Abstract
Background and aims
Available data suggest that the use of IVUS for guidance of percutaneous coronary interventions (PCIs) improves the prognosis of patients undergoing complex interventions. We aimed to examine how the utilization of intravascular ultrasound (IVUS) affects patient survival irrespective of procedure complexity.
Methods
The present analysis is based on the longitudinal ECAD registry of consecutive patients undergoing coronary angiography between 2004 and 2019. The incidence of death due to any cause was evaluated during a mean follow-up of 3.4 years. Cox regression analysis was used to determine the association of IVUS utilization with incident mortality.
Results
Overall, data from 30,814 coronary angiography exams (mean age 64.9 ± 12.5 years, 70.3% male) were included, among which 4991 procedures (16.2%) were guided by IVUS. Utilization of IVUS was associated with a 35% reduction in mortality, independent of traditional risk factors (0.64(0.58–0.71), p < 0.0001). The effect of IVUS on mortality was equally present in patients undergoing IVUS-guided coronary interventions (0.75[0.67–0.84], p < 0.0001) as well as purely diagnostic coronary angiography exams (0.62[0.56–0.72], p < 0.0001). In patients without coronary intervention, IVUS utilization led to a higher frequency of aspirin (82.6% vs. 61.9% for IVUS vs. no IVUS, p < 0.0001) and statin therapy (74.9% vs. 62.5%, p < 0.0001).
Conclusions
In a large longitudinal registry cohort of patients undergoing invasive coronary angiography, IVUS utilization was associated with lower long-term mortality. The beneficial role of IVUS utilization on survival was equally present for coronary interventions and diagnostic coronary angiograms. Our results support the use of intravascular imaging for decision making in interventional cardiology.
Keywords: Intravascular ultrasound, Coronary angiography, Coronary artery disease, ECAD registry
Highlights
-
•
IVUS utilization is associated with improved survival after coronary angiography.
-
•
IVUS improves prognosis in patients undergoing percutaneous coronary interventions.
-
•
IVUS improves prognosis in patients undergoing diagnostic coronary angiography.
-
•
Higher usage of aspirin and statin therapy after IVUS evaluation.
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- IVUS
intravascular ultrasound
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- NSTEMI
non-ST segment elevation myocardial infarction
- PCI
percutaneous coronary intervention
- STEMI
ST-Elevation myocardial infarction
Introduction
The use of intravascular ultrasound (IVUS) for guidance of percutaneous coronary interventions (PCIs) impacts the interventional strategy by providing important information on the target lesion and reference vessel characteristics in patients undergoing complex coronary procedures [1,2]. This leads to a reduction in cardiovascular mortality by lowering the rate of repeat revascularization, in-stent thrombosis, and recurrent myocardial infarction in IVUS-controlled PCI [[3], [4], [5], [6], [7]]. While available randomized controlled trials and observational registries have focused on short-term cardiovascular or composite endpoints, the ability of IVUS to improve overall long-term survival is less well documented [8].
In addition to its value in interventional therapy, randomized controlled trials document that IVUS enables quantification of nonstenotic coronary plaque burden that can effectively be reduced by intensified risk factor modification, associating with reduced atherosclerotic cardiovascular disease risk [9,10]. However, for patients with nonobstructive coronary artery disease (CAD), the association of the assessment of coronary plaque burden via IVUS with survival remains unknown. Therefore, we aimed to examine how the utilization of IVUS affects patient survival in a longitudinal registry of patients undergoing invasive coronary angiography. Specifically, we stratified our analysis by patients with and without PCI as part of the procedure to assess whether the information on overall plaque burden in addition to degree of stenosis alone improves outcome.
Methods
Study cohort
The present analysis is based on a retrospective registry of consecutive patients undergoing invasive coronary angiography at the West German Heart and Vascular Center, Essen, between 2004 and 2019 (the Essen Coronary Artery Disease [ECAD]-registry). Data from 40,461 coronary procedures are included in the ECAD registry (dataset as of July 2019). Data from 6483 examinations were excluded due to missing follow-up information. In addition, datasets of exams of 3117 noncoronary interventions were excluded. Finally, information on IVUS was missing for 47 procedures, leading to an overall dataset of 30,814 examinations (Online Fig. 1). The local ethics committee (19-8956-BO) approved the present analysis. The ECAD registry consists of a heterogeneous cohort of patients with various indications for coronary angiography examinations. The distribution of primary discharge diagnoses is provided in Online Table 1.
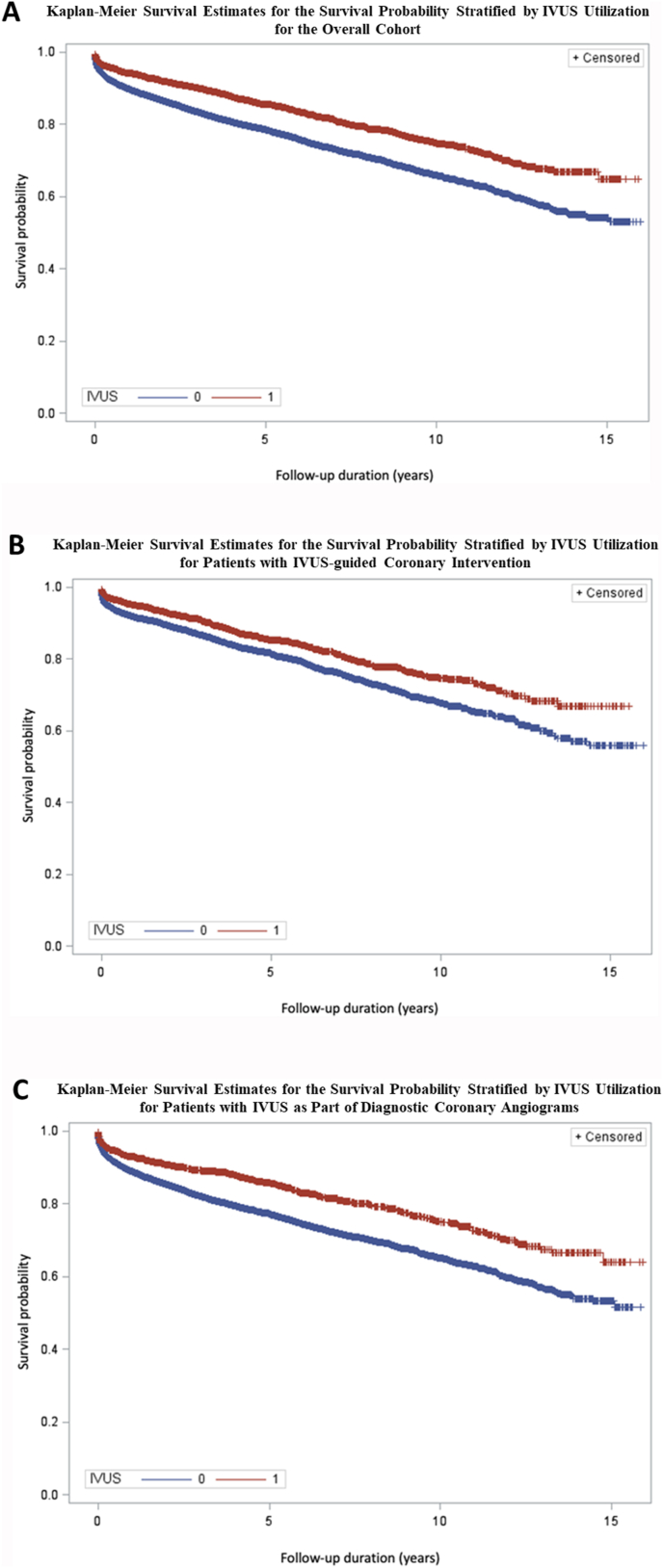
Fig. 1.
Kaplan-Meier Survival Estimates for the survival probability stratified by IVUS utilization for the overall cohort (A), for patients with IVUS-guided coronary intervention (B) and for patients with IVUS as part of diagnostic coronary angiograms (C)·
IVUS = intravascular ultrasound.
Table 1.
Baseline characteristics.
| Overall (n = 30,814) | Angiography with IVUS (n = 4991) | Angiography without IVUS (n = 25,823) | p-value (IVUS vs. no IVUS) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 64.9 ± 12.5 | 64.4 ± 11.3 | 65.0 ± 12.7 | 0.0005 |
| Male | 21,665 (70.3) | 3810 (76.3) | 17,855 (69.1) | <0.0001 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus | 2964 (9.6) | 548 (11.0) | 2416 (9.4) | 0.001 |
| Family CAD history | 4592 (14.9) | 811 (16.3) | 3781 (14.6) | <0.0001 |
| Current smoker | 3465 (11.2) | 537 (10.8) | 2928 (11.3) | <0.0001 |
| Systolic blood pressure | 136.4 ± 22.5 | 137.0 ± 20.9 | 136.3 ± 22.8 | 0.2 |
| Laboratory measurements | ||||
| LDL, mg/dl | 106.9 ± 39.3 | 103.2 ± 38.1 | 107.8 ± 39.5 | <0.0001 |
| HDL, mg/dl | 48.7 ± 16.3 | 47.9 ± 14.9 | 48.9 ± 16.5 | 0.0007 |
| LP(a), mg/dl | 33.3 ± 40.3 | 33.2 ± 39.0 | 33.4 ± 40.7 | 0.85 |
| BNPa, pg/mL | 94.9 (36.0; 272.5) | 56.8 (24.9; 129.5) | 122.1 (45.5; 350.2) | <0.0001 |
| NTproBNPa, pg/mL |
526.5 (141.0; 245.0) |
238 (90; 907) |
594 (150; 2628) |
<0.0001 |
|
Clinical presentation |
N = 23,001 |
N = 4083 |
N = 18,917 |
|
| Coronary artery disease | 13,114 (57.0) | 3116 (76.3) | 9997 (52.9) | <0.0001 |
| Chronic coronary syndrome | 7065 (30.7) | 1921 (47.0) | 5144 (27.2) | |
| Unstable angina | 3604 (15.7) | 791 (19.4) | 2813 (14.9) | |
| NSTEMI | 1725 (7.5) | 275 (6.7) | 1450 (7.7) | |
| STEMI | 719 (3.13) | 129 (3.2) | 590 (3.1) | |
| Other cardiac diagnosis | 5803 (25.2) | 386 (9.5) | 5417 (28.6) | |
| Noncardiac |
4084 (17.8) |
581 (14.2) |
3503 (18.5) |
|
|
Medication |
N = 18,633 |
N = 2488 |
N = 16,145 |
|
| Statin | 12,933 (70.4) | 2042 (83.0) | 10,891 (68.4) | <0.0001 |
| Nonstatin cholesterol-lowering therapy | 636 (4.5) | 91 (5.7) | 545 (4.4) | 0.01 |
| Antihypertensive therapy | 17,468 (93.8) | 2391 (96.1) | 15,077 (93.4) | <0.0001 |
| Aspirin | 12,848 (73.3) | 2144 (85.1) | 10,703 (65.4) | <0.0001 |
| P2Y12 inhibitors | 8214 (43.3) | 1613 (64.1) | 6601 (40.1) | <0.0001 |
| Oral anticoagulation | 3987 (21.4) | 360 (14.4) | 3627 (22.4) | <0.0001 |
Values of continuous variables are reported as mean ± SD if normally distributed, and median (interquartile range) if not normally distributed. Categorical variables are reported as n (%).
BNP = brain natriuretic peptide; CAD = coronary artery disease; IVUS = intravascular ultrasound; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; LP(a) = Lipoprotein(a); NSTEMI = Non-ST-Elevation Myocardial Infarction; STEMI = ST-Elevation Myocardial Infarction.
BNP values available in 2330 patients, NT-proBNP values available in 5694 patients.
Clinical characteristics and covariate assessment
Information on traditional cardiovascular risk factors from the same hospital stay was drawn from the hospital information system and merged into the database. Laboratory variables were assessed using standardized enzymatic methods (low- and high-density lipoprotein cholesterol, lipoprotein(a)) and automatically imported. Diabetes was defined as HbA1c ≥ 6.5%. Self-reported information on current smoking status and family history of premature CAD was classified as present, absent, or unknown. Primary discharge diagnoses were obtained from the hospital information system according to the International Statistical Classification of Disease (ICD 10). Primary diagnosis of CAD was defined as ICD codes from I20.0 to I25.9. Acute coronary syndrome was defined as ICD codes I20.0 to 24.9, whereas ICD codes I25.0 to 25.9 were defined as chronic coronary syndrome. Medication information was drawn from discharge letters and was limited to 18,633 cases. Antihypertensive therapy was defined as medication with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, beta blockers, and/or alpha blockers. Nonstatin lipid-lowering therapy was defined as medication with fibrate, ezetimibe, niacin, bile acid sequestrates, and/or PCSK-9 inhibitors.
Endpoint definition
All-cause mortality was defined as the primary endpoint variable. Information on survival status was assessed from all available hospital records (including partner healthcare facilities) as well as insurance information. Any ambulatory or inpatient presentation to the West German Heart and Vascular Center, the University Hospital Essen or any partner healthcare facility after the coronary exam was used for confirmation of survival status. Patients without confirmed death but no recurrent presentation to the healthcare provider were considered lost to follow-up and excluded from the present analysis.
Statistical analysis
The baseline characteristics are presented as the mean ± standard deviation for continuous variables and as frequency and percentages for categorical variables. Two-sided t-tests were used for normally distributed continuous variables, and chi-square tests were used for categorical variables for comparison of procedures with and without IVUS utilization. The incidence of death from any cause during follow-up was recorded. Kaplan-Meier analysis was used to depict the survival probability, stratified by group of patients with and without IVUS utilization as part of the coronary angiography examination. Differences between the groups were evaluated using the log rank test. Subgroup analysis was performed in groups with and without PCI as part of the coronary angiography exam. Cox regression analysis was used to determine the association of IVUS utilization with incident mortality. Adjustment sets were defined as follows: (1) unadjusted; (2) age- and sex-adjusted; and (3) ancillary adjustment for low-density lipoprotein (LDL-) cholesterol, systolic blood pressure, diabetes, family history of premature CAD, present smoking status, discharge diagnosis (coronary artery disease, other cardiovascular diagnosis, non-cardiovascular diagnosis), and experience of the interventional cardiologists (<vs. ≥ 1000 coronary angiography examinations); (4) model 3 + medication use (statin, aspirin, P2Y12-inhibitors, antihypertensive medication) and left main stenosis. Again, subgroup analysis was performed for patients with and without PCI and complemented by subgroup analyses stratified by chronic vs. acute coronary syndrome. Sensitivity analysis was performed, excluding patients with a primary discharge diagnosis of heart failure. Missing data on LDL-cholesterol and systolic blood pressure for the Cox regression analysis were imputed using multivariable multiple imputation by fully conditional specification with ten datasets [11]. The frequency of medical therapy with aspirin, statins, and nonstatin lipid-lowering agents after coronary angiography as well as discharge diagnosis of CAD was assessed in the group of patients without PCI and compared between groups with and without utilization of IVUS. The chi-square test was used to determine the difference between the groups. All analyses were performed using SAS software (version 9.4, SAS Institute Inc.). A p-value <0.05 indicated statistical significance.
Results
Overall, patient data from 30,814 coronary angiography exams (mean age 64.9 ± 12.5 years, 70.3% male) were included in our analysis. Of those, PCI was performed as part of the exam in 10,995 procedures (35.7%), while 19,819 exams (64.3%) were performed without the need for coronary intervention. Overall, 4991 procedures (16.2%) were guided by IVUS (3018 with PCI, 1973 without PCI) (Online Fig. 1). In contrast, 7977 PCIs were performed without IVUS. Patients undergoing IVUS were slightly younger, were more frequently male, had lower LDL-, HDL-cholesterol and BNP/NT-proBNP levels, and had a higher frequency of diabetes and family history of premature CAD than patients not receiving IVUS as part of coronary angiography (Table 1).
IVUS and mortality
During a mean follow-up of 3.4 ± 3.6 years (range 0.1–15.5 years), 5316 deaths (17.3%) occurred. Mortality was significantly lower following IVUS-guided coronary procedures than following procedures without IVUS (13.9% vs. 17.9% for IVUS-guided vs. non-IVUS-guided examinations, respectively, p < 0.0001, Fig. 1A). Kaplan-Meier analysis confirmed the improved survival of IVUS-guided examinations for both procedures including coronary interventions and purely diagnostic coronary angiography examinations (Fig. 1B and C). In Cox unadjusted regression analysis, utilization of IVUS was associated with a 36% reduction in mortality in the overall cohort (Table 2). Effect sizes remained stable upon adjustment for age and sex, with ancillary control for traditional risk factors and further adjustment for medication and left main stenosis.
Table 2.
Cox regression analysis for the association of IVUS utilization with all-cause mortality.
| IVUS |
IVUS with intervention |
IVUS without intervention |
||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Unadjusted | 0.64 (0.59, 0.70) | <0.0001 | 0.71 (0.63, 0.80) | <0.0001 | 0.64 (0.57, 0.72) | <0.0001 |
| Model 1 | 0.64 (0.59, 0.70) | <0.0001 | 0.72 (0.64, 0.80) | <0.0001 | 0.65 (0.58, 0.73) | <0.0001 |
| Model 2 | 0.64 (0.58, 0.71) | <0.0001 | 0.71 (0.62, 0.81) | <0.0001 | 0.62 (0.53, 0.71) | <0.0001 |
| Model 3 | 0.57 (0.50, 0.65) | <0.0001 | 0.68 (0.57, 0.82) | <0.0001 | 0.53 (0.43, 0.64) | <0.0001 |
Model 1: Adjusted for age, sex.
Model 2: Adjusted for age, sex, low-density lipoprotein (LDL)-cholesterol, systolic blood pressure, diabetes, family history of premature coronary artery disease (CAD), smoking status, and discharge diagnosis, and experience of the interventional cardiologists.
Model 3: Adjusted for age, sex, LDL-cholesterol, systolic blood pressure, diabetes, family history of premature CAD, smoking status, statin use, aspirin use, P2Y12-inhibitors use, antihypertensive medication use, left main stenosis, discharge diagnosis, and experience of the interventional cardiologists.
CAD = coronary artery disease; CI = confidence interval; IVUS = intravascular ultrasound; LDL = low-density lipoprotein.
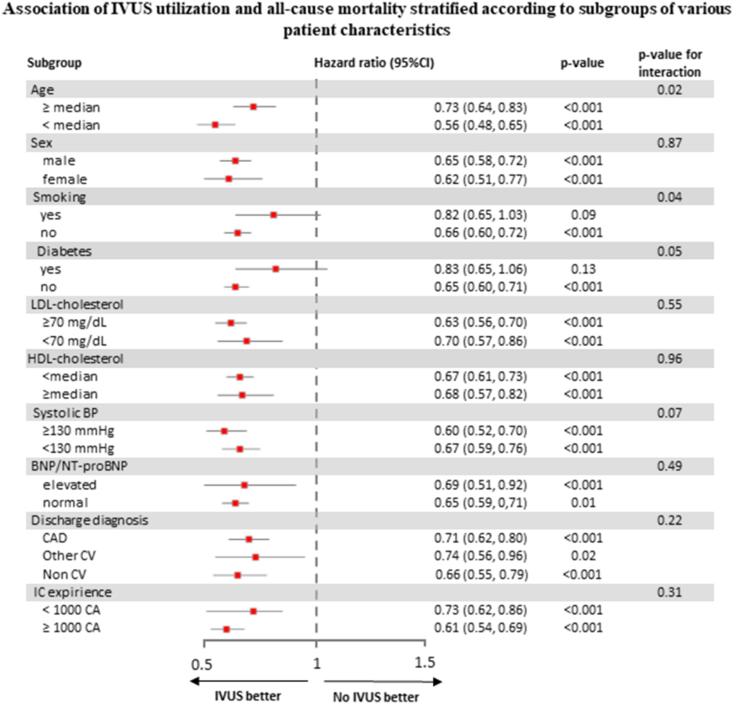
Fig. 2 describes the fully adjusted multivariable model of the relationship between IVUS utilization and all-cause mortality stratified according to subgroups of various patient characteristics. Effect sizes were more pronounced in younger patients, non-smokers, and patients without diabetes. In addition, there was the trend towards a stronger association for procedures that were performed by experienced interventional cardiologist, however, without reaching statistical significance in interaction. Utilization of IVUS was significantly associated with all-cause mortality, irrespective of sex, LDL-C, HDL-C, BNP/NT-proBNP-levels, systolic blood pressure, and discharge diagnosis.
Fig. 2.
Association of IVUS utilization and all-cause mortality stratified according to subgroups of various patient characteristics.
BNP = brain natriuretic peptide; BP = blood pressure; CA = coronary angiography; CAD = coronary artery disease; CV = cardiovascular; HDL-C = high-density lipoprotein cholesterol; IC = interventional cardiologist; IVUS = intravascular ultrasound; LDL-C = low-density lipoprotein cholesterol.
For procedures with IVUS-guided PCI, a 29% reduction in all-cause mortality was observed in unadjusted Cox regression analysis. Again, a significant negative association was observed when adjusting for risk factors. Likewise, for purely diagnostic coronary angiography examinations, utilization of IVUS was associated with improved prognosis independent of age, sex, and traditional risk factors (Table 2). Furthermore, we evaluated the influence of IVUS utilization on long-term survival, stratifying by indication of coronary angiography exam (chronic vs. acute coronary syndrome) showing that the beneficial effect of IVUS was present in chronic coronary syndrome and in acute coronary syndrome patients (Table 3, p-value for interaction: 0,43).
Table 3.
Cox regression analysis for the association of IVUS utilization with all-cause mortality for patients with chronic vs. acute coronary syndrome.
| IVUS utilization in Chronic Coronary Syndrome |
IVUS utilization in Acute Coronary Syndrome |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Unadjusted | 0.69 (0.58, 0.81) | <0.0001 | 0.78 (0.65, 0.94) | 0.008 |
| Model 1 | 0.70 (0.59, 0.82) | <0.0001 | 0.81 (0.67, 0.96) | 0.02 |
| Model 2 | 0.74 (0.62, 0.88) | 0.0005 | 0.80 (0.67, 0.96) | 0.01 |
| Model 3 | 0.69 (0.54, 0.88) | 0.003 | 0.77 (0.59, 1.00) | 0.05 |
Model 1: Adjusted for age, sex.
Model 2: Adjusted for age, sex, low-density lipoprotein (LDL)-cholesterol, systolic blood pressure, diabetes, family history of premature coronary artery disease (CAD), smoking status, and discharge diagnosis, and experience of the interventional cardiologists.
Model 3: Adjusted for age, sex, LDL-cholesterol, systolic blood pressure, diabetes, family history of premature CAD, smoking status, statin use, aspirin use, P2Y12-inhibitors use, antihypertensive medication use, left main stenosis, discharge diagnosis, and experience of the interventional cardiologists.
CAD = coronary artery disease; CI = confidence interval; IVUS = intravascular ultrasound; LDL = low-density lipoprotein.
Again, for both entities, associations of IVUS utilization with mortality were independent of traditional cardiovascular risk factors. Sensitivity analysis, excluding patients with heart failure manifestation, confirmed the risk factor-independent association of IVUS utilization with mortality (Online Table 2). Likewise, we performed sensitivity analysis, excluding patients with left main disease (n = 63), which did not alter the results (Online Table 2).
IVUS utilization and risk factor modification in nonobstructive CAD
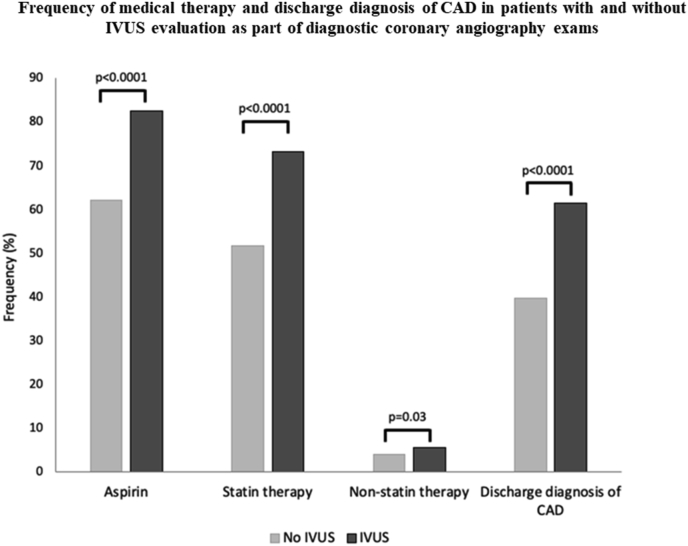
To further evaluate how the utilization of IVUS may have impacted the prognosis in patients without the need for PCI, we evaluated the differences in discharge diagnosis as well as aspirin and lipid-lowering therapy in this group. Patients undergoing IVUS as part of diagnostic coronary angiography more frequently received medical therapy with aspirin (82.6% vs. 61.9% for IVUS vs. no IVUS, p < 0.0001), statins (74.9% vs. 62.5%, p < 0.0001), and nonstatin lipid-lowering drugs (5.7% vs. 3.9%, p = 0.04). Likewise, patients with IVUS examinations were approximately 1.5-fold more likely to be diagnosed with CAD (61.5% vs. 39.6%, p < 0.0001, Fig. 3). Overall, the frequency of IVUS-utilization decreased over time, going in hand with a lower rate of statin and aspirin therapy, and a higher mortality rate (Online Fig. 2).
Fig. 3.
Frequency of medical therapy and discharge diagnosis of CAD in patients with and without IVUS evaluation as part of diagnostic coronary angiography exams.
CAD = coronary artery disease; IVUS = intravascular ultrasound.
Discussion
In the present large longitudinal observational registry on consecutive patients undergoing coronary angiography, we demonstrated that the utilization of IVUS was associated with improved long-term survival. The beneficial effect of IVUS utilization on patient outcome was equally present in diagnostic as well as therapeutic procedures. In patients with nonobstructive CAD, intracoronary imaging via IVUS was followed by a more frequent utilization of secondary prevention therapy. Therefore, our results support the hypothesis that detailed assessment of coronary anatomy and overall coronary plaque burden via IVUS leads to improved patient prognosis for diagnostic and therapeutic coronary procedures.
IVUS-guided PCI
The pivotal role of IVUS during PCI, particularly in complex lesions, is well documented in the literature. Utilization of IVUS leads to implantation of larger stents, more frequent adjunct poststent balloon dilation, and greater postprocedural minimum lumen diameter [7,12,13]. Numerous clinical studies have evaluated the impact of IVUS imaging on PCI and coronary and cardiovascular outcomes [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. While the follow-up in the existing literature extended to up to 10 years, most studies included patients with specific inclusion criteria with respect to anatomically complex coronary lesions. In contrast, our results are based on an all-comers cohort of consecutive patients over a time period of 16 years. We here confirm existing literature, reporting an improvement of mortality in patients undergoing PCI when IVUS is used as part of the procedure. In contrast, data suggesting a benefit on all-cause mortality are controversial [8]. A recent meta-analysis of randomized controlled trials confirmed that IVUS had a major impact on PCI-related outcomes for PCI cohorts [26]. However, the authors underlined the need for further efforts to promote this evidence-based PCI strategy among the global interventional cardiology community. These insights are in accordance with our findings of a reduced mortality rate of patients undergoing IVUS-guided PCI. Our data complement the existing literature by its long-term follow-up, documenting that the beneficial effect of IVUS-guided PCI extends to a follow-up of up to 15 years and was observed for all-cause mortality rather than coronary endpoints only. Stratified by acute vs. chronic coronary syndrome, we observed slightly higher effect sizes for nonacute settings. This finding may be explained by the greater impact of acute coronary syndromes on long-term survival, leaving less room for improvement by secondary prevention strategies.
While the ECAD registry reflects an overall heterogeneous cohort of patients undergoing invasive coronary angiography at a tertiary care university hospital, we observed strong and independent improvement in survival for patients receiving intracoronary imaging. Together with the finding that the association of IVUS with survival was similar for patients with CAD, other cardiovascular disease, or non-cardiovascular disease as leading diagnosis, his supports the hypothesis that intensive risk factor modification, tailored to the patients atherosclerosis burden, improves patient's outcome, irrespective of comorbidity.
IVUS for assessment of plaque burden
Assessment of atherosclerosis is increasingly performed via electron beam or multi-detector computed tomography to develop individualized treatment strategies [27,28]. A large-scale cohort study found that only patients with underlying atherosclerosis as detected by computed tomography were likely to benefit from cardiovascular prevention strategies [29]. This finding follows the idea that intensified risk factor modification should be applied to those patients with the highest risk and greatest therapy-associated benefit [30]. Likewise, for patients with diabetes, computed tomography coronary angiography was found to improve long-term prognosis [31]. For IVUS-guided assessment of coronary artery plaque burden, extensive literature documents that plaque burden can be affected by risk factor-modifying therapy, ultimately improving patient prognosis [9,[32], [33], [34], [35], [36]]. Our analysis supports these findings, as we found that IVUS-guided evaluation of coronary plaque burden led to a higher frequency of detection of CAD, was followed by higher utilization of prevention therapies, and ultimately improved long-term prognosis. Our results therefore suggest that detection of coronary plaque burden via IVUS compels treating physician towards a more aggressive medical therapy regime.
Clinical implications
Here, we provide evidence that utilization of IVUS as part of invasive coronary angiography is associated with improved long-term survival. We observed similar effect sizes as described in available randomized controlled trials and meta-analyses [4,37]. However, compared to existing evidence, certain conditions of the present cohort have to be taken into account: in the German healthcare system, utilization of IVUS imaging as part of invasive coronary angiography exams is of no extra cost to the patient. This fact leads to an overall liberal utilization of IVUS with limited influence of socioeconomic status but higher usage according to specific angiographic findings. Likewise, long-term medical therapy following the procedures is of no financial burden to the patients, increasing the willingness of treating physicians to initiate and maintain intensified medical risk factor modification. For IVUS-guided PCI, our data confirm the findings in the existing literature, complementing the evidence with long-term follow-up and all-cause mortality data. In nonobstructive CAD, we found that patients undergoing IVUS evaluation more frequently received aspirin, statins, and nonstatin lipid-lowering therapy, which may explain the improved outcome in this group. This improvement is most likely caused by the elevated awareness of the underlying disease burden when using IVUS imaging. Therefore, our results support the hypothesis that IVUS utilization as part of invasive coronary angiography exams, providing detailed assessment of coronary anatomy and overall coronary plaque burden, leads to altered patient management and ultimately improves the patient's prognosis. Therefore, intravascular imaging supports not only decision making for interventional cardiology but also long-term patient management in nonobstructive CAD.
Study limitations
Our results are based on a single-center experience. While the database includes coronary angiography exams performed by 74 different interventional cardiologists, our results need to be confirmed in cohorts from other centers and different heath care systems. In addition, we cannot rule out that patients with diagnostic coronary angiography underwent later PCI at other centers, which may have biased the difference between interventional and noninterventional coronary angiography. However, given the large database and the high frequency of patients returning to our center, this effect may not have relevantly affected our results. While we observed a relevantly increased frequency of secondary prevention therapy applied to patients receiving IVUS evaluation, by study design, we cannot establish causality according to IVUS findings but can only descriptively assess differences between the groups. Likewise, as the patients were not randomized to utilization of IVUS, a selection bias of factors that were not accounted for in adjusted regression analysis may have influenced the results. However, the observed effect sizes were very stable and only marginally altered by adjustment for established cardiovascular risk factors and when stratifying by stable vs. acute setting as well as when excluding patients with heart failure, supporting a causal effect of IVUS utilization on long-term prognosis. As by the design of the ECAD registry and the limited information included, we were not able to evaluate, how the amount of plaque burden has affected our results. Further studies are needed to determine, whether or not the observed effect applies to the complete spectrum of CAD. Last, our analysis is based on a predominantly Caucasian population; hence, generalization to other ethnic groups remains uncertain.
Conclusions
In a large registry cohort of patients undergoing invasive coronary angiography, utilization of IVUS was associated with a 36% reduction in all-cause mortality during longitudinal follow-up. The beneficial role of IVUS utilization on long-term survival was present for coronary interventions and diagnostic coronary angiograms. For nonobstructive CAD, utilization of IVUS was followed by an increased frequency of intensified preventive therapy. Our results suggest that the detailed assessment of coronary anatomy and overall coronary plaque burden leads to altered procedural as well as long-term patient management and ultimately improves the patient's prognosis.
Financial support
Iryna Dykun was supported by the German Research Foundation (DY149/2).
Author contributions
We can assure that all authors have 1) provided conception and design or analysis and interpretation of data, or both; 2) been drafting of the manuscript or revising it critically for important intellectual content; and 3) provided final approval of the manuscript submitted.
Declaration of competing interest
The authors have no relationship with industry, no conflict of interest to declare.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2021.07.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bavishi C., Sardar P., Chatterjee S., Khan A.R., Shah A., Ather S., et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. doi: 10.1016/j.ahj.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Ye Y., Yang M., Zhang S., Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound: a meta-analysis. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0179756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X.F., Wang Z.M., Wang F., Gu Y., Ge Z., Kong X.Q., et al. Intravascular ultrasound guidance reduces cardiac death and coronary revascularization in patients undergoing drug-eluting stent implantation: results from a meta-analysis of 9 randomized trials and 4724 patients. Int J Cardiovasc Imag. 2019;35(2):239–247. doi: 10.1007/s10554-019-01555-3. [DOI] [PubMed] [Google Scholar]
- 4.Steinvil A., Zhang Y.J., Lee S.Y., Pang S., Waksman R., Chen S.L., et al. Intravascular ultrasound-guided drug-eluting stent implantation: an updated meta-analysis of randomized control trials and observational studies. Int J Cardiol. 2016;216:133–139. doi: 10.1016/j.ijcard.2016.04.154. [DOI] [PubMed] [Google Scholar]
- 5.Darmoch F., Alraies M.C., Al-Khadra Y., Moussa Pacha H., Pinto D.S., Osborn E.A. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(5) doi: 10.1161/JAHA.119.013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik A.H., Yandrapalli S., Aronow W.S., Panza J.A., Cooper H.A. Intravascular ultrasound-guided stent implantation reduces cardiovascular mortality - updated meta-analysis of randomized controlled trials. Int J Cardiol. 2020;299:100–105. doi: 10.1016/j.ijcard.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Mentias A., Sarrazin M.V., Saad M., Panaich S., Kapadia S., Horwitz P.A., et al. Long-term outcomes of coronary stenting with and without use of intravascular ultrasound. JACC Cardiovasc Interv. 2020;13(16):1880–1890. doi: 10.1016/j.jcin.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buccheri S., Franchina G., Romano S., Puglisi S., Venuti G., D'Arrigo P., et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. 2017;10(24):2488–2498. doi: 10.1016/j.jcin.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 9.Puri R., Nissen S.E., Shao M., Ballantyne C.M., Barter P.J., Chapman M.J., et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur Heart J. 2013;34(41):3182–3190. doi: 10.1093/eurheartj/eht260. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls S.J., Hsu A., Wolski K., Hu B., Bayturan O., Lavoie A., et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55(21):2399–2407. doi: 10.1016/j.jacc.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Sterne J.A., White I.R., Carlin J.B., Spratt M., Royston P., Kenward M.G., et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y.J., Pang S., Chen X.Y., Bourantas C.V., Pan D.R., Dong S.J., et al. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2015;15:153. doi: 10.1186/s12872-015-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S.J., Kim B.K., Shin D.H., Nam C.M., Kim J.S., Ko Y.G., et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. J Am Med Assoc. 2015;314(20):2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 14.Jakabcin J., Spacek R., Bystron M., Kvasnak M., Jager J., Veselka J., et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Cathet Cardiovasc Interv. 2010;75(4):578–583. doi: 10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- 15.Chieffo A., Latib A., Caussin C., Presbitero P., Galli S., Menozzi A., et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165(1):65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.S., Kang T.S., Mintz G.S., Park B.E., Shin D.H., Kim B.K., et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6(4):369–376. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Tian N.L., Gami S.K., Ye F., Zhang J.J., Liu Z.Z., Lin S., et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10(12):1409–1417. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- 18.Kim B.K., Shin D.H., Hong M.K., Park H.S., Rha S.W., Mintz G.S., et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8(7) doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 19.Tan Q., Wang Q., Liu D., Zhang S., Zhang Y., Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36(5):549–553. doi: 10.15537/smj.2015.5.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Gao X., Kan J., Ge Z., Han L., Lu S., et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Ali Z.A., Maehara A., Genereux P., Shlofmitz R.A., Fabbiocchi F., Nazif T.M., et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388(10060):2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 22.Choi K.H., Song Y.B., Lee J.M., Lee S.Y., Park T.K., Yang J.H., et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12(7):607–620. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 23.Andell P., Karlsson S., Mohammad M.A., Gotberg M., James S., Jensen J., et al. Intravascular ultrasound guidance is associated with better outcome in patients undergoing unprotected left main coronary artery stenting compared with angiography guidance alone. Circ Cardiovasc Interv. 2017;10(5) doi: 10.1161/CIRCINTERVENTIONS.116.004813. [DOI] [PubMed] [Google Scholar]
- 24.Hong S.J., Mintz G.S., Ahn C.M., Kim J.S., Kim B.K., Ko Y.G., et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13(1):62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Gao X.F., Ge Z., Kong X.Q., Kan J., Han L., Lu S., et al. 3-Year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14(3):247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Nagaraja V., Kalra A., Puri R. Improving outcomes with IVUS guidance during percutaneous coronary interventions. Curr Treat Options Cardiovasc Med. 2020;5(22):10. [Google Scholar]
- 27.Yeboah J., McClelland R.L., Polonsky T.S., Burke G.L., Sibley C.T., O'Leary D., et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. Jama. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahabadi A.A., Mohlenkamp S., Lehmann N., Kalsch H., Dykun I., Pundt N., et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. JACC Cardiovasc Imag. 2017;10(2):143–153. doi: 10.1016/j.jcmg.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell J.D., Fergestrom N., Gage B.F., Paisley R., Moon P., Novak E., et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72(25):3233–3242. doi: 10.1016/j.jacc.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahabadi A.A., Nasir K., Rassaf T. Routine CAC-scoring prior to initiation of statin therapy - a European perspective. Eur J Prev Cardiol. 2019;26(14):1559–1561. doi: 10.1177/2047487319839188. [DOI] [PubMed] [Google Scholar]
- 31.Newby D.E., Adamson P.D., Berry C., Boon N.A., Dweck M.R., Flather M., et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 32.Puri R., Ballantyne C.M., Hoogeveen R.C., Shao M., Barter P., Libby P., et al. Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: insights from SATURN. Atherosclerosis. 2017;263:137–144. doi: 10.1016/j.atherosclerosis.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Puri R., Nissen S.E., Ballantyne C.M., Barter P.J., Chapman M.J., Erbel R., et al. Factors underlying regression of coronary atheroma with potent statin therapy. Eur Heart J. 2013;34(24):1818–1825. doi: 10.1093/eurheartj/eht084. [DOI] [PubMed] [Google Scholar]
- 34.Puri R., Nissen S.E., Shao M., Ballantyne C.M., Barter P.J., Chapman M.J., et al. Antiatherosclerotic effects of long-term maximally intensive statin therapy after acute coronary syndrome: insights from Study of Coronary Atheroma by Intravascular Ultrasound: effect of Rosuvastatin versus Atorvastatin. Arterioscler Thromb Vasc Biol. 2014;34(11):2465–2472. doi: 10.1161/ATVBAHA.114.303932. [DOI] [PubMed] [Google Scholar]
- 35.Clark D., 3rd, Nicholls S.J., St John J., Elshazly M.B., Kapadia S.R., Tuzcu E.M., et al. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur Heart J. 2018;39(27):2551–2558. doi: 10.1093/eurheartj/ehy209. [DOI] [PubMed] [Google Scholar]
- 36.Stegman B., Shao M., Nicholls S.J., Elshazly M., Cho L., King P., et al. Coronary atheroma progression rates in men and women following high-intensity statin therapy: a pooled analysis of REVERSAL, ASTEROID and SATURN. Atherosclerosis. 2016;254:78–84. doi: 10.1016/j.atherosclerosis.2016.09.059. [DOI] [PubMed] [Google Scholar]
- 37.Nerlekar N., Cheshire C.J., Verma K.P., Ihdayhid A.R., McCormick L.M., Cameron J.D., et al. Intravascular ultrasound guidance improves clinical outcomes during implantation of both first- and second-generation drug-eluting stents: a meta-analysis. EuroIntervention. 2017;12(13):1632–1642. doi: 10.4244/EIJ-D-16-00769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.