Abstract
Background and aims
Urine ketone bodies have been considered as predictors of stroke in diabetic patients, however, the role of urine ketone bodies in the prognosis of stroke has not been investigated well. This study aimed to investigate the association between urine ketone bodies and adverse outcomes in patients with acute ischemic stroke (AIS) or transient ischemic attack (TIA).
Methods
This study enrolled 14 015 patients with AIS or TIA who screened for urine ketone bodies from the Third China National Stroke Registry. Status of urine ketone bodies were classified into negative, suspicious positive and positive. The outcomes were all-cause death and poor functional outcomes (modified Rankin Scale [mRS] 2–6/3-6) at 3 months and 1 year. Multivariable Cox proportional hazards regressions and logistic regressions were adopted to explore the associations.
Results
Participants with negative, suspicious positive and positive urine ketone bodies were 12979 (92.61%), 480 (3.42%) and 556 (3.97%). After multivariate adjustment, patients with positive urine ketone bodies had a higher risk of all-cause death (hazard ratio, 1.74; confidence interval [CI], 1.07–2.83), a higher proportion of mRS score 2–6 (Odds ratio [OR], 1.85; 95% CI, 1.51–2.27), mRS score 3–6 (OR, 2.00; 95% CI, 1.61–2.48) at 3 months, compared to those with negative urine ketone bodies. Significant associations persisted at 1 year. Furthermore, there was no significant interaction of diabetes status and alcohol use with urine ketone bodies.
Conclusions
Positive urine ketone bodies can independently predict all-cause death and poor functional outcomes in patients with AIS or TIA.
Keywords: Urine ketone bodies, All-cause death, Poor functional outcomes, Acute ischemic stroke, Transient ischemic attack
Highlight
-
•
Positive urine ketone bodies can independently predict all-cause death in patients with AIS or TIA.
-
•
Positive urine ketone bodies can independently predict poor functional outcomes in patients with AIS or TIA.
-
•
The associations were robust in stratified by the diabetes status and alcohol use.
-
•
Stroke patients with positive urine ketone bodies warrant particular vigilance in clinical practice.
Introduction
Ketone bodies are the metabolic intermediates of human fat by liver and composed of three molecules: 3-hydroxybutyrate, acetoacetate, and acetone [1]. They play an important role in providing energy to the tissues in the brain, heart, muscle, and kidney in the conditions of glucose insufficiency and uncontrolled diabetes [2]. Although ketone bodies serve as substrates for energy, excessive amounts of them may lead to acidosis, causing acid-base balance, which was significantly associated with mortality and morbidity [3]. Derangements of ketone body metabolism occur in numerous disease states, including types 1, 2 diabetes, heart failure, cerebral infarction, and ketone body metabolism changes over the course of normal aging [[4], [5], [6], [7], [8], [9]]. Previous studies reported that ketone bodies were related to the incidence and prognosis of some diseases, such as specific viral infections, intractable epilepsy, drug poisoning, thus ketone bodies may have an impact on the prognosis of some certain disease under physiological and pathological conditions [10].
Although it has been reported that ketoacidosis was a predictor of stroke, these studies mainly focused on patients with diabetes [8,11,12]. Furthermore, evidence from large sample study on the association between urine ketone bodies and prognosis of stroke remains limited up to now. Therefore, the purpose of this study was to investigate the association between urine ketone bodes and the risk of adverse outcomes in patients with acute ischemic stroke (AIS) or transient ischemic attack (TIA), using data from the cohort of the Third China National Stroke Registry (CNSR-III), and further to explore whether the associations were modified by diabetes status and alcohol use.
Materials and methods
Study population
Data were derived from CNSR-III, which was a nationwide, consecutive, multicenter, prospective registry for patients with AIS or TIA presented to hospitals between 2015 and March 2018 in China. Participants were consecutively enrolled if meeting the following criteria: (1) >18 years old; (2) diagnosis of ischemic stroke or TIA within 7 days; (3) informed consent from participant or legally authorized representative. Finally, 15 166 patients were enrolled from 201 hospitals of 22 provinces. The detailed, rationale, and basic descriptions of the CNSR-III have been published previously [13].
Baseline data collection
Trained research coordinators collected baseline data at each site via a direct interview or medical records, including age, sex, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared, kg/m2), smoking status, self-reported alcohol use (never, current [drinking within the past 6 months], and past [cessation of more than 6 months]), National Institutes of Health Stroke Scale (NIHSS) assessed by trained neurologists on admission, time from onset from symptom to admission, medical history of ischemic stroke, TIA, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation/flutter (AF), carotid artery stenosis, epilepsy, and infection; laboratory test (including fasting plasma glucose [FPG], hemoglobin A1c [HbA1c], estimated glomerular filtration rate [eGFR] calculated by the creatinine-based Chronic Kidney Disease Epidemiological Collaboration equation, high-sensitivity C-reactive protein [hs-CRP], leukocytes, and hematocrit); stroke type (IS or TIA); the etiology classification of ischemic stroke according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [14], admission diagnosis (hypertension, diabetes, and hyperlipidemia), and medications in hospital (cholesterol-lowering agents, antihypertensive agents, hypoglycemic agents, antiplatelet agents, and anticoagulant agents).
Diabetes status was assessed by FPG or HbA1c levels, admission diagnosis of diabetes, and use of glucose-lowering medications as follows: 1) diabetes was defined as HbA1c ≥ 6.5%, or FPG ≥7.0 mmol/L, or receiving a diagnosis of diabetes, and/or taking antidiabetic medication; 2) prediabetes or impaired glucose tolerance was defined as HbA1c 5.7%–6.4% and/or FPG 5.6–7.0 mmol/L; or 3) no diabetes was defined as HbA1c <5.7% and FPG <5.6 mmol/L.
Sample collection and measurements of urine ketone bodies
Fasting blood samples and random urine samples were collected within 24 h of admission, then were preserved and processed in a manner recommended by the clinical site laboratory's policies and procedures in every hospital [13]. All the blood and urine samples were collected and then frozen in cryotube at −80 °C refrigerator until testing was performed. Urine ketone bodies measurements were performed by urine dipstick (Siemens Multistix 10SG). The results of the urine strip test were semiquantified as negative (−), suspicious positive (±) or positive (+, 2+, or ≥3+). Laboratory technicians were blinded to the clinical outcomes of patients. All measurements were performed according to the manufacturers' recommendations.
Follow-up and adverse outcomes
Patients were interviewed face-to face at 3 months and contacted over the telephone by trained research coordinators at 1 year. Adverse outcomes in our study include all-cause death and poor functional outcomes, these information were collected by trained research coordinators who were blinded to subjects’ baseline characteristics. All-cause death was defined as death from any cause and confirmed by a death certification from the attended hospital or the local citizen registry. Poor functional outcome was defined as modified Rankin Scale (mRS) score ranged 2–6 or 3–6.
Statistical analysis
Patients were categorized into 3 groups by urine ketone bodies states: negative, suspicious positive, positive. Continuous variables were presented as medians and interquartile ranges because of skewed distribution, differences among groups were compared with nonparametric Wilcoxon or Kruskal-Wallis test. Categories variables were presented as frequencies with percentages, and were compared with chi-square test. The Kaplan-Meier method and log-rank test were used to estimate the cumulative incidence of all-cause mortality. Ordinal logistic regression was applied to estimate the common odds ratio (OR) for a shift in the direction of a worse outcome on the mRS score.
Cox proportional hazard models and logistic regression models were used to explore the associations of urine ketone bodies states with all-cause death and poor functional outcomes, respectively, with patients with negative urine ketone bodies as the reference. Because 201 hospitals participated in the study, the hospitals were treated as clusters in the model and the sandwich estimator was used to account for the correlations. Variables were adjusted in the multivariable analyses if established as traditional predictors for outcomes or associated with urine ketone bodies in univariate analysis with a value of p < 0.2. Unadjusted and adjusted hazard ratios (HRs) or ORs and their 95% confidence intervals (CIs) were calculated. Three models were constructed. Model 1 was unadjusted; Model 2 was adjusted for age and gender; Model 3 was further adjusted for BMI, alcohol use, NIHSS score, medical history of AF, carotid artery stenosis, epilepsy, infection, stroke type, TOAST, diabetes status, diabetes types, FPG, eGFR, hs-CRP, hypoglycemic agents, antiplatelet agents, and anticoagulant agents. To test the robust of the finding, we further adjusted for time from onset of symptom to admission, leukocytes, and hematocrit in the sensitivity analysis. Additionally, we used C statistics, integrated discrimination (IDI), and net reclassification index (NRI) to evaluate the incremental predictive value of urine ketone bodies beyond conventional risk factors. We further tested the interactions of diabetes status and alcohol use with urine ketone bodies by adding their product terms into the full adjusted model using likelihood ratio test for significance.
All tests were two-sided, and a P value of 0.05 was considered statistically significant. All statistical analyses were performed by SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
After the exclusion of patients with missing available data on urine ketone bodies (n = 746), and missing available data on mRS at 3 months or 1 year (n = 405), 14 015 patients were included in our analysis. Participants with negative, suspicious positive and positive urine ketone bodies were 12979 (92.61%), 480 (3.42%) and 556 (3.97%), respectively. Compared to patients with negative urine ketone bodies, those with positive urine ketone bodies were more likely to have a lower BMI, a higher NIHSS score, shorter time from onset of symptom to admission, a higher prevalence of diabetes mellitus, AF, epilepsy, infection, have higher levels of FPG, eGFR, hsCRP, and leukocytes, a larger proportion of diabetes, especially type 1 diabetes on admission, large-artery atherosclerosis subtype, more likely to take hypoglycemic agents, antiplatelet agents and anticoagulant agents (Table 1).
Table 1.
Baseline characteristics according to urinary ketone bodies.
| Variable | Overall | Urinary ketone bodies |
|||
|---|---|---|---|---|---|
| Negative | Suspicious positive | Positive | P value | ||
| No. of patients | 14015 | 12979(92.61) | 480(3.42) | 556(3.97) | |
| Demographic information | |||||
| Age, y | 62(54–70) | 62(54–70) | 63(54–71) | 62(54–71) | 0.9773 |
| Men | 9567(68.26) | 8854(68.22) | 341(71.04) | 372(66.91) | 0.3337 |
| BMI, kg/m2 | 24.49(22.60–26.57) | 24.49(22.66–26.57) | 24.22(22.56–26.30) | 23.88(22.04–25.95) | <0.0001 |
| Current smoker | 4382(31.27) | 4062(31.30) | 151(31.46) | 169(30.40) | 0.9004 |
| Current alcohol drinking | 2272(16.21) | 2083(16.05) | 89(18.54) | 100(17.99) | 0.1773 |
| NIHSS score on admission | 3.00(1.00–6.00) | 3.00(1.00–5.00) | 4.00(2.00–8.00) | 6.00(3.00–10.00) | <0.0001 |
| Time from onset of symptoms to admission, hour | 14.00(3.00–44.00) | 15.00(3.00–45.00) | 10.00(2.00–30.00) | 10.00(3.00–34.00) | 0.0022 |
| Medical history, n (%) | |||||
| Ischemic stroke | 3082(21.99) | 2869(22.10) | 94(19.58) | 119(21.40) | 0.4001 |
| TIA | 396(2.83) | 373(2.87) | 9(1.88) | 14(2.52) | 0.3903 |
| Hypertension | 8774(62.60) | 8131(62.65) | 300(62.50) | 343(61.69) | 0.9000 |
| Diabetes mellitus | 3252(23.20) | 2900(22.34) | 158(32.92) | 194(34.89) | <0.0001 |
| Hyperlipidemia | 1112(7.93) | 1021(7.87) | 43(8.96) | 48(8.63) | 0.5649 |
| Atrial fibrillation/flutter | 920 (6.56) | 806 (6.21) | 51 (10.63) | 63 (11.33) | <0.0001 |
| Peripheral vascular disease | 107 (0.76) | 101 (0.78) | 1 (0.21) | 5 (0.90) | 0.3456 |
| Laboratory index | |||||
| FPG, mmol/L | 5.52(4.90–6.88) | 5.50(4.89–6.73) | 6.38(5.23–9.44) | 6.10(5.02–9.53) | <0.0001 |
| eGFR, mL/min/1.73 m2 | 93.03(81.42–101.84) | 92.94(81.34–101.71) | 94.52(81.15–102.60) | 94.88(84.45–104.49) | 0.0006 |
| hsCRP, mg/L | 1.74(0.81–4.56) | 1.65(0.79–4.26) | 2.78(1.03–7.33) | 4.95(1.49–17.44) | <0.0001 |
| Leukocytes, × 109/L | 6.90(5.71–8.40) | 6.84(5.70–8.31) | 7.60(6.19–9.30) | 7.80(6.32–9.61) | <0.0001 |
| Hematocrit, % | 41.10(36.90–44.50) | 41.10(36.90–44.50) | 41.70(37.00–45.60) | 41.20(36.80–44.70) | 0.0613 |
| Alb | |||||
| Stroke subtypes, n (%) | |||||
| Ischemic stroke | 13058(93.17) | 12041(92.77) | 467(92.29) | 550(98.52) | <0.0001 |
| TIA | 957(6.83) | 938(7.23) | 13(2.71) | 6(1.08) | |
| TOAST | <0.0001 | ||||
| Large-artery atherosclerosis | 3537(25.24) | 3174(24.45) | 161(33.54) | 202(36.33) | |
| Cardioembolism | 829(5.92) | 737(5.68) | 37(7.71) | 55(9.89) | |
| Small-vessel occlusion | 2956(21.09) | 2834(21.84) | 65(13.54) | 57(10.25) | |
| Other determined etiology | 173(1.23) | 164(1.26) | 3(0.63) | 6(1.08) | |
| Undetermined etiology | 6520(46.52) | 6070(46.77) | 214(44.58) | 236(42.45) | |
| Admission diagnosis, n (%) | |||||
| Hypertension | 10275(73.31) | 9524(73.38) | 343(71.46) | 408(73.38) | 0.6456 |
| Diabetes mellitus | 4506(32.15) | 4052(31.22) | 212(44.17) | 242(43.53) | <0.0001 |
| Hyperlipidemia | 5155(36.78) | 4770(36.75) | 187(38.96) | 198(35.61) | 0.5193 |
| Medication in hospital | |||||
| Cholesterol-lowering agents | 13423(96.42) | 12438(96.44) | 455(95.39) | 530(96.89) | 0.3979 |
| Antihypertensive agents | 6477(46.53) | 6010(46.60) | 215(45.07) | 252(46.07) | 0.7871 |
| Hypoglycemic agents | 3519(25.28) | 3127(24.25) | 182(38.16) | 210(38.39) | <0.0001 |
| Antiplatelet agents | 13530(97.19) | 12552(97.32) | 454(95.18) | 524(95.80) | 0.0027 |
| Anticoagulant agents | 1396(10.03) | 1259(9.76) | 59(12.37) | 78(14.26) | 0.0006 |
Abbreviations: BMI =Body Mass Index; eGFR = estimated glomerular filtration rate; FPG = fasting plasma glucose; hs-CRP = high sensitivity C-reactive protein; NIHSS = National Institutes of Health Stroke Scale.
Urine ketone bodies and adverse outcomes
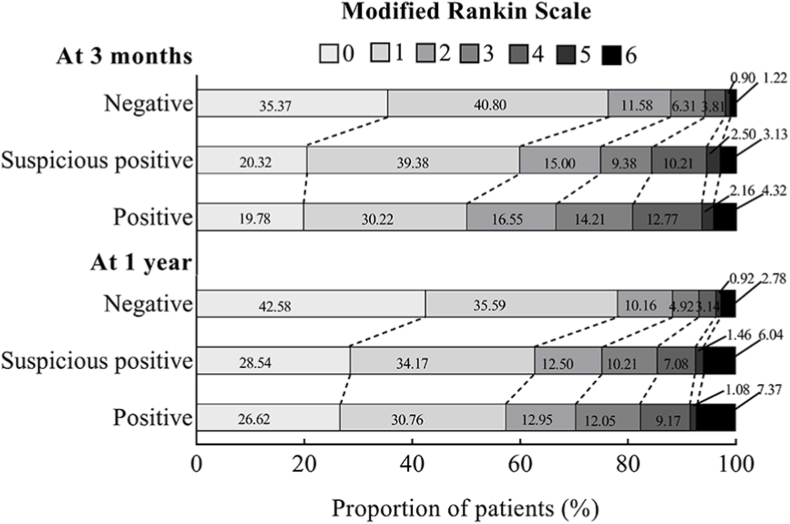
At 3-months assessment, death occurred in 197 (1.41%) patients, mRS score 2–6 occurred in 3563 (25.42%) patients, and mRS score 3–6 occurred in 1896 (13.53%) patients. At 1-year assessment, death occurred in 431 (3.08%) patients, mRS score 2–6 occurred in 3262 (23.28%) patients, and mRS score 3–6 occurred in 1811 (12.92%) patients. The Kaplan-Meier curves showed patients with positive urine ketone bodies had a higher incidence of all-cause death at both 3-months and 1-year follow-up (log-rank test p < 0.01, Fig. 1A and B). There were significant shifts in the distributions of the mRS scores according to the status of urine ketone bodies, the common OR was 1.49 (95% CI, 1.27–1.75) at 3-months assessment and 1.31 (95% CI, 1.12–1.54) at 1-year assessment (Fig. 2).
Fig. 1.
Kaplan-Meier curves of the incidence rate of all-cause death by status of urine ketone bodies. A: Death at 3 months; B: Death at 1 year.
Fig. 2.
The distribution of Modified Rankin Scale Scores Stratified by status of urine ketone bodies.
The figure is shown the distribution of scores on the modified Rankin Scale. Scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability and 6 death.
After adjusting for potential covariates, positive urine ketone bodies was associated with elevated risk of all-cause mortality (HR, 1.74; 95% CI, 1.07–2.83) at 3 months, compared to negative urine ketone bodies. Additionally, both suspicious positive and positive urine ketone bodies were significantly related to high risk of poor functional outcomes, the adjusted ORs were 1.53 (95% CI, 1.23–1.90) and 1.85 (95% CI, 1.51–2.27) for mRS score 2–6, 1.65 (95% CI, 1.29–2.12) and 2.00 (95% CI, 1.61–2.48) for mRS score 3–6, respectively. These associations persisted in the sensitivity analysis and when the time point was set up at 1 year (Table 2).
Table 2.
HRs (95% CIs) for outcomes according to urinary ketone bodies status.
| Outcomes | Urinary ketone bodies |
P for trend | ||
|---|---|---|---|---|
| Negative | Suspicious positive | Positive | ||
| At 3 month | ||||
| Death | 158 (1.22) | 15 (3.13) | 24 (4.32) | |
| Model 1 | Reference | 1.98(1.15–3.41) | 2.93(1.88–4.59) | <0.0001 |
| Model 2 | Reference | 1.86(1.08–3.22) | 3.15(2.01–4.94) | <0.0001 |
| Model 3 | Reference | 1.32(0.75–2.32) | 1.74(1.07–2.83) | 0.0202 |
| Sensitivity analysis | Reference | 1.29(0.73–2.27) | 1.87(1.14–3.05) | 0.0113 |
| mRS score 2-6 | 3092 (23.82) | 193 (40.21) | 278 (50.00) | |
| Model 1 | Reference | 2.03(1.66–2.48) | 2.92(2.43–3.50) | <0.0001 |
| Model 2 | Reference | 2.05(1.68–2.51) | 3.00(2.49–3.60) | <0.0001 |
| Model 3 | Reference | 1.53(1.23–1.90) | 1.85(1.51–2.27) | 0.0169 |
| Sensitivity analysis | Reference | 1.72(1.39–2.12) | 2.37(1.96–2.87) | 0.0252 |
| mRS score 3-6 | 1589 (12.24) | 121 (25.21) | 186 (33.45) | |
| Model 1 | Reference | 2.22(1.77–2.79) | 3.23(2.66–3.93) | <0.0001 |
| Model 2 | Reference | 2.26(1.80–2.85) | 3.32(2.72–4.05) | <0.0001 |
| Model 3 | Reference | 1.65(1.29–2.12) | 2.00(1.61–2.48) | 0.0237 |
| Sensitivity analysis | Reference | 1.84(1.45–2.34) | 2.57(2.08–3.16) | 0.0247 |
| At 1 year | ||||
| Death | 361 (2.78) | 29 (6.04) | 41 (7.37) | |
| Model 1 | Reference | 2.00(1.36–2.96) | 2.36(1.68–3.30) | <0.0001 |
| Model 2 | Reference | 1.89(1.28–2.80) | 2.49(1.77–3.50) | <0.0001 |
| Model 3 | Reference | 1.48(0.99–2.22) | 1.62(1.14–2.32) | 0.0024 |
| Sensitivity analysis | Reference | 1.55(1.03–2.31) | 1.81(1.27–2.59) | <0.0001 |
| mRS score 2-6 | 2846 (21.93) | 179 (37.29) | 237 (42.63) | |
| Model 1 | Reference | 2.05(1.68–2.51) | 2.44(2.03–2.93) | |
| Model 2 | Reference | 2.08(1.70–2.56) | 2.52(2.09–3.03) | <0.0001 |
| Model 3 | Reference | 1.63(1.31–2.02) | 1.63(1.33–1.98) | <0.0001 |
| Sensitivity analysis | Reference | 1.80(1.45–2.22) | 2.01(1.66–2.44) | <0.0001 |
| mRS score 3-6 | 1527 (11.77) | 119 (24.79) | 165 (29.68) | |
| Model 1 | Reference | 2.34(1.87–2.94) | 2.86(2.34–3.50) | |
| Model 2 | Reference | 2.40(1.90–3.02) | 3.00(2.44–3.68) | <0.0001 |
| Model 3 | Reference | 1.84(1.44–2.37) | 1.90(1.52–2.38) | 0.0147 |
| Sensitivity analysis | Reference | 2.07(2.62–2.63) | 2.37(1.90–2.94) | 0.0160 |
Abbreviations: CIs = confidence intervals; HRs = hazard ratios; mRS = modified Rankin Scale.
Model 1: unadjusted.
Model 2: adjusted for age and gender.
Model 3: further adjusted for BMI, current alcohol use, NIHSS score, medical history of diabetes mellitus, atrial fibrillation/flutter, stroke type, TOAST, FPG, eGFR, hs-CRP, diagnosis of diabetes mellitus, hypoglycemic agents, antiplatelet agents, and anticoagulant agents during hospitalization.
Sensitivity analysis was further adjusted for time from onset of symptom to admission, leukocytes, and hematocrit.
Incremental predictive value of urine ketone bodies
We evaluated whether urine ketone bodies would further increase the predictive value of convention risk factors. For mRS score 3–6 at 3 months as the outcome of interest, the C statistics by the conventional model significantly improve with the addition of urine ketone bodies (from 0.782 to 0.786, p < 0.0001), furthermore, the discriminatory power and risk reclassification appeared to be substantially better (IDI 0.74%, P < 0.0001; NRI 2.33%, p = 0.0023). Similar results were found for mRS score 2–6 and when the time point was set up as 1 year. However, insignificant results were observed when all-cause death as the outcome of interest (Table 3).
Table 3.
Reclassification and discrimination statistics for adverse outcomes at 3 months and 1 year by urine ketone bodies.
| C statistics |
IDI |
NRI (continuous) |
NRI (categorical)a |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI), % | P value | Estimate (95% CI), % | P value | Estimate (95% CI), % | P value | |
| Outcomes at 3 months | ||||||||
| Death | ||||||||
| Conventional modelb | 0.821 (0.792–0.851) | Reference | Reference | Reference | ||||
| Conventional model + urine ketone bodies | 0.824 (0.795–0.854) | 0.2197 | 0.24 (−0.06–0.56) | 0.1325 | 6.65 (−5.41–18.72) | 0.3536 | 0.06 (−4.16–3.02) | 0.7544 |
| mRS score 2–6 | ||||||||
| Conventional modelb | 0.764 (0.755–0.773) | Reference | Reference | Reference | ||||
| Conventional model + urine ketone bodies | 0.767 (0.758–0.775) | 0.0004 | 0.54 (0.38–0.70) | <0.0001 | 1.78 (−0.97–4.52) | 0.3596 | 1.02 (0.32–1.72) | 0.0044 |
| mRS score 3–6 | ||||||||
| Conventional modelb | 0.781 (0.771–0.792) | Reference | Reference | Reference | ||||
| Conventional model + urine ketone bodies | 0.786 (0.775–0.796) | 0.0001 | 0.75 (0.51–0.99) | <0.0001 | 4.00 (0.24–7.75) | 0.0106 | 2.20 (0.66–3.75) | 0.0053 |
| Outcomes at 1 year | ||||||||
| Death | ||||||||
| Conventional modelb | 0.788 (0.765–0.810) | Reference | Reference | Reference | ||||
| Conventional model + urine ketone bodies | 0.790 (0.768–0.812) | 0.0899 | 0.18 (−0.02–0.39) | 0.0794 | 5.88 (−2.02–13.79) | 0.2291 | 0.99 (−1.38–3.36) | 0.4125 |
| mRS score 2–6 | ||||||||
| Conventional modelb | 0.737 (0.728–0.747) | Reference | Reference | Reference | ||||
| Conventional model + urine ketone bodies | 0.739 (0.730–0.749) | 0.0064 | 0.40 (0.26–0.53) | <0.0001 | 0.88 (−1.98–3.75) | 0.6589 | 0.71 (−0.14–1.55) | 0.1029 |
| mRS score 3–6 | ||||||||
| Conventional modelb | 0.771 (0.760–0.783) | Reference | Reference | Reference | ||||
| Conventional model + urine ketone bodies | 0.776 (0.764–0.787) | 0.0002 | 0.63 (0.41–0.85) | <0.0001 | 6.10 (2.26–9.94) | 0.0154 | 1.31 (−0.13–2.75) | 0.0741 |
Abbreviations: CI = confidence interval; IDI = integrated discrimination improvement; NRI = net reclassification index; mRS = modified Rankin Scale.
Patients were divided into 3 risk categories by urine ketone bodies.
Conventional model: adjusted for age, gender, BMI, current alcohol use, NIHSS score, medical history of diabetes mellitus, atrial fibrillation/flutter, stroke type, TOAST, FPG, eGFR, hs-CRP, diagnosis of diabetes mellitus, hypoglycemic agents, antiplatelet agents, and anticoagulant agents during hospitalization.
Subgroup analysis
There was no significant interaction between urine ketone bodies and diabetes status when all-cause mortality (p for interaction = 0.9140), mRS score 2–6 (p for interaction = 0.1168), mRS score 3–6 (p for interaction = 0.1171) at 3 months were considered as the outcomes of interest. The associations between urine ketone bodies and adverse outcomes were also consistent in current drinkers and non-drinkers, all P for interactions were >0.05. Similar results were observed for 1 year follow-up (Tables S1–S2 in the online-only Data supplement).
Discussion
Results from this prospective study conducted in the CNSR-III showed that positive urine ketone bodies were significantly associated with the risk of all-cause death and poor functional outcomes in patients with AIS or TIA at 3 months and 1 year. Furthermore, the associations were not significantly modified by diabetes status and alcohol use.
There are very limited data in the literature as to whether urine ketone bodies is associated with the risk of all-cause death and poor functional outcomes in patients with AIS or TIA. The role of ketone bodies or ketoacidosis was paid more attention on patients with diabetes. Diabetic patients not only have increased ketone production but their ketone clearance also significantly goes down [15], which can be attributed to their inherently low insulin levels as insulin is required for efficient ketone clearance [16]. A review demonstrated that hyperketonemia and ketosis would increase the risk of complications in type1 diabetes [17], especially would increase the risk of diabetic ketoacidosis (DKA), a serious complication in these patients [18]. Previous studies reported that DKA was often complicated with cerebral edema in children, extremely in those with newly diagnosed diabetes and in very young adults under 20 years of age [19,20]. Cerebral edema associated with hyperketonemia has been implicated to intracerebral complications and neurological deterioration [21]. A retrospective nationwide population-based cohort study with 3572 type 2 diabetes patients with DKA and 7144 controls found that type 2 diabetes patients with previous DKA had a higher risk of stroke, especially ischemic stroke, compared to those without DKA [22].
The association between abnormal urine ketone bodies and disease outcomes in non-diabetes individuals was mainly conducted among alcoholics. Alcoholic ketoacidosis occurred when the levels of ketones in the blood elevated causing by alcohol abuse, which was common in the emergency room in alcoholics [7]. Most commonly this condition was associated with normal blood glucose levels. However, the patients may become hypoglycemic and are rendered unconscious with potential brain damage when accompanied with malnourishment (low carbohydrate intake) [23,24], which indicated the potential negative effect of ketone bodies on nervous system in non-diabetic patients. Thus ketoacidosis may contribute to the pathology of adverse outcomes after stroke.
To our best knowledge, there has been no study evaluating the associations between urine ketone bodies and adverse outcomes in patients with AIS or TIA. The role of urine ketone bodies was extended to patients with AIS or TIA as an independent predictor of adverse outcomes after stroke, the associations were independent of hematocrit and leukocytes, which reflect inflammatory process and were also reported to be associated with stroke prognosis [25,26]. In addition, our study also found all the associations were consistent in different diabetes status, and in current drinker and non-drinkers. The detection of urine ketone bodies in urine is easily accessible, inexpensive, rapid and high repeatability, and it is convenient for clinical doctors to make decisions and treatment according to the results. Stroke patients with positive urine ketone may warrant particular vigilance and should be follow-up closely for the prevention of adverse outcomes.
Although the mechanisms responsible for the association between urine ketone bodies and adverse outcomes of stroke are still unknown, several possibilities have been proposed. First, the ketoacidosis was associated with a systemic inflammatory response mediated by signaling molecules, and was characterized by an increase of inflammatory markers (such as C-reactive protein), cytokines [interleukin (IL)-6, IL-1 IL-1β, tumour necrosis factor (TNF)-α] and complement activation [27,28]. Second, ketones were shown to accelerate oxidative stress in cardiomyocytes, erythrocytes, and endothelial cells, and oxidative stress induced by ketosis may further contribute to the inflammatory reaction and result in diffuse vascular injury [29,30]. It was becoming clear that the elevated levels of oxidative stress can increase the oxidation of low-density lipoprotein and thereby promote or increase the risk for atherosclerosis, that may be proportional to the degree of severity of stroke, and influenced the prognosis of stroke [31]. The clear mechanisms still needed further investigations.
The strength of our study is that it is a multicenter prospective registry with a large sample size, which supports sufficient statistical power. However, there were still several limitations to our study. First, the ketone bodies assessment by urine dipstick was considered only semiquantified as negative, suspicious positive or positive. Further consideration of trace/small ketones versus moderate/large ketones may provide more detailed information. Additionally, quantitative measurement of ketone bodies and information of albuminuria were not available, which warrants further investigation. Second, urine dipsticks react more with acetoacetate and acetone, but less with β-hydroxybutyrate, the test result could also reflect the real metabolism status of urine ketone bodies in our body. Furthermore, the method is rapid, easily accessible, inexpensive, and has excellent sensitivity for DKA in clinical practice. Third, this study only monitored baseline ketone bodies and did not examine the dynamic changes in urine ketone bodies, which may provide more valuable information to understand the mechanism of the associations. Fourth, equipment heterogeneity at research centers may lead to biased estimates of results, but this may have little impact, because the hospital was treated as clusters in the model and the sandwich estimated were used to account for the correlations in our study. Fifth, oral glucose tolerance test (OGTT) has not been used for diabetes diagnosis so that there might be misclassification of diabetes patients, however, given that HbA1c is more reliable compared with an OGTT, the misclassification is likely to be only moderate [32]. Finally, because all the patients were from China, the findings should be extrapolated cautiously to other populations. Further prospective studies conducted among different populations are needed to replicate our findings.
In conclusion, we found that positive urine ketone bodies were significantly associated with elevated risk of all-cause mortality and poor functional outcomes in patients with AIS or TIA at 3 months and 1 year, regardless of the diabetes status and alcohol use. The results suggested that stroke patients with positive urine ketone bodies warrant particular vigilance in clinical practice.
Ethics approval
The ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2015-001-01) and all study centers gave ethical approval of the study protocol. Written consents were obtained from all participants or their legal representatives.
Author contributions
Y. W. contributed to the conception and design of the study; A.W. contributed to manuscript drafting; A.W., X.T., Y.Z. and Q.X. contributed to the statistics analysis; XM., P.C. and H.L. contributed to the acquisition of data; A.W. and Y.W. contributed to critical revisions of the manuscript.
Funding
This study is supported by grants from the Capital's Funds for Health Improvement and Research (2020-1-2041), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), National Natural Science Foundation of China (81870905, U20A20358).
Data availability
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Declaration of competing interest
These authors declare no conflict of interest.
Acknowledgment
We thank all the participating hospitals, their physicians and nurses, and CNSR III steering Committee members and all the participants in the present study.
Footnotes
This work was supported by Beijing Municipal Administration of Hospitals Incubating Program (PX2020021), National Key R&D Program of China (2018YFC1312903), National Science and Technology Major Project (2017ZX09304018), Beijing Municipal Science & Technology Commission (D171100003017002, Z181100001818001), Beijing Excellent Talents Training Program (2018000021469G234), and Young Elite Scientists Sponsorship Program by CAST (2018QNRC001).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2022.03.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sasaki R., Taura N., Miyazoe Y., Yamamichi S., Nakashiki S., Yamashima M., et al. Ketone bodies as a predictor of prognosis of hepatocellular carcinoma after transcatheter arterial chemoembolization. Nutrition. 2018;50:97–103. doi: 10.1016/j.nut.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Garcia E., Shalaurova I., Matyus S., Oskardmay D., Otvos J., Dullaart R., et al. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J Clin Med. 2020;9 doi: 10.3390/jcm9020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilici M., Tavil B., Dogru O., Davutoglu M., Bosnak M. Diabetic ketoasidosis is associated with prothrombotic tendency in children. Pediatr Hematol Oncol. 2011;28:418–424. doi: 10.3109/08880018.2011.558568. [DOI] [PubMed] [Google Scholar]
- 4.Soeters M., Sauerwein H., Faas L., Smeenge M., Duran M., Wanders R., et al. Effects of insulin on ketogenesis following fasting in lean and obese men. Obesity. 2009;17:1326–1331. doi: 10.1038/oby.2008.678. [DOI] [PubMed] [Google Scholar]
- 5.Lommi J., Kupari M., Koskinen P., Näveri H., Leinonen H., Pulkki K., et al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S., Peterson T., Laplante M., Oh S., Sabatini D. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama A., Yokoyama T., Mizukami T., Matsui T., Shiraishi K., Kimura M., et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol. 2014;49:618–625. doi: 10.1093/alcalc/agu048. [DOI] [PubMed] [Google Scholar]
- 8.Nyenwe E., Kitabchi A. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metab Clin Exp. 2016;65:507–521. doi: 10.1016/j.metabol.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Lee H., Hwang J. Cerebral infarction associated with transient visual loss in child with diabetic ketoacidosis. Diabet Med : J British Diabetic Assoc. 2011;28:516–518. doi: 10.1111/j.1464-5491.2011.03241.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukao T., Mitchell G., Sass J., Hori T., Orii K., Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541–551. doi: 10.1007/s10545-014-9704-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Weng S., Yang C., Wang J., Tien K. Long-term risk of stroke in type 2 diabetes patients with diabetic ketoacidosis: a population-based, propensity score-matched, longitudinal follow-up study. Diabetes Metabol. 2017;43:223–228. doi: 10.1016/j.diabet.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura R., Tanaka Y., Koiwai K., Ishida K., Salsali A., Kaspers S., et al. Effect of empagliflozin on free fatty acids and ketone bodies in Japanese patients with type 2 diabetes mellitus: a randomized controlled trial. Adv Ther. 2019;36:2769–2782. doi: 10.1007/s12325-019-01045-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Jing J., Meng X., Pan Y., Wang Y., Zhao X., et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke and vascular neurology. 2019;4:158–164. doi: 10.1136/svn-2019-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Hall S., Wastney M., Bolton T., Braaten J., Berman M. Ketone body kinetics in humans: the effects of insulin-dependent diabetes, obesity, and starvation. J Lipid Res. 1984;25:1184–1194. [PubMed] [Google Scholar]
- 16.Sherwin R., Hendler R., Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes. 1976;25:776–784. doi: 10.2337/diab.25.9.776. [DOI] [PubMed] [Google Scholar]
- 17.Kanikarla-Marie P., Jain S. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radical Biol Med. 2016;95:268–277. doi: 10.1016/j.freeradbiomed.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser N., Barnett P., McCaslin I., Nelson D., Trainor J., Louie J., et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The pediatric emergency medicine collaborative research committee of the American academy of pediatrics. N Engl J Med. 2001;344:264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 20.Silver S.M., Clark E.C., Schroeder B.M., Sterns R.H. Pathogenesis of cerebral edema after treatment of diabetic ketoacidosis. Kidney Int. 1997;51:1237–1244. doi: 10.1038/ki.1997.169. [DOI] [PubMed] [Google Scholar]
- 21.Wolfsdorf J., Glaser N., Sperling M. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–1159. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y.L., Weng S.F., Yang C.Y., Wang J.J., Tien K.J. Long-term risk of stroke in type 2 diabetes patients with diabetic ketoacidosis: a population-based, propensity score-matched, longitudinal follow-up study. Diabetes Metab. 2017;43:223–228. doi: 10.1016/j.diabet.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Jain H., Beriwal S., Singh S. Alcohol induced ketoacidosis, severe hypoglycemia and irreversible encephalopathy. Med Sci Mon Int Med J Exp Clin Res : Int Med J Experiment Clin Res. 2002;8:CS77–CS79. [PubMed] [Google Scholar]
- 24.Matsuzaki T., Shiraishi W., Iwanaga Y., Yamamoto A. Case of alcoholic ketoacidosis accompanied with severe hypoglycemia. J UOEH. 2015;37:43–47. doi: 10.7888/juoeh.37.43. [DOI] [PubMed] [Google Scholar]
- 25.Stavropoulos K., Imprialos K.P., Bouloukou S., Boutari C., Doumas M. Hematocrit and stroke: a forgotten and neglected link? Semin Thromb Hemost. 2017;43:591–598. doi: 10.1055/s-0037-1602663. [DOI] [PubMed] [Google Scholar]
- 26.Tian C., Ji Z., Xiang W., Huang X., Wang S., Wu Y., et al. Association of lower leukocyte count before thrombolysis with early neurological improvement in acute ischemic stroke patients. J Clin Neurosci : J Neurosurg Soc Australia. 2018;56:44–49. doi: 10.1016/j.jocn.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Karavanaki K., Kakleas K., Georga S., Bartzeliotou A., Mavropoulos G., Tsouvalas M., et al. Plasma high sensitivity C-reactive protein and its relationship with cytokine levels in children with newly diagnosed type 1 diabetes and ketoacidosis. Clin Biochem. 2012;45:1383–1388. doi: 10.1016/j.clinbiochem.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman W., Cudrici C., Zafranskaia E., Rus H. Complement activation in diabetic ketoacidosis brains. Exp Mol Pathol. 2006;80:283–288. doi: 10.1016/j.yexmp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Kanikarla-Marie P., Jain S. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, ICAM-1, and monocyte adhesivity in endothelial cells, Cellular physiology and biochemistry. Int J Experiment Cell Phys Biochecm Pharmcol. 2015;35:364–373. doi: 10.1159/000369702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelletier A., Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2007;292:E1325–E1332. doi: 10.1152/ajpendo.00186.2006. [DOI] [PubMed] [Google Scholar]
- 31.Stentz F., Umpierrez G., Cuervo R., Kitabchi A. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 32.Chamnan P., Simmons R.K., Forouhi N.G., Luben R.N., Khaw K.T., Wareham N.J., et al. Incidence of type 2 diabetes using proposed HbA1c diagnostic criteria in the european prospective investigation of cancer-norfolk cohort: implications for preventive strategies. Diabetes Care. 2011;34:950–956. doi: 10.2337/dc09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.