Abstract
Background and aims
The prognostic impact of non-obstructive coronary artery disease (CAD) has long been underestimated due to its mild stenosis (<50% stenosis). We aim to investigate the prognostic value of atherosclerotic extent in DM patients with non-obstructive CAD.
Methods
The analysis was based on a single center cohort of DM patients referred for coronary computed tomography angiography (CCTA) due to suspect CAD in 2015–2017. Based on coronary stenosis combined with segment involvement score (SIS), the study population were divided into four groups: normal (0% stenosis), non-obstructive SIS<3, non-obstructive SIS≥3 and obstructive (≥50% stenosis). The intra-class correlation (ICC) was used to test the inter-and intra-reviewer agreement. Multivariate Cox model and Kaplan-Meier method were used to evaluate the effect size of atherosclerotic extent on the prognosis.
Results
In total, 1241 patients (age 60.2 ± 10.4 years, 54.1% male) were included, of which 50.2% were non-obstructive. During a median follow-up of 2.6 years, 131 MACEs (10.6%) were adjudicated, including 17 cardiovascular deaths, 28 non-fatal myocardial infarctions, 64 unstable anginas requiring hospitalization and 22 strokes. Incremental event rates could be observed across the four groups. After adjustment for age, gender, hyperlipidemia and presence of high-risk plaque, Hazard Ratio (HR) for non-obstructive SIS<3, non-obstructive SIS≥3 and the obstructive group was 1.84 (95%CI: 0.70–4.79), 3.71 (95%CI: 1.37–10.00) and 5.46 (95%CI: 2.18–13.69), respectively. Compared with non-obstructive SIS<3, non-obstructive SIS≥3 showed a significantly higher risk (HR:2.02 95%CI:1.11–3.68, p = 0.021). Similar results were demonstrated when Leiden risk score was used for sensitivity analysis.
Conclusion
In DM patients with non-obstructive CAD, atherosclerotic extent was associated with higher risk of major adverse cardiac events at long-term follow-up. Efforts should be made to determine risk stratification for the management of DM patients with non-obstructive CAD.
Keywords: Coronary computed tomography angiography, Non-obstructive coronary artery disease, Risk stratification, Atherosclerosis, Diabetes mellitus
Highlights
-
•
Combined with the more extensive atherosclerosis, Non-obstructive CAD conferred a relatively poor prognosis in DM patients.
-
•
Segmental involvement score can be reliably used to risk stratification in DM patients with non-obstructive CAD.
-
•
The presence of High-risk plaque remained independently associated with clinical outcome in current population.
-
•
Leidon score was another potential predictor of clinical outcome in DM patients with non-obstructive CAD.
Introduction
Coronary computed tomography angiography (CCTA) has been proved effective in providing comprehensive information on coronary artery disease (CAD) for the presence and constituents of atherosclerotic plaque even in the absence of flow-limiting disease [1,2]. On one hand, obstructive CAD (>50% stenosis by CCTA) indicates a demand for further functional assessment and intensive treatment. On the other hand, though commonly detected by CCTA, the optimal treatment strategy for non-obstructive CAD remains to be determined. In addition, increasing evidence have consistently demonstrated the prognostic impact of non-obstructive CAD, indicating that the cardiac risk of this kind can no longer be ignored [3,4]. The extent of CAD derived from CCTA has been shown to provide more prognostic information among patients without obstructive CAD, and may act as a potential approach for risk stratification in non-obstructive CAD [5,6].
The prevalence of diabetes mellitus (DM) is increasing rapidly due to a growing obesity epidemic and an aging population. With the extensive vascular disease and lack of typical symptoms in the early stage, DM patients were more likely to suffer from cardiac events [7,8]. However, little relevant supporting evidence existed for the prognostic value of DM patients with non-obstructive CAD, and it remains uncertain if atherosclerotic extent derived from CCTA can be applied to this special DM population. Therefore, we sought to investigate the impact of the atherosclerotic extent detected by CCTA on clinical outcome in DM patients with non-obstructive CAD.
Patients and methods
Study population
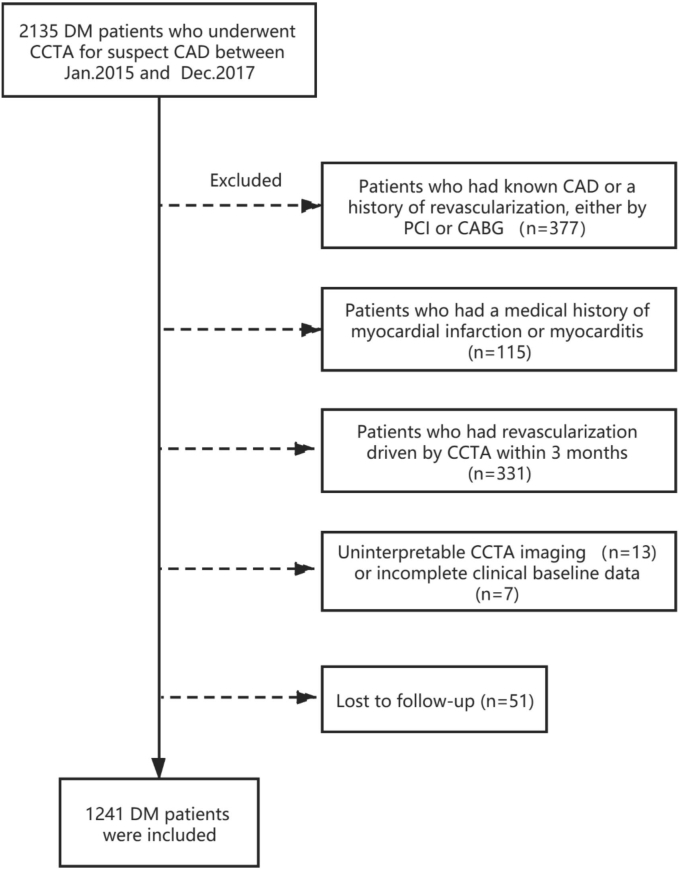
Our study was a prospective single-center study. Patients who underwent CCTA in PLA General Hospital because of suspect CAD between Jan. 2015 and Dec. 2017 were included. Exclusion criteria: 1) known CAD or a history of revascularization, either percutaneous coronary intervention or coronary artery bypass grafting (n = 377), 2) a medical history of myocardial infarction or myocarditis (n = 115), 3) revascularization driven by CCTA results within 3 months (n = 331), 4) uninterpretable CCTA imaging because of poor image quality (n = 13), 5) incomplete clinical baseline data for further analysis (n = 7), 6) lost to follow-up (n = 51) (Fig. 1). Finally, 1241 patients were included for further analysis.
Fig. 1.
Flow chart of the study population.
CAD, coronary artery disease; DM, diabetes mellitus; CCTA, coronary computed tomography angiography; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and the ethics approval of this prospective observational study was obtained from the Ethics Committee of Chinese PLA General Hospital. Informed consent was obtained from all participants.
Clinical data
Demographic characteristics and cardiac risk factors were collected retrospectively by review of medical records or patient interviews. According to 2019 ADA guidelines [9], DM was defined as fasting blood glucose ≥ 7.0 mmol/L, 2-h plasma glucose ≥ 11.1 mmol/L during oral glucose tolerance test, A1C ≥ 6.5% (48 mmol/mol) or the use of oral hypoglycemic agents/insulin. Patients were given advices on healthy lifestyles and diet plans when they visited the doctor. Diet was self-reported by patients in follow-up phone calls but was conducted under the guidance of physicians. Risk factors were recorded as follows: 1) presence of hypertension (defined as systolic blood pressure≥140 mmHg or diastolic blood pressure≥90 mmHg or receiving antihypertensive therapy), 2) hypercholesterolemia (serum total cholesterol ≥230 mg/dl or serum triglycerides ≥200 mg/dl or treatment with lipid-lowering medication), 3) family history of CAD (presence of CAD in first-degree family members at <55 years in men and <65 years in women), 4) smoking (current smoking or previous smoking within the last 3 months of CCTA).
CCTA acquisition and image analysis
All CCTA scans were performed on a dual-source CT scanner (Somatom Definition Flash CT, Siemens Medical Solutions, Forchheim, Germany). Coronary lesions were assessed according to the 17-segment model from the modified American Heart Association classification. The CAD-RADS system was introduced as a quantitative index of lesion severity [16]. Stenosis severity was assessed for each plaque visually, and was categorized as: normal, minimal (1%–24%), mild (25%–49%), moderate (50%–69%), severe (70%–99%) and total occlusion. Non-obstructive lesion was defined as lumen stenosis less than 50%. Plaque composition was recognized as calcified plaque (plaques with high density of >130HU), noncalcified plaque (having lower density compared with the contrast-enhanced lumen) and mixed plaque (both calcified and noncalcified elements existed). For further analysis, high-risk plaque (HRP) was defined as the coexistence of at least two high-risk characteristics [[10], [11], [12]], including spotty calcification (a <3 mm calcified plaque surrounded by non-calcified plaque) [13], low CT attenuation plaques (<30 HU average attenuation) [14], napkin ring sign (a low attenuation central area surrounded by a ring-like comparative higher attenuation plaque tissue) [15] and positive remodeling (the diameter of outer vessel where atherosclerotic exists was 10% greater than the mean of the diameter of the segments immediately proximal and distal to the plaque) [14,15].
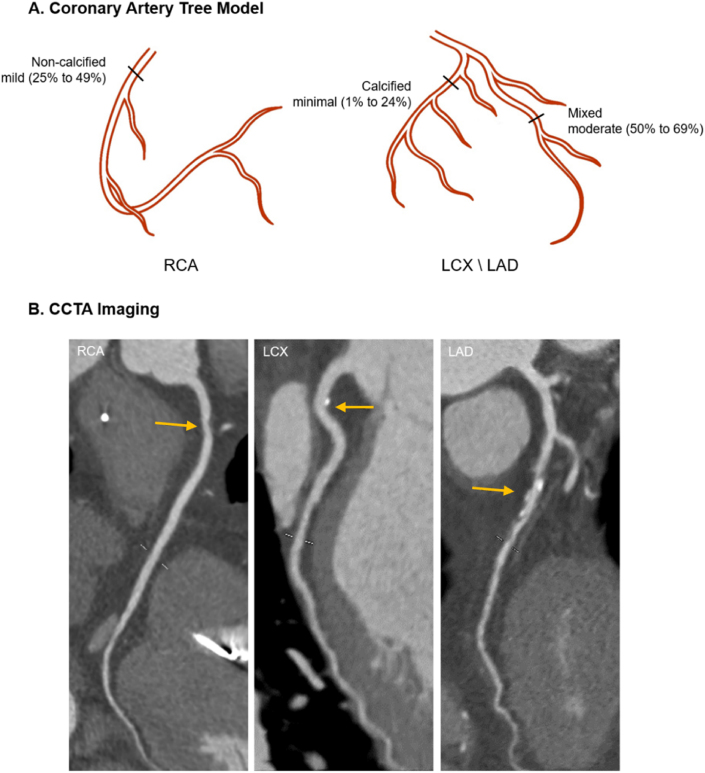
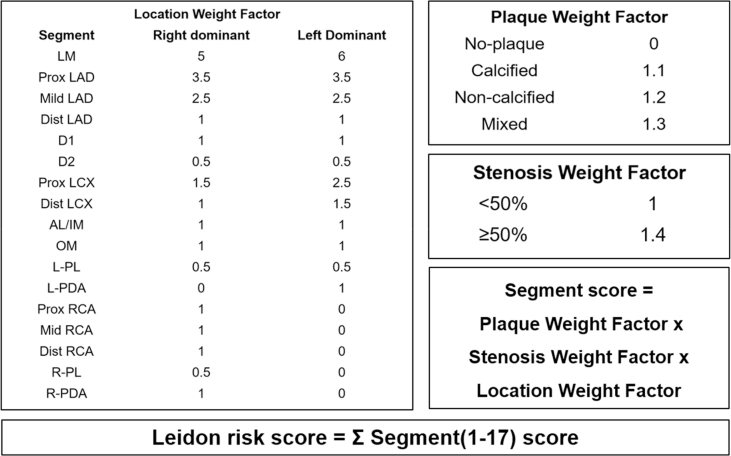
The segment involvement score (SIS) was obtained to quantify the atherosclerotic extent as Min. et al. described [17]. It was calculated as the sum of the number of coronary artery segments that exhibit any plaque within each segment, irrespective of the degree of stenosis (ranging from 0 to 16). In line with a previous study [18], atherosclerotic extents were stratified by SIS: SIS <3 and SIS ≥3. For sensitivity analysis, Leiden risk score was used based on the presence and component (plaque weighting factor, range 0–1.3), stenosis (stenosis weighting factor, range 1.0–1.4) and location (location weighting factor, range 0–6) depending on different system dominance [19]. For each segment, the score was calculated as the multiplication of the three weighting factors and summed to a total Leiden score (range 0–42) (Fig. 2) (Supplement Fig 1). Furthermore, this continuous variable was also stratified into Leiden score <5 and Leiden score ≥5 to represent atherosclerotic extent. Two experienced readers were responsible for the CCTA imaging and both of them were blind to clinical outcomes. When disagreements existed, the final decision would be made through consultation or the intervention of a third experienced physician.
Fig. 2.
A schematic representation of the risk score computation.
(A) is a schematic illustration of coronary artery tree model. Three marked lesions correspond to the vascular lesion (arrow) respectively in CCTA images(B). SIS was calculated by summation of the segments exhibiting plaque, i.e., the proximal right coronary artery (1) +proximal left circumflex artery (1) +middle left anterior descending artery (1). So, in this example, the segment involvement score is 3 out of a possible 16. Leiden score is calculated by summation of segment score quantified as plaque weight factor x stenosis weight factor x location weight factor, i.e., a right dominant system with a non-calcified plaque with <50% stenosis in the proximal right coronary artery(1 × 1.2 × 1)+ a calcified plaque with <50% stenosis in the proximal left circumflex artery(1.5 × 1.1 × 1)+ a mixed plaque with >50% stenosis in the middle left anterior descending artery(2.5 × 1.3 × 1.4), so the Leiden score is 7.4. CCTA = coronary computed tomography angiography; RCA = right coronary artery; LCX = left circumflex coronary artery; LAD = left anterior descending coronary artery.
Follow-up and study endpoint
The survival status of the patients was obtained by reviewing the electronic medical record system or patient interview at least 90 days after CCTA examination. MACEs were recorded as the outcome endpoint of the present study, including cardiovascular death, non-fatal myocardial infarction, stroke and unstable angina requiring hospitalization. Each event was judged independently by two physicians and the categorization of MACEs was performed blinded to the CCTA result or other study data.
Statistical analysis
All statistical analysis was performed with R version 3.6.3 and SPSS version 26.0 (SPSS, IL, USA). The continuous variables were represented as mean ± standard deviation or median (interquartile range, IQR) while categorical variables were represented as percentage and frequency. The intra-class correlation (ICC) statistic was applied to assess intra- and inter-reviewer reproducibility of CCTA findings and scoring results. The criteria of ICC: poor agreement (0.01–0.20); fair agreement (0.21–0.40); moderate agreement (0.41–0.60); good agreement (0.61–0.80) and excellent agreement (0.81–1.0). Patients were divided into 4 groups by CCTA results with risk score as normal, non-obstructive SIS<3, non-obstructive SIS≥3 and obstructive. Cumulative event rates were estimated using the Kaplan-Meier method and compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards method. P-value <0.05 was rendered as statistical significance.
Results
Baseline characteristics
A total of 1241 patients were finally included, with a mean age of 60.2 ± 10.4 years and 54.1%(671) were male. Non-obstructive CAD was observed in 50.2% of the CCTA examinations while 34.5% had obstructive CAD. Baseline characteristics and CCTA results were summarized in Table 1. The prevalence of hypertension, hyperlipidemia, smoking and family history of CAD were 66.4%, 53.6%, 27.4% and 23.8% respectively. In 248 patients, blood glucose levels were controlled by diet only, 901 patients received oral hypoglycemic agent, and the others benefited from insulin. Examined by CCTA, 66 high-risk plaques were detected and 428 subjects were rendered as obstructive CAD.
Table 1.
Baseline characteristics.
| Characteristic | Total (n = 1241) | Event |
P-value | |
|---|---|---|---|---|
| No (n = 1110) | Yes (n = 131) | |||
| Age, years | 60.2 ± 10.4 | 59.9 ± 10.2 | 63.3 ± 11.4 | 0.001 |
| Male | 671 (54.1%) | 592 (53.3%) | 79 (60.3%) | 0.130 |
| Body mass index, kg/m2 | 26.2 ± 3.6 | 26.2 ± 3.6 | 26.2 ± 3.8 | 0.779 |
| Cardiac risk factors | ||||
| Hypertension | 824 (66.4%) | 732 (65.9%) | 92 (70.2%) | 0.326 |
| Hyperlipidemia | 665 (53.6%) | 578 (52.1%) | 87 (66.4%) | 0.002 |
| Current smoking | 340 (27.4%) | 295 (26.6%) | 45 (34.4%) | 0.059 |
| Family history of CAD | 295 (23.8%) | 267 (24.1%) | 28 (21.4%) | 0.496 |
| CCTA findings | ||||
| High-risk plaque | 66 (5.3%) | 43 (3.9%) | 23 (17.6%) | <0.001 |
| CAD-RADS | ||||
| 0 | 190 (15.3%) | 185(16.7%) | 5 (3.8%) | <0.001 |
| 1 | 121 (9.8%) | 117 (10.5%) | 4 (3.1%) | 0.006 |
| 2 | 502 (40.5%) | 461 (41.5%) | 41 (31.3%) | 0.024 |
| 3 | 215 (17.3%) | 188 (16.9%) | 27 (20.6%) | 0.293 |
| 4 | 189 (15.2%) | 140 (12.6%) | 49 (37.4%) | <0.001 |
| 5 | 24 (1.9%) | 19 (1.7%) | 5 (3.8%) | 0.098 |
| Segment Involvement Score | 2 (1–3) | 1 (1–3) | 3 (2–5) | <0.001 |
| Leiden Risk Score | 5.1 (2.8–8.9) | 4.6 (2.3–8.1) | 10.6 (6.4–13.4) | <0.001 |
| Medication | ||||
| Anti-platelet | 480 (38.7%) | 435 (39.2%) | 45 (34.4%) | 0.280 |
| Beta blocker | 408 (32.9%) | 368 (33.2%) | 40 (30.5%) | 0.550 |
| ACEI/ARB | 287 (23.1%) | 252 (22.7%) | 35 (26.7%) | 0.300 |
| Statin | 482 (38.8%) | 418 (37.7%) | 64 (48.9%) | 0.013 |
| Calcium channel blocker | 262 (21.1%) | 230 (20.7%) | 32 (24.4%) | 0.330 |
| Diabetic treatment | ||||
| Diet only | 248 (20.0%) | 226 (20.4%) | 22 (16.8%) | 0.330 |
| Oral hypoglycemic agent | 901 (72.6%) | 806 (72.6%) | 95 (72.5%) | 0.980 |
| Insulin | 300 (24.2%) | 266 (24.0%) | 34 (26.0%) | 0.610 |
Values are mean ± SD or n (%).
CAD, coronary artery disease; CCTA, coronary computed tomography angiography.
Inter- and intra-reviewer agreement for CCTA findings and risk score
Inter-reviewer agreement for stenosis evaluation revealed by CAD-RADS system was excellent agreement (ICC = 0.882–0.961) as well as the intra-reviewer agreement (ICC = 0.921–0.986). For HRP, an excellent agreement was observed both in inter- and intra-reviewer agreement (ICC = 0.843 and 0.926, respectively). As to the risk score, both SIS and Leiden score showed excellent agreement (ICC of inter-reviewer agreement: 0.987 and 0.942; of intra-reviewer agreement: 0.991 and 0.957 respectively).
Predictors of outcome events
Of the univariate analysis, age, hyperlipidemia, the presence of HRP and SIS score were associated with outcome events (Table 2). Compared with normal coronary artery patency, HR was 2.07 (95%CI:0.80–5.39, p = 0.136) for the group of non-obstructive SIS<3, 4.65 (95%CI:1.74–12.46, p = 0.002) for non-obstructive SIS≥3 and 7.63 (95%CI:3.09–18.84, p < 0.001) for obstructive, respectively (Table 2).
Table 2.
Univariate and multivariate analysis of SIS x CAD for major cardiovascular events.
| Univariate HR (95%CI) | p-Value | SIS x CAD |
||
|---|---|---|---|---|
| Multivariate HR (95%CI) | p-Value | |||
| Age, yrs | 1.03 (1.01–1.05) | <0.001 | 1.02 (1.00–1.04) | 0.021 |
| Male | 1.31(0.92–1.85) | 0.136 | 1.17 (0.81–1.68) | 0.409 |
| BMI (kg/m2) | 1.00(0.95–1.05) | 0.994 | ||
| Cardiac risk factors | ||||
| Hypertension | 1.19(0.82–1.73) | 0.372 | ||
| Hyperlipidemia | 1.69 (1.18–2.43) | 0.004 | 1.83 (1.27–2.63) | 0.001 |
| Current smoker | 1.42(0.99–2.04) | 0.057 | ||
| Family history of CAD | 0.84(0.55–1.27) | 0.401 | ||
| CCTA findings | ||||
| High-risk plaque | 4.71 (3.00–7.40) | <0.001 | 3.15 (1.97–5.04) | <0.001 |
| Segment involvement score | 1.22 (1.15–1.30) | <0.001 | ||
| Leiden Risk Score | 1.08 (1.06–1.10) | <0.001 | ||
| CAD severity (SIS x CAD) | ||||
| Normal | 1 | 1 | ||
| Non-obstructive SIS<3 | 2.07 (0.80–5.39) | 0.136 | 1.84 (0.70–4.79) | 0.214 |
| Non-obstructive SIS≥3 | 4.65 (1.74–12.46) | 0.002 | 3.71 (1.37–10.00) | 0.010 |
| Obstructive | 7.63 (3.09–18.84) | <0.001 | 5.46 (2.18–13.69) | <0.001 |
CAD, coronary artery disease; CCTA, coronary computed tomography angiography.
After adjustment of age, gender, hyperlipidemia and presence of HRP, SIS remained a significant predictor of outcome event risk (HR:1.17; 95%CI:1.10–1.25; p < 0.001) (Table 2). Compared to normal group, non-obstructive CAD SIS≥3 group had a higher hazard ratio (HR:3.71; 95%CI:1.37–10.00; p = 0.010) and a much higher risk was observed in obstructive CAD (HR: 5.46; 95%CI:2.18–13.69; p < 0.001) (Table 2). Furthermore, non-obstructive SIS≥3 group was associated with a higher risk of outcome events in comparison to non-obstructive SIS<3 group (HR:2.02; 95%CI:1.11–3.68; p = 0.021). Survival analysis based on medication (Supplement Table 1) was performed as well and the glucose lowering therapies did not have an effect on the outcomes (p=0.453∼0.968).
Survival analysis
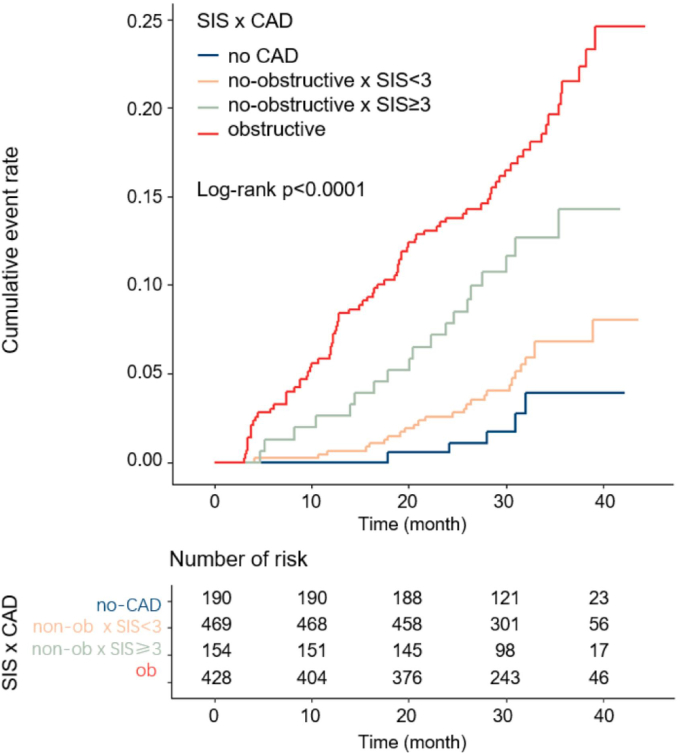
A similar incremental trend of event rate was demonstrated in Fig. 3. During a median follow-up duration of 31 months (IQR 27.6–37.3), 131 MACEs occurred, including 28 non-fatal MI, 64 unstable anginas requiring hospitalization, 22 strokes and 17 cardiac deaths. The annual MACE rate among patients in the normal group was 0.99 events per 100 person-years. The annual MACE rates among non-obstructive SIS<3 and non-obstructive SIS≥3 were 2.06 events and 4.66 events per 100 person-years, respectively, while the rate for obstructive CAD was 7.55 events per 100 person-years (p < 0.01).
Fig. 3.
Cumulative risk of the composite endpoint on the basis of CAD severity with segment involvement score. CAD, coronary artery disease; SIS, segment involvement score.
Sensitivity analysis
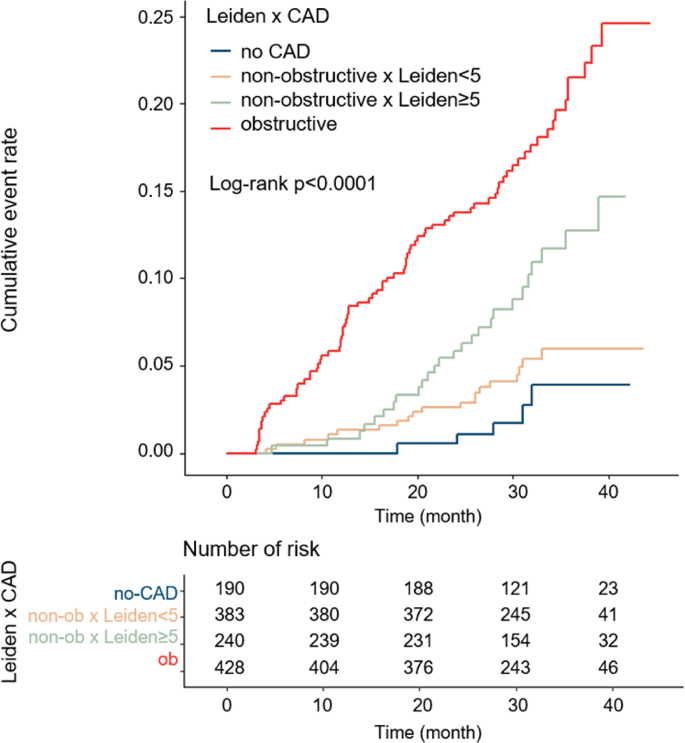
For further analysis, SIS was replaced by Leiden risk score to quantify the atherosclerotic burden. A similar distribution of event rate has been noticed (Fig. 4), which were 2.63%, 4.96%, 10.83% and 18.93% respectively. After adjustment for age, gender, hyperlipidemia and HRP, Leiden score was found a significant predictor as well (Table 2). In an adjusted Cox analysis, an increase was conferred in MACEs risk over those with normal CCTA results (non-obstructive Leiden score<5 HR: 1.69; 95%CI: 0.63–4.52; p = 0.299; non-obstructive Leiden score≥5 HR:3.24; 95%CI: 1.24–8.47; p = 0.017; obstructive HR: 5.46; 95%CI: 2.18–13.68; p < 0.001) (Table 3). Furthermore, non-obstructive Leiden score≥5 group was associated with a higher risk of outcome events in comparison to non-obstructive Leiden score<5 group (HR:1.92; 95%CI:1.06–3.48; p = 0.032).
Fig. 4.
Cumulative risk of the composite endpoint on the basis of CAD severity with Leiden score. CAD, coronary artery disease.
Table 3.
Univariate and multivariate analysis of Leiden x CAD for major cardiovascular events.
| Univariate HR (95%CI) | p-Value | Leiden x CAD |
||
|---|---|---|---|---|
| Multivariate HR (95%CI) | p-Value | |||
| Age, yrs | 1.03 (1.01–1.05) | <0.001 | 1.02 (1.00–1.04) | 0.017 |
| Male | 1.31 (0.92–1.85) | 0.136 | 1.18 (0.82–1.71) | 0.364 |
| BMI (kg/m2) | 1.00 (0.95–1.05) | 0.994 | ||
| Cardiac risk factors | ||||
| Hypertension | 1.19 (0.82–1.73) | 0.372 | ||
| Hyperlipidemia | 1.69 (1.18–2.43) | 0.004 | 1.82 (1.26–2.63) | 0.001 |
| Current smoker | 1.42 (0.99–2.04) | 0.057 | ||
| Family history of CAD | 0.84 (0.55–1.27) | 0.401 | ||
| CCTA findings | ||||
| High-risk plaque | 4.71 (3.00–7.40) | <0.001 | 3.08 (1.93–4.91) | <0.001 |
| CAD severity (Leiden x CAD) | ||||
| Normal | 1 | 1 | ||
| Non-obstructive Leiden<5 | 1.86 (0.70–4.99) | 0.216 | 1.69 (0.63–4.52) | 0.299 |
| Non-obstructive Leiden≥5 | 4.04 (1.55–10.52) | 0.004 | 3.24 (1.24–8.47) | 0.017 |
| Obstructive | 7.63 (3.09–18.83) | <0.001 | 5.46 (2.18–13.68) | <0.001 |
CAD, coronary artery disease; CCTA, coronary computed tomography angiography.
Discussion
The present study found that atherosclerotic extent was associated with long-term major adverse cardiovascular outcome in DM patients with non-obstructive CAD, and an incremental prognostic value could be conferred even after adjustment for traditional risk factors and high-risk plaque profiles. Our results further supported the notion that greater risk-stratification efforts should be needed to promote management in DM patients with non-obstructive CAD.
Among patients with angina, the absence of significant coronary artery stenosis (>50%) is a relatively common finding. A comparatively lighter stenosis prevents non-obstructive CAD from stronger downstream treatment and management, intensive medical therapy or revascularization, with a consideration that it is a low-risk disease with no flow-limiting. However, increasing data revealed a potential high-risk population with non-obstructive CAD, associated with worse outcome, even comparable to obstructive CAD [2,20]. Some studies dedicated to discriminate these patients using further stratification with combined risk factors including clinical risk factors, demographic characteristics and other CCTA findings like involved segments, calcium burden and epicardial adipose tissue thickness [[21], [22], [23], [24]]. These all indicated that non-obstructive CAD should no longer be ignored in the setting of various risk factors and it is worth further identification.
For DM patients, multi-vessel coronary artery disease and microcirculation disturbance are more likely to occur on the basis of vasculopathy. Previous studies confirmed that it is appropriate for further cardiac risk stratification according to the degree of diffusion detected by CCTA [23]. The assessment of atherosclerotic extent may also produce incremental prognostic value over the evaluation of plaque stenosis, especially in DM patients [25]. Of the current CCTA derived risk scores, SIS is a simple and effective approach to represent the extent of lesion, and is feasible in clinical practice. Meanwhile, Leiden comprehensive risk score, combining the information of both plaque location and stenosis composition, was introduced for sensitivity analysis. Those results supported that the measurement of atherosclerotic extent works in risk stratification of cardiovascular outcome, especially for DM patient with non-obstructive CAD.
Previous findings indicated a potential loss of information and missed opportunity of preventive management in patients with non-obstructive disease, especially in the presence of DM. In the SCOT-HEART (Scottish Computed Tomography of the Heart) trial, a hazard reduction of non-fatal myocardial infarction or cardiac death was observed in patients assigned to an anatomic strategy versus functional strategy (2.4% vs. 3.9%) [4], which may be associated with detection of non-obstructive coronary atherosclerosis and subsequent early-management. The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial revealed that 67% of cardiac deaths or myocardial infarctions occurred in patients with non-obstructive disease found by cardiac CT and normal stress test at baseline [26]. Our results seem to confirm this notion and further emphasized the importance of early management in DM patients with extensive non-obstructive coronary artery disease. Thus, cardiac CT should be rendered as an significant opportunity of earlier prevention or intensive treatment in the process of disease, which has been proved effective in reducing MACEs [27].
Our findings were broadly similar with previous study [2], however, several disparities must be noted. A higher ratio of non-obstructive/obstructive CAD was observed in the present cohort, approximately half of them were non-obstructive, presenting a comparative low-risk population, unlike the previous study [28]. This may be ascribed to a direct referral to the invasive examination or revascularization driven by CCTA within 3 months, which has met the exclusion criteria, in high-risk population. Nonetheless, a slightly higher MACEs rate was presented, compared with an annual events rate ranged from 1.5% to 16.9% as shown in a meta-analysis [28], in which DM patients examined by CCTA were investigated. One possibility is that we broadened enrollment to MACEs with stroke and extended follow-up to a median of 31 months, which was a sufficient duration to capture more events. Moreover, up to 4/5 patients received hypoglycemic therapy in baseline, indicating a potential long duration of DM and higher vascular risk. Another important observation from our study is that in risk-adjusted hazard analysis, the presence of HRP was found an independent predictor with a high HR of 3.15 (95%CI:1.97–5.04). This corresponds to the result from ICONIC study [29] that stressed the importance of HRP lesions in non-obstructive CAD, which exhibited comparable risk of becoming a culprit lesion to obstructive HRP-free lesions. In view of this, we bring it into analysis, which has been done by little research before. However, after adjustment for HRP, extensive non-obstructive CAD was still found a significant indicator. This finding may inform future trials to determine the potential role of non-obstructive CAD in the setting of DM.
Study limitation
Firstly, as a prospective single center study, referral decision for CCTA was made by physicians independently and some patients were excluded finally due to various reasons, which may introduce selection bias. Secondly, lack of the DM duration and biochemical information on baseline might introduce bias into the research as longer duration of DM is associated with increased CAD burden and higher rate of MACE in CAD patients. Previous published studies also failed to report the diabetes disease vintage [2,18,23]. However, nearly 75% of the patients only had a diet control or oral hypoglycemic agent to lower glucose, indicating a generally moderate condition of DM and less heterogeneity in the present study population. Furthermore, because patients are more likely to have relatively good compliance of treatment on traditional risk factors due to follow-up visit in our clinic, a guideline-recommended target of such biochemical information can be expected. Thirdly, although some downstream treatments and management were recorded, other treatments, such as diuretics and sacubitril/valsartan, were not recorded in the final analysis, which may bring in potential confounders and impact the effect size of target variables. Finally, the functional tests were not included in present study, especially for the patients with microcirculation dysfunction, which has been demonstrated to be independently associated with MACEs in DM patients.
Conclusion
Atherosclerotic extent was associated with higher risk of MACEs, and relevant risk score derived by CT imaging could allow improving risk stratification for the management of DM patients with non-obstructive CAD.
Authors’ contribution
The first two authors contribute to the work equally.
Sources of funding
This work was supported by grants from National Key R&D Program of China (2016YFC1300304), Beijing NOVA Program (Z181100006218055), and Medical big data program of PLAGH (2019MBD-035).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2021.07.021.
Contributor Information
Yundai Chen, Email: cyundai@vip.163.com.
Junjie Yang, Email: fearlessyang@126.com.
Abbreviations
- DM
diabetes mellitus
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- MACEs
major adverse cardiac events
- HRP
high-risk plaque
- SIS
segment involvement score
- SSS
segment stenosis score
- HR
hazard ratio
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Lee K.Y., Hwang B.H., Kim T.H., Kim C.J., Kim J.J., Choo E.H., Choi I.J., Choi Y., Park H.W., Koh Y.S., et al. Computed tomography angiography images of coronary artery stenosis provide a better prediction of risk than traditional risk factors in asymptomatic individuals with type 2 diabetes: a long-term study of clinical outcomes. Diabetes Care. 2017;40(9):1241–1248. doi: 10.2337/dc16-1844. [DOI] [PubMed] [Google Scholar]
- 2.Blanke P., Naoum C., Ahmadi A., Cheruvu C., Soon J., Arepalli C., Gransar H., Achenbach S., Berman D.S., Budoff M.J., et al. Long-term prognostic utility of coronary CT angiography in stable patients with diabetes mellitus. JACC Cardiovasc Imag. 2016;9(11):1280–1288. doi: 10.1016/j.jcmg.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Radico F., Zimarino M., Fulgenzi F., Ricci F., Di Nicola M., Jespersen L., Chang S.M., Humphries K.H., Marzilli M., De Caterina R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39(23):2135–2146. doi: 10.1093/eurheartj/ehy185. [DOI] [PubMed] [Google Scholar]
- 4.Investigators S.-H., Newby D.E., Adamson P.D., Berry C., Boon N.A., Dweck M.R., Flather M., Forbes J., Hunter A., Lewis S., et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 5.van Rosendael A.R., Bax A.M., Smit J.M., van den Hoogen I.J., Ma X., Al'Aref S., Achenbach S., Al-Mallah M.H., Andreini D., Berman D.S., et al. Clinical risk factors and atherosclerotic plaque extent to define risk for major events in patients without obstructive coronary artery disease: the long-term coronary computed tomography angiography CONFIRM registry. Eur Heart J Cardiovasc Imag. 2020;21(5):479–488. doi: 10.1093/ehjci/jez322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jespersen L., Hvelplund A., Abildstrom S.Z., Pedersen F., Galatius S., Madsen J.K., Jorgensen E., Kelbaek H., Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 7.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41(2):255–323. doi: 10.1093/eurheartj/ehz486. 2020. [DOI] [PubMed] [Google Scholar]
- 8.Emerging Risk Factors C., Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 10.Williams M.C., Moss A.J., Dweck M., Adamson P.D., Alam S., Hunter A., Shah A.S.V., Pawade T., Weir-McCall J.R., Roditi G., et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol. 2019;73(3):291–301. doi: 10.1016/j.jacc.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferencik M., Mayrhofer T., Bittner D.O., Emami H., Puchner S.B., Lu M.T., Meyersohn N.M., Ivanov A.V., Adami E.C., Patel M.R., et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest Pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018;3(2):144–152. doi: 10.1001/jamacardio.2017.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motoyama S., Ito H., Sarai M., Kondo T., Kawai H., Nagahara Y., Harigaya H., Kan S., Anno H., Takahashi H., et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337–346. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 13.Ehara S., Kobayashi Y., Yoshiyama M., Shimada K., Shimada Y., Fukuda D., Nakamura Y., Yamashita H., Yamagishi H., Takeuchi K., et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110(22):3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 14.Motoyama S., Kondo T., Sarai M., Sugiura A., Harigaya H., Sato T., Inoue K., Okumura M., Ishii J., Anno H., et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50(4):319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwagi M., Tanaka A., Kitabata H., Tsujioka H., Kataiwa H., Komukai K., Tanimoto T., Takemoto K., Takarada S., Kubo T., et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imag. 2009;2(12):1412–1419. doi: 10.1016/j.jcmg.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Cury R.C., Abbara S., Achenbach S., Agatston A., Berman D.S., Budoff M.J., Dill K.E., Jacobs J.E., Maroules C.D., Rubin G.D., et al. Coronary artery disease - reporting and data system (CAD-RADS): an expert consensus document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imag. 2016;9(9):1099–1113. doi: 10.1016/j.jcmg.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Min J.K., Shaw L.J., Devereux R.B., Okin P.M., Weinsaft J.W., Russo D.J., Lippolis N.J., Berman D.S., Callister T.Q. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 18.Kang S.H., Park G.M., Lee S.W., Yun S.C., Kim Y.H., Cho Y.R., Park H.W., Suh J., Yang D.H., Kang J.W., et al. Long-term prognostic value of coronary CT angiography in asymptomatic type 2 diabetes mellitus. JACC Cardiovasc Imag. 2016;9(11):1292–1300. doi: 10.1016/j.jcmg.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 19.van Rosendael A.R., Shaw L.J., Xie J.X., Dimitriu-Leen A.C., Smit J.M., Scholte A.J., van Werkhoven J.M., Callister T.Q., DeLago A., Berman D.S., et al. Superior risk stratification with coronary computed tomography angiography using a comprehensive atherosclerotic risk score. JACC Cardiovasc Imag. 2019;12(10):1987–1997. doi: 10.1016/j.jcmg.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddox T.M., Stanislawski M.A., Grunwald G.K., Bradley S.M., Ho P.M., Tsai T.T., Patel M.R., Sandhu A., Valle J., Magid D.J., et al. Nonobstructive coronary artery disease and risk of myocardial infarction. J Am Med Assoc. 2014;312(17):1754–1763. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rosendael A.R., Bax A.M., Smit J.M., van den Hoogen I.J., Ma X., Al'Aref S., Achenbach S., Al-Mallah M.H., Andreini D., Berman D.S., et al. Clinical risk factors and atherosclerotic plaque extent to define risk for major events in patients without obstructive coronary artery disease: the long-term coronary computed tomography angiography CONFIRM registry. Eur Heart J Cardiovasc Imag. 2020;21(5):479–488. doi: 10.1093/ehjci/jez322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam M.S., Green R., de Kemp R., Beanlands R.S., Chow B.J. Epicardial adipose tissue thickness as a predictor of impaired microvascular function in patients with non-obstructive coronary artery disease. J Nucl Cardiol. 2013;20(5):804–812. doi: 10.1007/s12350-013-9739-6. [DOI] [PubMed] [Google Scholar]
- 23.Hadamitzky M., Hein F., Meyer T., Bischoff B., Martinoff S., Schomig A., Hausleiter J. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care. 2010;33(6):1358–1363. doi: 10.2337/dc09-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortensen M.B., Dzaye O., Steffensen F.H., Botker H.E., Jensen J.M., Ronnow Sand N.P., Kragholm K.H., Sorensen H.T., Leipsic J., Maeng M., et al. Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis. J Am Coll Cardiol. 2020;76(24):2803–2813. doi: 10.1016/j.jacc.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 25.van den Hoogen I.J., van Rosendael A.R., Lin F.Y., Lu Y., Dimitriu-Leen A.C., Smit J.M., Scholte A., Achenbach S., Al-Mallah M.H., Andreini D., et al. Coronary atherosclerosis scoring with semiquantitative CCTA risk scores for prediction of major adverse cardiac events: propensity score-based analysis of diabetic and non-diabetic patients. J Cardiovasc Comput Tomogr. 2020;14(3):251–257. doi: 10.1016/j.jcct.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann U., Ferencik M., Udelson J.E., Picard M.H., Truong Q.A., Patel M.R., Huang M., Pencina M., Mark D.B., Heitner J.F., et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest Pain: insights from the PROMISE trial (prospective multicenter imaging study for evaluation of chest Pain) Circulation. 2017;135(24):2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferraro R., Latina J.M., Alfaddagh A., Michos E.D., Blaha M.J., Jones S.R., Sharma G., Trost J.C., Boden W.E., Weintraub W.S., et al. Evaluation and management of patients with stable Angina: beyond the ischemia paradigm: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(19):2252–2266. doi: 10.1016/j.jacc.2020.08.078. [DOI] [PubMed] [Google Scholar]
- 28.Celeng C., Maurovich-Horvat P., Ghoshhajra B.B., Merkely B., Leiner T., Takx R.A. Prognostic value of coronary computed tomography angiography in patients with diabetes: a meta-analysis. Diabetes Care. 2016;39(7):1274–1280. doi: 10.2337/dc16-0281. [DOI] [PubMed] [Google Scholar]
- 29.Ferraro R.A., van Rosendael A.R., Lu Y., Andreini D., Al-Mallah M.H., Cademartiri F., Chinnaiyan K., Chow B.J.W., Conte E., Cury R.C., et al. Non-obstructive high-risk plaques increase the risk of future culprit lesions comparable to obstructive plaques without high-risk features: the ICONIC study. Eur Heart J Cardiovasc Imag. 2020;21(9):973–980. doi: 10.1093/ehjci/jeaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.