Abstract
Background and aims
Proprotein convertase subtilisin/kexin type 9 (PCSK9) circulates as mature and furin-cleaved forms, but their biological functions are uncertain. We investigated whether their levels associate with prognosis in patients with acute ST elevation myocardial infarction (STEMI).
Methods
We enrolled 160 statin-naïve patients with acute STEMI and followed for 3 years. PCSK9 subtype levels were determined by an enzyme-linked immunosorbent assay before and at five timepoints up to 48 h after emergent coronary intervention. The occurrence of coronary and cardiac events was compared between subjects stratified by the PCSK9 level.
Results
One hundred and twenty-six patients completed 3 years of follow-up. In the acute phase, both PCSK9 subtype levels decreased, and thereafter increased from 6 to 48 h (mature: from 198 ± 67 to 334 ± 116 ng/mL, furin-cleaved: from 20 ± 7 to 39 ± 16 ng/mL, both p < 0.01). Major cardiac events occurred in 46 patients. The furin-cleaved/mature PCSK9 ratio at 48 h after coronary intervention predicted the likelihood of experiencing of events; patients in the third tertile had lower event-free survival than those in the first and second tetiles in Kaplan–Meier analysis (p = 0.004). Multivariate Cox regression analysis revealed that this ratio had a greater impact (HR: 1.92; 95% CI: 1.06–3.45, p = 0.03) on events than other known atherosclerosis risk factors.
Conclusions
The furin-cleaved/mature PCSK9 ratio was associated with 3-year cardiovascular events in statin-naïve patients with acute STEMI, suggesting a potential link between furin cleavage process of PCSK9 and its effect on prognosis. (249 words)
Keywords: Proprotein convertase subtilisin/kexin type 9 (PCSK9), Acute myocardial infarction, Prognosis
Highlights
-
•
PCSK9 levels changed during the acute phase of STEMI in statin-naïve patients.
-
•
Changes of fc- and m-PCSK9 levels over time differed.
-
•
The fc/m-PCSK9 ratio was independently associated with subsequent MACE.
1. Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), the ninth member of the proprotein convertase family, is a serine protease mainly synthesized by the liver. It plays a key role in lipid metabolism and atherosclerosis by degrading low-density lipoprotein (LDL) receptor and thus elevating the plasma LDL-cholesterol (LDL-C) level [1,2]. In addition to receptor modulation, PCSK9 reportedly has pleiotropic effects and thereby directly promotes inflammatory activities, oxidative stress, and atheroma formation [[3], [4], [5], [6], [7]].
PCSK9 circulates as two forms: matured PCSK9 (m-PCSK9) and furin-cleaved PCSK9 (fc-PCSK9). In hepatocytes, m-PCSK9 is synthesized by the endoplasmic reticulum. It consists of a pro-domain, a catalytic domain, and a C-terminal domain. Furin cleaves part of m-PCSK9 from the prodomain, resulting in fc-PCSK9 generation [8,9]. Hepatocytes secrete both forms of PCSK9 into the circulation. Although m-PCSK9 functions in LDL receptor degradation, the biological function of fc-PCSK9 is unknown [10]. Various proteins cleaved by furin promotes inflammation and atherosclerosis [11,12]; however, it remains unclear whether fc- PCSK9 has the same functions.
Furthermore, the role of PCSK9 in the very early phase of acute myocardial infarction (AMI) is largely unknown. It has been reported that the serum PCSK9 level rapidly and significantly increases on day 1, and the maximum increase is observed on day 2 in acute coronary syndrome (ACS) patients. PCSK9 levels plateau thereafter and are positively associated with the severity of coronary artery lesions [13]. Another study of non-ST-elevation myocardial infarction (STEMI) patients also reported that the PCSK9 level increases during the early phase and peaks toward the end of day 2, and that these increases are modestly associated with neutrophil counts and the presence of hypercholesterolemia [14]. However, from a prognostic viewpoint, high initial PCSK9 plasma levels are not reportedly associated with 1 year mortality of ACS patients [15]. In addition, changes of m-PCSK9 and fc-PCSK9 were not investigated in these previous studies.
Based on these findings, we hypothesized that the circulating levels of the two molecular forms of PCSK9 may be differentially altered by AMI. To test this hypothesis, we conducted a prospective study to evaluate serial changes of m-PCSK9 and fc-PCSK9 in patients with acute STEMI, paying special attention to the relationship with adverse events during follow-up.
2. Materials and methods
2.1. Study design
To evaluate changes of serum PCSK9 levels in the acute phase of STEMI, we conducted a single-center, prospective study. Circulating PCSK9 levels are higher in statin users than in nonusers [16], and therefore statin users were excluded. The study protocol conformed with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of our institution. Written informed consent was obtained from all patients before enrollment.
2.2. Population
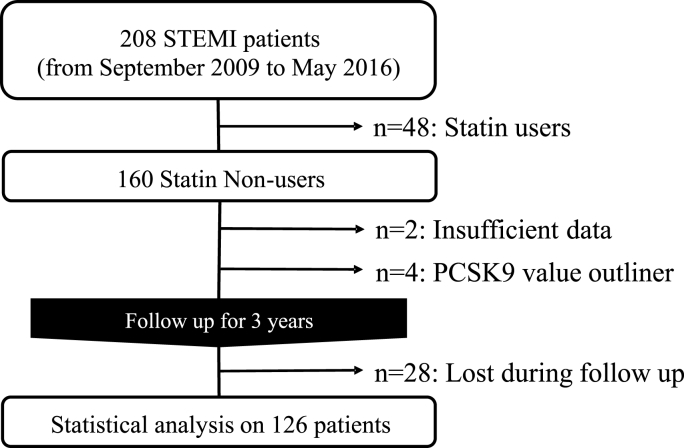
A total of 208 STEMI patients were admitted to our hospital between September 2009 and May 2016. Of these, 160 statin-naïve patients with STEMI met our inclusion criteria. Two were excluded due to insufficient data, and four were excluded because they were considered outliners with values that deviated at a significance level of 0.01 according to the Smirnov-Grubbs test. A total of 126 patients were followed up for 3 years after STEMI, during which 28 patients were dropped out (Fig. 1.).
Fig. 1.
Flow diagram of the inclusion of study subjects.
2.3. Definition of STEMI
STEMI was defined as an increased level of creatine kinase-myocardial isoform (CK-MB) with the following symptoms: typical chest pain lasting more than 20 min, and an electrocardiogram (ECG) showing new ST-segment elevation ≥2 mm in at least two contiguous precordial ECG leads or ≥1 mm in at least two contiguous limb ECG leads or a newly appeared left bundle branch block [17].
2.4. Blood sampling protocol and measurement
Peripheral blood samples to measure circulating serum levels of serum m-PCSK9, fc-PCSK9, and high-sensitivity C-reactive protein (hs-CRP) were collected before coronary angiography (CAG) and at 3, 6, 12, 24, and 48 h after percutaneous coronary intervention (PCI). In cases with no coronary occlusion, samples were collected at the same timepoints. Serum was separated by centrifugation (2500×g) at 4°C for 10 min and stored at −80°C until use. m- and fc-PCSK9 levels were determined using an enzyme-linked immunosorbent assay kit (BML Inc., Tokyo, Japan) [18]. The inter- and intra-assay coefficients of variance were 7.7% and 2.2% for m-PCSK9, respectively, and 5.6% and 2.1% for fc-PCSK9, respectively. The lower and upper detection limits were 3.9 and 20,000 ng/mL for m-PCSK9, respectively, and 0.7 and 300 ng/mL for fc-PCSK9, respectively. The intra- or interassay variability of measurement (CV) was less than 5%. Other laboratory measurements described in Table 1 were obtained using standard methods at each timepoint.
Table 1.
Characteristics of study subjects and baseline data.
| Age | (years) | 64.7 ± 12.4 |
| Sex (men/women) | 112/14 | |
| Past medical history | ||
| Myocardial infarction | 7 | (5.6%) |
| Unstable angina pectoris | 3 | (2.4%) |
| Angina pectoris | 10 | (7.9%) |
| Prior coronary artery bypass grafting | 2 | (1.6%) |
| Prior percutaneous coronary intervention | 7 | (5.6%) |
| Atrial fibrillation | 5 | (4.0%) |
| Cerebral infarction | 6 | (4.8%) |
| Lower limb ischemia | 2 | (1.6%) |
| Diabetes mellitus | 37 | (29.4%) |
| Dyslipidemia | 21 | (16.7%) |
| Hypertension | 61 | (48.4%) |
| Hemodialysis | 2 | (1.6%) |
| Family history of ischemic heart disease | 12 | (9.5%) |
| Smoking | 61 | (48.4%) |
| Alcohol consumption | 36 | (28.6%) |
| Medication | ||
| Antiplatelets | 22 | (17.5%) |
| Aspirin | 17 | (13.5%) |
| Calcium channel blocker | 43 | (34.1%) |
| ACEI/ARB | 32 | (25.4%) |
| Beta blocker | 6 | (4.8%) |
| Loop diuretics | 4 | (3.2%) |
| Nitrates | 11 | (8.7%) |
| Antiarrhythmic agents | 5 | (4.0%) |
| Statin | 0 | (0%) |
| Fibrate | 2 | (1.6%) |
| Ezetimibe | 1 | (0.8%) |
| Insulin | 6 | (4.8%) |
| Sulfonylurea or glinide | 13 | (10.3%) |
| Thiazolidine | 4 | (3.2%) |
| Biguanide | 5 | (4.0%) |
| DPP-4 inhibitor or incretin | 4 | (3.2%) |
| BMI (kg/m2) | 23.8 ± 3.4 | |
| Blood pressure (mmHg) | Systolic | 133 ± 32 |
| Diastolic | 81 ± 20 | |
| Laboratory data | ||
| Hemoglobin | (mg/dL) | 13.7 ± 2.0 |
| Glucose | (mg/dL) | 199 ± 98 |
| Hemoglobin A1c | (%) | 6.6 ± 1.5 |
| Total cholesterol | (mg/dL) | 189 ± 43 |
| Triglyceride | (mg/dL) | 118 ± 75 |
| HDL-cholesterol | (mg/dL) | 42 ± 10 |
| LDL-cholesterol | (mg/dL) | 125 ± 35 |
| Non HDL-cholesterol | (mg/dL) | 147 ± 42 |
| BNPa | (pg/mL) | 42 (18, 122) |
| Creatininea | (mg/dL) | 0.9 (0.8, 1.1) |
| γ-Glutamyl transpeptidasea | (IU/L) | 31 (18, 48) |
| Aspartate aminotransferasea | (IU/L) | 38 (23, 87) |
Values are the number of subjects in each category or data expressed as mean ± SD.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; DPP-4 inhibitor, dipeptidyl peptidase-4 inhibitor; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Due to skewed distribution, median (25%, 75% tile) are shown.
2.5. PCI procedure
Emergent CAG was performed in all patients. In the cases with multiple coronary occlusions, the lesion that was consistent with the electrocardiographic and echocardiographic changes was considered as the culprit. PCI was immediately performed conventionally. The exact procedures depended on the interventional cardiology staff (T-AT, MK, and YK). A loading dose of aspirin and P2Y12 inhibitors was given orally, and 70–100 IU/kg unfractionated heparin was administered intravenously before the procedure. During PCI, the activated clotting time was kept higher that 250 s with additional heparin administration. Successful primary PCI was defined as achievement of TIMI flow higher than grade 3 antegrade coronary blood flow with residual stenosis <20%. Dual antiplatelet therapy was maintained during the deferred period and for at least 1 year after PCI.
2.6. Clinical follow-up
All patients were followed up at an outpatient clinic in our university hospital. Standard medications of antiplatelets, a beta blocker, an angiotensin-converting-enzyme inhibitor or angiotensin II receptor blocker, and statins, were given to ensure that the clinical and laboratory values reached the targets for secondary prevention after myocardial infarction, if tolerable. Statin was introduced on from Day 1 to Day 4, depending on the length of the coronary care unit stay, renal/liver function, and previous drug adverse events information, but it started until Day 2 in most of all patients. The clinical endpoint was defined as the incidence of major adverse cardiovascular events (MACE), defined as a composite of all-cause death, nonfatal myocardial infarction and stroke, and new angina pectoris (AP). Cases of new AP were defined as those in which the culprit of AP was different from that of STEMI. Restenosis requiring revascularization was not included in MACE.
2.7. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as numbers and percentages. The median and interquartile ranges were calculated for asymmetrically distributed data. Repeated measure of one-way analysis of variance, with covariate if necessary, was used to assess serial changes of the biomarker levels on pre- and post-PCI, and paired t-test with Bonferroni correction for multiple tests was applied in the comparison of single time points. In case of variable with asymmetrical distribution, inter-group difference was analyzed using Kruskal-Wallis test and Scheffe's multiple comparison. To analyze between-biomarkers data, Pearson's correlation coefficient (R) and Spearman's rank correlation coefficient (SRCC) were used for data with a normal distribution and non-parametric measures, respectively. The Chi-square test was used to analyze between-group characteristics. Univariate and multivariable analyses using the Cox proportional hazards model were performed. The model for time to MACE was evaluated using following variables: age; gender; current smoking; presence of hypertension; diabetes mellitus, and dyslipidemia; multivessel disease; and peak values of creatine kinase (CK), CK-MB and hs-CRP. Multivariate model was built by including variables showing p value ≤ 0.1 in univariate analysis. Outcomes were estimated using the Kaplan-Meier method and compared using the log-rank test. Statistical analyses were performed using BellCurve for Excel ver. 3.21 (Social Survey Research Information Co., Ltd., Tokyo, Japan). All statistical tests were two-sided. p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
Table 1 summarizes the baseline characteristics of statin-naïve patients with acute STEMI who completed 3 years of follow-up. The majority of patients were male (88.9%), and half were smokers, and treated for hypertension (both 48.4%). Regarding lipid-modifying treatment, two patients were treated with fibrate and one was treated with ezetimibe. Although some patients had a history of prior cardiovascular events, none were treated with statins. Baseline clinical and laboratory data are also shown in Table 1. The mean levels ± SD of total cholesterol, triglyceride, and LDL-C (calculated using the Friedewald formula) were 189 ± 43 mg/dL, 118 ± 75 mg/dL, and 125 ± 35 mg/dL, respectively.
3.2. Characteristics of STEMI
Three patients did not receive PCI because coronary stenosis was resolved by injection of isosorbide dinitrate into coronary arteries, suggesting that STEMI was caused by coronary vasospasm (Supplementary Table 1). The remaining subjects subsequently received primary PCI. PCI was successful in 119 patients (94.4%). The most common culprit vessel was the left anterior descending artery (71 patients, 56.3%). The culprit vessel was the left main coronary trunk in three patients, all of whom received primary PCI. Sixty patients had multivessel diseases, but primary PCI was only performed on the culprit lesion. The majority of patients received coronary stenting, but 24 patients (19%) only received balloon dilatation. The median (25% and 75% tiles) of the medians (25%, 75% tile) of peak CK, CK-MB, and hs-CRP levels were 2473 (1454, 4683) IU/L, 208 (144, 405) IU/L, and 58650 (16800, 96475) ng/mL, respectively.
3.3. Serial biomarker changes and their interrelations
The changes of serum levels of fc-PCSK9, m-PCSK9, CK, CK-MB, and hs-CRP are shown in Table 2 fc-PCSK9 levels initially decreased significantly up to 12hr, and then increased above the pre-PCI levels. The initial decrease of the m-PCSK9 level was not significant (212–198 ng/mL), but this level was significantly higher at 48 h than at baseline. The mean hs-CRP level increased gradually, and the CK and CK-MB levels both initially increased, peaked at 6 h, and then gradually decreased (these changes were both statistically significant). The fc-PCSK9 level increased from baseline to 48 h and showed a significant and positive association with the m-PCSK9 levels at baseline (R = 0.655, p < 0.001) and 48 h (R = 0.658, p < 0.001). Furthermore, these changes in fc-PCSK9 levels were also associated with peak, but not baseline, hs-CRP values (R = −0.0014, p = 0.876). Peak m- and fc-PCSK9 levels had modest and positive associations with peak CK levels (m-PCSK9: SRCC = 0.292; p < 0.01, fc-PCSK9: SRCC = 0.270; p < 0.01), but only peak fc-PCSK9 levels had a positive association with CK-MB levels (SRCC = 0.253; p < 0.01).
Table 2.
m-PCSK9, fc-PCSK9, hs-CRP, CK, and CK-MB Levels Pre- and Post-PCI.
| Baseline | 3 h | 6 h | 12 h | 24 h | 48 h | Repeated measure of ANOVAb | |
|---|---|---|---|---|---|---|---|
| m-PCSK9, ng/ml | 212.0 ± 74.8 | 197.6 ± 67.2 | 197.9 ± 68.4 | 200.4 ± 71.6 | 226.9 ± 78.0 | 334.1 ± 115.5∗∗ | <0.001 |
| fc-PCSK9, ng/ml | 25.1 ± 8.4 | 21.7 ± 7.7∗∗ | 19.7 ± 6.7∗∗ | 20.7 ± 8.1∗∗ | 24.9 ± 9.8 | 39.1 ± 15.9∗∗ | <0.001 |
| hs-CRP, ng/mL a | 1095 (398, 5840) | 1150 (364, 5810) | 1895 (799, 6523) | 6425 (2680, 14825) | 24600 (8785, 52550)∗∗ | 58650 (16800, 96475) ∗∗ | <0.001 |
| CK, IU/L a | 237 (110, 637) | 2139 (1085, 4238)∗∗ | 2470 (1454, 4683)∗∗ | 2221 (1241, 3884)∗∗ | 1287 (758, 2164)∗∗ | 503 (302, 776) | <0.001 |
| CK-MB, IU/L a | 20 (10, 59) | 193 (103, 413)∗∗ | 208 (144, 405)∗∗ | 181 (103, 301)∗∗ | 77 (50, 125)∗ | 20 (11, 31) | <0.001 |
| Fc/m-PCSK9 ratio a | 0.12 (0.10, 0.14) | 0.11 (0.09, 0.13) ∗∗ | 0.10 (0.08, 0.12) ∗∗ | 0.10 (0.09, 0.12) ∗∗ | 0.11 (0.09, 0.13) ∗∗ | 0.12 (0.10, 0.14) | <0.001 |
Mean values ± SD at each timepoint are shown.
CK, creatine kinase; CK-MB, creatine kinase MB; fc-PCSK9, furin-cleaved proprotein convertase subtilisin/kexin type 9; hs-CRP, high-sensitivity C-reactive protein; m-PCSK9, mature proprotein convertase subtilisin/kexin type 9; PCI, percutaneous coronary intervention. ∗p < 0.05, ∗∗p < 0.01 vs. baseline value.
Due to skewed distribution, median (25%, 75% tile) are shown.
All p-values remain same in case that age (10-yr age group) or sex was included as covariate (ANCOVA).
3.4. Associations among biomarker candidates
To explore possible determinants of PCSK9 levels, we investigated associations between peak m- and fc-PCSK9 levels and sex, age, the peak aspartate aminotransferase level, the peak alanine aminotransferase level, the white blood cell (WBC) count, and the hemoglobin level. Peak m-PCSK9 levels had a modest and positive association with the WBC count (SRCC = 0.353; p < 0.01). Other variables were not associated with the m- or fc-PCSK9 levels.
3.5. Adverse events during follow-up
Forty-six patients developed MACE during follow-up. Of the ten patient who died, eight died from heart disease, and two died from non-cardiac diseases (cancer and unspecified cause). New AP was recorded in 24 cases, two of whom had unstable conditions. No patient had non-fatal myocardial infarction. Seven and three patients had heart failure requiring hospitalization or embolic events (two patients had cerebral infarction and one patient had critical limb ischemia), respectively.
3.6. The fc/m-PCSK9 ratio and prognostic outcome
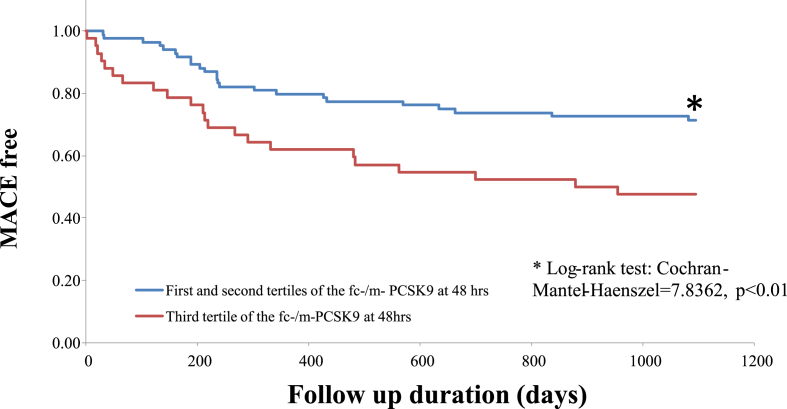
The proportional change of the fc-PCSK9 level from baseline to 48 h after PCI was greater than that of m-PCSK9 (+73% vs. + 64%), although the levels of both fc- and m-PCSK9 peaked at 48 h after PCI (Table 2). Therefore, we focused on the ratio of fc-PCSK9 to m-PCSK9 (fc/m-PCSK9) at 48 h after PCI based on the hypothesis that the pathophysiological condition of acute STEMI might influence furin-cleavage of m-PCSK9 to generate fc-PCSK9. When patients were divided into tertiles according to the fc/m-PCSK9 ratio, the prevalence of MACE was higher among patients in the third tertile, but was comparable between patients in the other two tertiles (Supplementary Tables 2 and 3), except for potential relation with rGTP values. Based on these observations, we combined patients in the first and second tertiles, and compared them with patients in the third tertile. Patients in the third tertile were significantly more susceptible to subsequent MACE than patients in the first and second tertiles (log-rank test χ2: 7.8362, p < 0.01) (Fig. 2).
Fig. 2.
Cumulative freedom from the risk of MACE during 3 years of follow-up.
Patients classified into the first and the second tertiles of the fc-/m-PCSK9 ratio at 48 h showed significantly favorable outcomes compared with those classified in to the third tertile in terms of all-cause death, nonfatal myocardial infarction and stroke, or new AP (log-rank test χ2: 7.8362, p<0.01).
Univariate analysis showed that the tertiles of the fc/m-PCSK9 ratio, diabetes mellitus, or presence of multivessel lesions were significantly associated with MACE during 3 years of follow-up (Table 3). The presence of hypertension and the value of log-transformed hs-CRP value were potentially associated with MACE. Multivariate Cox regression analysis of these five factors showed that the tertiles of the fc/m-PCSK9 ratio were the strongest predictor of MACE (HR: 1.92; 95% CI: 1.06–3.45, p = 0.03). There was no significant relation between fc/m-PCSK9 ratio and any specific type of events (Supplementary Table 4). Neither fc- nor m-PCSK9 levels showed significant association with outcome (data not shown).
Table 3.
Univariate and multivariate analyses to predict MACE.
| Variable | Univariate Cox regression |
Multivariate Cox regression |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| Tertile of fc/m PCSK9 | 2.24 | 1.25 to 3.99 | <0.01b | 1.92 | 1.06 to 3.45 | 0.03a |
| Age | 0.75 | 0.78 to 1.41 | 0.75 | |||
| Gender | 0.95 | 0.41 to 2.61 | 0.95 | |||
| Smoking | 0.93 | 0.78 to 1.10 | 0.39 | |||
| Peak CK-MB | 1.00 | 0.99 to 1.00 | 0.48 | |||
| Ln hs-CRP | 1.23 | 0.96 to 1.57 | 0.10 | 1.16 | 0.90 to 1.51 | 0.25 |
| Diabetes mellitus | 1.90 | 1.07 to 3.40 | 0.03a | 1.49 | 0.81 to 2.73 | 0.20 |
| Dyslipidemia | 0.84 | 0.38 to 1.88 | 0.67 | |||
| Hypertension | 1.75 | 1.01 to 3.04 | 0.05 | 1.72 | 0.96 to 3.05 | 0.07 |
| Multivessel disease | 1.82 | 1.06 to 3.06 | 0.03a | 1.34 | 0.75 to 2.39 | 0.32 |
Multivariate analysis was performed of four variables with significant associations (p ≤ 0.10) in univariate analysis.
CI, confidence interval; CK-MB, creatine kinase MB; fc/m-PCSK9, ratio of furin-cleaved to mature proprotein convertase subtilisin/kexin type 9; HR, hazard ratio; Ln hs-CRP, natural logarithm of the values of high-sensitivity C-reactive protein; MACE, major adverse cardiovascular events.
p < 0.05.
p < 0.01.
4. Discussion
In the present study, we found that the circulating PCSK9 levels, especially that of fc-PCSK9, decreased up to 12 h after PCI and then increased up to 48 h in statin-naïve acute STEMI patients. We also demonstrated for the first time the potential prognostic significance of the fc/m-PCSK9 ratio at 48 h after PCI for subsequent MACE.
A previous study that measured PCSK9 levels in ACS patients reported that serum PCSK9 concentrations significantly increased from day 0 to day 4 in both statin-naïve and -treated patients, and the maximum increase was observed on day 2 (+34% in statin-naïve patients and +31% in statin-treated patients), after which PCSK9 levels plateaued [13]. That study did not examine the molecular forms of PCSK9. Another study, in which the two molecular forms of PCSK9 were measured separately, showed that both fc- and m-PCSK9 levels gradually increased, peaked on day 3, and returned to the baseline by day 20 [19]. However, that study also enrolled both statin-naïve and -treated patients. The current study is the first to investigate the clinical significance of both fc- and m-PCSK9 levels in statin-naïve STEMI patients.
In addition, the present study evaluated serial changes of PCSK9 levels from the very early phase of STEMI. A recent study reported that the levels of PCSK9 and pentraxin-related protein 3 (PTX3), a newly discovered acute phase protein upon inflammation belonging to the hs-CRP superfamily, are positively associated in STEMI patients undergoing primary PCI (r = 0.302, p < 0.003) [21]. Another study suggested that gene expression of PTX3 and neutrophil CD11b, a member of the B2 integrin subfamily, in the infarcted myocardium significantly decreases at 24 h after PCI, causing suppression of the inflammatory response and thereby relieving myocardial injury [20]. Based on these observations, the initial decrease of PCSK9 observed in the present study may be attributed to successful revascularization induced by PCI.
In terms of the prognostic value of circulating PCSK9 levels, the results of previous studies using large-scale cohorts in both primary and secondary prevention settings are inconsistent [[22], [23], [24]]. In ACS patients, high initial PCSK9 plasma levels were reported to be associated with the inflammatory condition in the acute phase and hypercholesterolemia, but did not predict mortality at 1 year [15]. All these studies applied ELISAs that can only measure the total PCSK9 concentration and therefore did not investigate the differential roles of the two molecular forms of PCSK9. In addition, a significant number of patients enrolled in these studies had already been treated with statins at baseline [15,22,23]. Statin administration significantly increase circulating PCKS9 levels [16]. Variability in patient background, especially in terms of prior statin therapy, and the measured molecular form of PCSK9 might explain the inconsistencies of previous studies.
A recent cohort study investigated whether PCSK9 subtypes are associated with atherosclerotic cardiovascular events in 1436 statin-naïve Japanese subjects without any cardiovascular disease. During follow-up (average 13.6 years), the furin-cleaved form, but not the total or mature form, of PCSK9 was associated with both LDL cholesterol and hs-CRP levels and predicted future coronary events [25]. In the present study, we did not find any association between the baseline levels of fc- or m-PCSK9 and MACE during 3 years of follow-up. A difference in the coronary risk status (i.e., primary or secondary prevention setting) might explain these inconsistencies. A larger study is necessary to investigate this issue.
In our study, there is a positive association between m- and fc-PCSK9 levels, consistent with a previous study [25]. In addition, fc-PCSK9 changes were significantly associated with peak, but not baseline, hs-CRP levels, and neither were associated with subsequent MACE. This might be partly due to variability in the time course from STEMI onset in the enrolled subjects. The fact that the basal fc/m-PCSK9 ratio was also significantly associated with MACE at 3 years in the present study (data not shown) indicates that the best timepoint to evaluate the molecular subtype of PCSK9 should be confirmed by a future study. As shown in Supplementary Table 3, rGTP levels might be associated with the fc/m-PCSK9 ratio, while aspartate aminotransferase did not. Potential explanation for this discrepancy should be investigated by future study.
The aforementioned Japanese cohort study reported a positive relationship of hs-CRP levels with fc-PCSK9, but not m-PCSK9, levels and discussed that fc-PCSK9 might harbor more proatherogenic properties that could drive coronary events [25]. A laboratory investigation reported that furin and proprotein convertases 5 (PC5) are expressed in human peripheral blood mononuclear cells and colocalize with MT1-matrix metalloproteinases in macrophages in atherosclerotic plaques, suggesting they are potential treatment targets for atherosclerosis [11]. Another immunohistochemical analysis demonstrated that furin is upregulated in plaque lymphocytes and macrophages, implying that this enzyme has a role in plaque pathology, and that a furin inhibitor in arteries may modulate the course of atherosclerosis. Although there are no data providing mechanistic insights into the proinflammatory effect of fc-PCSK9, our observation that hs-CRP levels showed a significant and positive association with fc-PCSK9, but not m-PCSK9, levels indicates that fc-PCSK9 may have a proatherogenic role.
The clinical implications of our results could suggest the potential for the level of fc/m-PCSK9 ratio at 48 h after PCI to be a prognostic biomarker in patients with STEMI. Other clinical implications include the potential to identify novel therapeutic approaches that can improve the long-term outcome of STEMI patients. It is controversial whether treatment with evolocumab, a fully human monoclonal antibody that inhibits PCSK9, in the acute phase of AMI is beneficial. In a previous report, the plasma levels of m-PCSK9 and fc-PCSK9 significantly increased and decreased, respectively, after day 1 in AMI patients treated with evolocumab after primary PCI [19]. This report suggested that evolocumab may reduce the fc/m-PCSK9 ratio. Based on these previous results and our findings, treatment with an anti-PCSK9 antibody in the acute phase may modulate the furin-cleavage of PCSK9, leading to influence prognosis of STEMI patients. A prospective interventional study is required to investigate this important clinical issue.
This study has several limitations. First, it was carried out at a single center, meaning there was potentially unrecognized bias. In addition, regarding MACE criteria, we included new AP because a small number of patients exhibited a hard end point to sufficiently examine the prognostic value of the fc/m-PCSK9 ratio. Although, new AP is an atherosclerotic cardiovascular event, we believe its incidence may be influenced by the fc/m-PCSK9 ratio. A larger multicenter trial would resolve this issue. Second, the present study only enrolled STEMI patients, and it is unclear whether similar results would be obtained in non-STEMI or unstable AP patients. In addition, the influence of the amount of time from STEMI onset on the obtained results is unclear. Although it is possible that the severity of myocardial damages influences the occurrence of subsequent MACE, the lack of an association between well-known prognostic variables of STEMI, such as peak CK levels or the Killip classification, and the fc/m-PCSK9 ratio suggests that this ratio has an independent role. Introduction of more sensitive assay for myocardial damage such as troponin T or I might also provide more detailed results. Third, most patients enrolled in this study had been on statins the day after primary PCI, and thus the possibility that statins affected subsequent changes of PCSK9 levels in the acute phase could not be fully excluded. The beneficial effects of statin treatment after coronary events have been established, and therefore it is ethically difficult not to prescribe these drugs.
In conclusion, the fc/m-PCSK9 ratio in the circulation at 48 h after PCI in statin-naïve acute STEMI patients was associated with the occurrence of MACE within 3 years. Detailed evaluation of the molecular form of PCSK9 can reveal its potential utility with prognostic significance of atherosclerotic cardiovascular diseases.
Data availability
The data used to support the findings of the present study are available from the corresponding author upon request.
Funding sources
This work was supported by funding from The Vehicle Racing Commemorative Foundation (2013–2015), a Grant for Collaborative Research from Kanazawa Medical University (C2015-4), and Grants-in-Aid for Scientific Research (C) (18K08051 and 21K08139) from the Japan Society for the Promotion of Science awarded to Dr. Kouji Kajinami.
Authors contributions
Dr. Sawaguchi: principal investigator (overall study design, clinical data collection, data analysis and interpretation, and manuscript draft preparation).
Dr. Saeki: researcher (clinical data collection and interpretation).
Drs. Takamura, Kitayama, and Kawai: coronary interventionalists (performance of PCI procedures and patient management).
Drs. Oda, Fujibayashi, Wakasa, and Akao: staff cardiologists (support of PCI procedures, patient management, and data interpretation)
Dr Kajinami: Director (overall study design and manuscript finalization).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2022.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Urban D., Poss J., Bohm M., Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62:1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Glerup S., Schulz R., Laufs U., Schluter K.D. Physiological and therapeutic regulation of PCSK9 activity in cardiovascular disease. Basic Res Cardiol. 2017;112:32. doi: 10.1007/s00395-017-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C.Y., Tang Z.H., Jiang L., Li X.F., Jiang Z.S., et al. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 2012;359:347–358. doi: 10.1007/s11010-011-1028-6. [DOI] [PubMed] [Google Scholar]
- 4.Ding Z., Liu S., Wang X., Deng X., Fan Y., et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxidants Redox Signal. 2015;22:760–771. doi: 10.1089/ars.2014.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z., Jiang L., Peng J., Ren Z., Wei D., et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kB activation in THP-1-derived macrophages. Int J Mol Med. 2012;30:931–938. doi: 10.3892/ijmm.2012.1072. [DOI] [PubMed] [Google Scholar]
- 6.Dubuc G., Tremblay M., Paré G., JoséeHamelin H., Benjannet S., et al. A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res. 2010;51:140–149. doi: 10.1194/jlr.M900273-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis M., Marcinkiewicz J., Zaid A., Gauthier D., Poirier S., et al. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012;125:894–901. doi: 10.1161/CIRCULATIONAHA.111.057406. [DOI] [PubMed] [Google Scholar]
- 8.Benjannet S., Rhainds D., Hamelin J., Nassoury N., Seidah N.G. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J Biol Chem. 2006;281:30561–30572. doi: 10.1074/jbc.M606495200. [DOI] [PubMed] [Google Scholar]
- 9.Essalmani R., Susan-Resiga D., Chamberland A., Abifadel M., Creemers J.W., et al. In vivo evidence that furin from hepatocytes inactivates PCSK9. J Biol Chem. 2011;286:4257–4263. doi: 10.1074/jbc.M110.192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B., Eacho P.I., Knierman M.D., Troutt J.S., Konrad R.J., et al. Isolation and characterization of the circulating truncated form of PCSK9. J Lipid Res. 2014;55:1505–1514. doi: 10.1194/jlr.M049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philipp S., Heike M., Dietger S., Núbia B.P.S., Mattias R., et al. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation. 2005;31(21):2820–2827. doi: 10.1161/CIRCULATIONAHA.104.502617. 111. [DOI] [PubMed] [Google Scholar]
- 12.Hannu T., Emma R., Anna O., Niku O., Mari L., et al. Proprotein convertases in human atherosclerotic plaques: the overexpression of FURIN and its substrate cytokines BAFF and APRIL. Atherosclerosis. 2011;219(2):799–806. doi: 10.1016/j.atherosclerosis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Cariou B., Guerin P., Mayc C.L., Letocart V., Arnaud L., et al. Circulating PCSK9 levels in acute coronary syndrome: results from the PC-SCA-9 prospective study. Diabetes Metab. 2017;43:529–535. doi: 10.1016/j.diabet.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Ueland T., Kleveland O., Michelsen A.E., Wiseth R., Damås J.K., et al. Serum PCSK9 is modified by interleukin-6 receptor antagonism in patients with hypercholesterolaemia following non-ST-elevation myocardial infarction. Open Heart. 2018;5 doi: 10.1136/openhrt-2017-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baris G., Fabrizio M., David N., Federico C., Roland K., et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J. 2016;37:546–553. doi: 10.1093/eurheartj/ehv637. [DOI] [PubMed] [Google Scholar]
- 16.Nihar R.D., Robert P.G., Scott M.W., John P.G., Thomas L., et al. Association between circulating baseline proprotein convertase subtilisin kexin type 9 levels and efficacy of Evolocumab. JAMA Cardiol. 2017;2(5):556–560. doi: 10.1001/jamacardio.2016.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werf F.V., Bax J., Betriu A., Blomstrom-Lundqvist C., Crea F., et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 18.Hori M., Ishihara M., Yuasa Y., Makino H., Yanagi K., et al. Removal of plasma mature and furin-cleaved proprotein convertase subtilisin/kexin 9 by low-density lipoprotein-apheresis in familial hypercholesterolemia: development and application of a new assay for PCSK9. J Clin Endocrinol Metab. 2015;100:E41–E49. doi: 10.1210/jc.2014-3066. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura A., Kanazawa M., Kagaya Y., Kondo M., Sato K., et al. Plasma kinetics of mature PCSK9, furin-cleaved PCSK9, and Lp(a) with or without administration of PCSK9 inhibitors in acute myocardial infarction. J Cardiol. 2020;76:395–401. doi: 10.1016/j.jjcc.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu S.H., Huo Y.E., Jia X.W., Li Y. Effects of ischemic postconditioning on expressions of pentraxin-related protein 3 and neutrophil CD11b in the plasma of patients with acute myocardial infarction after percutaneous coronary intervention. Pakistan J Med Sci. 2016;32(2):427–430. doi: 10.12669/pjms.322.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X., Song L., Wang Y., Li J., Zhou J., et al. Proprotein convertase subtilisin/kexin type 9 and systemic inflammatory biomarker pentraxin 3 for risk stratification among STEMI patients undergoing primary PCI. J Inflamm Res. 2021 Oct 14;14:5319–5335. doi: 10.2147/JIR.S334246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y.M., Anderson T.J., Sikdar K., Fung M., McQueen M.J., et al. Association of proprotein convertase subtilisin/kexin type 9 (PCSK9) with cardiovascular risk in primary prevention. Arterioscler Thromb Vasc Biol. 2015;35:2254–2259. doi: 10.1161/ATVBAHA.115.306172. [DOI] [PubMed] [Google Scholar]
- 23.Leander K., Mälarstig A., Van’t Hooft F.M., Hyde C., Hellénius M.L., et al. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. 2016;133:1230–1239. doi: 10.1161/CIRCULATIONAHA.115.018531. [DOI] [PubMed] [Google Scholar]
- 24.Ridker P.M., Rifai N., Bradwin G., Rose L. Plasma proprotein convertase subtilisin/kexin type 9 levels and the risk of first cardiovascular events. Eur Heart J. 2016;37:554–560. doi: 10.1093/eurheartj/ehv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kataoka Y., Harada-Shiba M., Hori M., Watanabe M., Kokubo Y., et al. Circulating furin-cleaved proprotein convertase subtilisin/kexin type 9 concentration predicts future coronary Events in Japanese subjects. JACC (J Am Coll Cardiol): Asia. 2021 doi: 10.1016/j.jacasi.2021.09.003. (online before print) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of the present study are available from the corresponding author upon request.