Abstract
Background
Multifocal atherosclerosis has dramatically increased annual risk of adverse cardiovascular events than single artery affected. Triglyceride-rich lipoprotein (TRL) has been implicated in the early development of atherosclerosis.
However, evidence on the effect of remnant cholesterol, a major atherogenic component of TRL, on multifocal atherosclerosis in Chinese health asymptomatic subjects is insufficient. This study aimed to investigate the association of remnant cholesterol with intra- and extra-cranial atherosclerosis in Chinese population.
Methods
This study enrolled 3665 participants (median age 52.31 years) from the Asymptomatic Polyvascular Abnormalities Community study. Parameters of intra- and extra-cranial atherosclerosis includes intracranial artery stenosis (ICAS), carotid plaque, carotid artery stenosis (CAS), and carotid hypertrophy (intima-media thickness >0.9 mm). Logistic regression was used to assess these associations.
Results
The prevalence of vascular atherosclerosis significantly increased with increasing remnant cholesterol quartiles (P for trend <0.0001). In the multivariable-adjusted model, the odds ratio with 95% confidence interval comparing participants in Q4 versus Q1 of remnant cholesterol was 1.73 (1.29–2.31) for ICAS, 1.54 (1.22–1.94) for carotid plaque, 1.47 (1.17–1.84) for CAS, and 1.93 (1.48–2.52) for carotid hypertrophy, respectively. Furthermore, multivariable-adjusted spline regression showed S-shaped associations between remnant cholesterol and these outcomes.
Conclusion
Individuals with a high level of remnant cholesterol had a higher risk of intra- and extra-cranial atherosclerosis in Chinese population. Interventions aimed at reducing remnant cholesterol to prevent atherosclerotic diseases warrant further investigations.
Keywords: Remnant cholesterol, Intracranial atherosclerosis, Extracranial atherosclerosis, Plaque, Stenosis
Highlights
-
•
High remnant cholesterol was associated with high risk of intracranial atherosclerosis.
-
•
High remnant cholesterol was associated with high risk of extracranial atherosclerosis.
-
•
There was a S-shaped association of remnant cholesterol with intra- and extracraninal atherosclerosis.
-
•
Interventions aimed at reducing remnant cholesterol to prevent atherosclerotic diseases warrant further investigations.
Introduction
Atherosclerotic diseases occurring in extracranial carotid arteries and intracranial arteries are the major etiology of ischemic cerebrovascular events, such as stroke and transient ischemic attack [1]. As a systemic disease, atherosclerosis commonly involves multiple vascular beds simultaneously and the coexisting atherosclerosis in the intracranial and extracranial carotid arteries has been found to be prevalent in stroke patients [2,3]. It has been reported that the prevalence of multifocal atherosclerotic disease is approximately ranged from 16% to 40% [4,5]. Therefore, early detection and reduction of risk factors of atherosclerosis has significant implications in the prevention of cerebrovascular diseases.
Remnant cholesterol in triglyceride-rich lipoprotein (TRL) has been considered as the residual risk of atherosclerotic cardiovascular disease after lowering low-density lipoprotein cholesterol (LDL-C) to the recommended level [[6], [7], [8], [9], [10], [11], [12]]. Remnant cholesterol is defined as total cholesterol (TC) minus LDL-C minus high-density lipoprotein cholesterol (HDL-C) [6], which is composed of very-low-density lipoprotein and intermediate-density lipoprotein in the fasting state, and these two lipoproteins together with chylomicron remnants in the non-fasting state [13,14]. TRLs are heterogeneous and it is not likely that all species are atherogenic, since human cells can generally degrade triglycerides (TGs) but not cholesterol, accumulation in the arterial wall of the remnant cholesterol may play a causal role in atherosclerosis development [15]. Indeed, prior studies have linked remnant lipoproteins components to atherosclerosis, while these studies were mainly conducted in north European [[16], [17], [18]]. Limited studies have investigated the relationship between remnant cholesterol and early signs of multifocal atherosclerosis in Chinese healthy asymptomatic subjects. Therefore, we conducted the current study to assess the association of remnant cholesterol with intra- and extra-cranial atherosclerosis based on the Asymptomatic Polyvascular Abnormalities in Community (APAC) study in China.
Methods
Study population
The study subjects were recruited from the APAC study, which targets on the epidemiology of asymptomatic polyvascular abnormalities in Chinese adults. The details of the study design can be referred to previous descriptions [19,20]. A random sample of 5440 participants were included in the APAC study according to the following inclusion criteria: (1) aged ≥40 years; (2) no history of stroke, transient ischemic attack, neurological deficits, and coronary heart disease. Besides, 1775 participants with incomplete information on TC, LDL-C, HDL-C, or vascular examination results were further excluded. Finally, a total of 3665 participants were available in the current study. The study was performed according to the guidelines from the Helsinki Declaration and was approved by the Ethics Committees of the Kailuan General Hospital and the Beijing Tiantan Hospital. Written informed consent was obtained from all participants.
Laboratory measurements
Fasting blood samples were obtained from antecubital vein after an 8- to 12-h overnight fast in EDTA tubes. All the biochemical parameters were measured using Hitachi 747 auto-analyzer (Hitachi, Tokyo, Japan) in the central laboratory of Kailuan Hospital. Serum TC and TG were measured using the enzymatic colorimetric method, HDL-C and LDL-C were measured by direct test method with the inter-assay coefficient of variation <10% (Mind Bioengineering Co.Ltd., Shanghai, China). LDL-C was calculated by the Friedewald equation when TGs were ≤4 mmol/L, otherwise LDL-C was measured directly [21]. Remnant cholesterol was calculated as total cholesterol minus LDL-C minus HDL-C.
Assessment of intra- and extra-cranial atherosclerosis
Intracranial artery stenosis (ICAS) was assessed using trans-cranial Doppler Ultrasonography by two experienced neurologists who were blinded to the baseline information of all participants (EME Company, Nicolet, Madison, WI, USA). The definition of ICAS was according to the peak flow velocity criteria [22,23]: (1) >140 cm/s for the middle cerebral artery; (2) >120 cm/s for the anterior cerebral artery and internal carotid siphon; and (3) >100 cm/s for the posterior cerebral artery and vertebral-basilar. In addition to the above mentioned criteria, the patients’ age, the presence of turbulence sound or disturbance in echo frequency, and whether the abnormal velocity was segmental were also taken into account for ICAS diagnosis. Subjects without a good temporal window were considered without stenosis. ICAS was diagnosed if at least one of the studied arteries showed evidence of stenosis or occlusion.
Extracranial atherosclerosis was defined as any presence of carotid plaque, stenosis, and carotid hypertrophy. All participants underwent a bilateral carotid duplex ultrasound (Philips iU-22 ultrasound system, Philips Medical Systems, Bothell, WA) in the supine position of structures including the common carotid artery, internal carotid artery, external carotid artery, vertebral artery and subclavian artery. Both sides of carotid artery were assessed for the presence of carotid artery stenosis (CAS). Carotid plaque was demonstrated as a thickness of 1.5 mm from the intima–lumen interface to the media–adventitia interface, or defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding intima-media thickness (IMT) value. The initial measurement of carotid IMT was performed at the far wall of the CCA proximal to the bifurcation, along a plaque-free segment of ≥10 mm long at each side, with a quality index of ≥ 0.60. IMT was defined as the distance from the leading edge of the lumen-intima interface to the leading edge of the media-adventitia interface. IMT >0.9 mm was deemed as carotid hypertrophy [24].
Assessment of covariates
Information on demographic variables (age, sex, educational level, income), lifestyle (smoking status, drinking status, physical activity), and medical history (hypertension, diabetes, dyslipidemia, and medications on these diseases) were collected using standardized questionnaires. Biochemical indicators were assessed at Kailuan General Hospital (Hitachi, Tokyo, Japan), including fasting blood glucose (FBG), TC, TG, LDL-C, HDL-C, and high-sensitivity C-protein (hs-CRP).
Educational level was classified as illiteracy or primary school, middle school, and high school or above. Income was categorized into >3000 and ≤3000 yuan/month. Smoking was defined as at least 1 cigarette per day for more than 1 year. Drinking was defined as alcohol intake of at least 90 or 45 g of liquor per day for more than 1 year for men or women, respectively. Physical activity was classified as ≥4 times per week and ≥20 min at a time, <80 min per week, or none. BMI was calculated as the ratio of body weight (kg) divided by the square of body height (m2). Blood pressure was measured in the in the seated position using a mercury sphygmomanometer, and the average of three readings was calculated as systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Hypertension was defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg, any use of antihypertensive drugs, or a self-reported history of hypertension. Diabetes was defined as FBG levels ≥7.0 mmol/L, any use of glucose-lowing drugs, or any self-reported history of diabetes. Dyslipidemia was defined as any self-reported history or use of lipid-lowering drugs, or TC levels ≥5.17 mmol/L or TG levels ≥1.69 mmol/L or LDL-C levels ≥3.62 mmol/L or HDL-C level ≤1.04 mmol/L.
Statistical analyses
Participants were divided into 4 categories according to quartiles of remnant cholesterol. Continuous variables were described as median with interquartile range due to the skewed distribution, and were compared with Wilcoxon or Kruskal–Wallis test. Categorical variables were described as frequency with percentage, and were compared with chi-square test. Logistic regression models were constructed to assess the unadjusted and adjusted odds ratios (ORs) for the association of remnant cholesterol with intra- and extra-cranial atherosclerosis. In the multivariable analysis, we adjusted for age, sex, BMI, SBP, DBP, education, income, smoking status, drinking status, physical activity, history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive agents, antidiabetic agents, lipid-lowering agents, FBG, TC, HDL-C, and hs-CRP. P-values for trend were computed using quartiles of remnant cholesterol as ordinal variables. We also analyzed the effect of remnant cholesterol on the outcomes as a continuous variables using the restricted cubic spline with 5 knots (at the 5th, 25th, 50th, 75th, and 95th percentiles). Subgroup analyses were stratified by age (<60 years and ≥60 years), sex, history of hypertension, diabetes, and dyslipidemia (no and yes), where the interaction of remnant cholesterol with stratified variables was examined by likelihood ratio tests. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 3665 enrolled participants, the median age was 52.31 years (interquartile range: 45.63–61.94), the median concentration of remnant cholesterol was 0.50 mmol/L (interquartile range, 0.22–1.01). Baseline characteristics of enrolled participants according to quartiles of remnant cholesterol are presented in Table 1. Compared with participants in the Q1 group, those with a higher level of remnant cholesterol were more likely to be older, men, well educated, have a higher income, more current smokers, drinkers, active physical activity, a higher prevalence of hypertension, diabetes, dyslipidemia, more likely to take antihypertensive agents, antidiabetic agents, have a higher level of BMI, SBP, DBP, FBG, TC, TG, hs-CRP, and a lower level of LDL-C and HDL-C. Furthermore, the levels of remnant cholesterol were significantly higher in patients with intra- or extra-cranial atherosclerosis, as compared to their counterparts (P < 0.0001, Fig. 1).
Table 1.
Baseline characteristics of participants according to quartiles of remnant cholesterol.
| Characteristics | Remnant cholesterol, mmol/L |
P value | ||||
|---|---|---|---|---|---|---|
| Overall (N = 3665) | Q1 (<0.22) | Q2 (0.22–0.50) | Q3 (0.50–1.01) | Q4 (≥1.01) | ||
| Age, years | 52.31(45.63–61.94) | 50.87(45.13–57.64) | 52.50(45.77–60.48) | 53.05(45.83–64.74) | 53.37(46.17–67.11) | <0.0001 |
| Men, n (%) | 2387 (65.13) | 498 (54.25) | 563 (61.46) | 649 (71.08) | 677 (73.75) | <0.0001 |
| High school or above, n (%) | 1549 (42.26) | 374 (40.74) | 332 (36.24) | 412 (45.13) | 431 (46.95) | <0.0001 |
| Income >3000 RMB/m, n (%) | 412 (11.24) | 72 (7.84) | 76 (8.29) | 116 (12.71) | 148 (16.12) | <0.0001 |
| BMI, kg/m2 | 24.77(22.77–27.06) | 24.46(22.29–26.67) | 24.49(22.67–26.73) | 25.01(23.03–27.18) | 25.04(22.89–27.46) | <0.0001 |
| SBP, mm Hg | 130.00(120.00–143.33) | 130.00(120.00–140.00) | 130.67(120.00–146.67) | 130.00(120.00–146.33) | 130.00(120.00–143.67) | 0.0017 |
| DBP, mm Hg | 81.33(79.33–90.00) | 80.67(78.00–90.00) | 82.00(79.33–91.33) | 80.67(78.67–90.00) | 80.67(79.33–90.00) | 0.0005 |
| Current smoker, n (%) | 1222 (33.34) | 254 (27.67) | 298 (32.53) | 336 (36.80) | 334 (36.38) | 0.0001 |
| Current alcohol use, n (%) | 1254 (34.22) | 261 (28.43) | 282 (30.79) | 342 (37.46) | 369 (40.20) | <0.0001 |
| Active physical activity, n (%) | 1239 (33.81) | 278 (30.28) | 334 (36.46) | 325 (35.60) | 302 (32.90) | 0.0460 |
| Hypertension, n (%) | 927 (25.29) | 197 (21.46) | 216 (23.58) | 239 (26.18) | 275 (29.96) | 0.0002 |
| Diabetes Mellitus, n (%) | 281 (7.67) | 50 (5.45) | 63 (6.88) | 81 (8.87) | 87 (9.48) | 0.0040 |
| Dyslipidemia, n (%) | 329 (8.98) | 44 (4.79) | 70 (7.64) | 88 (9.64) | 127 (13.83) | <0.0001 |
| Antihypertensive agents, n (%) | 675 (18.42) | 141 (15.36) | 155 (16.92) | 178 (19.50) | 201 (21.90) | 0.0017 |
| Antidiabetic agents, n (%) | 217 (5.92) | 35 (3.81) | 50 (5.46) | 65 (7.12) | 67 (7.30) | 0.0043 |
| Lipid-lowering agents, n (%) | 35 (0.95) | 7 (0.76) | 8 (0.87) | 12 (1.31) | 8 (0.87) | 0.6285 |
| FBG, mmol/L | 5.29(4.86–5.88) | 5.10(4.75–5.64) | 5.27(4.90–5.82) | 5.36(4.90–6.00) | 5.40(4.91–6.00) | <0.0001 |
| TC, mmol/L | 4.90(4.30–5.58) | 4.30(3.90–4.77) | 4.79(4.27–5.30) | 5.00(4.51–5.66) | 5.61(4.98–6.35) | <0.0001 |
| TG, mmol/L | 1.27(0.94–1.83) | 1.16(0.85–1.52) | 1.08(0.81–1.48) | 1.39(1.06–2.01) | 1.63(1.10–2.42) | <0.0001 |
| HDL-C, mmol/L | 1.55(1.31–1.88) | 1.72(1.49–1.97) | 1.55(1.32–1.84) | 1.50(1.28–1.84) | 1.45(1.25–1.78) | <0.0001 |
| LDL-C, mmol/L | 2.70(2.27–3.13) | 2.73(2.35–3.07) | 2.81(2.40–3.27) | 2.70(2.30–3.26) | 2.50(1.91–2.96) | <0.0001 |
| Hs-CRP, mg/dL | 0.99(0.50–2.15) | 0.80(0.38–1.31) | 1.02(0.50–2.15) | 1.05(0.50–2.30) | 1.40(0.63–3.40) | <0.0001 |
Abbreviation: BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Fig. 1.
Comparing the differences in remnant cholesterol concentrations between the event-group and non-event group.
Abbreviations: ICAS, intracranial artery stenosis; CAS, carotid stenosis.
Association of remnant cholesterol with intra- and extra-cranial atherosclerosis
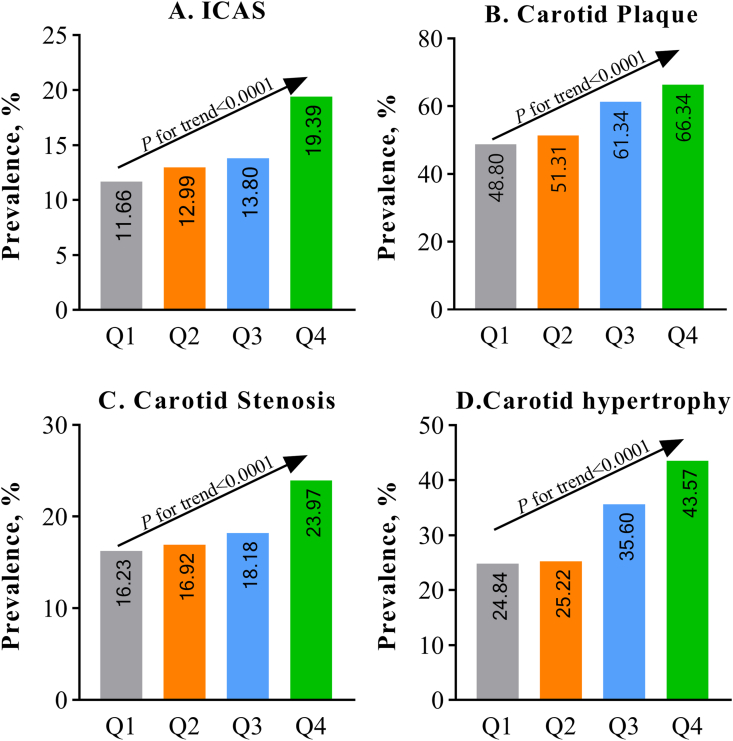
With increasing remnant cholesterol quartiles, the prevalence of ICAS, carotid plaque, CAS, and carotid hypertrophy increased significantly (P for trend <0.0001, Fig. 2). Without adjustment for confounders, as shown in Table 2, compared with the Q1 group, the Q4 group was associated with an increasing OR for ICAS (OR, 1.82; 95% confidence interval [CI], 1.41–2.36; P for trend<0.0001); carotid plaque (OR, 2.07; 95% CI, 1.71–2.50; P for trend<0.0001); CAS (OR, 1.97; 95% CI, 1.63–2.37; P for trend<0.0001); and carotid hypertrophy (OR, 2.34; 95% CI, 1.92–2.85; P for trend<0.0001). After adjusted for potential confounders, the associations between remnant cholesterol and the outcomes remained significant, the adjusted OR in the Q4 group versus the Q1 group was 1.61 (95% CI, 1.21–2.15) for ICAS, 1.60 (95% CI, 1.27–2.01) for carotid plaque, 1.51 (95% CI, 1.21–1.90) for CAS, and 1.73 (95% CI, 1.33–2.25) for carotid hypertrophy, respectively.
Fig. 2.
Prevalence of intra- and extra-cranial atherosclerosis stratified by quartiles of remnant cholesterol.
Abbreviations: ICAS, intracranial artery stenosis.
Table 2.
Odds ratio and 95% confidence interval for the association between remnant cholesterol and atherosclerosis.
| Outcomes | Remnant cholesterol, mmol/L |
P for trend | ||||
|---|---|---|---|---|---|---|
| Q1 (<0.22) | Q2 (0.22–0.50) | Q3 (0.50–1.01) | Q4 (≥1.01) | Per 1 mmol/L increase | ||
| ICAS | ||||||
| Unadjusted | Reference | 1.13(0.86–1.50) | 1.21(0.92–1.60) | 1.82(1.41–2.36) | 1.28(1.15–1.42) | <0.0001 |
| Adjusted | Reference | 1.04(0.77–1.40) | 1.08(0.81–1.46) | 1.61(1.21–2.15) | 1.23(1.10–1.38) | <0.0001 |
| Carotid plaque | ||||||
| Unadjusted | Reference | 1.11(0.92–1.33) | 1.66(1.38–2.00) | 2.07(1.71–2.50) | 1.32(1.21–1.43) | 0.0695 |
| Adjusted | Reference | 0.93(0.75–1.16) | 1.30(1.04–1.62) | 1.60(1.27–2.01) | 1.14(1.02–1.26) | <0.0001 |
| CAS | ||||||
| Unadjusted | Reference | 1.06(0.89–1.28) | 1.60(1.33–1.92) | 1.97(1.63–2.37) | 1.31(1.20–1.42) | 0.0295 |
| Adjusted | Reference | 0.88(0.71–1.09) | 1.24(1.00–1.54) | 1.51(1.21–1.90) | 1.14(1.04–1.26) | <0.0001 |
| Carotid hypertrophy | ||||||
| Unadjusted | Reference | 1.02(0.83–1.26) | 1.67(1.37–2.05) | 2.34(1.92–2.85) | 1.47(1.34–1.60) | <0.0001 |
| Adjusted | Reference | 0.79(0.60–1.04) | 1.12(0.86–1.46) | 1.73(1.33–2.25) | 1.30(1.16–1.44) | <0.0001 |
Abbreviations: CAS, carotid artery stenosis; ICAS, intracranial arterial stenosis.
Adjusted for age and sex, body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive agents, antidiabetic agents, lipid-lowering agents, fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, and high sensitivity C-reactive protein.
When remnant cholesterol was treated as a continuous variable, the adjusted OR for per 1 mmol/L increased of remnant cholesterol was 1.23 (95% CI, 1.10–1.38) for ICAS, 1.14 (95% CI, 1.02–1.26) for carotid plaque, 1.14 (1.04–1.26) for CAS, and 1.30 (95% CI, 1.16–1.44) for carotid hypertrophy. The multivariable-adjusted spline regression showed there were S-shaped association between remnant cholesterol and the outcomes (Fig. 3).
Fig. 3.
Multivariable-adjusted association of remnant cholesterol with intra- and extra-cranial atherosclerosis based on restricted cubic spines with 5 knots at 5th, 25th, 50th, 75th, and 95th percentiles of remnant cholesterol.
Abbreviations: ICAS, intracranial artery stenosis; CAS, carotid artery stenosis.
Adjusted for age and sex, body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive agents, antidiabetic agents, lipid-lowering agents, fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, and high sensitivity C-reactive protein.
Subgroup analyses
Age, sex and other selected risk factors were stratified for further analysis of the association between remnant cholesterol levels and the outcomes. The results showed that there was no significant interaction between remnant cholesterol and the subgroup factors (P for interaction >0.05; Fig. 4), indicating the association between remnant cholesterol and the outcomes were consistent across different subgroups.
Fig. 4.
Subgroup analyses for the association of remnant cholesterol with intra- and extra-cranial atherosclerosis.
Abbreviations: ICAS, intracranial artery stenosis; CAS, carotid artery stenosis
Adjusted for age and sex, body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive agents, antidiabetic agents, lipid-lowering agents, fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, and high sensitivity C-reactive protein other than variables for stratification.
Discussion
In this study, we found that remnant cholesterol was an independent risk factor of atherosclerosis. Step-wise higher remnant cholesterol concentrations were associated with step-wise higher risk of intra- and extra-cranial atherosclerosis parameters, including ICAS, carotid plaque, CAS, carotid hypertrophy among Chinese healthy asymptomatic adults in the APAC study.
TG and remnant cholesterol are highly correlated, and high TGs are a marker of high remnant cholesterol concentrations. Therefore, associations found for remnant cholesterol will be similar for TGs. High TGs have previously been shown to be play a causal role in the pathway of atherosclerosis [[25], [26], [27], [28], [29], [30]]. However, because TGs, but not cholesterol, can be readily metabolized by most cells of the body, TGs per se are unlikely to be causal. Importantly, few studies conducted among Chinese healthy asymptomatic have examined the role of remnant cholesterol in the development multifocal atherosclerosis, making the present findings novel.
ICAS is the result of atherosclerosis that affects large intracranial arteries, which was more common in Asian countries than that in Western nations [31]. The independent role of remnant cholesterol in the development of ICAS has been rarely investigated. Results from the Trial of cilOstazol in Symptomatic intracranial Stenosis 2 (TOSS-2) failed to show an independent relationship between remnant cholesterol and progression of symptomatic ICAS, while remnant cholesterol along with HDL-C was significantly associated with symptomatic ICAS [32]. Another cross-sectional study included 658 consecutive patients with ischemic stroke found that remnant cholesterol/HDL-C ratio showed a significant association with ICAS [33]. To address the knowledge limitation, we conducted the current study, our results showed that remnant cholesterol was significantly associated with ICAS, independently of TC, HDL-C and other cardiovascular risk factors. These findings indicated that early detection and treatment of high remnant cholesterol are necessary to delay the progression of ICAS and help prevent ischemic events.
Carotid artery stenosis, carotid plaque, as well as IMT, are important indices on assessing the extracranial atherosclerosis level of vascular. Increased IMT is a predictive marker for the atherosclerosis and is always associated with end-organ disease, carotid plaque is the marker of early stages of atherosclerosis and advanced arterial injury, which largely reflects the pathological process in the intima [5,34,35]. The association with remnant cholesterol and early signs of extracranial atherosclerosis has been investigated in several studies. Another study consisted of forty-five patients undergoing carotid endarterectomy provided evidence that TGL remnants significantly affect carotid plaque cellular composition, in particular macrophages content [18]. Another Stockholm study investigated the correlation of remnant-like particle cholesterol levels with subclinical atherosclerosis and demonstrated that remnant-like particle cholesterol was significantly related to increased carotid artery IMT in white middle-age men [17]. In line with these studies, our findings showed that remnant cholesterol was significantly associated with CAS, carotid plaque, and increased IMT, even after adjusting all the mentioned vascular risk factors, these relationships still exist significant. Extracranial atherosclerosis is a one of the most common contributors to ischemic stroke and worthy of attention, although the prevalence of extracranial atherosclerosis was lower in Asia than Caucasians [36]. Individuals with high remnant cholesterol warrant particular vigilance for the existence and development of atherosclerosis and the occurrence of stroke.
The plausible path-physiological mechanisms linking remnant cholesterol to atherosclerosis are summarized as follows. The components of remnant cholesterol includes very-low-density lipoprotein and intermediate-density lipoprotein, chylomicron remnants penetrate the arterial wall and appear to be selectively retained within the arterial intima, leading to accumulation of cholesterol, foam cell formation, and development of atherosclerotic plaque [37,38]. Low-grade inflammation caused by remnant cholesterol may be another reason for the association [15]. Remnant cholesterol could increase the expression of inflammatory protein levels of adhesion molecules and coagulation factors in endothelial cells, enhance the recruitment and attachment of monocytes, promote the formation of foam cells, ultimately resulting in atherosclerosis progression [39,40].
The current study has several limitations. First, this is a cross-sectional observational study rather than an interventional study, which limits what we can infer about the causality of the results. Second, we did not measure remnant cholesterol directly, the indirect calculation of remnant cholesterol might have overestimated its value in comparison to direct measurement. However, the calculated remnant cholesterol is an affordable and inexpensive method that could provide valuable data for clinical management, and it is therefore widely available [14,[41], [42], [43], [44]]. Finally, although we have adjusted for all the potential covariates, we cannot exclude the possibility of residual or unmeasured confounding, given the observational study design of the present analysis.
Conclusions
In conclusion, we found a positive association of remnant cholesterol with the risk of intra- and extra-cranial atherosclerosis in Chinese healthy asymptomatic adults. These results indicated that randomized clinical trials with remnant cholesterol lowering in individuals with high concentrations, with the aim of reducing atherosclerosis and preventing ischemic cerebrovascular diseases, are needed.
Funding
CAMS Fund for Medical Sciences (2019-I2M-5-029); Beijing Municipal Committee of Science and Technology (Z201100005620010); Beijing Natural Science Foundation (Z200016); National Natural Science Foundation of China (81870905, U20A20358); Beijing Municipal Science & Technology Commission (D171100003017002); Young Elite Scientists Sponsorship Program by CAST (2018QNRC001); Beijing Municipal Administration of Hospitals Incubating Program (PX2020021); Beijing Excellent Talents Training Program (2018000021469G234). Supported by: Research Unit of Artificial Intelligence in Cerebrovascular Disease, Chinese Academy of Medical Sciences, Beijing, China.
Declaration of competing interest
These authors declare that they have no conflicts of interests.
Acknowledgments
We thank all the participants of the APAC study for their invaluable contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2021.10.002.
Contributor Information
Yongjun Wang, Email: yongjunwang@ncrcnd.org.cn.
Xingquan Zhao, Email: zxq@vip.163.com.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wang Y., Zhao X., Liu L., Soo Y.O., Pu Y., Pan Y., et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–669. doi: 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- 2.Yang F., Liu L., Li M., Li M., Yin Q., Guo R., et al. Pattern of cerebrovascular atherosclerotic stenosis in older Chinese patients with stroke. J Clin Neurosci : official journal of the Neurosurgical Society of Australasia. 2013;20:979–983. doi: 10.1016/j.jocn.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Liu H.M., Tu Y.K., Yip P.K., Su C.T. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996;27:650–653. doi: 10.1161/01.str.27.4.650. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Friera L., Peñalvo J.L., Fernández-Ortiz A., Ibañez B., López-Melgar B., Laclaustra M., et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (progression of early subclinical atherosclerosis) study. Circulation. 2015;131:2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Wang A., Zhang S., Li N., Chen S., Zhang Y., et al. Asymptomatic polyvascular disease and the risks of cardiovascular events and all-cause death. Atherosclerosis. 2017;262:1–7. doi: 10.1016/j.atherosclerosis.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Langsted A., Madsen C.M., Nordestgaard B.G. Contribution of remnant cholesterol to cardiovascular risk. J Intern Med. 2020;288:116–127. doi: 10.1111/joim.13059. [DOI] [PubMed] [Google Scholar]
- 7.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. 2014. [DOI] [PubMed] [Google Scholar]
- 8.Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., et al. ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson T.A., Ito M.K., Maki K.C., Orringer C.E., Bays H.E., Jones P.H., et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. Journal of clinical lipidology. 2015;9:129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Chapman M.J., Ginsberg H.N., Amarenco P., Andreotti F., Borén J., Catapano A.L., et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruchart J.C., Sacks F., Hermans M.P., Assmann G., Brown W.V., Ceska R., et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1k–34k. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari R., Aguiar C., Alegria E., Bonadonna R.C., Cosentino F., Elisaf M., et al. Current practice in identifying and treating cardiovascular risk, with a focus on residual risk associated with atherogenic dyslipidaemia. Eur Heart J Suppl : J Eur Soc Cardiol. 2016;18:C2–c12. doi: 10.1093/eurheartj/suw009. [DOI] [PubMed] [Google Scholar]
- 13.Nordestgaard B., Benn M., Schnohr P., Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen A., Frikke-Schmidt R., West A., Grande P., Nordestgaard B., Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 15.Varbo A., Benn M., Tybjærg-Hansen A., Nordestgaard B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 16.Hodis H.N., Mack W.J., Dunn M., Liu C., Liu C., Selzer R.H., et al. Intermediate-density lipoproteins and progression of carotid arterial wall intima-media thickness. Circulation. 1997;95:2022–2026. doi: 10.1161/01.cir.95.8.2022. [DOI] [PubMed] [Google Scholar]
- 17.Karpe F., Boquist S., Tang R., Bond G.M., de Faire U., Hamsten A. Remnant lipoproteins are related to intima-media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res. 2001;42:17–21. [PubMed] [Google Scholar]
- 18.Zambon A., Puato M., Faggin E., Grego F., Rattazzi M., Pauletto P. Lipoprotein remnants and dense LDL are associated with features of unstable carotid plaque: a flag for non-HDL-C. Atherosclerosis. 2013;230:106–109. doi: 10.1016/j.atherosclerosis.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y., Li Y., Xu L., Xu J., Wang A., Gao X., et al. Asymptomatic polyvascular abnormalities in community (APAC) study in China: objectives, design and baseline characteristics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0084685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan C., Zhang Q., Zhang S., Wang A., Bi X., Chen S., et al. Association of newly found asymptomatic intracranial artery stenosis and ideal cardiovascular health metrics in Chinese community population. Sci Rep. 2020;10:7200. doi: 10.1038/s41598-020-63927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen M., Nordestgaard B. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet (London, England) 2020;396:1644–1652. doi: 10.1016/S0140-6736(20)32233-9. [DOI] [PubMed] [Google Scholar]
- 22.Meseguer E., Lavallée P.C., Mazighi M., Labreuche J., Cabrejo L., Olivot J.M., et al. Yield of systematic transcranial Doppler in patients with transient ischemic attack. Ann Neurol. 2010;68:9–17. doi: 10.1002/ana.21921. [DOI] [PubMed] [Google Scholar]
- 23.Wong K.S., Huang Y.N., Yang H.B., Gao S., Li H., Liu J.Y., et al. A door-to-door survey of intracranial atherosclerosis in Liangbei County, China. Neurology. 2007;68:2031–2034. doi: 10.1212/01.wnl.0000264426.63544.ee. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S., Yu S., Chi C., Fan X., Tang J., Ji H., et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95. doi: 10.1186/s12933-019-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batluk J., Leonards C.O., Grittner U., Lange K.S., Schreiber S.J., Endres M., et al. Triglycerides and carotid intima-media thickness in ischemic stroke patients. Atherosclerosis. 2015;243:186–191. doi: 10.1016/j.atherosclerosis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Hussain A., Ballantyne C.M., Saeed A., Virani S.S. Triglycerides and ASCVD risk reduction: recent insights and future directions. Curr Atherosclerosis Rep. 2020;22:25. doi: 10.1007/s11883-020-00846-8. [DOI] [PubMed] [Google Scholar]
- 27.Handelsman Y., Shapiro M.D. TRIGLYCERIDES, atherosclerosis, and cardiovascular outcome studies: FOCUS ON OMEGA-3 fatty acids. Endocr Pract Off J Am College Endocrinol Am Assoc Clin Endocrinol. 2017;23:100–112. doi: 10.4158/EP161445.RA. [DOI] [PubMed] [Google Scholar]
- 28.Dron J.S., Hegele R.A. Genetics of triglycerides and the risk of atherosclerosis. Curr Atherosclerosis Rep. 2017;19:31. doi: 10.1007/s11883-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Generoso G., Janovsky C., Bittencourt M.S. Triglycerides and triglyceride-rich lipoproteins in the development and progression of atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2019;26:109–116. doi: 10.1097/MED.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 30.Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. 2017;14:401–411. doi: 10.1038/nrcardio.2017.31. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Wang A., Wang J., Wu J., Wang D., Gao X., et al. Association between high-density-lipoprotein-cholesterol levels and the prevalence of asymptomatic intracranial arterial stenosis. Sci Rep. 2017;7:573. doi: 10.1038/s41598-017-00596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D., Kim J., Jeong S., Cho Y., Park J., Lee J., et al. Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study. Stroke. 2012;43:1824–1830. doi: 10.1161/STROKEAHA.112.653659. [DOI] [PubMed] [Google Scholar]
- 33.Yang W., Li R., Shen Y., Wang X., Liu Q., Wang H., et al. Importance of lipid ratios for predicting intracranial atherosclerotic stenosis. Lipids Health Dis. 2020;19:160. doi: 10.1186/s12944-020-01336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leng X.Y., Chen X.Y., Chook P., Xiong L., Lin W.H., Liu J.Y., et al. Correlation of large artery intracranial occlusive disease with carotid intima-media thickness and presence of carotid plaque. Stroke. 2013;44:68–72. doi: 10.1161/STROKEAHA.112.675652. [DOI] [PubMed] [Google Scholar]
- 35.Stein J.H., Korcarz C.E., Hurst R.T., Lonn E., Kendall C.B., Mohler E.R., et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. ; quiz 189-190. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.S., Nah H.W., Park S.M., Kim S.K., Cho K.H., Lee J., et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43:3313–3318. doi: 10.1161/STROKEAHA.112.658500. [DOI] [PubMed] [Google Scholar]
- 37.Proctor S.D., Vine D.F., Mamo J.C. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr Opin Lipidol. 2002;13:461–470. doi: 10.1097/00041433-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Tabas I., Williams K.J., Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 39.Khetarpal S.A., Rader D.J. Triglyceride-rich lipoproteins and coronary artery disease risk: new insights from human genetics. Arterioscler Thromb Vasc Biol. 2015;35:e3–9. doi: 10.1161/ATVBAHA.114.305172. [DOI] [PubMed] [Google Scholar]
- 40.Fujioka Y., Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atherosclerosis Thromb. 2009;16:145–154. doi: 10.5551/jat.e598. [DOI] [PubMed] [Google Scholar]
- 41.Varbo A., Nordestgaard B. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85:550–559. doi: 10.1002/ana.25432. [DOI] [PubMed] [Google Scholar]
- 42.Varbo A., Freiberg J., Nordestgaard B. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the copenhagen general population study. Clin Chem. 2018;64:219–230. doi: 10.1373/clinchem.2017.279463. [DOI] [PubMed] [Google Scholar]
- 43.Varbo A., Benn M., Tybjærg-Hansen A., Jørgensen A.B., Frikke-Schmidt R., Nordestgaard B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 44.Varbo A., Nordestgaard B. Remnant cholesterol and ischemic heart disease. Curr Opin Lipidol. 2014;25:266–273. doi: 10.1097/MOL.0000000000000093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.