Abstract
Background and aims
We have recently proposed urinary pteridine level as a useful biomarker of oxidative stress in a general population. However, the significance of urinary pteridines in patients with diabetes is unknown.
Methods
The relationships of the level of urinary pteridine derivatives with d-dimer, ankle-brachial pressure index (ABI), and known cardiovascular risk factors were investigated in patients with type 2 diabetes.
Results
Urinary pteridine level showed significant positive correlations with urinary15-isoprostane F2t, female gender, history of smoking and d-dimer and significant inverse correlations with history of alcohol drinking, body mass index (BMI) and ABI. ABI was significantly lower and d-dimer was significantly higher in the highest tertile group of pteridines than in the lowest tertile group. The odds ratios of the highest vs. lowest tertiles for low ABI and high d-dimer were significantly higher than the reference level. The above relationships of urinary pteridines with ABI and d-dimer were not altered when age, gender, BMI, hemoglobin A1c and history of alcohol drinking were used as explanatory variables in multivariable analyses. History of smoking confounded the relation of pteridines with ABI but not that with d-dimer. However, in logistic regression analysis, the association between pteridines and ABI remained significant with adjustment for history of smoking.
Conclusion
Urinary pteridine level was associated with d-dimer and ABI, which reflect blood coagulability and arterial flow to the lower extremities, respectively, and is thus thought to be a useful discriminator of thromboatherosclerotic risk in patients with diabetes.
Keywords: Blood coagulation, Diabetes, Oxidative stress, Peripheral arterial disease, Pteridines
Highlights
-
•
Relation of urinary pteridines with cardiovascular risk in DM was investigated.
-
•
Urinary pteridine level was positively associated with d-dimer.
-
•
Urinary pteridine level was inversely associated with ABI.
-
•
The above associations were independent of known cardiovascular risk factors.
-
•
Urinary pteridines may be a discriminator of cardiovascular risk in DM patients.
1. Introduction
Atherosclerotic cardiovascular disease is a major cause of death and disability in patients with diabetes [1,2], in whom progression of atherosclerosis is prone to be accelerated compared with that in non-diabetic people [3,4]. A variety of mechanisms including hyperglycemia, insulin resistance and/or hyperinsulinemia, dyslipidemia, inflammation, increased reactive oxygen species, endothelial dysfunction, hypercoagulability, and vascular calcification are known to contribute to the acceleration of atherosclerosis in patients with diabetes [5]. A common element in the pathophysiological mechanisms of cardiovascular disorders in diabetes is prolonged increases in production of reactive oxygen species in diabetic cardiovascular cells [6]. Thus, evaluation of oxidative stress and a strategy for its reduction are important for predicting and preventing future cardiovascular events.
Recently, we have developed a convenient method for evaluating oxidative forms of pteridine derivatives, e.g., biopterin and neopterin, by spectrophotometry [7]. Oxidized forms of pteridine derivatives, but not their reduced forms, emit fluorescence. Thus, only oxidized forms of pteridines are detected by spectrofluorometry. Urinary fluorometric pteridine levels were strongly associated with urinary levels of known oxidative stress markers such as DNA/RNA oxidation products and 15-isoprostane F2t. In general people, urinary pteridine level showed a stronger association with smoking, which causes oxidative stress in vivo, than did DNA/RNA oxidation products and 15-isoprostane F2t [7]. This suggests that urinary pteridine level is more useful than the other two biomarkers for evaluating oxidative stress in vivo. However, the significance of urinary pteridines in patients with diabetes is unknown. Since increased oxidative stress is deeply involved in the pathogenesis of thromboatherosclerotic complications in diabetes [6], it would be interesting to investigate the relationships of urinary pteridine level, as a marker of oxidative stress in vivo, with atherosclerotic risk in patients with diabetes.
The purpose of this study was therefore to clarify the relationship between urinary pteridines and cardiovascular risk in patients with diabetes. We evaluated cardiovascular risk by using ankle-brachial pressure index (ABI) as an indicator of atherosclerosis in the lower extremities [8,9] and d-dimer as an indicator of blood coagulability [10] in addition to classical cardiovascular risk factors including adiposity, blood pressure, blood lipids and glycemic status.
2. Subjects and methods
2.1. Subjects
The subjects of this study were 257 outpatients (158 men and 99 women) who had been diagnosed as having type 2 diabetes mellitus. This study was approved by the ethics committees of Kobe Tokushukai Hospital (number: TGE00313-014) and Hyogo College of Medicine (number: 1766). Individual histories of medication, cigarette smoking and alcohol drinking were surveyed by questionnaires. The subjects were divided by average alcohol consumption into three groups (nondrinkers; occasional drinkers, less than 2 days per week; regular drinkers, 2 days or more per week). The subjects were also divided into four groups by average cigarette consumption (nonsmokers; ex smokers; light smokers, < 20 cigarettes per day; heavy smokers, ≥ 20).
2.2. Measurements of urinary pteridine derivatives, 15-isoprostane F2t and creatinine
Levels of oxidized-form pteridine derivatives in urine were estimated by spectrofluorometry as described previously [7]. The excitation and emission wavelengths used were 360 nm and 450 nm, respectively. No pretreatment including oxidization of urine samples was performed. Levels of 15-isoprostane F2t were measured by an enzyme-linked immunoassay using a commercial kit, Urinary Isoprostane EIA Kit (Oxford Biochemical Research Inc., Oxford, MI, USA).
2.3. Measurements of cardiovascular risk factors
Height and body weight were measured with each subject wearing light clothes at a health checkup. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
After each subject had rested in the supine position, ABI was measured by an oscillometric method using an automatic ABI device (VaSera VS-1500, Fukuda Denshi, Tokyo, Japan) at rest. The lower value measured at the right and left legs in each individual was used for analysis of ABI. The cut-off value used for low ABI was 0.9 [11,12]. The threshold of low ABI (0.9 or lower) is usually used for diagnosis of peripheral arterial disease and is recommended in the ACC/AHA Practice Guidelines [12,13]. Arterial pressure of the right brachial artery was also recorded using Vasera VS-1500. Since high ABI (>1.3) reflects arterial calcification and is associated with an increase in prevalence of peripheral arterial disease in patients with diabetes [14], subjects showing ABI higher than 1.3 (n = 3) were excluded in the analysis of ABI. In meta-analysis studies, all-cause mortality and cardiovascular mortality have been shown to be increased in individuals with abnormally low ABI and high ABI in a general population and in a cohort of patients with diabetes [15,16]. Thus, there is a U-shaped relationship between ABI and cardiovascular disease mortality in patients with diabetes.
Fasting blood was collected from each patient in the morning, and serum and plasma samples were separated by centrifugation and were stored at −20 °C until measurements. Serum HDL cholesterol, LDL cholesterol and triglyceride concentrations were measured by enzymatic methods using commercial kits. Blood glucose was measured by using an automatic glucose analyzer based on the GOD/hydrogen peroxide electrode method. Hemoglobin A1c was measured by using an automatic glycol-hemoglobin analyzer based on high-performance liquid chromatography. Since the standards of hemoglobin A1c used for measurement are different in the NGSP (National Glycohemoglobin Standardization Program) method and the JDS (Japan Diabetes Society) method, hemoglobin A1c values were calibrated by using a formula proposed by the JDS [17]: hemoglobin A1c (NGSP) (%) = 1.02 × hemoglobin A1c (JDS) (%) + 0.25%. Subjects with diabetes were defined as those receiving drug therapy for diabetes and/or those showing high hemoglobin A1c levels (≥6.5%), according to the criteria for diagnosis of diabetes by the American Diabetes Association [18]. Serum creatinine was measured by an enzymatic method using a commercial kit, CRE-CL (Serotec Co., Ltd., Sapporo, Japan). Estimated glomerular filtration rate (eGFR) was calculated by using the following equation (cr: creatinine) developed by the Japanese Society of Nephrology [19]: eGFR (ml/min/1.73 m2) = 194 × cr(mg/dl)−1.094 × age (years)−0.287. Albumin concentrations in the urine were measured by the immune-nephelometry using an auto-analyzer (JCA-BM8000 series, JEOL Ltd., Tokyo, Japan).
D-dimer in plasma was measured with a latex agglutination assay using a commercial kit (Rapid chip d-dimer, Sekisui Medical Co., Ltd, Tokyo, Japan). An abnormally high level of d-dimer was defined as a level ≥1.0 μg/ml [20]. We referred to a previous study [20] in which d-dimer levels less than 1000 ng/ml safely excluded deep vein thrombosis and pulmonary embolism.
2.4. Statistical analysis
In our preliminary analysis, we estimated a suitable sample size for this study. Since there has been no study on the relation of urinary pteridines with atherosclerotic risk, we referred to a previous study on the relation of urinary isoprostanes with ABI [21]. Urinary isoprostane level is a marker of oxidative stress and has been shown to be correlated strongly with urinary pteridines [7]. The level of urinary 8-isoprostane was reported to be significantly correlated with ABI (correlation coefficient: 0.229 [p = 0.043]) [21]. Then, under the condition that α was 0.05, β was 0.1, and ρ was −0.229, the sample size was calculated to be 242. Thus, the sample size of this study (N = 257) was estimated to be large enough for investigation of the relationship between urinary pteridines and atherosclerotic risk represented by ABI. On the other hand, the subject numbers (158 men and 99 women) are not large enough for analysis of male and female subjects separately. Therefore, we performed analysis for men and women together. Statistical analyses were performed using a computer software program (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). The urinary pteridine levels were arranged in ascending order; then the subjects were divided into three tertile groups of equal sizes. The means of each variable were compared among the groups of the 1st, 2nd, and 3rd tertiles for pteridine levels using analysis of variance (ANOVA) followed by Scheffé’s F-test in univariable analysis and analysis of covariance (ANCOVA) followed by Student's t-test after Bonferroni correction in multivariable analysis. In logistic regression analysis, the odds ratios of subjects of the 2nd or 3rd tertiles versus the 1st tertile for pteridine levels were estimated before and after adjustment for other explanatory variables. Since d-dimer levels as well as pteridine levels did not show a normal distribution, their logarithmic transformation was necessary to normalize them for performing parametric analyses including linear regression analysis, ANOVA and ANCOVA. Spearman's rank correlation coefficients or Pearson's correlation coefficients were calculated in univariable correlation analysis. In the multivariable analyses, age, gender, BMI, hemoglobin A1c and history of alcohol consumption were used as other explanatory variables in the regression analyses or covariates in ANCOVA (Multivariable 1). In some analyses, history of smoking was also added to the explanatory variables or covariates (Multivariable 2). How well urinary pteridine level for prognostic risk prediction could separate those who did and did not have low ABI alone, high d-dimer alone or both low ABI and high d-dimer was evaluated using the receiver-operating characteristic (ROC) curve. Sensitivity and specificity are the basic measures of accuracy of a diagnostic test: The sensitivity is the probability of a positive test result, while the specificity is the probability of a negative test result. ROC is a plot of sensitivity versus 1-specificity that offers a summary of sensitivity and specificity across a range of cut points for a continuous predictor. The optimal cutoff point was selected by maximizing Youden's index, which is the difference between the true positive rate (sensitivity) and the false positive rate (1-specificity) in the ROC curve. Discrimination was measured by the area under the ROC curve (area under the curve [AUC]). AUC and 95% confidence interval were estimated empirically. Probability (p) values less than 0.05 were defined as significant.
3. Results
3.1. Characteristics of the participants
Table 1 shows the profile of the subjects. Their mean age was 69.2 years and about 30% and 45% of them were smokers and alcohol drinkers, respectively. The percentages of subjects receiving insulin therapy and anti-coagulant therapy were 14.4% and 38.6%, respectively. About one fifth and one fourth of the subjects showed low ABI and high d-dimer, respectively. The type of oral glucose-lowering drugs taken by the subjects was as follows: sulfonylureas, 113 (44.0%); dipeptidyl peptidase-4 (DPP-4) inhibitor 141 (54.9%); biguanide 81 (31.5%); thiazolidinediones, 14 (5.4%); α-glucosidase inhibitor 33 (12.8%); sodium glucose cotransporter type 2 (SGLT2) inhibitor, 12 (4.7%). About one seventh of the subjects were receiving insulin injection.
Table 1.
Characteristics of the subjects.
| Variables | Values |
|---|---|
| Number | 257 (men 158; women 99) |
| Age (years) | 69.2 ± 10.4 [35–91] |
| Smokers (%) | 29.2 |
| Alcohol drinkers (%) | 44.7 |
| Oral glucose-lowering drug (%) | 80.9 |
| Insulin therapy (%) | 14.4 |
| Anti-coagulation therapy (%) | 38.6 |
| Mean BMI (kg/m2) | 24.9 ± 4.5 [15.5–46.8] |
| Mean systolic BP (mmHg) | 142.6 ± 21.2 [92–239] |
| Mean diastolic BP (mmHg) | 85.0 ± 11.0 [57–124] |
| Mean ABI | 1.07 ± 0.14 [0.44–1.35] |
| Low ABI (%) | 19.8 |
| Mean fasting blood glucose (mg/dl) | 134.9 ± 44.5 [54–339] |
| Mean hemoglobin A1c (%) | 7.15 ± 1.23 [5.30–14.20] |
| Mean HDL cholesterol (mg/dl) | 56.7 ± 18.7 [21–156] |
| Mean LDL cholesterol (mg/dl) | 110.8 ± 32.8 [43–223] |
| Mean triglycerides (mg/dl) | 115 (78.5, 168.5) [34–1079] |
| Median d-dimer (μg/ml) | 0.60 (0.40, 0.90) [0.16–9.08] |
| High d-dimer (%) | 22.6 |
| Mean eGFR (mL/min/1.73m2) | 77.7 ± 28.2 [9.8–154.9] |
| Median urinary creatinine (mg/dl) | 101.9 (63.5, 159.1) [12.8–500.2] |
| Median urinary albumin (g/g creatinine) | 1.85 (0.64, 7.32) [0–481.1] |
| Median urinary pteridine (μM/g creatinine) | 13.81 (10.47, 19.26) [4.96–67.25] |
| Median urinary 15-isoprostane F2t (μM/g creatinine) | 1.37 (1.06, 1.97) [0.31–15.27] |
Shown are numbers, percentages, means with standard deviations and ranges in square brackets, and medians with interquartile ranges in parentheses and ranges in square brackets.
3.2. Distribution of urinary pteridine levels
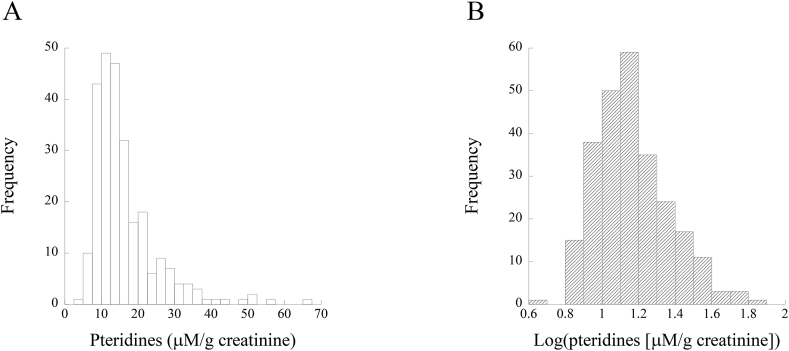
Histograms of urinary pteridine levels and log-transformed urinary pteridine levels are shown in Fig. 1A and B, respectively. Urinary pteridine levels display a normal distribution not before but after their logarithmic transformation (Fig. 1B).
Fig. 1.
Histograms of urinary pteridines (A) and log-transformed pteridines (B).
3.3. Correlations of urinary pteridines with urinary 15-isoprostane F2t and creatinine
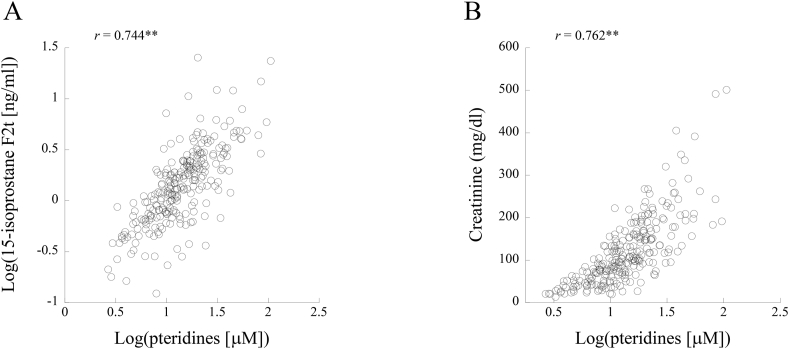
Fig. 2 shows scatter plots of the relationships of log-transformed urinary pteridine level with log-transformed 15-isoprostane F2t level (A) and creatinine (B). Log-transformed urinary pteridine level was significantly correlated with log-transformed 15-isoprostane F2t (Pearson's correlation coefficient: 0.744) and with creatinine level (Pearson's correlation coefficient: 0.762). Thus, urinary pteridine level was strongly associated with urinary creatinine level, and then individual urinary pteridine level was used after correction by urinary creatine level in the following analyses for relationships of urinary pteridines with other variables.
Fig. 2.
Correlations of log-transformed pteridines with log-transformed 15-isoprostane F2t (A) and urinary creatinine (B). Pearson's correlation coefficients are given in the figures. Asterisks denote significant correlations (∗∗, p < 0.01).
3.4. Correlations of pteridines, ABI and d-dimer with atherosclerotic risk factors in univariable analysis
Table 2 shows Spearman's rank correlation coefficients of urinary pteridines, ABI and d-dimer with each variable. Pteridine level showed significant positive correlations with urinary 15-isoprostane F2t, female gender, history of smoking and d-dimer and showed significant inverse correlations with history of alcohol drinking, BMI and ABI. ABI showed significant positive correlations with history of alcohol drinking and diastolic blood pressure and showed significant inverse correlations with age, female gender and systolic blood pressure. D-dimer showed significant positive correlations with age and triglycerides and showed significant inverse correlations with history of alcohol drinking, HDL cholesterol and eGFR.
Table 2.
Correlations of each variable with urinary pteridines, ABI and d-dimer in univariable analysis.
| Pteridines/cr | ABI | d-dimer | |
|---|---|---|---|
| Pteridine/cr | – | −0.164∗∗ | 0.157∗ |
| 15-isoprostane F2t/cr | 0.381∗∗ | −0.069 | −0.044 |
| Age | −0.011 | −0.233∗∗ | 0.362∗∗ |
| Gender | 0.132∗ | −0.254∗∗ | 0.074 |
| Smoking | 0.141∗ | −0.047 | −0.054 |
| Alcohol | −0.163∗∗ | 0.148∗ | −0.147∗ |
| Insulin therapy | 0.115 | −0.041 | 0.032 |
| Anticoagulation therapy | 0.078 | 0.089 | −0.023 |
| BMI | −0.158∗ | 0.104 | 0.099 |
| Systolic BP | 0.039 | −0.188∗∗ | 0.067 |
| Diastolic BP | −0.004 | 0.171∗∗ | −0.068 |
| Fasting blood sugar | 0.017 | 0.004 | 0.047 |
| Hemoglobin A1c | −0.042 | 0.055 | 0.012 |
| HDL cholesterol | −0.028 | 0.027 | −0.381∗∗ |
| LDL cholesterol | −0.031 | 0.015 | 0.108 |
| Triglycerides | 0.036 | 0.006 | 0.426∗∗ |
| eGFR | 0.033 | 0.095 | −0.197∗∗ |
| Urinary albumin/cr | 0.183∗∗ | −0.127∗ | 0.096 |
Spearman's rank correlation coefficients of each variable with urinary pteridines, ABI and d-dimer are shown. Gender, women vs. men (men as a reference); cr, creatinine; BP, blood pressure. Asterisks denote significant correlation coefficients (∗, p < 0.05; ∗∗, p < 0.01).
3.5. Correlations of pteridines with ABI and d-dimer in multivariable analysis
The results of univariable and multivariable analyses for correlations of ABI and d-dimer with log-transformed pteridines are shown in Table 3. Age, gender (women vs. men), BMI, hemoglobin A1c and history of alcohol drinking were used as other explanatory variables (Multivariable 1). In addition, history of smoking was added to the explanatory variables in another analysis (Multivariable 2). In univariable analysis, log-transformed pteridines showed significant inverse and positive correlations with ABI and d-dimer, respectively. In multivariable analysis adjusting for the above variables except for history of smoking (Multivariable 1), log-transformed pteridines showed significant inverse and positive correlations with ABI and d-dimer, respectively. When history of smoking was also added to the explanatory variables (Multivariable 2), log-transformed pteridine level was significantly correlated with d-dimer but not with ABI. The correlations of the variables, including urinary pteridines, with ABI and d-dimer were significant but rather weak. Thus, in linear regression analysis, we confirmed just statistical significance of the relations of cardiovascular risk factors and pteridines with ABI and d-dimer.
Table 3.
Correlations of each variable with ABI and d-dimer in univariable and multivariable analyses.
| ABI |
Log(d-dimer) |
|||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable 1 | Multivariable 2 | Univariable | Multivariable 1 | Multivariable 2 | |
| Log(pteridine) | −0.155∗ | −0.122∗ | −0.076 | 0.171∗∗ | 0.179∗∗ | 0.166∗∗ |
| Age | – | −0.205∗∗ | −0.256∗∗ | – | 0.411∗∗ | 0.426∗∗ |
| Gender | – | −0.144∗ | −0.229∗∗ | – | −0.045 | −0.021 |
| BMI | – | 0.058 | 0.057 | – | 0.139∗ | 0.139∗ |
| Hemoglobin A1c | – | −0.016 | −0.041 | – | 0.012 | 0.019 |
| Alcohol | – | 0.074 | 0.097 | – | −0.131∗ | −0.138∗ |
| Smoking | – | – | −0.222∗∗ | – | – | 0.062 |
Shown are Pearson's correlation coefficients in univariable analysis and standardized partial regression coefficients (β) in multivariable analysis. Asterisks denote significant correlation coefficients (∗, p < 0.05; ∗∗, p < 0.01).
3.6. Comparison of means of ABI and d-dimer among tertile groups for pteridines in uni- and multivariable analyses
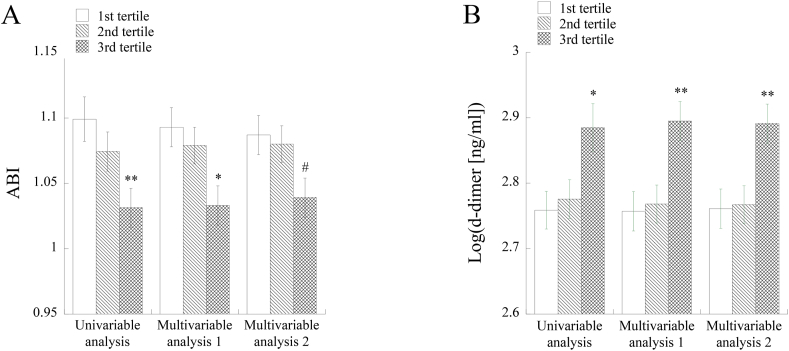
Both in uni- and multivariable analyses, ABI was significantly lower and d-dimer was significantly higher in the 3rd tertile group for pteridines than in the 1st tertile group except for the comparison of mean ABI values in the multivariable analysis using history of smoking as an explanatory variable (Fig. 3A and B). In the multivariable analysis with adjustment for history of smoking in addition to age, gender, BMI, hemoglobin A1c and history of alcohol drinking, there was a marginally significant difference in ABI values (p = 0.077) of the 1st and 3rd tertiles for pteridines (Fig. 3A).
Fig. 3.
Comparison of mean levels of ABI (A) and log-transformed d-dimer (B) in the tertile groups of urinary pteridines. In multivariable analysis, age, gender, BMI, hemoglobin A1c and history of alcohol drinking were adjusted (Multivariable analysis 1 and 2). In addition, history of smoking was adjusted in Multivariable analysis 2. Asterisks denote significant differences from the 1st tertile for pteridine (∗, p < 0.05; ∗∗, p < 0.01). #, a marginally significant difference from the 1st tertile for pteridine (p = 0.077).
3.7. Odds ratios vs the. 1st tertile group of pteridines for low ABI and high d-dimer in uni- and multivariable analyses
The results of logistic regression analysis for the relationships of pteridine level with low ABI and high d-dimer are shown in Table 4. In multivariable analysis, we used the same explanatory variables as those used in multivariable linear regression analysis (Table 3). The odds ratios for low ABI and high d-dimer of the 3rd vs. 1st tertile groups of pteridines were significantly higher than the reference level in both univariable and multivariable analyses with adjustment for history of smoking as well as age, gender, BMI, hemoglobin A1c and history of alcohol drinking.
Table 4.
Odds ratios for low ABI and high d-dimer of the 2nd and 3rd tertiles vs. the 1st tertile for urinary pteridine in univariable and multivariable logistic regression analyses.
| 1st tertile | 2nd tertile | 3rd tertile | |
|---|---|---|---|
| Low ABI | |||
| univariable | 1.00 | 2.06 (0.82–5.16) | 4.81 (2.04–11.35)∗∗ |
| multivariable 1 | 1.00 | 2.00 (0.77–5.20) | 5.07 (2.04–12.63)∗∗ |
| multivariable 2 | 1.00 | 1.85 (0.70–4.86) | 4.70 (1.85–11.92)∗∗ |
| High d-dimer | |||
| univariable | 1.00 | 1.61 (0.74–3.52) | 2.55 (1.21–5.40)∗ |
| multivariable 1 | 1.00 | 1.52 (0.65–3.56) | 2.76 (1.20–6.31)∗ |
| multivariable 2 | 1.00 | 1.51 (0.64–3.56) | 2.90 (1.25–6.72)∗ |
Odds ratios and their confidence intervals are shown. In multivariable analysis, age, gender, BMI, hemoglobin A1c and history of alcohol drinking were adjusted (Multivariable analysis 1 and 2). In addition, history of smoking was adjusted in Multivariable analysis 2. Asterisks denote significant differences from the reference level of 1.00. (∗, p < 0.05; ∗∗, p < 0.01).
3.8. ROC analysis
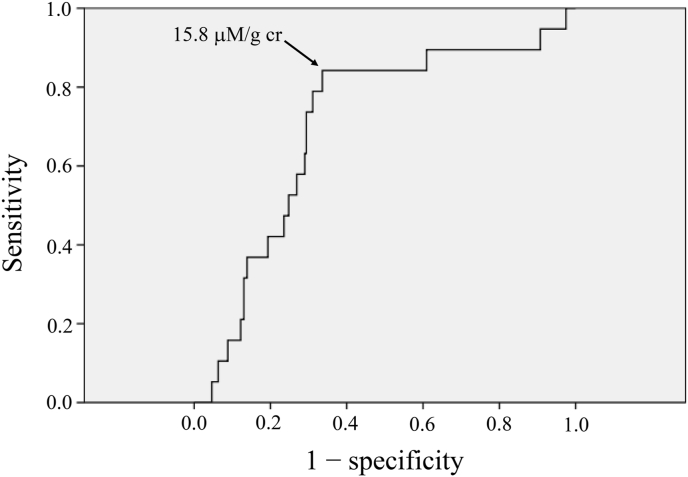
ROC analysis was performed for the relationships of urinary pteridine levels with low ABI alone, high d-dimer alone, and both low ABI and high d-dimer. The area under the curve (AUC) was larger in the relationship with both low ABI and high d-dimer (AUC, 0.701 [0.581–0.821], p = 0.004) than in the relationships with low ABI alone (AUC, 0.643 [0.559–0.728], p = 0.002) and high d-dimer alone (AUC, 0.600 [0.516–0.685], p = 0.020). Then, we performed further analysis for the relationship of pteridine levels with prevalence of both low ABI and high d-dimer (Fig. 4). The optimal cutoff point was selected by maximizing Youden's index, which is the difference between the true positive rate (sensitivity) and the false positive rate (1-specificity) in the ROC curve. The cutoff point of urinary pteridine level, yielding maximal sensitivity plus specificity for predicting low ABI and high d-dimer, in the ROC curves was determined. The optimal cutoff point (the point with an arrow in Fig. 4) was determined to be 15.8 μM/g creatinine. Specificity and sensitivity of the cutoff were 66.4% and 84.2%, respectively.
Fig. 4.
ROC curves for urinary pteridine level to predict low ABI and high d-dimer. Cutoff points of urinary pteridine level, yielding maximal sensitivity plus specificity for predicting low ABI and high d-dimer, in the ROC curves were determined. The value with arrow, indicating the cutoff points in the figures, are cut-off values. Specificity and sensitivity of the cutoffs were 66.4% and 84.2%, respectively.
4. Discussion
This study clearly demonstrated that urinary pteridine level is associated with d-dimer and ABI. D-dimer is a representative marker of blood coagulation [10], which is increased with progression of cardiovascular complications in patients with diabetes [22], [23]. Measurement of ABI is a standard examination for diagnosis of peripheral arterial disease [8,9], which is a major atherosclerotic complication of diabetes [11,12]. Therefore, urinary pteridine level is thought to be a useful biomarker for discriminating the risk of atherosclerotic complications in patients with diabetes. This is the first study showing the clinical significance of urinary pteridines in patients with diabetes. There was no significant difference in log-transformed pteridine levels among the three groups of subjects without medication therapy, with oral glucose-lowering drugs alone, and with insulin injection (data not shown).
In our previous study, urinary pteridine level was shown to reflect oxidative stress in a general population [7]. In fact, urinary pteridine level was strongly correlated with urinary 15-isoprostane F2t, a known biomarker of oxidative stress, in the present study. Formation of reactive oxygen species is increased in the presence of cardiovascular risk factors including smoking, dyslipidemia, hypertension and diabetes [24]. Since oxidative stress is deeply involved in atherogenesis through various mechanisms including lipoprotein modification, endothelial dysfunction, vascular smooth muscle cell proliferation and leukocyte migration [25], it is reasonable that urinary pteridine level is associated with ABI and d-dimer, which are representative markers for atherosclerotic complications in patients with diabetes. Although there was a significant correlation between levels of urinary pteridines and 15-isoprostane F2t, 15-isoprostane F2t level did not show significant correlations with ABI and d-dimer level (Table 2). This suggests that urinary pteridine level is a more useful biomarker of atherosclerotic disease in patients with diabetes than is urinary 15-isoprostane F2t.
Smoking causes an increase in oxidative stress. In general people, urinary pteridine level was significantly higher in smokers than in nonsmokers [5]. Similarly, urinary pteridine level was significantly correlated with smoking in patients with diabetes (Table 2). Smoking as well as diabetes is a major risk factor of peripheral arterial disease [26,27], and smoking causes an increase in blood coagulability through various mechanisms including endothelial dysfunction, increased platelet aggregation, elevation of fibrinogen in blood, and formation of thrombolysis-resistant fibrin clots [28,29]. Thus, smoking is a possible confounder for the relationships of urinary pteridines with ABI and d-dimer. However, in multivariable analyses, the relation of urinary pteridines to d-dimer remained significant after adjustment for history of smoking (Table 3, Table 4, Fig. 3). In linear regression analysis and ANCOVA, urinary pteridines did not show significant relationships with ABI when history of smoking was adjusted (multivariable 2 in Table 3 and Fig. 3). However, in multivariable logistic regression analysis with adjustment for smoking, the odds ratio for low ABI of the 3rd vs. 1st tertile groups of pteridines remained significantly high in comparison with the reference level (Table 4). In addition, there was a marginally significant difference in ABI of the subjects in the 1st and 3rd tertile groups for pteridine levels when smoking was adjusted (Fig. 3). Therefore, urinary pteridine level is associated with both ABI and d-dimer independently of smoking. The results of multivariable analyses also suggest that the associations of urinary pteridines with ABI and d-dimer are independent of age, gender, adiposity, glycemic status and alcohol drinking.
Ten subjects (3.9%) had a history of cancer. However, the associations of pteridines with ABI and d-dimer were not altered when a history of cancer was added as an explanatory variable in ANCOVA (data not shown). Therefore, it was thought that cancer history did not confound the relationships of pteridines with ABI and d-dimer.
Urinary albumin level was significantly correlated with urinary pteridine levels [Spearman's rank correlation coefficient: 0.183 (p = 0.003)]. Then, ABI and d-dimer levels were compared among the three tertile groups for pteridines with adjustment for urinary albumin level in addition to age, gender, hemoglobin A1c and alcohol drinking. ABI was significantly lower in the 3rd tertile group than in the 1st tertile group, and log-transformed d-dimer level was significantly higher in the 2nd and 3rd tertile groups than in the 1st tertile group (data not shown). Thus, the associations of urinary pteridines with ABI and d-dimer were not confounded by urinary albumin. The correlation between pteridines and d-dimer was significant in the 1st and 2nd tertile groups for urinary albumin but not in the 3rd tertile group (data not shown). Therefore, it seems that there is an association between pteridines and d-dimer in the early stage of renal damage but not in its late stage, suggesting that urinary pteridine level cannot be used in patients with severe renal damage.
There are limitations of this study. The number of subjects was not large enough for analyzing men and women separately. Urinary pteridine level and ABI were significantly higher and lower, respectively, in women than in men (data not shown). These results agree with the previous finding that women with diabetes have a higher risk of cardiovascular disease than do men with diabetes [2]. The mean age of the subjects was relatively old, and further studies are needed to confirm the findings of this study in young people. The mean hemoglobin A1c was 7.15% and 14.4% of the subjects were receiving insulin therapy, suggesting that glycemic status of the subjects in this study was generally not so poor. Thus, it remains to be determined whether urinary pteridine level can be used for discrimination of atherosclerotic risk in patients with severe diabetes. In multivariable analyses, age, gender, BMI, hemoglobin A1c and histories of smoking and alcohol drinking were used as explanatory variables and covariates. However, there are other possible confounders, e.g., diet, physical activity, education, and occupation, for which information was not available in this study. It is not easy to know the correct time of onset of type 2 diabetes in each patient. Unfortunately, we had no information on the duration of diabetes in the subjects of this study, although it would be interesting to investigate the relation of the disease vintage with urinary pteridine levels. Finally, the design of this study is cross-sectional, and thus further studies are needed to discuss causal relationships of urinary pteridines with atherosclerotic complications in patients with diabetes.
In conclusion, in patients with diabetes, urinary pteridine level was strongly correlated with urinary 15-isoprostane F2t, a representative marker of oxidative stress, and was associated with ABI and d-dimer, which reflect blood flow to the lower extremities and blood coagulability, respectively. Therefore, urinary pteridine level reflects oxidative stress and is proposed to be a useful biomarker that discriminates the risk of atherosclerotic disease in patients with diabetes. In addition to the convenience of urine as a clinical sample, another merit of urinary pteridines is the easiness of measurement by using spectrophotometry. Therefore, urinary pteridine level is expected to become a useful biomarker for a screening test of oxidative stress in the future.
Financial support
This study was supported by a Grant-in-Aid for Scientific Research (No. 21H03386) from the Japan Society for the Promotion of Science.
Authors contribution statement
I.W. conceived and designed the study. M.M. and K.E. collected urine and blood samples. M.M., K.E., and I.W. managed obtained data. I.W. performed the statistical analysis of the data. I.W. wrote the paper, and M.M. edited it.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Dr. Mamoru Nakanishi for his excellent technical support for measurements of urinary pteridines and 15-isoprostane F2t.
References
- 1.Gu K., Cowie C.C., Harris M.I. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., et al. Emerging Risk Factors Collaboration, Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virmani R., Burke A.P., Kolodgie F. Morphological characteristics of coronary atherosclerosis in diabetes mellitus. Can J Cardiol. 2006;22(Suppl B):81B–84B. doi: 10.1016/s0828-282x(06)70991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X., Song J., Watase H., Hippe D.S., Zhao X., et al. Differences in carotid plaques between symptomatic patients with and without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2019;39:1234–1239. doi: 10.1161/ATVBAHA.118.312092. CARE-II Investigators. [DOI] [PubMed] [Google Scholar]
- 5.Low Wang C.C., Hess C.N., Hiatt W.R., Goldfine A.B. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in Type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah M.S., Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi I., Nakanishi M., Ohki M., Suehiro A., Uchida K. A simple and useful method for evaluation of oxidative stress in vivo by spectrofluorometric estimation of urinary pteridines. Sci Rep. 2020;10:11223. doi: 10.1038/s41598-020-67681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guirguis-Blake J.M., Evans C.V., Redmond N., Lin J.S. Screening for peripheral artery disease using the ankle-brachial index: updated evidence report and systematic review for the US preventive services task force. J Am Med Assoc. 2018;320:184–196. doi: 10.1001/jama.2018.4250. [DOI] [PubMed] [Google Scholar]
- 9.Herraiz-Adillo Á., Cavero-Redondo I., Álvarez-Bueno C., Pozuelo-Carrascosa D.P., Solera-Martínez M. The accuracy of toe brachial index and ankle brachial index in the diagnosis of lower limb peripheral arterial disease: a systematic review and meta-analysis. Atherosclerosis. 2020;315:81–92. doi: 10.1016/j.atherosclerosis.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Weitz J.I., Fredenburgh J.C., Eikelboom J.W. A test in context: d-dimer. J Am Coll Cardiol. 2017;70:2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Jude E.B., Eleftheriadou I., Tentolouris N. Peripheral arterial disease in diabetes -a review. Diabet Med. 2010;27:4–14. doi: 10.1111/j.1464-5491.2009.02866.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch A.T., Haskal Z.J., Hertzer N.R., Bakal C.W., Creager M.A., et al. American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing, TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 13.Hiatt W.R. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 14.Potier L., Abi Khalil C., Mohammedi K., Roussel R. Use and utility of ankle brachial index in patients with diabetes. J. Vasc. Endovasc. Surg. 2011;41:110–116. doi: 10.1016/j.ejvs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Gu X., Man C., Zhang H., Fan Y. High ankle-brachial index and risk of cardiovascular or all-cause mortality: a meta-analysis. Atherosclerosis. 2019;282:29–36. doi: 10.1016/j.atherosclerosis.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Nie F., He J., Cao H., Hu X. Predictive value of abnormal ankle-brachial index in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2021 doi: 10.1016/j.diabres.2021.108723. (in press) [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi A., Kasuga M., Araki E., Oka Y., Hanafusa T., et al. Committee on the Standardization of diabetes mellitus - related laboratory testing of Japan diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: from Japan diabetes Society to National Glycohemoglobin Standardization program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japan Nephrology Society Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi. 2012;54:1034–1191. [PubMed] [Google Scholar]
- 20.Michiels J.J., Gadisseur A., van der Planken M., Schroyens W., De Maeseneer M., et al. Different accuracies of rapid enzyme-linked immunosorbent, turbidimetric, and agglutination D-dimer assays for thrombosis exclusion: impact on diagnostic work-ups of outpatients with suspected deep vein thrombosis and pulmonary embolism. Thromb. Hemost. 2006;32:678–693. doi: 10.1055/s-2006-951296. [DOI] [PubMed] [Google Scholar]
- 21.Cherneva R.V., Cherneva Z.V., Georgiev O.B., Petrova D.S., Petrova J.I. 8-isoprostanes and resistin as markers of vascular damage in non-hypersomnolent obstructive sleep apnoea patients. Clin Physiol Funct Imag. 2017;37:695–702. doi: 10.1111/cpf.12361. [DOI] [PubMed] [Google Scholar]
- 22.Alzahrani S.H., Ajjan R.A. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res. 2010;7:260–273. doi: 10.1177/1479164110383723. [DOI] [PubMed] [Google Scholar]
- 23.Nwose E.U., Richards R.S., Jelinek H.F., Kerr P.G. D-dimer identifies stages in the progression of diabetes mellitus from family history of diabetes to cardiovascular complications. Pathology. 2007;39:252–257. doi: 10.1080/00313020701230658. [DOI] [PubMed] [Google Scholar]
- 24.Kattoor A.J., Pothineni N.V.K., Palagiri D., Mehta J.L. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 25.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 26.Lu L., Mackay D.F., Pell J.P. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. 2014;100:414–423. doi: 10.1136/heartjnl-2013-304082. [DOI] [PubMed] [Google Scholar]
- 27.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 28.Leone A. Smoking, haemostatic factors, and cardiovascular risk. Curr Pharm Des. 2007;13:1661–1667. doi: 10.2174/138161207780831347. [DOI] [PubMed] [Google Scholar]
- 29.Barua R.S., Ambrose J.A. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol. 2013;33:1460–1467. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]