs to prevent fibrin formation or to enhance fibrinolysis increased mortality after CLP.
MATERIALS AND METHODS
Reagents.
Heparin (Liquemin) was purchased from Hoffmann-La Roche, Grenzach-Wyhlen, Germany; urokinase was purchased from Ribosepharm GmbH, Haan, Germany; ciprofloxacin (Ciprobay) and metronidazole (Clont) were purchased from Bayer AG, Leverkusen, Germany. Recombinant hirudin was a generous gift from Knoll AG, Ludwigshafen, Germany. Monoclonal rat anti-mouse TNF antibody V1q is described in reference 7; normal rat immunoglobulin G (IgG) was purchased from Sigma, Deisenhofen, Germany. Thioglycolate medium was purchased from Merck, Darmstadt, Germany, and Mueller-Hinton agar was purchased from Oxoid, Basingstoke, England.
Mice.
Male NMRI mice (25 to 30 g) were purchased from Charles River, Sulzfeld, Germany.
Citrate plasma and citrate serum.
NMRI mice were bled retro-orbitally into tubes containing 1 volume of citrate buffer (0.1 M sodium citrate [pH 8.0]) to which 9 volumes of blood was added (citrate serum). After centrifugation, the citrate plasma was frozen at −20°C.
CLP.
NMRI mice were anesthetized by intraperitoneal (i.p.) injection of Ketanest (75 mg/kg of body weight; Parke, Davis & Company, Munich, Germany) and Rompun (16 mg/kg; Bayer AG) in 0.2 ml of sterile pyrogen-free saline (Fresenius AG, Bad Homburg, Germany). The abdominal skin of the mice was shaved, and a 0.7-cm midline incision was made. The cecum was exteriorized and filled with feces by milking stool back from the ascending colon. The distal end of the cecum (about 40% of the total cecal length for sublethal CLP and 80% for lethal CLP) was ligated and punctured twice with a 0.9-mm needle. Gentle pressure was applied on the ligated cecum to exteriorize a small amount of feces. The cecum was then returned to the peritoneal cavity, and the incision was closed with clamps. After closure of the wound, the mice were injected i.p. with 0.5 ml of phosphate-buffered saline (PBS) or the same volume of other reagents dissolved in PBS. Mice were observed for 2 weeks (7, 28).
Heparin treatment after CLP.
Sublethal CLP was performed. Immediately after closure of the abdominal incision, mice were injected i.p. with 0.5 ml of PBS, with 150 U of heparin in 0.5 ml of PBS, or with 0.5 ml of PBS containing 150 U of heparin, ciprofloxacin (16.6 mg/kg), and metronidazole (41.6 mg/kg).
Treatment with heparin and TNF neutralization after CLP.
Sublethal CLP ensuring survival despite heparin treatment (30% of total cecal length and only one puncture) was performed. Immediately after closure of the abdominal incision, mice were injected i.p. either with 0.5 ml of PBS containing 100 U heparin and 50 μg of normal rat IgG or with 0.5 ml of PBS containing 100 U of heparin and 50 μg of monoclonal anti-mouse TNF antibody V1q, a quantity known to be sublethal for NMRI mice after a CLP of the above-mentioned severity.
Treatment with hirudin after CLP.
Immediately after sublethal CLP, mice were injected i.p. with 0.5 ml of PBS, with 500 μg of hirudin in 0.5 ml of PBS, or with 0.5 ml of PBS containing 500 μg of hirudin, ciprofloxacin (16.6 mg/kg), and metronidazole (41.6 mg/kg).
Treatment with urokinase after CLP.
Eight hours after sublethal CLP, mice were injected i.p. with 0.5 ml of PBS, with 10,000 U of urokinase in 0.5 ml of PBS, or with 0.5 ml of PBS containing 10,000 U of urokinase, ciprofloxacin (16.6 mg/kg), and metronidazole (41.6 mg/kg).
Treatment with plasma or serum after CLP.
Immediately after lethal CLP (80% of total cecal length and two punctures), mice were injected i.p. with 300 μl of mouse citrate plasma, with 300 μl of mouse citrate serum, or with 300 μl of PBS.
Determination of bacterial counts after CLP.
Based on previous experiments, the peritoneal cavities of the CLP mice were lavaged with 5 ml of thioglycolate medium 16 h after injection of heparin or hirudin or 11 h after injection of urokinase, and one liver lobe and one kidney were removed. These organs were homogenized in an Ultra-Turrax (IKA-Labortechnik, Staufen, Germany) at 8,000 rpm in 2 ml of thioglycolate medium for 5 s. Lavage fluids and homogenates were diluted serially with thioglycolate medium (30 g/liter) and incubated on Mueller-Hinton agar plates at 36°C for 24 h in aerobic atmosphere. The resulting bacterial colonies were counted and expressed as bacteria per milliliter.
Statistical analysis.
Significance of the differences in survival after CLP was assessed using the log rank statistic. The microbiological data were compared by the two-tailed Mann-Whitney U test.
RESULTS
Heparin treatment increases CLP mortality.
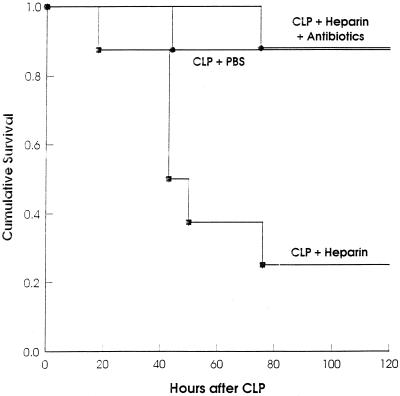
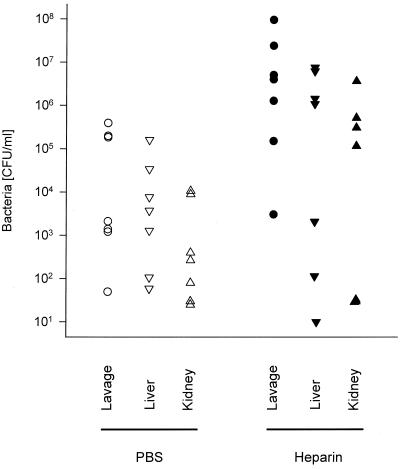
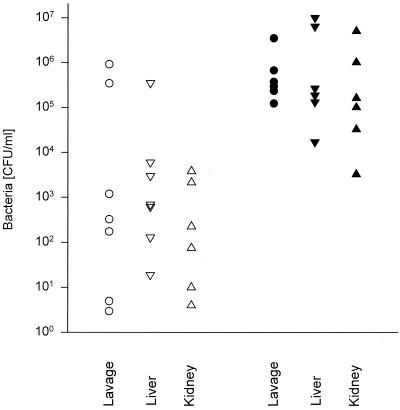
To determine whether the inhibition of fibrin formation influences the outcome of CLP, we treated mice after the operation with the widely used anticoagulant heparin. First, we determined the amount of heparin that does not cause lethal bleeding if administered after laparotomy without CLP. One thousand units of heparin injected i.p. caused lethal bleeding from the abdominal incision in laparotomized mice, whereas nonlaparotomized mice were not affected by this quantity of heparin. Injection of 10 to 150 U of heparin i.p. per 30-g mouse prolonged the ex vivo clotting time significantly (data not shown) but was lethal for neither laparotomized nor nonlaparotomized mice. Ten units had no significant influence on mortality after sublethal CLP. However, 75% of the mice died when treated with 150 U of heparin after sublethal CLP (Fig. 1). These experiments showed that mice treated with a dose of heparin which is anticoagulant in vivo do not bleed to death from the abdominal incision after CLP. The mice did also not bleed excessively into the peritoneal cavity or chest or from organs or tissues located in these compartments. This was checked 16 h after CLP and heparin injection, when the mice were sacrificed for quantification of bacteria. At this time, the ceca of these mice were also examined for adhesions. Adhesions had formed at the ceca of control mice but were absent in the heparin-treated mice. Significantly more bacteria were found in the lavage fluids of heparin-treated mice compared to PBS-treated control mice. The increase in bacterial numbers was statistically not significant for homogenates of liver and kidney, although the highest numbers were detected in livers and kidneys of the heparin-treated groups (Fig. 2). The seven heparin-treated mice that were examined microbiologically were the survivors among 15 mice subjected to CLP. Eight mice died earlier than 16 h after CLP and were not examined. Of 15 control mice, which all survived for 16 h, 7 were chosen randomly for determination of bacteria. It is likely that of the heparin-treated mice, the nonsurvivors died due to higher numbers of bacteria in their vital organs than in those of the survivors.
FIG. 1.
Heparin treatment after CLP increases mortality. After sublethal CLP, groups of mice (n = 8) were injected i.p. with PBS, heparin, or heparin plus antibiotics. (P < 0.015 for heparin versus PBS; P < 0.01 for heparin versus heparin plus antibiotics [log rank statistic].)
FIG. 2.
Heparin treatment after CLP increases bacterial spread. After CLP, groups of mice (n = 15) were injected i.p. with PBS or heparin; 16 h later, numbers of bacteria were determined in peritoneal lavage fluids, livers, and kidneys of the surviving heparin-treated mice (n = 7) and randomly chosen PBS-treated mice (n = 7). (Bacterial numbers after heparin versus after PBS: for lavage fluid, P < 0.018; for liver, P < 0.34; for kidney, P < 0.25 [two-tailed Mann-Whitney U test].)
To exclude any essential nonanticoagulant contribution to the death of heparin-treated mice after CLP, the animals were treated with heparin plus the antibiotics ciprofloxacin and metronidazole. This antimicrobial therapy protected both mice which had undergone only CLP (data not shown) and heparin-treated mice efficiently (Fig. 1), indicating that death after CLP and heparin treatment was caused by unrestricted release of bacteria, due to the prevention of fibrinous peritoneal adhesions.
Coagulation-independent protective TNF effects exist in CLP.
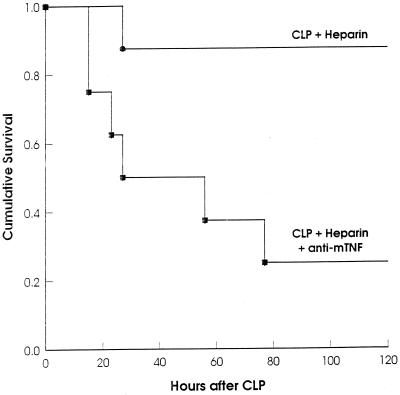
To find out whether other, coagulation-independent, protective TNF-induced mechanisms are active during septic peritonitis, a less severe CLP which was sublethal despite the administration of heparin was performed (Fig. 3). In this system, mortality of the heparin-treated mice could be further increased by neutralizing the endogenous TNF. This finding supports the idea of coagulation-independent protective TNF functions.
FIG. 3.
Nonlethal heparin treatment after sublethal CLP becomes lethal if followed by TNF neutralization. After sublethal CLP, groups of mice (n = 8) were injected i.p. with heparin plus normal rat IgG (control) or with heparin plus monoclonal anti-mouse TNF antibody. (P < 0.02 for control versus anti-TNF [log rank statistic].)
Hirudin treatment increases CLP mortality.
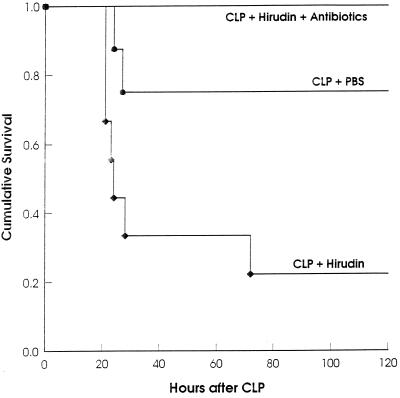
Heparin has additional, nonanticoagulant effects that might influence survival from CLP. To exclude the possibility that these side effects were partially or entirely responsible for the death of CLP-treated mice, we tested whether the specific thrombin inhibitor hirudin enhances mortality after CLP. As with heparin, we first determined a dose of hirudin that did not lead to lethal bleeding in laparotomized mice. A dose of 500 mg of hirudin per 30-g mouse was not lethal for laparotomized mice but was lethal for mice that underwent an otherwise sublethal CLP (Fig. 4). As observed after heparin treatment, this amount of hirudin caused no excessive bleeding and still prevented formation of adhesions at the ceca 16 h after CLP. Microbiological comparison of these mice treated after CLP with PBS or hirudin showed significantly increased numbers of bacteria in peritoneal lavage fluids and homogenates of liver and kidney after hirudin treatment (Fig. 5). Like after heparin treatment, only some (6 of 15) of these mice survived for 16 h, whereas all control mice survived. After CLP, hirudin-treated mice received the same antibiotic therapy as the heparin-treated mice represented in Fig. 1. All antibiotic-treated mice were protected from death, demonstrating that death after CLP and treatment with the thrombin-specific inhibitor hirudin was due to bacterial infection and not to unknown side effects of hirudin (Fig. 4).
FIG. 4.
Treatment with the thrombin inhibitor hirudin after CLP increases mortality. After sublethal CLP, groups of mice were injected i.p. with PBS (n = 8), hirudin (n = 8), or hirudin plus antibiotics (n = 10). (P < 0.03 for hirudin versus PBS; P < 0.001 for hirudin versus hirudin plus antibiotics [log rank statistic].)
FIG. 5.
Treatment with hirudin after CLP increases bacterial spread. After CLP, groups of mice (n = 15) were injected i.p. with PBS or hirudin; 16 h later, numbers of bacteria were determined in peritoneal lavage fluids, livers, and kidneys of the surviving hirudin-treated mice (n = 6) and randomly chosen PBS-treated mice (n = 7). (Bacterial numbers after hirudin versus after PBS: for lavage fluid, P < 0.011; for liver, P < 0.016; for kidney, P < 0.0045 [two-tailed Mann-Whitney U test].)
Protective effect of plasma on CLP mortality.
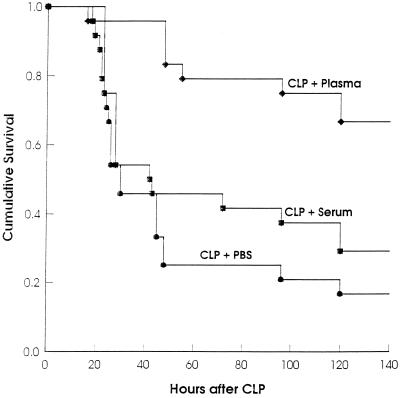
A consequence of peritonitis is the exudation of plasma components, e.g., fibrinogen into the peritoneal cavity. This is also true for peritonitis caused by CLP, as can be demonstrated by the enhanced exudation of intravenously injected Evans blue into the peritoneal cavity after CLP (data not shown). We tested the hypothesis that i.p. injection of plasma immediately after CLP, a treatment intented to provide fibrinogen, might protect mice from CLP-caused death. Indeed, plasma treatment after CLP significantly protected CLP-treated mice compared to PBS-treated mice (Fig. 6). In contrast, serum treatment did not protect mice significantly from consequences of CLP. However, examination of peritoneal cavities of plasma-treated CLP mice revealed no difference in adhesions compared to mice treated with PBS or serum.
FIG. 6.
Intraperitoneal injection of plasma after CLP increases survival. After lethal CLP, mice were injected i.p. with plasma (n = 8), serum (n = 8), or PBS (n = 8). The data are pooled from three independent experiments (n = 72). (P < 0.0001 for PBS versus plasma; P < 0.0033 for serum versus plasma; P > 0.3 for PBS versus serum [log rank statistic].)
Urokinase treatment increases CLP mortality.
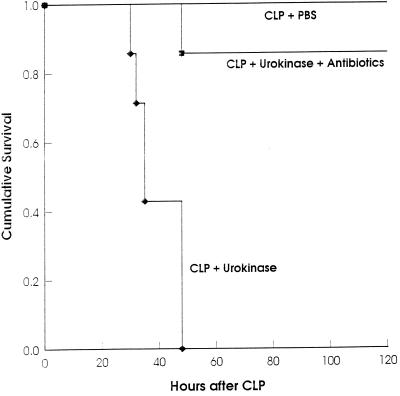
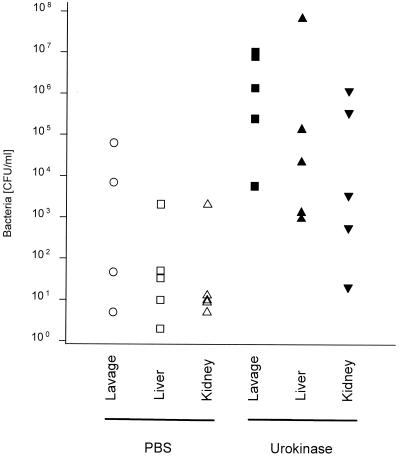
To determine the period after CLP during which fibrin-dependent adherence determines survival, we used urokinase as a fibrinolytic agent. While 5,000 or 10,000 U of urokinase injected i.p. into laparotomized mice did not increase bleeding, treatment of mice immediately after CLP with the same amount of urokinase increased mortality (one of seven control mice died, versus five of seven urokinase-treated mice). Even more striking was treatment with 10,000 U of urokinase 8 h after CLP (Fig. 7). Further delay of urokinase treatment until 16 h after CLP led to variable effects on mortality: in one experiment all mice died very rapidly (n = 8), whereas in a second experiment (n = 12) mortality was the same as for PBS-treated mice (data not shown). To assess the influence of urokinase treatment on bacterial spread, mice were subjected to CLP and injected i.p. with urokinase 8 h later; after an additional 11 h, the number of bacteria was determined in peritoneal lavage fluid, liver, and kidney. Bacterial numbers from the lavage fluids as well as from the organs were significantly increased after urokinase treatment (Fig. 8). No excessive bleeding was found in these mice, and adhesions at the ceca were strongly reduced or absent. Again, antibiotics protected 87% of the mice treated with urokinase 8 h after CLP (Fig. 7), indicating that death was due to infection and not to other urokinase effects.
FIG. 7.
Treatment with the plasminogen activator urokinase after CLP increases mortality. Eight hours after sublethal CLP, groups of mice (n = 7) were injected i.p. with PBS, urokinase, or urokinase plus antibiotics. (P < 0.0005 for urokinase versus PBS; P < 0.002 for urokinase versus urokinase plus antibiotics [log rank statistic].)
FIG. 8.
Treatment with urokinase after CLP increases bacterial spread. Eight hours after CLP, groups of mice (n = 5) were injected i.p. with PBS or hirudin; 11 h later, numbers of bacteria in peritoneal lavage fluids, livers, and kidneys were determined. (Bacterial numbers after hirudin versus after PBS: for lavage fluid, P < 0.028; for liver, P < 0.029, for kidney, P < 0.029 [two-tailed Mann-Whitney U test].)
DISCUSSION
In models of sepsis or septic shock, TNF-induced DIC was regarded as a major cause of morbidity and death of septic animals. The reported procoagulant activity of TNF was not considered potentially beneficial. The idea that the procoagulant activity of TNF might have a positive aspect arose when we observed that neutralization of TNF both increased mortality and was correlated with the lack of peritoneal adhesions. The same observation was made in TNF gene-deficient mice, which 27 h after CLP displayed no or at least strongly reduced adhesions to the cecum compared to normal control mice. In preliminary experiments cultivation of anaerobic bacteria from mice after CLP yielded very inconsistent results, whereas correlation of the numbers of aerobic bacteria in peritoneal lavage fluid and organs with mortality was high. For this reason, the numbers of bacteria were determined in an aerobic atmosphere on Mueller-Hinton agar only.
The question arose of whether prevention of fibrin formation by anticoagulants would be lethal for mice after CLP. Because of the pleiotropy of TNF activities, TNF-dependent processes other than the procoagulant effect might be far more important for the protection conferred by endogenous TNF. In confirmation of our hypothesis, the anticoagulant heparin prevented both the formation of peritoneal adhesions and increased mortality after CLP.
Besides its well-characterized activity as an anticoagulant, heparin has many nonanticoagulant activities (25). For example, it can bind and thereby inactivate endogenous TNF (17) and is able to inhibit mast cell responses (18), recently shown to be critical for survival of CLP (9). Heparin reduces the influx of neutrophils into the peritoneal cavity (22). Since prevention of the drainage of the peritoneal cavity via the stomata in the diaphragm protected animals from death in a peritonitis model (19), the spread of bacteria from the peritoneal cavity via lymphatics into the circulation, resulting in bacteremia, seems to be decisive for the lethal consequences of bacterial peritonitis. However, inhibited recruitment of neutrophils by heparin seems to be less important than the deleterious consequences of unimpaired bacterial spread due to lack of localization, because treatment of mice with antineutrophil antibodies reduced the blood neutrophil number by 90% but impaired survival less than the heparin treatment (data not shown).
To exclude effects on survival after CLP by nonanticoagulant heparin activities, we also used the more specific anticoagulant hirudin, with the same results. In contrast to heparin, hirudin is not known to have side effects that might influence the outcome of CLP by interfering with TNF-dependent mechanisms not related to coagulation. Hirudin directly interacts with thrombin by blocking its enzymatic activity.
Prevention of adhesion formation at the cecum and significantly increased bacterial numbers in various compartments after heparin or hirudin treatment made it likely that bacteria released from the injured cecum caused the high mortality. The idea that mainly living bacteria increase mortality after CLP is supported by our findings: antibiotic treatment does not prevent the release of bacteria from the cecum and their dissemination in mice, but it prevents bacterial growth. Because antibiotics were able to protect mice, death after CLP was certainly caused by bacterial growth, deleterious bacterial products released by living bacteria, or the host reaction to the bacterial infection.
In many reports, bacterial LPS, a constituent of the outer membrane of gram-negative bacteria, is claimed to be a major mediator of multiorgan failure. In addition, there are reports that antibiotics induce release of LPS from dying bacteria, with detrimental consequences for the host (21). Such LPS effects are most probably not a major reason for death after CLP because antibiotics protected CLP-treated mice. In support of this notion, we (unpublished data) and others (20) found no difference in mortality after CLP between normal and LPS low-responder mice.
Despite the fact that after CLP both heparin and hirudin increased mortality significantly, not all mice died. We investigated whether CLP-induced lethality after heparin treatment could further be increased by TNF neutralization, thus indicating additional TNF-dependent mechanisms for protection. The result of increased mortality after sublethal CLP in the presence of heparin and additional neutralization of endogenous TNF clearly showed that other coagulation-independent TNF functions, such as neutrophil recruitment and/or exudation of plasma components into the peritoneal cavity, are important in the CLP model as well.
Intraperitoneal application of plasma after CLP might provide more substrate for coagulation (fibrinogen), complement components, and immunoglobulin, allowing the opsonization of bacteria. Serum, on the other hand, containing opsonins but no fibrinogen, should improve opsonization without any additional effects on fibrin deposition. Whereas plasma treatment significantly protected mice from the lethal effect of CLP, a nonsignificant positive trend was also seen when CLP-treated mice received serum. In a clinical study, i.p. serum administration also showed a trend toward improved survival in patients with bacterial peritonitis (2). Because we did not find more pronounced peritoneal adhesions in plasma-treated CLP mice, improved adhesion formation does not seem to account for the protective effect of i.p. injection of plasma.
We made use of the fibrinolytic function of urokinase to test whether removal of the fibrinous adhesions at a later time would influence the outcome of CLP. Urokinase is not known to inhibit TNF production or other mechanisms possibly beneficial in CLP. Treatment with urokinase, which removed CLP-induced adhesions as verified by visual inspection, was lethal for all mice when given 8 h after CLP. The finding that urokinase treatment was less effective directly after CLP may be explained by the observation that some hours after CLP, the ligated and punctured cecum swells considerably due to the action of gas-producing bacteria. It is conceivable that after pressure has built up, degradation of the fibrin leads to the release of more bacteria from the cecum than immediately after CLP. At even later time points, the fibrin might be too abundant for the urokinase-dependent plasmin to dissolve. The findings that urokinase treatment increased bacterial counts significantly and that treatment with antibiotics after CLP, which was lethal only in combination with urokinase administration, was protective rule out major fibrinolysis-independent deleterious effects of urokinase.
The morbidity that may result from i.p. abscesses seems less relevant than the danger of death occurring early during the course of septic peritonitis if peritoneal adhesions are not formed. Some authors share this view insofar as they consider the formation of i.p. abscesses after bacterial peritonitis in humans to be beneficial (16, 29). In agreement with this view, our experimental data suggest that i.p. adhesions are prerequisite for localization of the bacterial infection and for survival from the fecal peritonitis resulting from intestinal puncture and leakage.
ACKNOWLEDGMENT
This work was supported by a grant from BMBF (01KI9473 project A3).
REFERENCES
- 1.Bagby G J, Plessala K J, Wilson L A, Thompson J J, Nelson S. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991;163:83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Billing A G, Fröhlich D, Konecny G, Schildberg F W, Machleidt W, Fritz H, Jochum M. Local serum application: restoration of sufficient host defense in human peritonitis. Eur J Clin Investig. 1994;24:28–35. doi: 10.1111/j.1365-2362.1994.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 3.Bone R C. Modulators of coagulation. A critical appraisal of their role in sepsis. Arch Intern Med. 1992;152:1381–1389. doi: 10.1001/archinte.152.7.1381. [DOI] [PubMed] [Google Scholar]
- 4.Dickneite G, Czech J. Combination of antibiotic treatment with the thrombin inhibitor recombinant hirudin for the therapy of experimental Klebsiella pneumoniae sepsis. Thromb Haemost. 1994;71:768–772. [PubMed] [Google Scholar]
- 5.Dickneite G, Czech J, Keuper H. Formation of fibrin monomers in experimental disseminated intravascular coagulation and its inhibition by recombinant hirudin. Circ Shock. 1994;42:183–189. [PubMed] [Google Scholar]
- 6.Dosne A M, Dubor F, Lutcher F, Parant M, Chedid L. Tumor necrosis factor (TNF) stimulates plasminogen activator inhibitor (PAI) production by endothelial cells and decreases blood fibrinolytic activity in the rat. Thromb Res Suppl. 1988;8:115–122. doi: 10.1016/0049-3848(88)90160-0. [DOI] [PubMed] [Google Scholar]
- 7.Echtenacher B, Falk W, Männel D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 8.Echtenacher B, Falk W, Männel D N, Krammer P H. Survival from cecal ligation and puncture and the formation of fibrous adhesions in the peritoneal cavity depend on endogenous tumor necrosis factor. In: Faist E, Meakins J L, Schildberg F W, editors. Host defense dysfunction in trauma, shock and sepsis. Heidelberg, Germany: Springer Verlag; 1993. pp. 755–758. [Google Scholar]
- 9.Echtenacher B, Männel D N, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 10.Eskandari K M, Bolgos G, Miller C, Nguyen D T, DeForge L E, Remick D G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 11.Fisher C J, Jr, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 12.Gibson F C, III, Onderdonk A B, Kasper D L, Tzianabos A O. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J Immunol. 1998;160:5000–5006. [PubMed] [Google Scholar]
- 13.Giroir B P. Mediators of septic shock: new approaches for interrupting the endogenous inflammatory cascade. Crit Care Med. 1993;21:780–789. [PubMed] [Google Scholar]
- 14.Gorbach S L. Good and laudable pus. J Clin Investig. 1995;96:2545. doi: 10.1172/JCI118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaidi A A, Gurchumelidze T, Nazzal M, Figert P, Vanterpool C, Silva Y. Tumor necrosis factor-alpha: a marker for peritoneal adhesion formation. J Surg Res. 1995;58:516–518. doi: 10.1006/jsre.1995.1081. [DOI] [PubMed] [Google Scholar]
- 16.Koperna T, Schulz F. Prognosis and treatment of peritonitis. Do we need new scoring systems? Arch Surg. 1996;131:180–186. doi: 10.1001/archsurg.1996.01430140070019. [DOI] [PubMed] [Google Scholar]
- 17.Lantz M, Thysell H, Nilsson E, Olsson I. On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Investig. 1991;88:2026–2031. doi: 10.1172/JCI115530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lucio J, D'Brot J, Guo C B, Abraham W M, Lichtenstein L M, Kagey-Sobotka A, Ahmed T. Immunologic mast cell-mediated responses and histamine release are attenuated by heparin. J Appl Physiol. 1992;73:1093–1101. doi: 10.1152/jappl.1992.73.3.1093. [DOI] [PubMed] [Google Scholar]
- 19.Maddaus M A, Ahrenholz D, Simmons R L. The biology of peritonitis and implications for treatment. Surg Clin North Am. 1988;68:431–443. doi: 10.1016/s0039-6109(16)44487-7. [DOI] [PubMed] [Google Scholar]
- 20.Mercer-Jones M A, Heinzelmann M, Peyton J C, Wickel D J, Cook M, Cheadle W G. The pulmonary inflammatory response to experimental fecal peritonitis: relative roles of tumor necrosis factor-alpha and endotoxin. Inflammation. 1997;21:401–417. doi: 10.1023/a:1027366403913. [DOI] [PubMed] [Google Scholar]
- 21.Morrison D C. Assessment of antibiotic-mediated endotoxin release. J Endotoxin Res. 1996;3:275–279. [PubMed] [Google Scholar]
- 22.Nelson R M, Cecconi O, Roberts W G, Aruffo A, Linhardt R J, Bevilacqua M P. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- 23.Stack A M, Saladino R A, Thompson C, Sattler F, Weiner D L, Parsonnet J, Nariuchi H, Siber G R, Fleisher G R. Failure of prophylactic and therapeutic use of a murine anti-tumor necrosis factor monoclonal antibody in Escherichia coli sepsis in the rabbit. Crit Care Med. 1995;23:1512–1518. doi: 10.1097/00003246-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Steinhauser L M, Hogaboam C M, Lukacs N W, Strieter R M, Kunkel S L. Multiple roles for IL-12 in a model of acute septic peritonitis. J Immunol. 1999;162:5437–5443. [PubMed] [Google Scholar]
- 25.Tyrell D J, Kilfeather S, Page C P. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci. 1995;16:198–204. doi: 10.1016/s0165-6147(00)89022-7. [DOI] [PubMed] [Google Scholar]
- 26.van der Poll T, Levi M, Buller H R, van Deventer S J, de Boer J P, Hack C E, ten Cate J W. Fibrinolytic response to tumor necrosis factor in healthy subjects. J Exp Med. 1991;174:729–732. doi: 10.1084/jem.174.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Hinsbergh V W, Kooistra T, Scheffer M A, Hajo van Bockel J, van Muijen G N. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood. 1990;75:1490–1497. [PubMed] [Google Scholar]
- 28.Wichterman K A, Baue A E, Chaudry I H. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 29.Wittmann D H, Schein M, Condon R E. Management of secondary peritonitis. Ann Surg. 1996;224:10–18. doi: 10.1097/00000658-199607000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]