Abstract
Background and aims
Preeclampsia (PE) is associated with life-long increased risk of cardiovascular disease. One of the main protective functions of high-density lipoprotein (HDL) is its role in reverse cholesterol transport. HDL-mediated cholesterol efflux capacity (CEC) is decreased during pregnancy in women with PE. Whether this persists postpartum is unknown.
Methods
Basal and transporter-specific CEC were determined 6 months postpartum in women who had a normotensive (n = 44) or a PE (n = 42) pregnancy. CEC was also measured in 23 normotensive and 20 PE women for whom samples were collected 24 months postpartum. Basal, ATP-binding cassette transporter-A1 (ABCA1)- and -G1 (ABCG1)-specific CEC were primarily determined using Chinese hamster ovary cells stably expressing human ABCA1 or ABCG1, and were also assessed using a J774 mouse macrophage cell line.
Results
ABCA1-specific CEC was significantly lower in women who had PE 6 months postpartum (0.57 ± 0.1 vs 0.53 ± 0.08; p < 0.05), whilst basal and ABCG1-specific efflux were not significantly different. cAMP-specific CEC in J774 cells was also lower 6 months after PE (0.85 ± 0.21 vs 0.75 ± 0.25, p < 0.05). Although apoA-I, apoE, plasminogen and PON-1 levels were not significantly different in women who had PE compared with controls, ABCA1 efflux did correlate with apoA-l, HDL-C and apoE levels after a normal, and with apoA-l and HDL-C levels after a PE pregnancy. ABCA1-specific efflux decreased in all women between 6 and 24 months postpartum, by 11 ± 1.6% in women who had a normotensive pregnancy and 9 ± 1.3% in women who had PE. After adjustment for apoA-I levels, there was no significant difference in ABCA1-specific efflux between the groups at 6 months postpartum and in normotensive women over time, but remained significantly different between 6 and 24 months in women who had PE.
Conclusions
ABCA1-mediated CEC is impaired 6 months postpartum after a PE pregnancy and decreases thereafter in both normotensive and PE pregnancies. ABCA1-mediated efflux is dynamic after pregnancy but is unlikely to explain the long-term increased CVD risk in women with PE.
Keywords: Preeclampsia, CVD, Cholesterol efflux capacity
Abbreviations: PE, preeclampsia; CEC, cholesterol efflux capacity; HDL, high-density lipoprotein; ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; apo, apolipoprotein; CVD, cardiovascular disease; RCT, reverse cholesterol transport; BMI, body mass index; PON1, paraoxonase 1; CHO, Chinese Hamster Ovary; LDL, Low-density lipoprotein
Highlights
-
•
Women with a history of preeclampsia display decreased HDL-mediated cholesterol efflux.
-
•
Impaired cholesterol efflux after preeclampsia is specific to the ABCA1-transporter.
-
•
HDL function is dynamic after pregnancy.
-
•
Impaired cholesterol efflux after preeclampsia does not explain life-long increased risk of CVD.
Introduction
Women with a history of preeclampsia (PE) have a long-term increased risk of cardiovascular disease (CVD) compared to women who had a normotensive pregnancy [[1], [2], [3]]. Meta-analysis and cohort studies indicate that this risk is increased 2-fold and is evident within 10 years after pregnancy, i.e. still at an early stage in life, interacts with established risk factors, and lasts a lifetime [1,[4], [5], [6], [7]]. Women who have experienced PE also show an increased incidence of hypertension, diabetes and dyslipidemia, all thought to contribute to the increased risk of CVD. Although it has been suggested that endothelial damage associated with PE may underly vascular disease, it is unclear whether PE independently increases cardiovascular risk or whether the unfavorable risk profile already exists in women who later develop PE.
The dyslipidemia of PE is similar to that of the metabolic syndrome with elevated triglycerides and low HDL-cholesterol (HDL-C) levels [[8], [9], [10]] although this is not observed in all populations. Some women with PE only show increased triglycerides, and others have similar lipid profiles to women with a normotensive pregnancy [11,12].
Recent studies have suggested that HDL function is a better predictor of CVD risk than HDL concentration [13]. The main protective function of HDL is believed to be its role in reverse cholesterol transport (RCT). In RCT, HDL removes cholesterol from macrophages within the arterial wall and delivers it to the liver for excretion via the bile and faeces, thereby inhibiting atherosclerotic plaque formation and progression. It is now recognized that the capacity of HDL to stimulate cholesterol efflux from macrophages is a strong predictor of CVD risk [14,15].
Cholesterol efflux from cholesterol-enriched macrophages is mediated by three processes: a) aqueous diffusion (“Basal efflux”), b) ATP-binding cassette transporter A1 (ABCA1)-mediated efflux, and c) ATP-binding cassette transporter G1 (ABCG1)-mediated efflux [16]. ABCA1-mediated transport functions most efficiently with small HDL species while the diffusion and ABCG1-mediated transport favour larger HDL particles [[17], [18], [19]]. One study has explored cholesterol efflux capacity (CEC) in relation to PE, examining total (whole serum) and HDL (apoB-depleted serum) CEC prior to delivery [20]. Total and HDL-mediated CEC were increased in women with PE whilst ABCA1-specific efflux was decreased, suggesting that pathways other than ABCA1 may play a part in HDL-mediated CEC in women with PE. Whether these observations persist long-term was not investigated.
In the present study we have investigated transporter-specific CEC in women after a pregnancy complicated by PE at 6 months and 24 months postpartum.
Patients and methods
Subjects
Women were selected from a larger cohort previously reported (The P4 study; [10]). All patients had anthropomorphic variables, clinical history, routine and 24hr blood pressure (BP) measures and routine biochemical analyses undertaken (Table 1, Table 3 and Supplementary Tables S1 and S2 and S3). Self-reported lifestyle and pharmacological data were obtained using questionnaires. None of the women included in this study were taking lipid-lowering medication such as statins or fibrates. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. All subjects were recruited with approval of the South Eastern Sydney Local Health District Human Research Ethics Committee (HREC ref no. 12/195 HREC/12/POWH/395), and written informed consent was obtained from all subjects. Based on sample size calculations (see below) CEC was determined 6 months postpartum in 44 women following a normotensive and 42 women following PE pregnancy (Group 1). Subgroups of 23 women who had a normotensive pregnancy and 20 women who had a PE pregnancy from whom blood samples had also been collected at 24 months postpartum were analysed for change between 6 and 24 months postpartum (Group 2).
Table 1.
Subject characteristics 6 months postpartum.
| Reference values | N (n = 44) | PE (n = 42) | p | |
|---|---|---|---|---|
| Age (yrs) | – | 32.4 ± 4.7 | 31.4 ± 4.8 | 0.29 |
| BMI | 18.5–24.9 | 23.5 [21.1–27.6] | 25.6 [22.1–28.2] | 0.11 |
| Cholesterol (mmol/L) | ≤5.5 | 4.7 ± 0.8 | 4.5 ± 0.9 | 0.35 |
| LDL-C (mmol/L) | ≤3.0 | 2.7 ± 0.8 | 2.6 ± 0.8 | 0.50 |
| Triglycerides (mmol/L) | ≤2.0 | 0.5 [0.4–0.8] | 0.7 [0.5.-1.0] | 0.08 |
| HDL-C (mmol/L) | ≥1.2 | 1.7 ± 0.4 | 1.5 ± 0.4 | 0.10 |
| Glucose (mmol/L) | 3.0–5.4 | 4.7 ± 0.4 | 4.8 ± 0.4 | 0.49 |
| Insulin (mU/L) | 2.6–24.9 | 5.2 [3.3–7.1] | 6.7 [4.3–9.5] | 0.06 |
| HbA1C (%) | ≥6.5 | 5.2 ± 0.4 | 5.2 ± 0.3 | 0.35 |
| HOMA score | n/a | 1.1 [0.8–1.5] | 1.1 [0.9–2.1] | 0.18 |
| Creatinine (μmol/L) | 45–90 | 64.7 ± 10.1 | 63.1 ± 10.2 | 0.47 |
| eGFR (mL/min) | ≥60 | 100 [100-100] | 100 [100-100] | 0.91 |
| Urate (mg/dL) | 0.14–0.34 | 0.27 [0.23–0.32] | 0.29 [0.25–0.32] | 0.14 |
| Urine albumin/creatinine (mg/g) | <3.5 | 0.0 [0–0.7] | 0.8 [0–2.2] | <0.001 |
| Haemoglobin (g/dL) | 115–165 | 132 ± 9 | 129 ± 11 | 0.17 |
| Plasminogena (μg/mL) | n/a | 62.5 ± 17.1 | 66.1 ± 19.9 | 0.16 |
| ApoA-I (g/L) | 1.05–2.05 | 1.5 ± 0.2 | 1.4 ± 0.2 | 0.07 |
| ApoEa (mg/dL) | n/a | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.19 |
| ApoE/HDL-Ca | n/a | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.58 |
| PON-1 (ng/mL)b | n/a | 3.4 [1.3–7.2] | 3.1 [2.4–4.1] | 0.97 |
N: normotensive pregnancy, PE: preeclampsia, BMI: body mass index, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, HbA1C: haemoglobin A1c, HOMA: homeostatic model assessment; eGFR: estimated glomerular filtration rate; apoA-I: apolipoprotein A-1; apoE: apolipoprotein E, PON-1: paraoxonase-1; n/a: not applicable. aplasminogen and apoE levels were determined in PEG-precipitated serum n = 30 for each group. bPON-1 levels were determined in PEG precipitated serum for all subjects. Data presented are Mean ± SD or median [first and third quartiles].
Table 3.
Subject characteristics 6 and 24 months.
| Reference values | N 6 months (n = 23) | N 24 months (n = 23) | PE 6 months (n = 20) | PE 24 months (n = 20) | |
|---|---|---|---|---|---|
| Age (yrs) | – | 33.1 ± 4.0 | 34.4 ± 4.0 | 31.5 ± 4.0 | 33.1 ± 3.9 |
| BMI | 18.5–24.9 | 24.2[22.3–28.5] | 24.9[21.6–28.3] | 25.6[21.2–28.3] | 24.7[20.9–28.4] |
| Cholesterol (mmol/L) | ≤5.5 | 4.8 ± 0.7 | 4.6 ± 0.8 | 4.8 ± 1.0 | 4.4 ± 0.8∗∗ |
| LDL-C (mmol/L) | ≤3.0 | 2.8 ± 0.8 | 2.7 ± 0.8 | 2.9 ± 0.9 | 2.5 ± 0.8∗∗ |
| Triglycerides (mmol/L) | ≤2.0 | 0.6[0.5–0.9] | 0.7[0.5–1.0] | 0.7[0.4–1.1] | 0.7[0.5–0.9] |
| HDL-C (mmol/L) | ≥1.2 | 1.7 ± 0.5 | 1.5 ± 0.4∗ | 1.6 ± 0.3 | 1.5 ± 0.3 |
| apoA-I (g/L) | 1.05–2.05 | 1.5 ± 0.3 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 |
| Glucose (mmol/L) | 3.0–5.4 | 4.7[4.3–5.0] | 4.7[4.5–5.0] | 4.7[4.4–4.9] | 4.6[4.3–4.9] |
| Insulin (mmol/L) | 2.6–24.9 | 5.8[3.3–7.5] | 7.0[5.2–8.7]∗ | 5.1[2.9–7.7] | 5.4[3.9–9.4] |
| HbA1C (%) | ≥6.5 | 5.1[4.9–5.4] | 5.2[4.9–5.4] | 5.2[5.0–5.4] | 5.1[5.0–5.4] |
| HOMA score | n/a | 1.2 [0.6–1.4] | 1.5[1.1–1.9]∗ | 1.0[0.9–2.4] | 1.1[0.8–2.0] |
| Creatinine (μmol/L) | 45–90 | 61.2 ± 7.4 | 63.5 ± 9.1 | 63.6 ± 10.2 | 61.3 ± 8.8 |
| eGFR (mL/min) | ≥60 | 100[100-100] | 100[100-100] | 100[100-100] | 100[100-100] |
| Urate (mg/dL) | 0.14–0.34 | 0.28 ± 0.1 | 0.28 ± 0.1 | 0.30 ± 0.1 | 0.27 ± 0.1 |
| Urine albumin/creatinine (mg/g) | <3.5 | 0[0–0.4] | 0[0–0.4] | 0.3[0–1.9] | 0.4[0–1.6] |
| Haemoglobin (g/dL) | 115–165 | 133 ± 9 | 131 ± 8 | 128 ± 7 | 130 ± 7 |
N: normotensive pregnancy, PE: preeclampsia, BMI: body mass index, LDL-C: low-density lipoprotein-cholesterol, HDL-C: high-density lipoprotein-cholesterol, apoA-I: apolipoprotein A-I, HbA1C: haemoglobin A1c. HOMA: homeostatic model assessment; eGFR: estimated glomerular filtration rate. All data is mean ± SD or median [first and third quartiles]. ∗p < 0.05, 6 months v 24 months, ∗∗p < 0.01, 6 months v 24 months; Wilcoxon test.
Laboratory analyses
Fasting peripheral blood samples were used for biochemical analysis. Serum glucose, cholesterol, triglycerides, HDL-C, urate and urine creatinine were analysed by autoanalyser on a Roche Cobas 8000. LDL cholesterol was calculated using the Friedewald equation [21]. Creatinine was analysed by the ID-MS traceable enzymatic method on the Roche Cobas 8000. Urine albumin and apoA-I (APOAT) were analysed by immunoturbidimetry. Insulin was measured by immunoassay on Roche Cobas e602. Glycated haemoglobin (HbA1c) was analysed by ion exchange HPLC on a BIO-RAD D-10. Estimated glomerular filtration rate (eGFR) was estimated with the CKD-EPI formula [22] and haemoglobin was measured on a Beckman DxH 800 analyser. The HOMA score was calculated from fasting insulin and glucose concentrations [23]. ApoE, plasminogen and paraoxonase 1 levels (PON1) in PEG precipitated sera were measured using an in house and commercial ELISAs from Abcam and Thermofisher, respectively.
Cholesterol efflux capacity (CEC)
ApoB-depletion from the serum was achieved as described previously [17,24]. Chinese hamster ovary (CHO) cells stably expressing tetracycline-inducible ABCA1 were used to determine ABCA1-specific cholesterol efflux [17,24]. ABCA1-dependent efflux was calculated as the difference in efflux rates between cells incubated with (ABCA1-expressing) and without tetracycline (basal efflux).
ABCG1-mediated efflux was determined using an ABCG1-CHO cell line which expresses human ABCG1 constitutively [25]. Efflux is compared to that from the parental CHO cell line, in which endogenous ABCG1 expression is not detectable. ABCG1-specific efflux was calculated as the difference in efflux rates between ABCG1-CHO cells and the non-transfected control parental cell line.
J774A.1 (ATCC TIB-67™) cells were maintained in DMEM containing 10% heat-inactivated foetal bovine serum. Cells were seeded into 24-well plates at 50 000 cells per well and left to adhere for 24 h. Cells were then incubated in complete medium containing 1μci/mL [3H]-cholesterol and 1 μM SZ58035 to inhibit acyl-coenzyme A:cholesterol acyltranferase (ACAT). After 24 h, cells were equilibrated for a further 24hr in serum-free medium containing 0.2% (w/v) BSA/ACAT inhibitor (SZ 58 035)/10 mM HEPES ± 0.3 mM cpt-cAMP (Sigma). Efflux was performed over 4 h in media containing ACAT inhibitor ± 0.3 mM cpt-cAMP. Total efflux is that measured from cells incubated with cAMP. cAMP-inducible efflux is the difference in efflux rates measured between cells incubated ± cAMP.
A standard reference serum was included on every plate to adjust for intra- and inter-assay variability. Intra-assay variability was 4.7%, 4.2%, 9.8%, 8.2%, 5.3% and 12.5% for basal-CHO, ABCA1-specific, ABCG1-specific, J774-basal, J774-total and J774-cAMP-specific efflux, respectively. Inter-assay variability was 12.0%, 4.1%, 16.3%, 7.9%, 15.3% and 26.9% for basal-CHO, ABCA1-specific, ABCG1-specific, J774-basal, J774-total and J774-cAMP-specific efflux, respectively. Efflux to individual patient samples was normalized to the efflux rate of this standard serum.
Statistical analysis
To determine the sample size required to detect a 10% difference in CEC, CEC (basal and ABCA1-specific) was measured in 10 normotensive women 6 months postpartum. Power calculations (error 0.05 and 80% power) indicated a group size of 37, and therefore a minimum of 40 normotensive and PE pregnancy samples were analysed.
Data were summarized as frequencies and percentages for categorical variables. Continuous variables were presented at mean ± SD or median [first and third quartiles] as appropriate. Comparison between previously normotensive and PE women was based on unpaired t-test (parametric distribution) or Mann-Whitney test (non-parametric distribution) as appropriate, and a paired t-test or Wilcoxon test was used for comparison between 6 and 24 months within each group. A two-tailed p value of <0.05 was used as a cut-off for statistical significance. Pearson or Spearman correlation coefficients were used as appropriate to assess associations between parameters. A general linear model was used to compare differences in CEC adjusted for apoA-I. All analyses were performed using Graphpad Prism 6 and SPSS.
Results
The subject characteristics of women 6 months postpartum who had a normotensive pregnancy and women who had a PE pregnancy are shown in Table 1. In this subcohort, women with PE were of same age, but, in contrast to the larger cohort [10], had a similar BMI and lipid profile to women with a normotensive pregnancy although there was a trend for higher triglycerides (p = 0.08) and plasma insulin levels (p = 0.06), and lower apoA-I levels (p = 0.07) in women who had had PE. It should be noted that BMI of women who had PE was slightly above the normal range (Table 1). These women also had higher systolic blood pressure (114 ± 13 vs. 106 ± 11 mmHg; p = 0.004), higher diastolic blood pressure (72 ± 8 vs. 65 ± 7; p < 0.001), gave birth earlier (37 ± 2 vs. 39 ± 1 weeks gestation, p < 0.0001), had more babies born preterm (31% vs. 2%, p < 0.001), and had smaller babies (2.7 ± 0.6 kg vs. 3.4 ± 0.5, p < 0.0001; Table S1), as observed in the larger cohort [10]. Lifestyle habits (smoking and exercise regime) were similar between the groups whilst more women with PE took medication to control blood pressure and a small percentage of women with a normotensive pregnancy were treated to prevent asthma and were taking fish oil (Table S1).
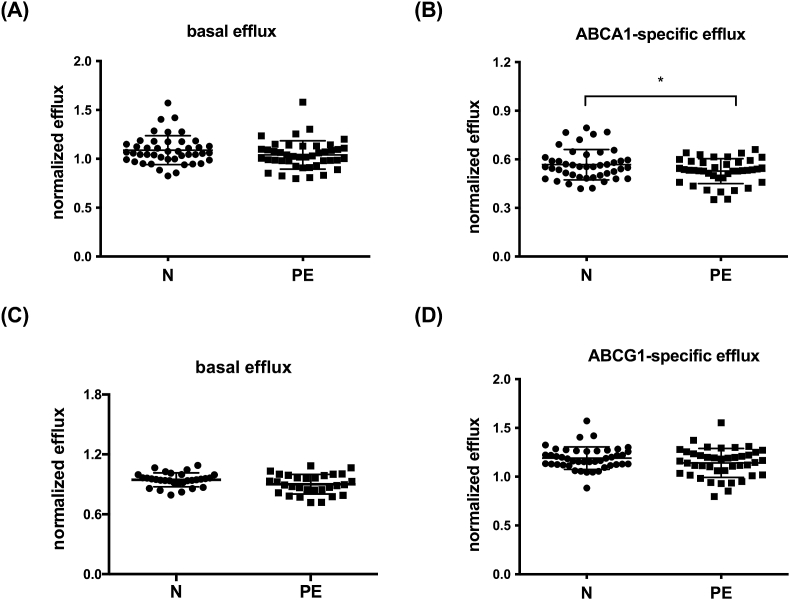
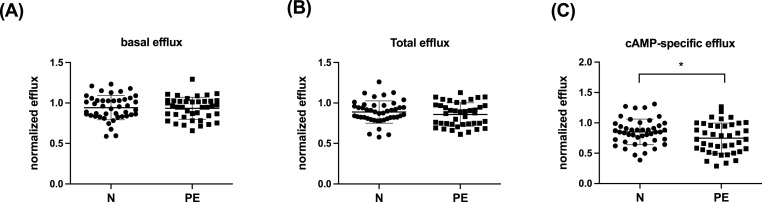
Circulating HDL-C levels at 6 months postpartum were similar (1.7 ± 0.4 vs. 1.5 ± 0.4 mmol/L, p = 0.10) in women who had a normotensive pregnancy and women who had PE (Table 1). Basal (Fig. 1A and C), and ABCG1-specific CEC (Fig. 1D) were also similar. ABCA1-specific cholesterol efflux however, was 7 ± 3% lower at 6 months postpartum (0.57 ± 0.09% vs 0.53 ± 0.08% p = 0.03) in women who had PE than in women who had a normotensive pregnancy (Fig. 1B). These findings were confirmed using J774A.1 macrophages, which use cAMP to induce ABCA1 expression [26] and demonstrated no difference in basal CEC but lower cAMP-specific efflux in women who had PE (0.85 ± 0.21 vs. 0.75 ± 0.25%, p = 0.04) (Fig. 2).
Fig. 1.
Six months after giving birth, ABCA1-specific efflux is impaired in women with a history of PE.3H-cholesterol efflux in women with a normotensive pregnancy (N; n = 45) and women with a PE pregnancy (PE; n = 42) was determined using ABCA1-or ABCG1-expressing CHO cells as described in Methods. Basal efflux (A and C) for both cell systems, ABCA1-specific efflux (B) and ABCG1-specific efflux (D). Data is mean ± SD. ∗p = 0.03. Note data in panels A and C represent basal data for panels B and D respectively.
Fig. 2.
Six months after giving birth, cAMP-specific efflux is impaired in women with a history of PE.3H-cholesterol efflux in women with a normotensive pregnancy (N; n = 45) and women with a PE pregnancy (PE; n = 42) was determined using J774A.1 as described in Methods. Basal efflux (A), total efflux (B) and cAMP-specific efflux. Data is mean ± SD. ∗p = 0.04.
As plasma plasminogen has been reported to contribute to ABCA1-mediated CEC [27], and some studies have investigated urinary plasmin/plasminogen as a marker or predictor of PE [28], plasminogen levels were determined in PEG sera used for CEC assays. Plasminogen levels did not significantly differ between women who had a normotensive pregnancy and women with PE (Table 1), nor did plasminogen levels correlate with ABCA1-specific efflux (r = 0.17; ns).
A previous study has linked increased maternal apoE concentrations to differences in CEC in women with PE [20], whilst others have suggested that apoE associated with HDL may inhibit ABCA1-specific efflux [29]. We therefore determined apoE levels in PEG-precipitated serum. We found that neither apoE levels nor apoE/HDL-C ratios differed significantly in women who had a normotensive pregnancy and women who had PE (Table 1).
ApoA-I levels were not different at between women who had a normotensive pregnancy compared to women who had PE (Table 1) but did correlate with ABCA1-specific efflux at 6 months within each group (Table 2). PEG-apoE levels correlated with ABCA1-specific efflux in women who had a normotensive pregnancy but not in women who had PE (Table 2).
Table 2.
Correlations between ABCA1-specific efflux and biochemical parameters 6 months postpartum.
| N |
PE |
|||
|---|---|---|---|---|
| coefficient | p | coefficient | p | |
| Age | 0.12 | 0.43 | −0.13 | 0.42 |
| BMI | −0.19 | 0.22 | −0.02 | 0.92 |
| Cholesterol | −0.03 | 0.82 | 0.19 | 0.24 |
| LDL-C | −0.08 | 0.63 | 0.14 | 0.38 |
| Triglycerides | −0.09 | 0.57 | −0.02 | 0.92 |
| HDL-C | 0.42 | 0.005 | 0.36 | 0.02 |
| Glucose | −0.23 | 0.12 | −0.20 | 0.21 |
| apoA-I | 0.54 | 0.0002 | 0.35 | 0.03 |
| Insulin | −0.26 | 0.08 | −0.18 | 0.27 |
| HbA1C | −0.05 | 0.76 | −0.01 | 0.94 |
| plasminogen | 0.03 | 0.87 | 0.31 | 0.10 |
| apoE | 0.42 | 0.02 | 0.22 | 0.24 |
| PON-1 | −0.12 | 0.46 | 0.05 | 0.77 |
N: normotensive pregnancy, PE: preeclampsia, BMI: body mass index, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, HbA1C: haemoglobin A1c; apoA-I: apolipoprotein A-1; apoE: apolipoprotein E, PON-1: paraoxonase-1; n/a: not applicable. Pearson and Spearman correlation coefficients are shown for parametric and non-parametric data, respectively.
Several studies indicate that increased oxidative stress, decreased antioxidant activity and endothelial dysfunction persist and are observed many years after giving birth in women who had a PE pregnancy [30,31]. Decreased anti-oxidant activity by PON-1, an HDL associated protein, has been observed in women with PE (38.39) and is associated with decreased CEC in women with diabetes [32]. We therefore investigated whether PON-1 was associated with reduced ABCA1-mediated cholesterol efflux in women who had PE. Levels of PON-1 were similar between women who had a normotensive pregnancy and women who had a pregnancy associated with PE 6 postpartum (Table 1) and did not correlate with ABCA1-specific CEC (Table 2).
In order to investigate whether ABCA1-specific CEC was dynamic after pregnancy, CEC was also determined at 24 months in a subgroup of women for whom both 6 and 24 months postpartum samples were available. Subject characteristics for these groups are shown in Tables 3, S2 and S3.
Although, HDL-C levels were similar between women with a previous normotensive pregnancy and women with previous PE at 6 and 24 months, they decreased in both groups over time (from 1.7 ± 0.5 to 1.5 ± 0.4 mmol/L, p = 0.02 in normotensive, and non-significantly from 1.6 ± 0.3 to 1.5 ± 0.3 mmol/L, p = 0.18 in the PE group) (Table 3). In contrast, apoA-I levels remained similar over time (1.5 ± 0.3 vs 1.5 ± 0.2 g/L, p = 0.44 in normotensive, and 1.5 ± 0.2 vs 1.5 ± 0.3 g/L, p = 0.83 in PE group). Insulin levels and HOMA score increased significantly between 6 and 24 months only in women who had a normotensive pregnancy (Table 3). Total plasma cholesterol and LDL-C levels were lower at 24 months compared to 6 months only in women who had PE (Table 3). Women who had a normotensive pregnancy were taking more supplements 6 months postpartum compared to women who had PE (Table S3). Over time, these women decreased their intake of supplements such as multivitamins and fish oil whilst women who had PE started to use supplements such as iron (Table S3). There were no differences between medication intake between the groups 6 months postpartum. At 24 months a few women who had PE were taking medication for asthma and allergies and compared to the group of normotensive women, more women in the PE group were on blood pressure medication and less women were taking oral contraceptives (Table S3).
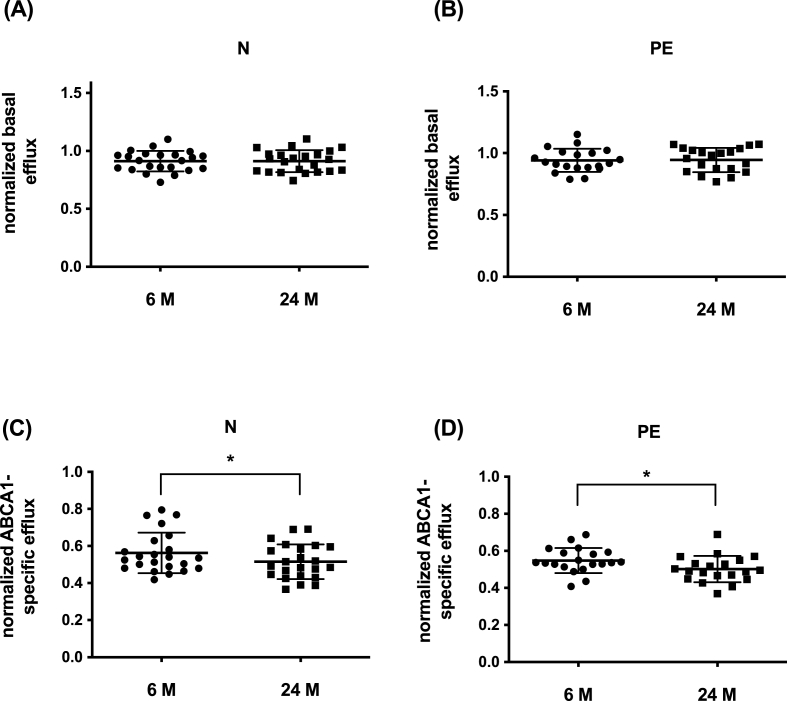
ABCA1-specific CEC decreased substantially between 6 and 24 months, by 11 ± 1.6% in women who had a previous normotensive pregnancy (p = 0.04), and by 9 ± 1.3% in women who had PE (p = 0.01) (Fig. 3). In this subgroup of 23 women, ABCA1-specific CEC was non-significantly different at 6 months (0.563 ± 0.11 vs 0.548 ± 0.07); p = 0.61), and was almost identical at 24 months (0.51 ± 0.09 vs 0.50 ± 0.07; p = 0.84). ABCA1-specific CEC showed moderate positive correlations at 6 and 24 months with HDL-C, which did not reach significance, and stronger correlations for apoA-I levels (Table 4). The change between 6 and 24 months in plasma HDL-C correlated significantly with the change in ABCA1-specific CEC in women with a normotensive pregnancy (r = 0.58; p = 0.004), and correlated more weakly and was not significant in women who had had PE (r = 0.37; p = 0.11). Basal efflux did not change over time in either group (Fig. 2) and was not different between the two groups (0.91 ± 0.09 vs 0.95 ± 1.0; p = 0.26).
Fig. 3.
Basal and ABCA1-specific efflux decreases over time in women who had a normotensive pregnancy and women who had a PE pregnancy.3H-cholesterol efflux in women who had a normotensive pregnancy (N; n = 23) and women who had a PE pregnancy (PE; n = 20) was determined using ABCA1-CHO cells as described in the method section. Basal (A and B) ABCA1-specific efflux (C and D) in women with a normotensive pregnancy (A and C) and women with PE (B and D). Data is mean ± SD. ∗p < 0.05, paired t-test.
Table 4.
Correlations between ABCA1-specific efflux and biochemical parameters (Group 2).
| N 6 months | N 24 months | PE 6 months | PE 24 months | |
|---|---|---|---|---|
| Age | 0.35 | 0.09 | −0.05 | −0.29 |
| BMI | 0.005 | −0.17 | 0.18 | −0.17 |
| Cholesterol | 0.33 | 0.34 | 0.35 | 0.55∗ |
| LDL-C | 0.07 | 0.10 | 0.20 | 0.34 |
| Triglycerides | −0.15 | 0.18 | 0.11 | 0.31 |
| HDL-C | 0.36 | 0.41 | 0.33 | 0.33 |
| ApoA-I | 0.43∗ | 0.45∗ | 0.42 | 0.69∗∗ |
| Glucose | −0.26 | −0.11 | 0.21 | 0.15 |
| Insulin | −0.50∗ | −0.17 | 0.13 | −0.15 |
| HbA1C | 0.29 | 0.16 | 0.04 | −0.09 |
N: normotensive pregnancy, PE: preeclampsia, BMI: body mass index, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, HbA1C: haemoglobin A1c; apoA-I: apolipoprotein A-1. Pearson and Spearman correlation coefficients are shown for parametric and non-parametric data, respectively. ∗p < 0.05, ∗∗p < 0.01.
After adjustment for apoA-I levels, ABCA1-specific CEC at 6 months postpartum remained lower in women who had PE, however the difference no longer reached statistical significance (0.56 ± 0.01 v 0.54 ± 0.01 p = 0.15). The comparison between ABCA1-specific CEC at 6 and 24 months after adjustment for apoA-I was 0.56 ± 0.02 v 0.52 ± 0.02 (p = 0.15) for women with a normotensive pregnancy and 0.55 ± 0.01 v 0.50 ± 0.01 p = 0.03 for women with a PE pregnancy. These results suggest that postpartum ABCA1-mediated CEC is at least in part related to apoA-I levels, especially in women who had a normotensive pregnancy. In women who had PE, other factors contribute to changes in postpartum ABCA1-specific efflux.
Discussion
Preeclampsia markedly increases the risk of cardiovascular disease later in life. Recently, it was shown that ABCA1-specific cholesterol efflux was decreased during pregnancy in women with PE [20], however whether this persists after pregnancy is unknown. We have identified that ABCA1-specific efflux, but not ABCG1-specific efflux, is impaired in women with PE 6 months after having given birth and decreases between 6 and 24 months postpartum in women who have had normotensive and PE pregnancies. These findings clarify that impaired HDL functionality in PE is specific to the ABCA1 transporter, persists for at least 6 months postpartum, and is dynamic.
Although pregnancy is associated with profound changes in lipoprotein levels and changes in LDL and HDL subclasses [33,34], very few studies have investigated these changes over time during and after birth, especially comparing PE pregnancies versus normotensive pregnancies. Women who had a PE pregnancy generally show higher BMI, elevated circulating cholesterol, triglycerides, LDL-C levels, low HDL-C and higher apoB/apoA-I ratios years after giving birth, though this is not observed in all studies [35]. The differences in reported findings are likely related to differences in prepregnancy BMI as most of the differences in lipids are attenuated when prepregnancy BMI is taken into account [9].
In our study, women who had had PE had similar BMI, triglycerides, total cholesterol, LDL-C and HDL-C levels to women who had a normotensive pregnancy, though these similarities may have reflected a type 2 error as differences existed in the larger cohort of the P4 study [10]. Although HDL-C levels were similar between the two groups, they decreased over time in both groups (from 1.7 ± 0.5 to 1.5 ± 0.4 mmol/L, p = 0.02 in normotensive, and non-significantly from 1.6 ± 0.3 to 1.5 ± 0.3 mmol/L, p = 0.18 in PE group), whilst apoA-I levels remained similar in both groups over time suggesting changes in HDL particle composition in women who had a normotensive pregnancy. ABCA1-specific efflux was impaired in women with previous PE at 6 months postpartum, extending previous findings by Mistry et al. [20], who observed impaired ABCA1-mediated efflux just before giving birth. ABCA1-mediated efflux decreased in all women between 6 and 24 months, and there was a correlation with the change in plasma HDL-C especially in women with a normotensive pregnancy, suggesting that changes in HDL levels may have contributed to the decrease in CEC. As HDL-C levels were similar between formerly normotensive and PE women at 6 months postpartum, the differences in ABCA1-efflux between these groups suggest HDL functional impairment in women with PE. Detailed analysis of HDL particle subpopulation over time after normotensive and PE pregnancies would be warranted for future studies.
We and others have shown that HDL size is a key determinant of ABCA1-mediated efflux in other patients groups [19,24]. Whether decreased ABCA1-mediated efflux in women with PE relates to changes in HDL composition or size is unclear. ABCA1-mediated cholesterol efflux is mainly driven by small lipid-poor preβ-HDL particles [36] which are formed in the initial steps in HDL synthesis and during remodelling of mature HDL particles by enzymes such as hepatic lipase (HL), lipoprotein lipase (LPL) and cholesteryl ester transfer protein (CETP). Lipid-poor preβ-HDL particles also accumulate under conditions of lecithin:cholesterol acyl-transferase (LCAT) deficiency due to impaired HDL maturation [37]. There are few studies investigating HDL subpopulations or remodelling enzymes in pregnancy. CETP activity is dynamic during pregnancy being highest during the second semester, decreasing during the third semester and reaching normal levels a few weeks after giving birth [[38], [39], [40]]. Lipase activity decreases during the second and third semester returning to normal levels postpartum [40] whilst LCAT activity increases in the third semester [41]. Additional studies show decreased LCAT activity within 48 h after birth in women with PE [42]. These studies also indicate a shift to larger HDL2 particles during pregnancy and a subsequent shift to smaller HDL3 particles during late pregnancy and within a few weeks of giving birth [34,39,40]. Alterations in HDL size, particular that of preβ-HDL have not been explored long-term after pregnancy nor in women with a pregnancy associated with PE. In the only study to have reported long term lipoprotein particle changes after pregnancy, PE was associated with increased large HDL particle concentrations up to 9 years after delivery [43]. However, since this analysis also included women with type 1 diabetes the effect of PE specifically on particle changes and levels of preβ-HDL remains unknown.
Our data using a CHO-ABCA1 overexpression system were confirmed using mouse J774A.1 macrophages. Previous studies, using this system, have linked total CEC (basal + cAMP-stimulated) with increased risk of CVD [44,45]. Basal and total CEC were similar between women who had a normotensive pregnancy and women who had PE 6 months postpartum, whilst cAMP-specific efflux was decreased in women with previous PE in line with our data using ABCA1-CHO cells. Basal efflux using J774A.1 cells correlated moderately with basal efflux using CHO cells (r = 0.56; p < 0.0001) and cAMP-specific efflux correlated with ABCA1-specific efflux (r = 0.41; p < 0.0001) indicating that cAMP-specific efflux is only partly explained by ABCA1-mediated efflux. These results point to a specific impairment of ABCA1-mediated efflux in women who had a PE pregnancy which is most likely not related to an increased risk of cardiovascular disease.
We investigated whether plasminogen or apoE might modulate ABCA1 efflux in women with PE. Previous studies have indicated that plasma plasminogen (non-HDL bound) is a strong mediator of ABCA1-specific efflux, and more recently Sacks et al. [46] reported that HDL subspecies containing plasminogen are associated with an increased risk of coronary artery disease. Very early reports observed decreased plasma levels of plasminogen in women with PE [47], however more recent studies have found that urine albumin has a stronger association with PE than does urinary plasmin/plasminogen [28]. We determined total plasminogen levels in PEG-precipitated serum used for CEC analysis, which included HDL-bound as well as non-HDL bound plasminogen, and found no difference between women with a normal and PE pregnancy (Table 1). A previous study has linked increased maternal apoE concentrations to differences in CEC in women with PE [20], whilst others have suggested that apoE associated with HDL may inhibit ABCA1-specific efflux [29]. In our cohort, apoE levels and apoE/HDL-C ratios in PEG-precipitated serum were similar between women who had a normotensive pregnancy and women with previous PE (Table 1). PEG-apoE levels, however were positively correlated with ABCA1-specific efflux in women who had a normotensive pregnancy but not in women who had PE pointing to differences in apoE distribution over HDL subclasses between the two groups. Taken together, these results suggest that impaired ABCA1-specific efflux in women with a history of PE is unlikely to be explained by differences in plasminogen or apoE concentrations.
PE is associated with increased oxidative stress and vascular endothelial dysfunction [31,48,49]. Recent studies indicate that HDL isolated from women with PE during pregnancy and just before birth exhibits oxidative damage to HDL-bound lipids and proteins, decreased HDL-associated antioxidant activity and decreased HDL anti-inflammatory capacity [50,51]. These differences have been related to changes in HDL protein composition especially that of the antioxidant activity by PON-1, sphingosine 1-phosphate (S1P) and apolipoprotein M (apoM). Interestingly, low levels of PON-1 have been associated with low cholesterol efflux in patients with diabetes [32], a known predisposing factor for PE. Several studies indicate that increased oxidative stress, decreased antioxidant activity and endothelial dysfunction persist and are observed many years after giving birth in women who had a PE pregnancy [30,31]. We investigated PON-1 levels and found that they were similar between the two groups 6 months (Table 1) and 24 months (data not shown) postpartum and were not related to differences in ABCA1-specific efflux.
In summary, we have found that ABCA1-mediated cholesterol efflux is impaired 6 months after pregnancy in women who had pre-eclampsia, and that ABCA1 efflux declines in both normotensive and PE women between 6 and 24 months after pregnancy. It is unlikely that impaired CEC is sustained or that this contributes to the long term cardiovascular sequelae of PE. The structural basis of the dynamic changes to HDL function during and after pregnancy warrants further investigation.
Strengths and limitations
In this study, CEC was determined in women with a history of PE, who were well matched to women with a previous normotensive pregnancy. Since this is an observational study, only associations between biochemical parameters and impaired CEC can be determined. We were unable to determine efflux capacity in our cohort prior to delivery and can therefore not confirm whether CEC was impaired in our cohort of PE women as published previously [20]. We investigated known determinants of CEC and our data indicated that decreased CEC in women with previous PE was in part related to differences in apoA-I levels and not related to plasminogen, apoE or PON-1 levels. A full extensive characterization of HDL was not feasible and could not be included. Whether differences in HDL proteome or lipidome may have contributed to reduced CEC in women who have had PE therefore remains unclear. It should be noted that apoE levels were determined in PEG precipitated serum and may include non-HDL bound apoE and that PON-1 levels do not necessarily reflect PON-1 activity. A final limitation of this study is the smaller numbers of women than in the overall cohort (8) which may have reduced the ability to find significant differences, e.g. we did not observe a metabolic syndrome like dyslipidemia in the women with previous PE. Similarly, we did not observe a difference in ABCA1-specific efflux in our subgroup of women who were studied 6 and 24 months postpartum, although a trend to decreased ABCA1-specific CEC was observed at 6 months. As our initial power calculations indicated the requirement for around 37 subjects per group to detect a 10% difference, the small sample size of this group may have precluded our ability to observe significant differences between the groups. Our evaluation at 6 months was therefore restricted to the larger cohort (n = 42–44). Paired analysis over time was robust and showed decreased ABCA1-specific efflux in both groups.
Funding
This study was funded by a New South Wales Health Cardiovascular Disease Grant to LK (2019–2021) and the P4 study was supported by The St George and Sutherland Medical Research Foundation and by a philanthropic donation from Emeritus Professor Richard Henry. There was no involvement of the funder in conducting the research or writing the paper.
Author contributions
MK: performed all experiments and analysis. Wrote manuscript.
LR: Conducted the P4 study, collected samples, critically reviewed the manuscript.
JW: assisted with J774A.1 efflux experiments and PON1 measurement, critically reviewed the manuscript.
CT: performed apoA-I measurements and critically reviewed the manuscript.
MAB: Designed and conducted the P4 study, critically reviewed the manuscript.
LK: Supervised the study, supervised the analysis, co-wrote manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Ms Tania Tsatralis for assistance with CEC experiments and Prof David Sullivan, from the department of Chemical Pathology at Royal Prince Alfred Hospital, Sydney for assistance with apoA-I analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2022.01.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bellamy L., Casas J.P., Hingorani A.D., et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dall'Asta A., D'Antonio F., Saccone G., et al. Cardiovascular events following pregnancy complicated by pre-eclampsia with emphasis on comparison between early- and late-onset forms: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:698–709. doi: 10.1002/uog.22107. [DOI] [PubMed] [Google Scholar]
- 3.Leon L.J., McCarthy F.P., Direk K., et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic Health records: a caliber study. Circulation. 2019;140:1050–1060. doi: 10.1161/CIRCULATIONAHA.118.038080. [DOI] [PubMed] [Google Scholar]
- 4.Smith G.N., Pudwell J., Walker M., et al. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. 2012;34:830–835. doi: 10.1016/S1701-2163(16)35381-6. [DOI] [PubMed] [Google Scholar]
- 5.Garovic V.D., White W.M., Vaughan L., et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–2334. doi: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnott C., Nelson M., Alfaro Ramirez M., et al. Maternal cardiovascular risk after hypertensive disorder of pregnancy. Heart. 2020;106:1927–1933. doi: 10.1136/heartjnl-2020-316541. [DOI] [PubMed] [Google Scholar]
- 7.Riise H.K.R., Sulo G., Tell G.S., et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol. 2019;282:81–87. doi: 10.1016/j.ijcard.2019.01.097. [DOI] [PubMed] [Google Scholar]
- 8.Stekkinger E., Zandstra M., Peeters L.L.H., et al. Early-onset preeclampsia and the prevalence of postpartum metabolic syndrome. Obstet Gynecol. 2009;114:1076–1084. doi: 10.1097/AOG.0b013e3181b7b242. [DOI] [PubMed] [Google Scholar]
- 9.Sattar N., Bendomir A., Berry C., et al. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol. 1997;89:403–408. doi: 10.1016/S0029-7844(96)00514-5. [DOI] [PubMed] [Google Scholar]
- 10.Brown M.A., Roberts L., Hoffman A., et al. Recognizing cardiovascular risk after preeclampsia: the P4 study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benschop L., Bergen N.E., Schalekamp-Timmermans S., et al. Maternal lipid profile 6 years after a gestational hypertensive disorder. J Clin Lipidol. 2018;12:428–436. doi: 10.1016/j.jacl.2017.12.010. e424. [DOI] [PubMed] [Google Scholar]
- 12.Mangos G.J., Spaan J.J., Pirabhahar S., et al. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. 2012;30:351–358. doi: 10.1097/HJH.0b013e32834e5ac7. [DOI] [PubMed] [Google Scholar]
- 13.Tall A.R., Rader D.J. Trials and tribulations of CETP inhibitors. Circ Res. 2018;122:106–112. doi: 10.1161/CIRCRESAHA.117.311978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A., Khera A., Berry J.D., et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera A.V., Cuchel M., de la Llera-Moya M., et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips M.C. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X.M., Kim M.J., Hou L., et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 18.Anastasius M., Luquain-Costaz C., Kockx M., et al. A critical appraisal of the measurement of serum 'cholesterol efflux capacity' and its use as surrogate marker of risk of cardiovascular disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1257–1273. doi: 10.1016/j.bbalip.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 19.He Y., Ronsein G.E., Tang C., et al. Diabetes impairs cellular cholesterol efflux from ABCA1 to small HDL particles. Circ Res. 2020;127:1198–1210. doi: 10.1161/CIRCRESAHA.120.317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mistry H.D., Kurlak L.O., Mansour Y.T., et al. Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in preeclampsia. J Lipid Res. 2017;58:1186–1195. doi: 10.1194/jlr.M071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Johnson D.W., Jones G.R., Mathew T.H., et al. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: new developments and revised recommendations. Med J Aust. 2012;197:224–225. doi: 10.5694/mja11.11329. [DOI] [PubMed] [Google Scholar]
- 23.Matthews D.R., Hosker J.P., Rudenski A.S., et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Luquain-Costaz C., Kockx M., Anastasius M., et al. Increased ABCA1 (ATP-Binding cassette transporter A1)-specific cholesterol efflux capacity in Schizophrenia. Arterioscler Thromb Vasc Biol. 2020;40:2728–2737. doi: 10.1161/ATVBAHA.120.314847. [DOI] [PubMed] [Google Scholar]
- 25.Larrede S., Quinn C.M., Jessup W., et al. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol. 2009;29:1930–1936. doi: 10.1161/ATVBAHA.109.194548. [DOI] [PubMed] [Google Scholar]
- 26.de la Llera-Moya M., Drazul-Schrader D., Asztalos B.F., et al. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pamir N., Hutchins P.M., Ronsein G.E., et al. Plasminogen promotes cholesterol efflux by the ABCA1 pathway. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen L.H., Kronborg C., Vittinghus E., et al. Is urinary excretion of plasminogen associated with development of pre-eclampsia? An observational, explorative case-control study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pamir N., Hutchins P., Ronsein G., et al. Proteomic analysis of HDL from inbred mouse strains implicates APOE associated with HDL in reduced cholesterol efflux capacity via the ABCA1 pathway. J Lipid Res. 2016;57:246–257. doi: 10.1194/jlr.M063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy M.S., Vignarajah M., Smith G.N. Increased microvascular vasodilation and cardiovascular risk following a pre-eclamptic pregnancy. Phys Rep. 2014;2 doi: 10.14814/phy2.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barr L.C., Pudwell J., Smith G.N. Postpartum microvascular functional alterations following severe preeclampsia. Am J Physiol Heart Circ Physiol. 2021;320:H1393–H1402. doi: 10.1152/ajpheart.00767.2020. [DOI] [PubMed] [Google Scholar]
- 32.Murakami H., Tanabe J., Tamasawa N., et al. Reduction of paraoxonase-1 activity may contribute the qualitative impairment of HDL particles in patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;99:30–38. doi: 10.1016/j.diabres.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 33.van Stiphout W.A., Hofman A., de Bruijn A.M. Serum lipids in young women before, during, and after pregnancy. Am J Epidemiol. 1987;126:922–928. doi: 10.1093/oxfordjournals.aje.a114729. [DOI] [PubMed] [Google Scholar]
- 34.Zeljkovic A., Vekic J., Spasic S., et al. Changes in LDL and HDL subclasses in normal pregnancy and associations with birth weight, birth length and head circumference. Matern Child Health J. 2013;17:556–565. doi: 10.1007/s10995-012-1031-x. [DOI] [PubMed] [Google Scholar]
- 35.Akhter T., Larsson A., Larsson M., et al. Sub-clinical atherosclerosis in the common carotid artery in women with/without previous pre-eclampsia: a seven-year follow-up. Atherosclerosis. 2019;290:206–213. doi: 10.1016/j.atherosclerosis.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Favari E., Calabresi L., Adorni M.P., et al. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48:11067–11074. doi: 10.1021/bi901564g. [DOI] [PubMed] [Google Scholar]
- 37.Rye K.A., Barter P.J. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24:421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 38.Iglesias A., Montelongo A., Herrera E., et al. Changes in cholesteryl ester transfer protein activity during normal gestation and postpartum. Clin Biochem. 1994;27:63–68. doi: 10.1016/0009-9120(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 39.Silliman K., Tall A.R., Kretchmer N., et al. Unusual high-density lipoprotein subclass distribution during late pregnancy. Metabolism. 1993;42:1592–1599. doi: 10.1016/0026-0495(93)90156-i. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez J.J., Montelongo A., Iglesias A., et al. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996;37:299–308. [PubMed] [Google Scholar]
- 41.Wallentin L., Fahraeus L. Cholesterol esterification rate and its relation to lipoprotein levels in plasma in normal human pregnancy. J Lab Clin Med. 1986;107:216–220. [PubMed] [Google Scholar]
- 42.Loukidi-Bouchenak B., Lamri-Senhadji M.Y., Merzouk S., et al. Serum lecithin: cholesterol acyltransferase activity, HDL2 and HDL3 composition in hypertensive mothers and their small for gestational age newborns. Eur J Pediatr. 2008;167:525–532. doi: 10.1007/s00431-007-0545-5. [DOI] [PubMed] [Google Scholar]
- 43.Amor A.J., Vinagre I., Valverde M., et al. Nuclear magnetic resonance lipoproteins are associated with carotid atherosclerosis in type 1 diabetes and pre-eclampsia. Diabetes Metab Res Rev. 2021;37 doi: 10.1002/dmrr.3362. [DOI] [PubMed] [Google Scholar]
- 44.Hunjadi M., Lamina C., Kahler P., et al. HDL cholesterol efflux capacity is inversely associated with subclinical cardiovascular risk markers in young adults: the cardiovascular risk in Young Finns study. Sci Rep. 2020;10:19223. doi: 10.1038/s41598-020-76146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mody P., Joshi P.H., Khera A., et al. Beyond coronary calcification, family history, and C-reactive protein: cholesterol efflux capacity and cardiovascular risk prediction. J Am Coll Cardiol. 2016;67:2480–2487. doi: 10.1016/j.jacc.2016.03.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacks F.M., Liang L., Furtado J.D., et al. Protein-defined subspecies of HDLs (High-Density lipoproteins) and differential risk of coronary heart disease in 4 prospective studies. Arterioscler Thromb Vasc Biol. 2020;40:2714–2727. doi: 10.1161/ATVBAHA.120.314609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayakawa M., Maki M. Coagulation-fibrinolytic and kinin-forming systems in toxemia of pregnancy. Gynecol Obstet Invest. 1988;26:181–190. doi: 10.1159/000293693. [DOI] [PubMed] [Google Scholar]
- 48.Chiarello D.I., Abad C., Rojas D., et al. Oxidative stress: normal pregnancy versus preeclampsia. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2020;1866:165354. doi: 10.1016/j.bbadis.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Patil S.B., Kodliwadmath M.V., Kodliwadmath M. Lipid peroxidation and antioxidant activity in complicated pregnancies. Clin Exp Obstet Gynecol. 2009;36:110–112. [PubMed] [Google Scholar]
- 50.Leon-Reyes G., Maida-Claros R.F., Urrutia-Medina A.X., et al. Oxidative profiles of LDL and HDL isolated from women with preeclampsia. Lipids Health Dis. 2017;16:90. doi: 10.1186/s12944-017-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Einbinder Y., Biron-Shental T., Agassi-Zaitler M., et al. High-density lipoproteins (HDL) composition and function in preeclampsia. Arch Gynecol Obstet. 2018;298:405–413. doi: 10.1007/s00404-018-4824-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.