Abstract
Background and aims
The increasing prevalence of diabetes mellitus is causing a massive growth of peripheral artery disease incidences, a disabling complication of diabetic atherosclerosis, which leads often to the amputation of the affected limb. Critical limb ischemia is the terminal disease stage, which requires a prompt intervention to relieve pain and save limbs. However, patients undergoing revascularization often suffer from cardiovascular, cerebrovascular and major adverse limb events with poor outcomes. Furthermore, the same procedure performed in apparently similar patients has various outcomes and lack of an outcome predictive support causes a high lower limb arterial revascularization rate with disastrous effects for patients. We collected the main risk factors of major adverse limb events in a more readable and immediate format of the topic, to propose an overview of parameters to manage effectively peripheral artery disease patients and to propose basics of a new predictive tool to prevent from disabling vascular complications of the disease.
Methods
Most recent and updated literature about the prevalence of major adverse limb events in peripheral artery disease was reviewed to identify possible main predictors.
Results
In this article, we summarized major risk factors of limb revascularization failure and disabling vascular complications collecting those parameters principally responsible for major adverse limb events, which provides physio-pathological explanation of their role in peripheral artery disease.
Conclusion
We evaluated and listed a panel of possible predictors of MALE (Major Adverse Limb Event) in order to contribute to the development of a predictive score, based on a summary of the main risk factors reported in scientific articles, which could improve the management of peripheral artery disease by preventing vascular accidents.
Keywords: Peripheral artery disease, Critical limb ischemia, Diabetes mellitus, Major adverse limb event (MALE), Predictors
Highlights
-
•
Patients with peripheral artery disease (PAD) undergoing revascularization could have severe complications.
-
•
New therapeutic targets play a fundamental role for the improvement of PAD clinical outcomes after revascularization.
-
•
We collected all modifiable and unmodifiable risk factors related with PAD complications.
1. Introduction
The growing global prevalence of diabetes mellitus with nearly 500 million affected people [1,2] will make atherosclerosis and its complications the leading cause of death in the near future. The chronic subclinical inflammation underlying diabetic atherosclerosis along with the oxidative stress of advanced glycation end-product (AGEs) are the main promoter of macrovascular complications such as PAD [[3], [4], [5], [6]]. The risk of developing cardio-cerebrovascular complications in patients with PAD is well known; however, these patients often suffer from disabling limb adverse events. Studies and knowledge on the causes of these complications are not as well-structured and organized as in the literature regarding major adverse cardiovascular events (MACE). Hence, this review is a comprehensive search of all relevant studies and a collection of available knowledge on possible predictors of MALE to provide a more organic, readable and immediate state of the art for future prospects. As a primary objective, this article provides a panel of parameters to identify new risk categories for adequate management of patients with PAD at risk of developing MALE. Moreover, as a secondary objective, this review introduces the basis for a predictive tool to locate our patients in risk categories and prevent the incidence of MALE in higher risk patients.
1.1. Diabetes mellitus: not only a matter of glucose
Diabetes mellitus is often described as a clinical syndrome characterized by hyperglycemia due to defective insulin secretion by pancreatic β-cells and reduced sensitivity of target tissues (muscle, liver and adipose tissue) to its action (insulin resistance) leading to the development of inappropriate hyperglycemia. The progression of oxidative stress due to an excess of reactive oxygen species (ROS) and the formation of AGEs induce harmful consequences to vessels leading to vascular complications [7]. Unlike type 1 diabetes mellitus which has a genetic and immune etiology [8], type 2 diabetes sees not-modifiable and acquired risk factors. The result of the coexistence of these factors causes the development of genetic and epigenetic changes, mitochondrial dysfunctions, intestinal dysbiosis and oxidative stress that lead to a metabolic memory characterized by reduced insulin production due to gradual loss of pancreatic beta cell function, and increased inflammatory response promoting insulin resistance [9]. Recently, a growing body of evidence is underlining the importance of obesity, nutritional habits, lifestyle and subclinical systemic inflammation as possible promoters of this disease and related complications. Unbalanced diets (excessive consumption of high-density calorie meals, refined sugars, animal proteins, saturated fats) and harmful behaviors (sedentary lifestyles and physical inactivity) are characterizing the lives of a substantial part of the population, involving all generations and increasing the incidence of diabetes mellitus, obesity, peripheral artery disease and metabolic syndrome [[10], [11], [12]]. Currently diabetes affects almost half a billion people with a worrying trend in the next future [2]. Additionally, globalization easily offers availability, attraction and addiction [13,14] to highly palatable, cheap and low quality junk food, with a preannounced epidemic of obesity [15] and a massive increase of type-2 diabetes mellitus (T2DM) prevalence 1,16,17, with enormous impacts on economics.
Individual inflammatory state has also been demonstrated to play an interesting role on glycemic control [18]. This enabled to further expand the function of adipose tissue as source of cytokines and related proinflammatory signaling pathways [[19], [20], [21], [22], [23], [24], [25]]. Moreover, new evidence is available on the close relationship between food and inflammation, and the effects of several dietary patterns (such as North America, the Northern Europe and the traditional Mediterranean diet) on systemic inflammation [26]. Interestingly, both insulin resistance (a preventable pre-diabetic condition) and type 1 diabetes mellitus (a condition characterized by hyperglycemia due to the immune-mediated destruction of pancreatic beta cells) trigger chronic inflammation, sustaining the metabolic dysfunction, insulin resistance, and promoting atherosclerosis [27]. Moreover, the early onset of hyperglycemia in type 1 DM determines a greater and lasting exposure to glycemic alterations, substantially increasing the risk of vascular complications [28]. Thus, hyperglycemia in DM is only an epiphenomenon of a fascinating relationship of factors already known, recently discovered and probably yet to be discovered.
1.2. Atherosclerosis: an old, but new protagonist
The exponential increase of T2DM is associated with a fast-growing incidence of atherosclerotic complications, which play a central role in global morbidity and mortality. The chronic exposure to high serum glucose levels determines a disruption of vascular homeostasis and endothelial dysfunction, inducing a subclinical inflammation, which precedes vessel injury [27]. Intracellular hyperglycemia stimulates the production of mitochondrial reactive oxygen species (ROS) along with the formation of AGEs, which enhance cytokine expression accelerating the diabetic atherosclerosis due to a chronic vascular inflammation [29]. Moreover, through the activation of ubiquitin molecular pathways, ROS induce insulin resistance perpetuating the entire process [27]. According to the World Health Organization, major vascular complications related to atherosclerosis will be the first cause of death in the next decades and scientific progress must contribute to prevent the otherwise inevitable dramatic effects on public health. Diabetic patients are often destined to suffer from complications of this atherosclerotic process 30–32 such as peripheral artery disease (PAD), which is an increasing macrovascular disabling disease 3–5 associated to chronic vessel inflammation [33]. PAD is a relatively new pathological expression of the “old” atherosclerotic disease, which deserves proper research to identify efficient therapeutic solutions.
1.3. Peripheral artery disease: focus on an emerging disabling complication: MALE-
Peripheral artery disease is a main risk factor for cardio- and cerebrovascular death and a clinical expression of atherosclerosis [34,35]. In fact, diabetic PAD patients are directly exposed to a high risk of major adverse cardiovascular events (MACE)―defined as composite of acute myocardial infarction, stroke, transient ischemic attack and cardiovascular death [35,36]―. Unfortunately, an elevated percentage of PAD patients remains unaware of their disease because of their absence of symptoms [37]. The underestimation of PAD might be a direct result of a disconcerting low sensibility of clinicians towards this pathology [38] with disastrous effects on patients who, both asymptomatic and symptomatic, are exposed to an equally high risk of MACE [39,40].
Additionally, evidences underlined the presence of major adverse limb event (MALE)―defined as composite of acute limb ischemia, major vascular amputations, limb-threatening ischemia leading to urgent revascularization [36,41,42]―which is another pathological consequence of this disease involving the short-term management of diabetic PAD patients and affecting negatively patients’ quality of life [43] and public economy [44,45]. The ineffectiveness of currently available treatments often derives from the clinicians’ inadequate capability to recognize complications and from lacking predictive support, which could guide physicians to prevent MALE. However, the scientific resonance of MALE is not comparable to the amount of information on cardiovascular complications (defined as MACE) that are associated with PAD. This lower sensitivity of the scientific community on this topic leads to a current lack of systematic articles that can highlight the poor short and long-term results related to PAD when a MALE occurs, but above all, that can describe the main causes underlying the incidence by MALE. More studies are needed to understand and prevent this disabling, often fatal complication.
1.4. Arterial revascularization: a complete success?
Adequate life style promotion, principal modifiable risk factor correction and medical therapy optimization are the first fundamental therapeutic steps in PAD treatment [[46], [47], [48], [49], [50]]. However, in severe symptomatic cases or at the very late stage of the illness, named critic limb ischemia, revascularization results indispensable [48,51,52]. Peripheral artery revascularization is a therapeutic approach that gives relief from invalidating symptoms of claudication, improving patients’ quality of life [53], and it becomes a limb salvage treatment in patients affected by critical limb ischemia. In fact, available pharmacological and not pharmacological therapy (such as the injection of stem cells or colony-forming endothelial cells of human cord blood) are not able to effectively manage invalidating symptoms of chronic threatening limb ischemia [54], in which impaired distal arterial blood flow provokes disabling tissue loss due to the necrosis and gangrene. In this setting, limb revascularization efficiently resolves ischemic pain and supports wound healing [55]. Balloon angioplasty is a safer revascularization technique compared to major vascular surgery. However, PAD patients, who underwent endovascular treatment often showed a high rate of MACE and MALE [51]. A retrospective observational study [55] demonstrated that 10% of PAD patients, who underwent limb revascularization, were within a year readmitted to the hospital for MALE [55]. Evidence suggests that the prognosis of patients with PAD strongly depends on the expression of traditional and non-traditional risk factors [51,56] (including previous revascularization treatments) and this manuscript is an attempt to provide a collection of these predictors in a unified work.
2. Review design, methods and results
We evaluated the recent evidence in the literature on the main risk factors of PAD and the possible correlation with the incidence of MALE. We conducted a literature review of key articles, narrative and systematic reviews, meta-analyzes, clinical studies of peripheral lower limb arterial disease, and major predictors of lower limb adverse events. We filtered the most relevant studies on PubMed and Google Scholar by using keywords and we selected 52 papers that served as a reference bibliography to list the main predictors of MALE in patients with PAD. We also collected the primary studies on the predictors of MALE in a summary table with corresponding bibliographic notes. After a careful review of the main scientific articles on the correlation between PAD risk factors and the incidence of MALE in order to identify the main predictors of adverse outcomes in the lower limbs, 52 articles and 13 main risk factors were selected. We have listed and divided all the predictors in 6 specific Macro Areas and reported all the corresponding bibliographic notes (Table 1). Age was supported by 3 articles and was included in the "Unmodifiable risk factors" Macro Area. Smoking was supported by 4 articles and was included in the "Modifiable Risk Factors" Macro Area. Diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, lower limb neuropathy, microangiopathy were supported by 5, 2, 12, 5, 4 and 2 articles respectively and were collected in the "disease and organic risk factors" Macro Area. Prior MACE, prior revascularization, pharmacological medications were supported by 2, 7 and 14 articles respectively and were collected in the "Individual background atherosclerosis" Macro Area. Serum cytokine levels were supported by 2 articles and were listed in the “serum cytokines” Macro Area. The ankle-arm index was supported by 3 articles and was listed in the "Diagnostic tools" Macro Area.
Table 1.
Predictors of MALE and corresponding references. List of the main predictors of MALE classified as Macro and Microarea, and references of the corresponding articles. MALE: Major Adverse Limb Event; LDL-c: Low-Density Lipoprotein cholesterol; Lp(a): Lipoprotein-a; MACE: Major Adverse Cardio/Cerebrovascular Event; IL-6: Interleukine-6; CRP: C Reactive Protein; TNF-a: Tumor Necrosis Factor-a; OPG: Osteoprotegerin; ABI: Ankle-Brachial Index.
| Macro area | Micro area | References |
|---|---|---|
| Unmodifiable risk factors | Age | [36,63,64] |
| Modifiable risk factors | Smoking | [72,[80], [81], [82]] |
| Disease and organic risk factors | Diabetes mellitus | [55,65,66,69,74] |
| Hypertension | [72,80] | |
| Dyslipidemia [LDL-c, Lp(a)] | [80,[89], [90], [91], [92],95,96,103,[106], [107], [108], [109]] | |
| Chronic kidney disease | [55,72,81,138,139] | |
| Lower limb neuropathy | [[147], [148], [149], [150]] | |
| Microangiopathy | [154,155] | |
| Atherosclerosis individual background | Prior MACE | [56,163] |
| Prior revascularization | [55,81,[188], [189], [190],212,213] | |
| Pharmacological Medications | [41,83,84,89,90,92,93,[178], [179], [180], [181], [182], [183],187] | |
| Serum cytokines | Serum levels cytokines (IL-6, CRP, TNF-a, OPG) | [36,231] |
| DIAGNOSTIC TOOL | ABI rest/post-exercise | [74,176,177] |
3. Predictors of MALE, why so important?
3.1. Age and MALE
Atherosclerosis is a chronic inflammatory process that slowly leads to an endothelial dysfunction, intramural thickening of the vessel subintima and vascular stenosis [27,29]. Atherosclerosis starts after birth and silently grows with us [57]. An older age means a longer exposure to inflammation along with age-related vascular remodeling due to dysfunctional endothelial progenitor cells [58]; therefore, age is an independent risk factor and cause of advanced atherosclerotic disease stage [36,[59], [60], [61], [62]]. Consequently, age is a fundamental unmodifiable risk factor of PAD causing complications such as MALE [36,63]. Indeed, age seems to be a determining factor for MALE both in elderly patients due to the advanced deterioration of the arteries, and in younger individuals with significantly severe atherosclerosis-related comorbidities by demonstrating a more aggressive progression of atherosclerotic disease [64].
3.2. Diabetes mellitus and MALE
Diabetes mellitus (DM) is the main risk factor of PAD alongside smoking [33,34]. PAD patients, additionally affected by DM, suffer from a higher rate of MALE. A linear correlation between MALE incidence and glycemic control (determined by the HbA1c value) was demonstrated, suggesting that a strict pre-operative glycemic control provides a better surgical outcome [65]. In fact, diabetic PAD patients with HbA1c values > 10% before a surgical revascularization are increasingly affected by MALE compared to nondiabetics [66]; the poorer diabetes is controlled, the higher the incidence of MALE. However, preoperative normal values of HbA1c, result of well-controlled long-term glycaemia, has no significant effect on MALE treatment outcomes. This could be explained by the close interdependence between glycemic control and individual inflammatory status [18]. HbA1c is a not very sensitive marker of individuals' glycemic variability that has been observed to be a major obstacle to neoangiogenesis by preventing the formation of new collateral vessels due to impaired VEGF pathway [67,68]. Type 1 diabetes mellitus often involves long-term exposure to high blood glucose values with an increased risk of developing macrovascular complications leading to a high incidence of MALE and mortality [69]. The increased susceptibility of patients with diabetes type 1 mellitus with large glycemic changes and increased accumulation of end products of advanced glycation [70] and growth factors [28] in arterial vessel walls due to an inflammatory trigger increases the incidence of vascular adverse events [28,70]. To the best of our knowledge, little evidence is available on the role of glycemic variability on patient lower limb outcomes, with the need for new dedicated studies. Furthermore, although the duration of diabetes and therefore exposure to hyperglycemia has a significant importance on the progression of micro and macrovascular damage, its effect on the incidence of MALE is still controversial. Duration of diabetes is associated with an increased risk of foot ulcer complications [71], but has not shown a significant correlation with amputation or other adverse limb events [72,73].
DM is also a major cause of higher second-amputation rate in patients with previous amputation due to dysvascular conditions [74]. Additionally, 10% of patients who have undergone revascularization procedures, requires a hospitalization for MALE with diabetes mellitus such as one of the main MALE-related predictors [55]. The treatment of diabetes is a fundamental element for the correct management of the patient with diabetic PAD. Patients should frequently monitor blood glucose values and treat diabetes to keep glycated hemoglobin values below 7%. This therapeutic target is strongly recommended and allows to effectively reduce the risk of MALE [75].
3.3. Smoking and MALE
Smoking is a strong risk factor for atherosclerosis [76]. The association between smoking and PAD is stronger than for coronary artery disease [33,34,77], with a linear correlation between exposure to smoking and PAD development [78]. Although a high incidence persists in former smokers [79], smoking duration and point in time of quitting smoking (early vs. late in life) reduces PAD risk [76]. Smoking influences disease location with more proximal vascular lesions in smokers compared with non-smokers, and more distal critical stenosis in diabetic smokers compared with non-diabetic smokers. The duration of chronic exposure to smoking has a documented higher incidence of MALE [80], in particular if associated to diabetes [72], which confirms the fundamental role of non-medical interventions, such as mitigating risk factors, in PAD management [80]. Smoking is a predictive factor of amputation and post-amputations outcomes demonstrating the importance of quitting smoking habits in order to prevent disabling morbidity related to cigarettes [81]. Interestingly, living in distressed communities is significantly associated with smoking habit. Moreover, stressed individuals are more frequently affected by several comorbidities are more reluctant to follow non-medical interventions or assume pharmacological therapies and show a higher rate of MALE [82]. The intensity of smoking determines a cumulative risk of MALE in patients who underwent a revascularization procedure. Personal smoking behavior is a basic screening for PAD patients and a detailed description with a quantification of the individual smoking exposure allows a more precise prediction of post procedural outcomes rather than the approximate classification in “non-smoker, current smoker or former smoker” [82].
3.4. Hypertension and MALE
MALE incidence has a direct correlation with uncontrolled blood pressure [80] and an adequate pharmacological treatment (e.g., angiotensin converting enzyme inhibitors and angiotensin II receptor blockers) achieves increased limb salvage rates and better outcomes in high-risk PAD patients [83,84]. Furthermore, the presence of hypertension in PAD patients with diabetic foot ulcers increases the risk of amputation and re-amputation operations leading to disabling outcomes [72]. The target systemic blood pressure values recommended by the guidelines are below 130/80 mmHg since adequate blood pressure control has shown evident benefits on disease progression and reduces the incidence of complications. However, recent new evidence suggests an increased risk of MALE even in patients with systolic blood pressure below 120 mmHg with the need for further studies to identify the best therapeutic approach to manage the hypertension of our patients [[85], [86], [87]].
3.5. Lipid profile and MALE
“The lower, the better”, is a valid statement regarding serum cholesterol concentrations in PAD patients affected by dyslipidemia [88]. A growing body of evidence confirms the importance of achieving the therapeutic goal for low-density lipoprotein cholesterol since there is a consistent linear correlation between lowering serum LDL-c levels and prevention of limb adverse events [89]. Additionally, reduction of non-HDL cholesterol at very low levels results in a significant beneficial impact on prevention of MALE [80] together with greater tendency to survival, in parallel with a fairly certain safety for patients with PAD [90]. Pharmacological reduction of low-density lipoprotein-cholesterol (LDL-c) is beneficial for PAD outcomes [91], and high-intensity statins are a mainstay of this treatment with proven effect on MALE incidence [91,92]. Moreover, patients, taking statins for secondary prevention after revascularization, showed a longer patency rate of treated lesions [93]. Unfortunately, an efficient educational program for patients supporting clinicians to manage correctly PAD, is still an unmet aim [80,94]. In fact, sensitizing both clinicians and patients to introduce statins in therapy, could reduce MALE incidence [95]. However, the reduction of LDL-c would miss a residual cardiovascular risk [96] due to the discovery of another molecule that has recently attracted the interest of researchers. Lipoprotein a or Lp(a) is a complex polymorphic lipoprotein produced by the liver and it is structurally similar to LDL-c. In fact, this molecule has a strong association with PAD [97,98] and MACE [97,[99], [100], [101], [102], [103], [104], [105]]. Furthermore, Lp(a) is an independent risk factor of MALE [96,103,[106], [107], [108], [109]] and showed a linear correlation between serum concentration and rate of vascular events [107]. Currently, there are a few therapeutic strategies to manage Lp(a)-related risk, such as apolipoprotein (a) targeted antisense therapy (RNA-targeted therapies) [100,110], the PCSK9 inhibitors already in use for the known cholesterol-lowering effect [110] and apheresis [103,110] which showed a significant effect of reducing MALE occurrence. New molecules (such as bempedoic acid [[111], [112], [113], [114], [115], [116]], niacin [[117], [118], [119], [120], [121], [122]] and carnitine [[123], [124], [125], [126], [127], [128]]) with promising results in amelioration of lipid profile has been introduced but they are not yet backed by solid scientific support for routine use in clinical practice. In recent years there has been a greater understanding of the cardiovascular risk of patients with PAD with the strong recommendation to take a more aggressive approach to the treatment of dyslipidemia. Previous indications on target LDL cholesterol [129] have been revised and patients diagnosed with PAD have been included in the risk class defined as "very high" with therapeutic goal LDL cholesterol values < 55 mg/dL [88],[[130], [131], [132]].While triglycerides must be kept below 150 mg/dL, the role of triglycerides on cardiovascular risk in PAD [88] remains controversial.
3.6. Chronic kidney disease and MALE
Aging, diabetes mellitus and chronic kidney disease (CKD) are known factors of vascular calcification. Vascular calcification is the result of the intimal and tunica media vessel calcification leading to complete vascular calciphylaxis, which is associated with increased morbidity and mortality. Several factors contribute to vascular calcification but new discoveries are needed to further understand this phenomenon. Bone morphogenetic proteins (BMPs) modulate bone production and their localization in vessels’ tunica media could be associated to vascular smooth muscle cells (VSMCs) osteoblastic differentiation mineralizing the extracellular matrix [133]. CKD reduces serum fetuin A levels [134] that inhibits calcification of vessel walls bonding the circulating calcium and phosphate ions, promotes bone mineral accretion and, despite controversial opinions about its vascular beneficial role [135], appears to be a protective factors of cardiovascular disease [136]. Diabetes mellitus and CKD promotes exponentially vessels calciphylaxis [137]. In fact, the association of DM and CKD accelerates the medial arterial calcification in symptomatic PAD patients resulting in a high incidence of MALE [138] and urgent treatment, slowing down the progression of kidney disease to ameliorate PAD outcomes, is required. Chronic kidney disease is an independent risk factor for revascularization failure and amputation, from the early stage of pathological proteinuria to the end stage of renal failure requiring dialysis [55,72,81,139]. PAD patients affected by CKD, who underwent a limb endovascular revascularization (LER), are often re-hospitalized for MALE, which confirms the predictive role of CKD in PAD outcomes [140]. Thus, PAD patient management must include the prevention of kidney disease and a rigorous follow-up since it contributes to atherosclerotic progression. Furthermore, the presence of CKD is related to a more severe PAD disease (e.g., advanced Leriche-Fontaine and Rutherford classification scores) and it is an independent risk factor for MALE at any stage of kidney disease [141]. A new challenge is to identify the best revascularization strategy for end-stage renal disease PAD population where MALEs are responsible of poor outcomes [139]. People affected by end-stage renal disease often suffer from PAD with a high risk of MALE but their frailty strongly restricts therapeutic options [142].
3.7. Lower limb neuropathy and MALE
Diabetes is the main cause of neuropathy [143] and this disabling complication often has a silent onset that in a large percentage of patients leads to serious foot complications such as traumatic foot ulcers, infections, inability to walk and non-traumatic amputations. Hyperglycemia together with various molecular pathways involved in neuro-trophism and oxidative stress, contribute to the dramatic degeneration of peripheral nerves and related complications [144,145]. A common cause of hospitalization of patients with diabetic neuropathy is foot ulcer infection but unfortunately specific therapeutic approaches and effective prevention programs are not yet available [146]. Amputation is a common failure outcome in patients with diabetic neuropathy and arterial disease [147]. Local neuropathic impairment of vascular auto-regulation has been shown to be a strong predictor of MALE by promoting the incidence of neuro-ischemic ulcers [148]. Diabetic neuropathy affects patient survival and quality of life, as well, due to the high risk of hospitalizations and amputation rates [149]. Therefore, all diabetic patients should be regularly screened for early diagnosis of diabetic neuropathy in order to intervene on risk factors and slow the progression of the disease preventing the onset of MALE [150].
3.8. Microangiopathy and MALE
Capillary microangiopathy is a disease characterized by a progressive occlusion of the small terminal vessels that supply organs and tissues and supply nourishment to the walls of the great arteries, contributing to macrovascular pathology. Diabetic microangiopathy is often associated with the involvement of precapillary arterioles, further complicating the healing of neuro-ischemic foot ulcers [151]. Currently we do not have effective diagnostic techniques to be used in the clinical setting to characterize the degree of microangiopathy [152], but we know their role in progression of peripheral arterial disease together with macrovascular involvement [153]. In fact, a growing body of evidence has shown that microangiopathy and macrovascular disease simultaneously participate in the deterioration of the vessels and both contribute together with ischemic damage of the tissues of the lower limbs which increases the risk of amputation [154].
Microangiopathy impairs tissue perfusion and nutrition, deteriorates the complex interaction between thermoregulation, arterial blood flow, neurogenic control of the vascular system and endothelial function. Therefore, microvascular damage is a fundamental component that increases the risk of MALE as it establishes a profound structural change and irreversible dysfunction of the vascularity [155]. The reduction of glycated hemoglobin values by improving the control of diabetes is the most effective therapeutic approach for slow the progression of microangiopathic damage and the risk of MALE [156].
3.9. Prior stroke, myocardial infarction or limb amputation and MALE
PAD is a main disabling expression of atherosclerosis as well as a risk factor for other atherosclerotic manifestations such as stroke, transient ischemic attack and myocardial infarction [41,51,157,158]. Conversely, patients hit by prior cardiovascular and cerebrovascular events or prior limb amputation, have a higher incidence of PAD, which suggests that the involvement of multiple vascular sites determines a more aggressive and advanced atherosclerotic phenotype [159]. Moreover, prior cardio-cerebrovascular events and limb amputation in PAD patients are predictors of poor outcomes [[160], [161], [162]] and an independent factor of MALEs [56,163]. PAD patients deserve a comprehensive management of every single atherosclerotic complication, regardless of their location, to delay the progression of the disease and to reduce the incidence of MALEs.
3.10. Ankle-Brachial Index and MALE
The patient with subclinical PAD is a high-risk individual who requires timely diagnosis to intercept the disease before it can develop late-stage complications. Despite the limitations that emerge from the literature [[164], [165], [166]], ABI remains the first non-invasive tool in the diagnosis of PAD [46,[166], [167], [168]] with an additional prognostic value on the incidence of death and MACE [[169], [170], [171], [172], [173], [174], [175]]. In addition, as regards its prognostic value, a higher incidence of revascularization procedures and lower limb amputations [74] was found in patients with altered ABI values both at rest and after exercise. Furthermore, the trend towards higher all-cause mortality as well as a higher rate of cardiovascular events and poorer limb outcomes were also confirmed in presence of abnormal ABI [176,177].
3.11. Pharmacological therapy and MALE
PAD’s best therapeutic strategies are still unknown and the urgency to discover new effective approaches is encouraging and stimulating new studies. However, the principal aim is a durable and effective improvement of modifiable risk factors [[46], [47], [48]]. Many therapeutic interventions, acquired from cardiovascular and cerebrovascular disease, have been applied to slow down PAD progression and to reduce the incidence of major adverse events. In fact, antiplatelet therapy and statin therapy are the mainstay of PAD treatment. These drugs are effectively used for primary and secondary prevention; while, lowering-lipid therapy (such as statin use) along with antiplatelet therapy effectively reduce MALE risk [93,95,[178], [179], [180], [181], [182]]. Additionally, the further decrease of serum cholesterol, promoted by new PCSK9 inhibitors, determines a significant reduction of MALE with a reliable safety profile [89,90]. Although the best pharmacological approach has not been defined yet, studies suggest new interesting treatment combinations. Symptomatic PAD patients who required LER often suffer from a higher MALE rate [36], but the use of a dual antiplatelet therapy (DAPT) for ≥6 months after the procedure seems to be a safe, correlated with a low bleeding rate, and effective intervention to reduce MALE incidence [183]. Evidences about anticoagulants in PAD encouraged new studies proving a safe combination with antiplatelet therapy and a superior efficacy in comparison with mono-antiplatelet therapy alone [184]. Anticoagulation protects from the incidence of ischemic events in PAD even in high risk patients with multiple comorbidities [185]. The principal limit of antithrombotic therapy is bleeding. However, its incidence is relatively low and mostly affects patients with a more advanced atherosclerotic disease stage because of vessel frailty [186]. Moreover, treatment cessation due to bleeding is an independent risk factor of subsequent ischemic events [186]. Therefore, the significant reduction in the incidence of MALE in individuals taking a combination of antiplatelet and low-dose anticoagulant therapies supports this optimization of antithrombotic treatment in patients with a history of symptomatic CAD or PAD [41,187]. Interestingly, angiotensin converting enzyme inhibitors and angiotensin receptor blockers in PAD demonstrated longer amputation-free survival due to their anti-remodeling effect on arterial vessels [83,84]. Risk factor correction, symptom control, serum lipid lowering and antithrombotic therapy are the mainstays of PAD management and the new challenge is to adopt new evidence-based therapeutic approaches to reduce disease progression and MACE and MALE incidence [94].
3.12. Prior revascularization, procedure performer or adopted technique and MALE
Symptomatic PAD is an advanced stage of atherosclerotic disease requiring often a prompt intervention. In this case, revascularization is an effective therapeutic approach to restore adequate blood flow to ischemic tissues. Generally, revascularization is the most effective treatment with unmatched efficiency in terms of limb salvage, pain relief, wound healing and speed of results. In past, narrowing of arterial vessels and the presence of vascular stenosis were the main criteria for performing revascularization. Therefore, the restoration of the patency of the arterial vessels guided the therapeutic process towards an invasive treatment. However, the relatively recent awareness of a progression of PAD with a consistent risk of MALE [55,81,[188], [189], [190]] has led to the fundamental recommendation to prioritize the correction of risk factors, optimize pharmacological therapy and increase exercise tolerance through the introduction of supervised exercise programs. Revascularization at any stage of PAD is an independent risk factor of worse long-term limb salvage outcomes, therefore, the real clinical need for prompt revascularization should guide the physician to seek such treatment.
Although, preliminary studies demonstrated that an endovascular and an open revascularization are comparable [[191], [192], [193], [194]], it remains still unclear, which technique is most effective and safe to reduce MALE [195], and which patient characteristics can be used to determine suitability of endovascular or open revascularization. Some individuals are more suited for an open surgical revascularization approach than an endovascular technique (and vice versa). Therefore, the next challenge is to identify the optimal revascularization strategy for each patient [196]. Additionally, the vascular anatomy, stenosis location [142], technique adoption and experience of the surgeon [166] are variables that should guide physicians towards the best revascularization approach. The gold standard for PAD revascularization is an open surgery that provides the best long-term results with lower MALE incidence. A longer vessel patency obtained with a surgical approach, correlates with a higher incidence of perioperative MACE [197,198] and this contributes to increased costs [142]. In particular, endovascular revascularization guarantees lower short-term adverse events, especially in PAD patients characterized by significant frailty related to comorbidities [[198], [199], [200], [201]]. First, surgical suitability should be always evaluated [202,203] and endovascular revascularization should be offered as a second valid option in patients with high surgical risks or unfavorable vascular characteristics [204], although a higher incidence of MALE was observed. The most suitable surgical strategy should be determined considering personal characteristics and the individual surgical risks [205]. In fact, in frail patients, (e.g., in diabetic PAD), acceptable patency outcomes, cost effective results, shorter hospitalization time [191] and lower short-term adverse events suggest endovascular revascularization as the best approach [192,206]. Moreover, in some comorbid patients, endovascular revascularization resulted in lower MALE rates [207]. Since LER is increasingly applied, several studies compared techniques to identify which technique and correlated factors resulted in better outcomes, like for example reducing MALE incidence [[208], [209], [210], [211]]. The performer experience seems to contribute fundamentally to patient outcomes after limb revascularization. An higher incidence of MALE was observed in procedures executed by non-cardiologists or non-revascularization specialist, suggesting that patients should be referred to high-volume revascularization centers [55]. Arterial limb revascularization is an effective symptomatic treatment, but whether it is always a worthy approach, is not clear yet. Therefore, prior revascularization is an independent risk factor for MALE [212,213] and determines an inflammatory burden, which could promote vascular inflammation and subsequent complications [[214], [215], [216]]. Currently, controversial opinions about the best revascularization approach exist and further studies are required to obtain stronger evidences [217] to provide guidelines, which could contribute to reduce significantly MALE incidence.
3.13. Serum cytokines and MALE
The prediction of MALE could be a fundamental strategy to prevent a treatment failure in diabetic PAD. The interest on predictive role of cytokines in PAD [218] to prevent vascular complications lead to expand further their function in vascular inflammation and degeneration [219], LDL cholesterol oxidation, endothelial cell dysfunction, regulation of calcium metabolism, modulation of foam cell activity and the influence on platelet adhesion [27,29,[220], [221], [222], [223], [224], [225], [226], [227], [228], [229]]. However, only a few of these molecules have shown clinical importance for monitoring disease progression or as predictors of adverse outcomes [230].
Pre-procedural inflammatory status of PAD patients should be routinely assessed as elevated pre-procedural serum cytokine levels are associated with a significant increase in rate of MALE [231]. Indeed, the interaction between individual proinflammatory status and the vulnerability of atherosclerotic plaques [232] could potentially explain the progression to harmful vascular complications.
The relevant interaction between cytokines and MALE is an interesting topic that requires further investigation in order to develop new therapeutic and preventive strategies for limb outcomes. A linear correlation between the increasing serum levels of these molecules and MALE rate was demonstrated [36]. Basal concentrations of serum high sensitivity C-Reactive Protein (hsCRP), C-Reactive Protein (CRP) [233,234], omentin-1 [235], High Mobility Group Box-1 [236], tumor necrosis factor-ɑ (TNF-ɑ) [237], interleukin-6 (IL-6) [227] and osteoprotegerin (OPG) [225,226,229,238] were measured in a population of diabetic PAD patients affected by a below-the-knee occlusive disease who were candidate to an angioplasty. MALE prediction was notably more precise and strongly enforced by the presence of higher serum levels of each cytokine [36]. Therefore, individual blood samples could predict suitability of diabetic patients for revascularization, support physicians to identify patients with higher risks of limb revascularization failure and their follow-up care to prevent MALE.
5. Discussion
PAD is certainly one of the most disabling complications of diabetes mellitus, although extensive guidelines have only recently been created for the correct management of patients at risk or affected by this disease. Unlike the more well-known cardiovascular adverse events of diabetes, the incidence of MALE is an underestimated complication that leads to disastrous clinical consequences. Unfortunately, patients come to the clinician when the disease is already in a very advanced stage, limiting therapeutic opportunities. The support of the current guidelines has made it possible to establish which are the priorities of the patient with PAD in terms of primary, secondary prevention and revascularization treatment [[239], [240], [241], [242]]. All patients with diabetes should undergo regular outpatient check-ups with vascular ultrasound. Moreover, the ABI measurement remains a cost-effective preliminary tool recommended as a first screening approach [243,244].
Once patients with asymptomatic and symptomatic PAD (in its different stages of clinical presentation) have been identified, it is essential to understand the risk of cardiovascular consequences to which this population is exposed. In fact, this category of patients is considered to be at “very high risk” of developing MACE and MALE with the urgent need to set up an effective secondary prevention to face the otherwise inevitable and disastrous outcomes. Diabetes management has the therapeutic objective of maintaining glycated hemoglobin values below 7% for most patients and recently new drug classes (SGLT2 inhibitors [4,[245], [246], [247], [248]] and Glucagon-Like Peptide-1 Receptor Agonists [249,250]) have shown an incredible contribution to the reduction of glycated hemoglobin values with additional beneficial effect on patients' risk of MALE and MACE [251].
The management of systemic arterial hypertension in PAD is still under study as the therapeutic range of blood pressure values is very narrow with risk of adverse events and mortality for blood pressure above 130/80 mmHg and systolic values below 120 mmHg [86].
The new therapeutic targets for the control of dyslipidemia represent a fundamental contribution for the improvement of clinical outcomes and the protection of the cardiovascular risk of patients with PAD. Indeed, the diagnosis of PAD, like CAD, is an intrinsic risk factor for the development of macrovascular complications. Therefore, PAD patients have been included in the highest risk category. Most “very high-risk” patients must reach LDL cholesterol values below 55 mg/dL demonstrating a consistent reduction in MACE and MALE occurrence [252]. A growing body of evidence confirms the safety of LDL cholesterol values even below 40 mg/dL in those with residual cardiovascular risk despite proper management of the other risk factors [131].
Finally, following scientific advances in CAD, it has been possible to improve the revascularization techniques of patients with threatening limb ischemia. The use of drug-coated balloon angioplasty and/or drug-eluting stents during revascularization procedures has led to enormous results in ensuring greater patency of the treated vessel and clinical benefits. The use of a double antiplatelet therapy for one month is also recommended after an endovascular revascularization procedure, although there is still a huge difference in knowledge from the cardiovascular world on this topic. However, the incredible results observed with the use of low-dose anticoagulants in CAD have already been confirmed in PAD which can therefore benefit from this additional therapeutic support to reduce the incidence of MALE and MACE. Currently, there are promising results on the treatment of the residual risk due to the systemic low-grade inflammation with the use of immunomodulatory therapies such as colchicine. In summary, the current European and American guidelines consider PAD among the main topics with growing scientific interest, epidemiological importance and therapeutic discoveries.
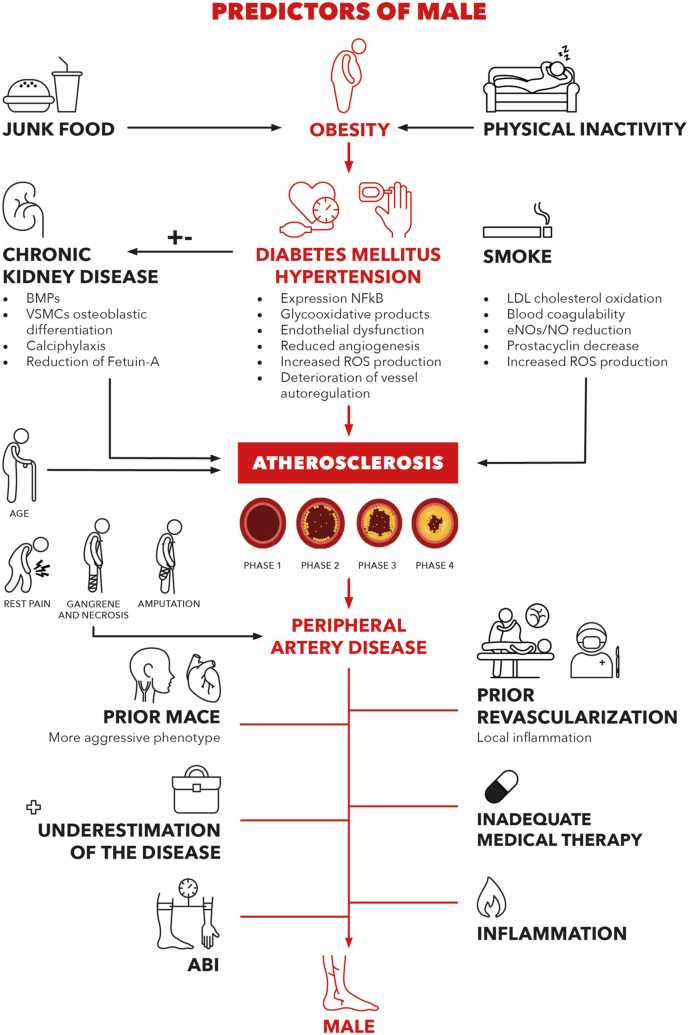
The growing prevalence of diabetes mellitus and its vascular disabling complications1 determined an increasing limb revascularization rate. Compared to the past, outpatient centers and hospitals offer increasingly revascularization and more patients are treated for chronic limb ischemia [55]. Not surprisingly, apparently similar patients who underwent the same revascularization procedure showed different results in terms of vessel patency, incidence of MALE and rate of cardio- and cerebrovascular events [36,55]. Follow-up programs after a revascularization often do not consider these individual differences hampering major adverse events prevention. Diabetic PAD patients are extremely frail, suffer from high complication rates and encounter prohibitive surgical risks; LER is suitable for diabetic PAD patients to achieve ischemic pain relief [192,206,207]. Unfortunately, a considerable part of patients who underwent LER is consequently affected by dramatic complications (e.g., MACE and MALE) determining the emerging doubt whether an invasive intervention is always worthy. In Fig. 1, all possible players of MALE pathogenesis are represented. Revascularization failure is responsible for disabling complications, additional costs and poor patient outcomes. We aimed at (Table 1) collecting all modifiable and unmodifiable risk factors related with PAD initiation and progression and, those related with increased MALE risk, to propose a set of main major adverse limb event and LER failure predictors to estimate the risk of MALE. Prompt identification of high-risk patients is critical to prevent disabling vascular complications through stricter follow-up programs, effective residual risk factor correction, anticipation of needed interventions. Furthermore, personalization of care is essential for this category of patients and very fragile individuals. Clinical practice is enriched by bed-side and predictive scores to guide physicians’ decisions like for example the CHA2DS2-VASc and HAS-BLED scores for atrial fibrillation, stroke [253] and bleeding risks [254]; the Padua, Geneva and Improve scores for thromboembolism risk assessment; or the ABCD2 score for stroke prediction after TIA [255]. However, the estimation of the incidence of MALE in PAD, especially after a revascularization procedure, is still an unmet need, which we wish to contribute to understand. Although this is a critical aspect of peripheral artery disease, a greater scientific contribute is needed to improve the management of our patients. Our goal is to provide a useful and immediately available synopsis of the latest knowledge on the factors that increase the incidence of MALE in PAD, in order to facilitate the creation of a predictive score that can be applied in the clinical setting.
Fig. 1.
Predictors of Male: Main risk factors promoting the atherosclerotic process underlying peripheral artery disease. All possible predictors of major adverse limb events. MALE: Major Adverse Limb Event; BMP’s: Bone Morphogenetic Proteins; VSMCs: Vascular Smooth Muscle Cell; NFkB: Nuclear Factor Kappa-light-chain-enhancer of activated B cells; ROS: Reactive Oxygen Species; LDL: Low-Density Lipoprotein; eNOs: endothelial Nitric Oxide Synthase; MACE: Major Adverse Cardio/Cerebrovascular Event; ABI: Ankle-Brachial Index.
Funding and financial support
Not applicable.
Author contributions
All the authors contributed to the conception and design of the work, to the collection of data, to the implementation of the research, to the discussion of the results and to the drafting of the manuscript with the final approval of the version to be published.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Franziska Lohmeyer for her English language assistance.
References
- 1.Thiruvoipati T., Kielhorn C.E., Armstrong E.J. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. Jul 2015;6(7):961–969. doi: 10.4239/wjd.v6.i7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9. Diabetes Res Clin Pract. Nov 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Beckman J.A., Duncan M.S., Damrauer S.M., et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 08 2019;140(6):449–458. doi: 10.1161/CIRCULATIONAHA.119.040672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dicembrini I., Tomberli B., Nreu B., et al. Peripheral artery disease and amputations with Sodium-Glucose co-Transporter-2 (SGLT-2) inhibitors: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. Jul 2019;153:138–144. doi: 10.1016/j.diabres.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Badjatiya A., Merrill P., Buse J.B., et al. Clinical outcomes in patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Circ Cardiovasc Interv. 12 2019;12(12) doi: 10.1161/CIRCINTERVENTIONS.119.008018. [DOI] [PubMed] [Google Scholar]
- 6.Hanssen N.M.J., Teraa M., Scheijen J.L.J.M., et al. Plasma methylglyoxal levels are associated with amputations and mortality in severe limb ischemia patients with and without diabetes. Diabetes Care. 01 2021;44(1):157–163. doi: 10.2337/dc20-0581. [DOI] [PubMed] [Google Scholar]
- 7.Lebovitz H.E. Etiology and pathogenesis of diabetes mellitus. Pediatr Clin. Jun 1984;31(3):521–530. doi: 10.1016/s0031-3955(16)34604-1. [DOI] [PubMed] [Google Scholar]
- 8.Saberzadeh-Ardestani B., Karamzadeh R., Basiri M., et al. Type 1 diabetes mellitus: cellular and molecular pathophysiology at A glance. Cell J. Oct 2018;20(3):294–301. doi: 10.22074/cellj.2018.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galicia-Garcia U., Benito-Vicente A., Jebari S., et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. Aug. 2020;30(17):21. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punthakee Z., Goldenberg R., Katz P., Diabetes Canada Clinical Practice Guidelines Expert Committee Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. Apr 2018;42(Suppl 1):S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Peterson N.E., Sirard J.R., Kulbok P.A., DeBoer M.D., Erickson J.M. Sedentary behavior and physical activity of young adult university students. Res Nurs Health. 02 2018;41(1):30–38. doi: 10.1002/nur.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X.Y., Han L.H., Zhang J.H., Luo S., Hu J.W., Sun K. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: a systematic review. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garber A.K., Lustig R.H. Is fast food addictive? Curr Drug Abuse Rev. Sep. 2011;4(3):146–162. doi: 10.2174/1874473711104030146. [DOI] [PubMed] [Google Scholar]
- 14.Filgueiras A.R., Pires de Almeida V.B., Koch Nogueira P.C., et al. Exploring the consumption of ultra-processed foods and its association with food addiction in overweight children. Appetite. 04 2019;135:137–145. doi: 10.1016/j.appet.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Datar A., Nicosia N. Junk food in schools and childhood obesity. J Pol Anal Manag. 2012;31(2):312–337. doi: 10.1002/pam.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elimam H., Abdulla A.M., Taha I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab Syndr. 2019 Jan - Feb 2019;13(1):800–804. doi: 10.1016/j.dsx.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M.I., Duncan B.B., Sharrett A.R., et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. May 1999;353(9165):1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 20.Donath M.Y. Inflammation and type 2 diabetes: from basic science to treatment. Semin Immunopathol. 07 2019;41(4):411–412. doi: 10.1007/s00281-019-00749-0. [DOI] [PubMed] [Google Scholar]
- 21.Yuan M., Konstantopoulos N., Lee J., et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. Aug 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 22.Uysal K.T., Wiesbrock S.M., Marino M.W., Hotamisligil G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. Oct 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg H.O., Chaker H., Leaming R., Johnson A., Brechtel G., Baron A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. Jun 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaftenaar F., Frodermann V., Kuiper J., Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 06 2016;27(3):209–215. doi: 10.1097/MOL.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 25.Duncan B.B., Schmidt M.I., Pankow J.S., et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. Jul 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 26.Galland L. Diet and inflammation. Nutr Clin Pract. Dec 2010;25(6):634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 27.Biscetti F., Nardella E., Cecchini A.L., Landolfi R., Flex A. The role of the microbiota in the diabetic peripheral artery disease. Mediat Inflamm. 2019;2019:4128682. doi: 10.1155/2019/4128682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martí-Carvajal A.J., Gluud C., Nicola S., et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. Oct 28 2015;(10):CD008548. doi: 10.1002/14651858.CD008548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan T., Yang T., Chen H., et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 01 2019;20:247–260. doi: 10.1016/j.redox.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signorelli S.S., Scuto S., Marino E., Xourafa A., Gaudio A. Oxidative stress in peripheral arterial disease (PAD) mechanism and biomarkers. Antioxidants (Basel) Sep 2019;8(9) doi: 10.3390/antiox8090367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowkes F.G., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. Oct 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 35.Aronow H., Hiatt W.R. The burden of peripheral artery disease and the role of antiplatelet therapy. Postgrad Med. Jul 2009;121(4):123–135. doi: 10.3810/pgm.2009.07.2038. [DOI] [PubMed] [Google Scholar]
- 36.Biscetti F., Ferraro P.M., Hiatt W.R., et al. Inflammatory cytokines associated with failure of lower extremity endovascular revascularization (LER): a prospective study of a population with diabetes. Diabetes Care. Aug 2019 doi: 10.2337/dc19-0408. [DOI] [PubMed] [Google Scholar]
- 37.Leibson C.L., Ransom J.E., Olson W., Zimmerman B.R., O'fallon W.M., Palumbo P.J. Peripheral arterial disease, diabetes, and mortality. Diabetes Care. Dec 2004;27(12):2843–2849. doi: 10.2337/diacare.27.12.2843. [DOI] [PubMed] [Google Scholar]
- 38.Stoffers H.E., Rinkens P.E., Kester A.D., Kaiser V., Knottnerus J.A. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. Apr 1996;25(2):282–290. doi: 10.1093/ije/25.2.282. [DOI] [PubMed] [Google Scholar]
- 39.McDermott M.M., Kerwin D.R., Liu K., et al. Prevalence and significance of unrecognized lower extremity peripheral arterial disease in general medicine practice. J Gen Intern Med. Jun. 2001;16(6):384–390. doi: 10.1046/j.1525-1497.2001.016006384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leng G.C., Lee A.J., Fowkes F.G., et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. Dec. 1996;25(6):1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 41.Anand S.S., Caron F., Eikelboom J.W., et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 05 2018;71(20):2306–2315. doi: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Miyata T., Higashi Y., Shigematsu H., et al. Evaluation of risk factors for limb-specific peripheral vascular events in patients with peripheral artery disease: a post hoc analysis of the season prospective observational study. Angiology. Jul. 2019;70(6):506–514. doi: 10.1177/0003319718814351. [DOI] [PubMed] [Google Scholar]
- 43.Domínguez-Olmedo J.M., Munuera-Martínez P.V., Sáez-Díaz A., Palomo-Toucedo I.C., Vázquez-Bautista C., Reina-Bueno M. Impact of peripheral artery disease on the quality of life of patients with diabetes mellitus. Foot (Edinb). Dec 2019;41:1–5. doi: 10.1016/j.foot.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Desai R., Singh S. Disparities in health care cost in peripheral arterial disease-related hospitalizations: a nationwide analysis stratified by age, sex, race, and type of admission. Atherosclerosis. 12 2019;291:132–133. doi: 10.1016/j.atherosclerosis.2019.06.917. [DOI] [PubMed] [Google Scholar]
- 45.Hasvold P., Nordanstig J., Kragsterman B., et al. Long-term cardiovascular outcome, use of resources, and healthcare costs in patients with peripheral artery disease: results from a nationwide Swedish study. Eur Heart J Qual Care Clin Outcomes. 01 2018;4(1):10–17. doi: 10.1093/ehjqcco/qcx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correction. Am Fam Physician. 07 2019;100(2):74. [PubMed] [Google Scholar]
- 47.Colantonio L.D., Muntner P. It is time for reducing global cardiovascular mortality. Circulation. 08 2019;140(9):726–728. doi: 10.1161/CIRCULATIONAHA.119.041653. [DOI] [PubMed] [Google Scholar]
- 48.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med. 2016;22(3):NP1–NP43. doi: 10.1177/1358863X17701592. 06 2017. [DOI] [PubMed] [Google Scholar]
- 49.Biscetti F., Pecorini G., Straface G., et al. Cilostazol promotes angiogenesis after peripheral ischemia through a VEGF-dependent mechanism. Int J Cardiol. Aug. 2013;167(3):910–916. doi: 10.1016/j.ijcard.2012.03.103. [DOI] [PubMed] [Google Scholar]
- 50.Biscetti F., Pecorini G., Arena V., et al. Cilostazol improves the response to ischemia in diabetic mice by a mechanism dependent on PPARγ. Mol Cell Endocrinol. Dec. 2013;381(1–2):80–87. doi: 10.1016/j.mce.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Biscetti F., Ferraro P.M., Hiatt W.R., et al. Inflammatory cytokines associated with failure of lower-extremity endovascular revascularization (LER): a prospective study of a population with diabetes. Diabetes Care. Oct 2019;42(10):1939–1945. doi: 10.2337/dc19-0408. [DOI] [PubMed] [Google Scholar]
- 52.Lichtenberg M. Peripheral artery disease: endovascular therapy. Med Monatsschr Pharm. Mar. 2017;40(3):102–106. [PubMed] [Google Scholar]
- 53.Petersohn S., Ramaekers B.L.T., Olie R.H., et al. Comparison of three generic quality-of-life metrics in peripheral arterial disease patients undergoing conservative and invasive treatments. Qual Life Res. Aug. 2019;28(8):2257–2279. doi: 10.1007/s11136-019-02166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaddi A.V., Cicero A.F. [Treatment of peripheral obstructive artery disease: a battle that could be winned also with drugs?] Minerva Cardioangiol. Dec. 2005;53(6):605–610. [PubMed] [Google Scholar]
- 55.Hess C.N., Rogers R.K., Wang T.Y., et al. Major adverse limb events and 1-year outcomes after peripheral artery revascularization. J Am Coll Cardiol. 08 2018;72(9):999–1011. doi: 10.1016/j.jacc.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 56.Kazakov Y.I., Lukin I.B., Sokolova N.Y., Ivanova O.V., Bakulina A.V. [Outcomes of revascularizing operations on lower-limb arteries in patients with critical ischaemia and multifocal atherosclerosis] Angiol Sosud Khir. 2019;25(3):114–121. doi: 10.33529/ANGIO2019317. [DOI] [PubMed] [Google Scholar]
- 57.de Nigris F., Cacciatore F., Mancini F.P., et al. Epigenetic hallmarks of fetal early atherosclerotic lesions in humans. JAMA Cardiol. 12 2018;3(12):1184–1191. doi: 10.1001/jamacardio.2018.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J.X., Pan Y.Y., Wang X.X., Qiu Y.G., Mao W. Endothelial progenitor cells in age-related vascular remodeling. Cell Transplant. 05 2018;27(5):786–795. doi: 10.1177/0963689718779345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. Apr 2015;116(9):1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 60.McDivitt J.D., Braun M., Kassop D. Cardiovascular disease: lower extremity peripheral artery disease. FP Essent. Apr. 2019;479:11–15. [PubMed] [Google Scholar]
- 61.Golledge J., Biros E., Bingley J., Iyer V., Krishna S.M. Epigenetics and peripheral artery disease. Curr Atheroscler Rep. Apr. 2016;18(4):15. doi: 10.1007/s11883-016-0567-4. [DOI] [PubMed] [Google Scholar]
- 62.Vlachopoulos C., Georgakopoulos C., Koutagiar I., Tousoulis D. Diagnostic modalities in peripheral artery disease. Curr Opin Pharmacol. 04 2018;39:68–76. doi: 10.1016/j.coph.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Costa R.H.R., Cardoso N.A., Procópio R.J., Navarro T.P., Dardik A., de Loiola Cisneros L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Syndr. Dec 2017;11(Suppl 2):S583–S587. doi: 10.1016/j.dsx.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Bruun C., Siersma V., Guassora A.D., Holstein P., de Fine Olivarius N. Amputations and foot ulcers in patients newly diagnosed with type 2 diabetes mellitus and observed for 19 years. The role of age, gender and co-morbidity. Diabet Med. Aug. 2013;30(8):964–972. doi: 10.1111/dme.12196. [DOI] [PubMed] [Google Scholar]
- 65.Singh N., Zeng C., Lewinger J.P., et al. Preoperative hemoglobin A1c levels and increased risk of adverse limb events in diabetic patients undergoing infrainguinal lower extremity bypass surgery in the Vascular Quality Initiative. J Vasc Surg. 10 2019;70(4):1225–1234. doi: 10.1016/j.jvs.2018.12.041. e1. [DOI] [PubMed] [Google Scholar]
- 66.McGinigle K.L., Kindell D.G., Strassle P.D., et al. Poor glycemic control is associated with significant increase in major limb amputation and adverse events in the 30-day postoperative period after infrainguinal bypass. J Vasc Surg. Mar. 2020 doi: 10.1016/j.jvs.2019.11.048. [DOI] [PubMed] [Google Scholar]
- 67.Biscetti F., Pitocco D., Straface G., et al. Glycaemic variability affects ischaemia-induced angiogenesis in diabetic mice. Clin Sci (Lond). Dec 2011;121(12):555–564. doi: 10.1042/CS20110043. [DOI] [PubMed] [Google Scholar]
- 68.Biscetti F., Gaetani E., Flex A., et al. Peroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vascular endothelial growth factor upregulation. J Vasc Res. 2009;46(2):103–108. doi: 10.1159/000143793. [DOI] [PubMed] [Google Scholar]
- 69.Mohammedi K., Potier L., Belhatem N., et al. Lower-extremity amputation as a marker for renal and cardiovascular events and mortality in patients with long standing type 1 diabetes. Cardiovasc Diabetol. Jan 07 2016;15:5. doi: 10.1186/s12933-015-0322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanc-Bisson C., Velayoudom-Cephise F.L., Cougnard-Gregoire A., et al. Skin autofluorescence predicts major adverse cardiovascular events in patients with type 1 diabetes: a 7-year follow-up study. Cardiovasc Diabetol. 06 08 2018;17(1):82. doi: 10.1186/s12933-018-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Rubeaan K., Al Derwish M., Ouizi S., et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sayiner Z.A., Can F.I., Akarsu E. Patients' clinical charecteristics and predictors for diabetic foot amputation. Prim Care Diabetes. 06 2019;13(3):247–251. doi: 10.1016/j.pcd.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Ugwu E., Adeleye O., Gezawa I., Okpe I., Enamino M., Ezeani I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: findings from MEDFUN, a multi-center observational study. J Foot Ankle Res. 2019;12:34. doi: 10.1186/s13047-019-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norvell D.C., Czerniecki J.M. Risks and risk factors for ipsilateral Re-amputation in the first year following first major unilateral dysvascular amputation. Eur J Vasc Endovasc Surg. 10 2020;60(4):614–621. doi: 10.1016/j.ejvs.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arya S., Binney Z.O., Khakharia A., et al. High hemoglobin A. J Vasc Surg. 01 2018;67(1):217–228. doi: 10.1016/j.jvs.2017.06.101. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sotoda Y., Hirooka S., Orita H., Wakabayashi I. [Recent knowledge of smoking and peripheral arterial disease in lower extremities] Nihon Eiseigaku Zasshi. 2015;70(3):211–219. doi: 10.1265/jjh.70.211. [DOI] [PubMed] [Google Scholar]
- 77.Lu L., Mackay D.F., Pell J.P. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. Mar. 2014;100(5):414–423. doi: 10.1136/heartjnl-2013-304082. [DOI] [PubMed] [Google Scholar]
- 78.He Y., Jiang Y., Wang J., Fan L., Li X., Hu F.B. Prevalence of peripheral arterial disease and its association with smoking in a population-based study in Beijing, China. J Vasc Surg. Aug 2006;44(2):333–338. doi: 10.1016/j.jvs.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 79.Conen D., Everett B.M., Kurth T., et al. Smoking, smoking cessation, [corrected] and risk for symptomatic peripheral artery disease in women: a cohort study. Ann Intern Med. Jun. 2011;154(11):719–726. doi: 10.7326/0003-4819-154-11-201106070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hageman S.H.J., de Borst G.J., Dorresteijn J.A.N., et al. Cardiovascular risk factors and the risk of major adverse limb events in patients with symptomatic cardiovascular disease. Heart. Mar. 2020 doi: 10.1136/heartjnl-2019-316088. [DOI] [PubMed] [Google Scholar]
- 81.Czerniecki J.M., Thompson M.L., Littman A.J., et al. Predicting reamputation risk in patients undergoing lower extremity amputation due to the complications of peripheral artery disease and/or diabetes. Br J Surg. 07 2019;106(8):1026–1034. doi: 10.1002/bjs.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hawkins R.B., Mehaffey J.H., Charles E.J., Kern J.A., Schneider E.B., Tracci M.C. Socioeconomically Distressed Communities Index independently predicts major adverse limb events after infrainguinal bypass in a national cohort. J Vasc Surg. 12 2019;70(6):1985–1993. doi: 10.1016/j.jvs.2019.03.060. e8. [DOI] [PubMed] [Google Scholar]
- 83.Khan S.Z., Montross B., Rivero M., et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers (ACEI/ARB) are associated with improved limb salvage after infrapopliteal interventions for critical limb ischemia. Ann Vasc Surg. Feb. 2020;63:275–286. doi: 10.1016/j.avsg.2019.08.093. [DOI] [PubMed] [Google Scholar]
- 84.Khan S.Z., O'Brien-Irr M.S., Rivero M., et al. Improved survival with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic limb-threatening ischemia. J Vasc Surg. 12 2020;72(6):2130–2138. doi: 10.1016/j.jvs.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 85.Parvar S.L., Fitridge R., Dawson J., Nicholls S.J. Medical and lifestyle management of peripheral arterial disease. J Vasc Surg. 11 2018;68(5):1595–1606. doi: 10.1016/j.jvs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 86.Itoga N.K., Tawfik D.S., Lee C.K., Maruyama S., Leeper N.J., Chang T.I. Association of blood pressure measurements with peripheral artery disease events. Circulation. 10 23 2018;138(17):1805–1814. doi: 10.1161/CIRCULATIONAHA.118.033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fudim M., Hopley C.W., Huang Z., et al. Association of hypertension and arterial blood pressure on limb and cardiovascular outcomes in symptomatic peripheral artery disease: the EUCLID trial. Circ Cardiovasc Qual Outcomes. 09 2020;13(9) doi: 10.1161/CIRCOUTCOMES.120.006512. [DOI] [PubMed] [Google Scholar]
- 88.Mach F., Baigent C., Catapano A.L., et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41(1):111–188. doi: 10.1093/eurheartj/ehz455. 01 2020. [DOI] [PubMed] [Google Scholar]
- 89.Bonaca M.P., Nault P., Giugliano R.P., et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk) Circulation. 01 2018;137(4):338–350. doi: 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed] [Google Scholar]
- 90.Creager M.A. Protecting life and limb in peripheral artery disease. Circulation. 01 2018;137(4):351–353. doi: 10.1161/CIRCULATIONAHA.117.032422. [DOI] [PubMed] [Google Scholar]
- 91.Golledge J., Ward N.C., Watts G.F. Lipid management in people with peripheral artery disease. Curr Opin Lipidol. 12 2019;30(6):470–476. doi: 10.1097/MOL.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 92.Kokkinidis D.G., Arfaras-Melainis A., Giannopoulos S., et al. Statin therapy for reduction of cardiovascular and limb-related events in critical limb ischemia: a systematic review and meta-analysis. Vasc Med. 04 2020;25(2):106–117. doi: 10.1177/1358863X19894055. [DOI] [PubMed] [Google Scholar]
- 93.Westin G.G., Armstrong E.J., Bang H., et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. Feb. 2014;63(7):682–690. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Govsyeyev N., Nehler M.R., Hiatt W.R., Bonaca M.P. Tackling elevated risk in PAD: focus on antithrombotic and lipid therapy for PAD. Curr Cardiol Rep. Jan. 2020;22(3):13. doi: 10.1007/s11886-020-1264-z. [DOI] [PubMed] [Google Scholar]
- 95.Pastori D., Farcomeni A., Milanese A., et al. Statins and major adverse limb events in patients with peripheral artery disease: a systematic review and meta-analysis. Thromb Haemost. May. 2020;120(5):866–875. doi: 10.1055/s-0040-1709711. [DOI] [PubMed] [Google Scholar]
- 96.Schwartz G.G., Steg P.G., Szarek M., et al. Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. May 2020;141(20):1608–1617. doi: 10.1161/CIRCULATIONAHA.120.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tmoyan N.A., Ezhov M.V., Afanasieva O.I., et al. The association of lipoprotein(a) and apolipoprotein(a) phenotypes with peripheral artery disease. Ter Arkh. Sep. 2018;90(9):31–36. doi: 10.26442/terarkh201890931-36. [DOI] [PubMed] [Google Scholar]
- 98.Laschkolnig A., Kollerits B., Lamina C., et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. Jul. 2014;103(1):28–36. doi: 10.1093/cvr/cvu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng S.W., Ting A.C. Lipoprotein (a) level and mortality in patients with critical lower limb ischaemia. Eur J Vasc Endovasc Surg. Aug 2001;22(2):124–129. doi: 10.1053/ejvs.2001.1431. [DOI] [PubMed] [Google Scholar]
- 100.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. Feb. 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 101.Gudbjartsson D.F., Thorgeirsson G., Sulem P., et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 12 2019;74(24):2982–2994. doi: 10.1016/j.jacc.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 102.Ferretti G., Bacchetti T., Johnston T.P., Banach M., Pirro M., Sahebkar A. Lipoprotein(a): a missing culprit in the management of athero-thrombosis? J Cell Physiol. 04 2018;233(4):2966–2981. doi: 10.1002/jcp.26050. [DOI] [PubMed] [Google Scholar]
- 103.Weiss N., Julius U. Lipoprotein(a) apheresis in patients with peripheral arterial disease: rationale and clinical results. Clin Res Cardiol Suppl. Apr. 2019;14(Suppl 1):39–44. doi: 10.1007/s11789-019-00097-1. [DOI] [PubMed] [Google Scholar]
- 104.Brandão J.A.M., Meireles-Brandão L.R., Coelho R., Rocha-Gonçalves F. Lipoprotein(a) as a key target in combined therapeutic approaches for cardiovascular disease. Rev Port Cardiol. 07 2019;38(7):485–493. doi: 10.1016/j.repc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Gurdasani D., Sjouke B., Tsimikas S., et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. Dec. 2012;32(12):3058–3065. doi: 10.1161/ATVBAHA.112.255521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kosmas C.E., Silverio D., Sourlas A., et al. Role of lipoprotein (a) in peripheral arterial disease. Ann Transl Med. Sep 2019;7(Suppl 6):S242. doi: 10.21037/atm.2019.08.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanchez Muñoz-Torrero J.F., Rico-Martín S., Álvarez L.R., et al. Lipoprotein (a) levels and outcomes in stable outpatients with symptomatic artery disease. Atherosclerosis. 09 2018;276:10–14. doi: 10.1016/j.atherosclerosis.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 108.Golledge J., Rowbotham S., Velu R., et al. Association of serum lipoprotein (a) with the requirement for a peripheral artery operation and the incidence of major adverse cardiovascular events in people with peripheral artery disease. J Am Heart Assoc. 03 2020;9(6) doi: 10.1161/JAHA.119.015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hishikari K., Hikita H., Nakamura S., et al. Usefulness of lipoprotein(a) for predicting clinical outcomes after endovascular therapy for aortoiliac atherosclerotic lesions. J Endovasc Ther. Dec. 2017;24(6):793–799. doi: 10.1177/1526602817728068. [DOI] [PubMed] [Google Scholar]
- 110.Gencer B., Kronenberg F., Stroes E.S. Mach F. Lipoprotein(a): the revenant. Eur Heart J. May. 2017;38(20):1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 111.Ballantyne C.M., Laufs U., Ray K.K., et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 04 2020;27(6):593–603. doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ballantyne C.M., Banach M., Mancini G.B.J., et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 10 2018;277:195–203. doi: 10.1016/j.atherosclerosis.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Banach M., Duell P.B., Gotto A.M., et al. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol. Oct 2020;5(10):1124–1135. doi: 10.1001/jamacardio.2020.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goldberg A.C., Leiter L.A., Stroes E.S.G., et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. 11 2019;322(18):1780–1788. doi: 10.1001/jama.2019.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laufs U., Banach M., Mancini G.B.J., et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 04 2019;8(7) doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ray K.K., Bays H.E., Catapano A.L., et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 03 2019;380(11):1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 117.Momtazi-Borojeni A.A., Katsiki N., Pirro M., Banach M., Rasadi K.A., Sahebkar A. Dietary natural products as emerging lipoprotein(a)-lowering agents. J Cell Physiol. 08 2019;234(8):12581–12594. doi: 10.1002/jcp.28134. [DOI] [PubMed] [Google Scholar]
- 118.Parhofer K.G. Lipoprotein(a): medical treatment options for an elusive molecule. Curr Pharmaceut Des. 2011;17(9):871–876. doi: 10.2174/138161211795428777. [DOI] [PubMed] [Google Scholar]
- 119.MacKay D., Hathcock J., Guarneri E. Niacin: chemical forms, bioavailability, and health effects. Nutr Rev. Jun. 2012;70(6):357–366. doi: 10.1111/j.1753-4887.2012.00479.x. [DOI] [PubMed] [Google Scholar]
- 120.Julius U. Niacin as antidyslipidemic drug. Can J Physiol Pharmacol. Dec 2015;93(12):1043–1054. doi: 10.1139/cjpp-2014-0478. [DOI] [PubMed] [Google Scholar]
- 121.Serban M.C., Sahebkar A., Mikhailidis D.P., et al. Impact of L-carnitine on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. Jan 12 2016;6:19188. doi: 10.1038/srep19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hiatt W.R., Hirsch A.T., Creager M.A., et al. Effect of niacin ER/lovastatin on claudication symptoms in patients with peripheral artery disease. Vasc Med. Jun. 2010;15(3):171–179. doi: 10.1177/1358863X09360579. [DOI] [PubMed] [Google Scholar]
- 123.Santos H.O., Kones R., Rumana U., Earnest C.P., Izidoro L.F.M., Macedo R.C.O. Lipoprotein(a): current evidence for a physiologic role and the effects of nutraceutical strategies. Clin Therapeut. 09 2019;41(9):1780–1797. doi: 10.1016/j.clinthera.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 124.Shakeri A., Tabibi H., Hedayati M. Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int. Oct. 2010;14(4):498–504. doi: 10.1111/j.1542-4758.2010.00476.x. [DOI] [PubMed] [Google Scholar]
- 125.Galvano F., Li Volti G., Malaguarnera M., Avitabile T., Antic T., Vacante M. Effects of simvastatin and carnitine versus simvastatin on lipoprotein(a) and apoprotein(a) in type 2 diabetes mellitus. Expert Opin Pharmacother. Aug. 2009;10(12):1875–1882. doi: 10.1517/14656560903081745. [DOI] [PubMed] [Google Scholar]
- 126.Hiatt W.R., Creager M.A., Amato A., Brass E.P. Effect of propionyl-L-carnitine on a background of monitored exercise in patients with claudication secondary to peripheral artery disease. J Cardiopulm Rehabil Prev. 2011 Mar-Apr 2011;31(2):125–132. doi: 10.1097/HCR.0b013e3181f1fd65. [DOI] [PubMed] [Google Scholar]
- 127.Goldenberg N.A., Krantz M.J., Hiatt W.R. L-Carnitine plus cilostazol versus cilostazol alone for the treatment of claudication in patients with peripheral artery disease: a multicenter, randomized, double-blind, placebo-controlled trial. Vasc Med. Jun. 2012;17(3):145–154. doi: 10.1177/1358863X12442264. [DOI] [PubMed] [Google Scholar]
- 128.Hiatt W.R. Carnitine and peripheral arterial disease. Ann N Y Acad Sci. Nov. 2004;1033:92–98. doi: 10.1196/annals.1320.008. [DOI] [PubMed] [Google Scholar]
- 129.Martin S.S., Blumenthal R.S., Miller M. LDL cholesterol: the lower the better. Med Clin North Am. Jan. 2012;96(1):13–26. doi: 10.1016/j.mcna.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 130.Atar D., Jukema J.W., Molemans B., et al. New cardiovascular prevention guidelines: how to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis. 02 2021;319:51–61. doi: 10.1016/j.atherosclerosis.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 131.Pedro-Botet J., Pintó X. LDL-cholesterol: the lower the better. Clín Invest Arterioscler. Dec 2019;31(Suppl 2):16–27. doi: 10.1016/j.arteri.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 132.Vogt A. [Hypercholesterolemia - the way to lower LDL-C < 55 mg/dl] MMW - Fortschritte Med. 11 2020;162(Suppl 3):36–42. doi: 10.1007/s15006-020-4373-0. [DOI] [PubMed] [Google Scholar]
- 133.Hruska K.A., Mathew S., Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. Jul. 2005;97(2):105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 134.Makulska I., Szczepańska M., Drożdż D., Polak-Jonkisz D., Zwolińska D. The importance of fetuin-A in vascular calcification in children with chronic kidney disease. Adv Clin Exp Med. Apr. 2019;28(4):499–505. doi: 10.17219/acem/82517. [DOI] [PubMed] [Google Scholar]
- 135.Ulutas O., Taskapan M.C., Dogan A., Baysal T., Taskapan H. Vascular calcification is not related to serum fetuin-A and osteopontin levels in hemodialysis patients. Int Urol Nephrol. Jan. 2018;50(1):137–142. doi: 10.1007/s11255-017-1740-6. [DOI] [PubMed] [Google Scholar]
- 136.Chen H.Y., Chiu Y.L., Hsu S.P., Pai M.F., Yang J.Y., Peng Y.S. Relationship between fetuin A, vascular calcification and fracture risk in dialysis patients. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0158789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krishnan P., Moreno P.R., Turnbull I.C., et al. Incremental effects of diabetes mellitus and chronic kidney disease in medial arterial calcification: synergistic pathways for peripheral artery disease progression. Vasc Med. 10 2019;24(5):383–394. doi: 10.1177/1358863X19842276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bourrier M., Ferguson T.W., Embil J.M., Rigatto C., Komenda P., Tangri N. Peripheral artery disease: its adverse consequences with and without CKD. Am J Kidney Dis. 05 2020;75(5):705–712. doi: 10.1053/j.ajkd.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 139.Zhang L.L., Saldana-Ruiz N., Elsayed R.S., et al. Predictors of major adverse limb events after open forefoot amputation in patients with chronic limb-threatening ischemia. Ann Vasc Surg. Jul 2020;66:614–620. doi: 10.1016/j.avsg.2020.01.099. [DOI] [PubMed] [Google Scholar]
- 140.Smilowitz N.R., Bhandari N., Berger J.S. Chronic kidney disease and outcomes of lower extremity revascularization for peripheral artery disease. Atherosclerosis. 03 2020;297:149–156. doi: 10.1016/j.atherosclerosis.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim H.O., Kim J.M., Woo J.S., et al. Effects of chronic kidney disease on clinical outcomes in patients with peripheral artery disease undergoing endovascular treatment: analysis from the K-VIS ELLA registry. Int J Cardiol. 07 2018;262:32–37. doi: 10.1016/j.ijcard.2018.03.108. [DOI] [PubMed] [Google Scholar]
- 142.Dayama A., Tsilimparis N., Kolakowski S., Matolo N.M., Humphries M.D. Clinical outcomes of bypass-first versus endovascular-first strategy in patients with chronic limb-threatening ischemia due to infrageniculate arterial disease. J Vasc Surg. 01 2019;69(1):156–163. doi: 10.1016/j.jvs.2018.05.244. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stino A.M., Smith A.G. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. Sep 2017;8(5):646–655. doi: 10.1111/jdi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dewanjee S., Das S., Das A.K., et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. Aug 15 2018;833:472–523. doi: 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 145.Calcutt N.A. Diabetic neuropathy and neuropathic pain: a (con)fusion of pathogenic mechanisms? Pain. 09 2020;161(Suppl 1):S65–S86. doi: 10.1097/j.pain.0000000000001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pitocco D., Spanu T., Di Leo M., et al. Diabetic foot infections: a comprehensive overview. Eur Rev Med Pharmacol Sci. Apr. 2019;23(2 Suppl):26–37. doi: 10.26355/eurrev_201904_17471. [DOI] [PubMed] [Google Scholar]
- 147.Volmer-Thole M., Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. Jun 10 2016;(6):17. doi: 10.3390/ijms17060917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schreuder S.M., Nieuwdorp M., Koelemay M.J.W., Bipat S., Reekers J.A. Testing the sympathetic nervous system of the foot has a high predictive value for early amputation in patients with diabetes with a neuroischemic ulcer. BMJ Open Diabetes Res Care. 2018;6(1) doi: 10.1136/bmjdrc-2018-000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Selvarajah D., Kar D., Khunti K., et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 12 2019;7(12):938–948. doi: 10.1016/S2213-8587(19)30081-6. [DOI] [PubMed] [Google Scholar]
- 150.Hicks C.W., Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diabetes Rep. 08 27 2019;19(10):86. doi: 10.1007/s11892-019-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fiordaliso F., Clerici G., Maggioni S., et al. Prospective study on microangiopathy in type 2 diabetic foot ulcer. Diabetologia. 07 2016;59(7):1542–1548. doi: 10.1007/s00125-016-3961-0. [DOI] [PubMed] [Google Scholar]
- 152.Labbé L., Maréchaud R., Hadjadj S. [Screening and treatment of diabetic microangiopathy] Rev Prat. Sep. 15 2007;57(13):1434–1443. [PubMed] [Google Scholar]
- 153.Portig I., Maisch B. [Noninvasive methods in the diagnosis of macro- and microangiopathy of peripherial and carotid arteries] Herz. Feb 2004;29(1):17–25. doi: 10.1007/s00059-004-2535-y. [DOI] [PubMed] [Google Scholar]
- 154.Avogaro A., Fadini G.P. Microvascular complications in diabetes: a growing concern for cardiologists. Int J Cardiol. 09 15 2019;291:29–35. doi: 10.1016/j.ijcard.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 155.Chao C.Y., Cheing G.L. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. Oct 2009;25(7):604–614. doi: 10.1002/dmrr.1004. [DOI] [PubMed] [Google Scholar]
- 156.Grimaldi A., Heurtier A. [Epidemiology of cardio-vascular complications of diabetes] Diabetes Metab. Jun. 1999;25(Suppl 3):12–20. [PubMed] [Google Scholar]
- 157.Anand S.S., Bosch J., Eikelboom J.W., et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 01 2018;391:219–229. doi: 10.1016/S0140-6736(17)32409-1. 10117. [DOI] [PubMed] [Google Scholar]
- 158.Hiatt W.R., Fowkes F.G., Heizer G., et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 01 2017;376(1):32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 159.Cavender M.A., Steg P.G., Smith S.C., et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 Years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation. Sep. 2015;132(10):923–931. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 160.Cordeiro F., Mateus P.S., Ferreira A., et al. Short-term prognostic effect of prior cerebrovascular and peripheral artery disease in patients with acute coronary syndrome: can we do better? Eur Heart J Acute Cardiovasc Care. Oct 2018;7(7):652–660. doi: 10.1177/2048872617716388. [DOI] [PubMed] [Google Scholar]
- 161.Sigvant B., Kragsterman B., Falkenberg M., et al. Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. J Vasc Surg. Oct. 2016;64(4):1009–1017. doi: 10.1016/j.jvs.2016.03.429. e3. [DOI] [PubMed] [Google Scholar]
- 162.McPhee J.T., Barshes N.R., Ho K.J., et al. Predictive factors of 30-day unplanned readmission after lower extremity bypass. J Vasc Surg. Apr 2013;57(4):955–962. doi: 10.1016/j.jvs.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 163.Bonaca M.P., Bhatt D.L., Storey R.F., et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. Jun 2016;67(23):2719–2728. doi: 10.1016/j.jacc.2016.03.524. [DOI] [PubMed] [Google Scholar]
- 164.Trevethan R. Subjecting the ankle-brachial index to timely scrutiny: is it time to say goodbye to the ABI? Scand J Clin Lab Invest. 2018 Feb - Apr 2018;78(1–2):94–101. doi: 10.1080/00365513.2017.1416665. [DOI] [PubMed] [Google Scholar]
- 165.Curry S.J., Krist A.H., Owens D.K., et al. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: US preventive services task force recommendation statement. JAMA. 07 2018;320(2):177–183. doi: 10.1001/jama.2018.8357. [DOI] [PubMed] [Google Scholar]
- 166.Nativel M., Potier L., Alexandre L., et al. Lower extremity arterial disease in patients with diabetes: a contemporary narrative review. Cardiovasc Diabetol. 10 2018;17(1):138. doi: 10.1186/s12933-018-0781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Casey S., Lanting S., Oldmeadow C., Chuter V. The reliability of the ankle brachial index: a systematic review. J Foot Ankle Res. 2019;12:39. doi: 10.1186/s13047-019-0350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alqahtani K.M., Bhangoo M., Vaida F., Denenberg J.O., Allison M.A., Criqui M.H. Predictors of change in the ankle brachial index with exercise. Eur J Vasc Endovasc Surg. Mar 2018;55(3):399–404. doi: 10.1016/j.ejvs.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gu X., Man C., Zhang H., Fan Y. High ankle-brachial index and risk of cardiovascular or all-cause mortality: a meta-analysis. Atherosclerosis. 03 2019;282:29–36. doi: 10.1016/j.atherosclerosis.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 170.Samba H., Guerchet M., Ndamba-Bandzouzi B., et al. Ankle Brachial Index (ABI) predicts 2-year mortality risk among older adults in the Republic of Congo: the EPIDEMCA-FU study. Atherosclerosis. 07 2019;286:121–127. doi: 10.1016/j.atherosclerosis.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 171.Abboud H., Monteiro Tavares L., Labreuche J., et al. Impact of low ankle-brachial index on the risk of recurrent vascular events. Stroke. 04 2019;50(4):853–858. doi: 10.1161/STROKEAHA.118.022180. [DOI] [PubMed] [Google Scholar]
- 172.Nishimura H., Miura T., Minamisawa M., et al. Clinical characteristics and outcomes of patients with high ankle-brachial index from the IMPACT-ABI study. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0167150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Otsuka K., Nakanishi K., Shimada K., et al. Ankle-brachial index, arterial stiffness, and biomarkers in the prediction of mortality and outcomes in patients with end-stage kidney disease. Clin Cardiol. Jul 2019;42(7):656–662. doi: 10.1002/clc.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miura T., Minamisawa M., Ueki Y., et al. Impressive predictive value of ankle-brachial index for very long-term outcomes in patients with cardiovascular disease: IMPACT-ABI study. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0177609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu L., He R., Hua X., et al. The value of ankle-branchial index screening for cardiovascular disease in type 2 diabetes. Diabetes Metab Res Rev. 01 2019;35(1) doi: 10.1002/dmrr.3076. [DOI] [PubMed] [Google Scholar]
- 176.Diehm C., Darius H., Pittrow D., et al. Prognostic value of a low post-exercise ankle brachial index as assessed by primary care physicians. Atherosclerosis. Feb 2011;214(2):364–372. doi: 10.1016/j.atherosclerosis.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 177.Hammad T.A., Strefling J.A., Zellers P.R., et al. The effect of post-exercise ankle-brachial index on lower extremity revascularization. JACC Cardiovasc Interv. Aug 2015;8(9):1238–1244. doi: 10.1016/j.jcin.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 178.Li Y.H., Yeh H.I., Hwang J.J. Antithrombotic treatment for symptomatic peripheral artery disease. Acta Cardiol Sin. Nov 2019;35(6):557–562. doi: 10.6515/ACS.201911_35(6).20190907A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.O'Donnell T.F.X., Deery S.E., Darling J.D., et al. Adherence to lipid management guidelines is associated with lower mortality and major adverse limb events in patients undergoing revascularization for chronic limb-threatening ischemia. J Vasc Surg. 08 2017;66(2):572–578. doi: 10.1016/j.jvs.2017.03.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu W., Wang B., Zhan B., et al. Statin therapy improved long-term prognosis in patients with major non-cardiac vascular surgeries: a systematic review and meta-analysis. Vasc Pharmacol. 10 2018;109:1–16. doi: 10.1016/j.vph.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 181.Stavroulakis K., Borowski M., Torsello G., Bisdas T., collaborators C. Association between statin therapy and amputation-free survival in patients with critical limb ischemia in the CRITISCH registry. J Vasc Surg. 11 2017;66(5):1534–1542. doi: 10.1016/j.jvs.2017.05.115. [DOI] [PubMed] [Google Scholar]
- 182.Parmar G.M., Novak Z., Spangler E., et al. Statin use improves limb salvage after intervention for peripheral arterial disease. J Vasc Surg. 08 2019;70(2):539–546. doi: 10.1016/j.jvs.2018.07.089. [DOI] [PubMed] [Google Scholar]
- 183.Cho S., Lee Y.J., Ko Y.G., et al. Optimal strategy for antiplatelet therapy after endovascular revascularization for lower extremity peripheral artery disease. JACC Cardiovasc Interv. 12 2019;12(23):2359–2370. doi: 10.1016/j.jcin.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 184.Gupta A., Lee M.S., Gupta K., Kumar V., Reddy S. A review of antithrombotic treatment in critical limb ischemia after endovascular intervention. Cardiol Ther. Dec 2019;8(2):193–209. doi: 10.1007/s40119-019-00153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chan Y.H., Lee H.F., Li P.R., et al. Effectiveness, safety, and major adverse limb events in atrial fibrillation patients with concomitant diabetes mellitus treated with non-vitamin K antagonist oral anticoagulants. Cardiovasc Diabetol. May 2020;19(1):63. doi: 10.1186/s12933-020-01043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Hattum E.S., Algra A., Lawson J.A., Eikelboom B.C., Moll F.L., Tangelder M.J. Bleeding increases the risk of ischemic events in patients with peripheral arterial disease. Circulation. Oct 2009;120(16):1569–1576. doi: 10.1161/CIRCULATIONAHA.109.858365. [DOI] [PubMed] [Google Scholar]
- 187.Kaplovitch E., Eikelboom J.W., Dyal L., et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease: a subanalysis of the compass randomized clinical trial. JAMA Cardiol. Jan 2021;6(1):21–29. doi: 10.1001/jamacardio.2020.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Madabhushi V., Davenport D., Jones S., et al. Revascularization of intermittent claudicants leads to more chronic limb threatening ischemia and higher amputation rates. J Vasc Surg. Mar 2021 doi: 10.1016/j.jvs.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 189.Golledge J., Moxon J.V., Rowbotham S., et al. Risk of major amputation in patients with intermittent claudication undergoing early revascularization. Br J Surg. 05 2018;105(6):699–708. doi: 10.1002/bjs.10765. [DOI] [PubMed] [Google Scholar]
- 190.Hess C.N., Wang T.Y., Weleski Fu J., et al. Long-term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol. 02 2020;75(5):498–508. doi: 10.1016/j.jacc.2019.11.050. [DOI] [PubMed] [Google Scholar]
- 191.Antonello M., Squizzato F., Bassini S., Porcellato L., Grego F., Piazza M. Open repair versus endovascular treatment of complex aortoiliac lesions in low risk patients. J Vasc Surg. 10 2019;70(4):1155–1165. doi: 10.1016/j.jvs.2018.12.030. e1. [DOI] [PubMed] [Google Scholar]
- 192.Mohapatra A., Henry J.C., Avgerinos E.D., et al. Bypass versus endovascular intervention for healing ischemic foot wounds secondary to tibial arterial disease. J Vasc Surg. 07 2018;68(1):168–175. doi: 10.1016/j.jvs.2017.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schindewolf M., Fuss T., Fink H., Gemperli A., Haine A., Baumgartner I. Efficacy outcomes of endovascular versus surgical revascularization in critical limb ischemia: results from a prospective cohort study. Angiology. Sep 2018;69(8):677–685. doi: 10.1177/0003319717750486. [DOI] [PubMed] [Google Scholar]
- 194.Premaratne S., Newman J., Hobbs S., Garnham A., Wall M. Meta-analysis of direct surgical versus endovascular revascularization for aortoiliac occlusive disease. J Vasc Surg. Mar 2020 doi: 10.1016/j.jvs.2019.12.035. [DOI] [PubMed] [Google Scholar]
- 195.Kolte D., Kennedy K.F., Shishehbor M.H., et al. Endovascular versus surgical revascularization for acute limb ischemia: a propensity-score matched analysis. Circ Cardiovasc Interv. Jan 2020;13(1) doi: 10.1161/CIRCINTERVENTIONS.119.008150. [DOI] [PubMed] [Google Scholar]
- 196.Iida O., Takahara M., Soga Y., et al. Three-year outcomes of surgical versus endovascular revascularization for critical limb ischemia: the SPINACH study (surgical reconstruction versus peripheral intervention in patients with critical limb ischemia) Circ Cardiovasc Interv. Dec 2017;10(12) doi: 10.1161/CIRCINTERVENTIONS.117.005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mohapatra A., Boitet A., Malak O., et al. Peroneal bypass versus endovascular peroneal intervention for critical limb ischemia. J Vasc Surg. 01 2019;69(1):148–155. doi: 10.1016/j.jvs.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Childers C.P., Lamaina M., Liu C., et al. 2019. Cost-effectiveness of Leg Bypass versus endovascular therapy for critical limb ischemia: a systematic review. [PubMed] [Google Scholar]
- 199.Kakkar A.M., Abbott J.D. Percutaneous versus surgical management of lower extremity peripheral artery disease. Curr Atherosclerosis Rep. 2015;17(2):479. doi: 10.1007/s11883-014-0479-0. [DOI] [PubMed] [Google Scholar]
- 200.Wiseman J.T., Fernandes-Taylor S., Saha S., et al. Endovascular versus open revascularization for peripheral arterial disease. Ann Surg. 02 2017;265(2):424–430. doi: 10.1097/SLA.0000000000001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Davis F.M., Albright J., Gallagher K.A., et al. Early outcomes following endovascular, open surgical, and hybrid revascularization for lower extremity acute limb ischemia. Ann Vasc Surg. Aug 2018;51:106–112. doi: 10.1016/j.avsg.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 202.Butt T., Lilja E., Örneholm H., et al. Amputation-free survival in patients with diabetes mellitus and peripheral arterial disease with heel ulcer: open versus endovascular surgery. Vasc Endovasc Surg. Feb 2019;53(2):118–125. doi: 10.1177/1538574418813746. [DOI] [PubMed] [Google Scholar]
- 203.Lucarelli P., Mantuano E., Schiattarella E., Palmarino R. Evidence for linkage equilibrium between two RFLPs associated with the human SST locus. Hum Genet. Mar 1988;78(3):291–292. doi: 10.1007/BF00291681. [DOI] [PubMed] [Google Scholar]
- 204.Conte M.S. Diabetic revascularization: endovascular versus open bypass--do we have the answer? Semin Vasc Surg. Jun 2012;25(2):108–114. doi: 10.1053/j.semvascsurg.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 205.Meyer A., Schilling A., Kott M., Rother U., Lang W., Regus S. Open versus endovascular revascularization of below-knee arteries in patients with end-stage renal disease and critical limb ischemia. Vasc Endovasc Surg. Nov 2018;52(8):613–620. doi: 10.1177/1538574418789036. [DOI] [PubMed] [Google Scholar]
- 206.Lin J.H., Brunson A., Romano P.S., Mell M.W., Humphries M.D. Endovascular-first treatment is associated with improved amputation-free survival in patients with critical limb ischemia. Circ Cardiovasc Qual Outcomes. 08 2019;12(8) doi: 10.1161/CIRCOUTCOMES.118.005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hess C.N., Huang Z., Patel M.R., et al. Acute limb ischemia in peripheral artery disease. Circulation. 08 2019;140(7):556–565. doi: 10.1161/CIRCULATIONAHA.119.039773. [DOI] [PubMed] [Google Scholar]
- 208.Gray W.A., Keirse K., Soga Y., et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 10 2018;392:1541–1551. doi: 10.1016/S0140-6736(18)32262-1. 10157. [DOI] [PubMed] [Google Scholar]
- 209.Zeller T., Baumgartner I., Scheinert D., et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. Oct 2014;64(15):1568–1576. doi: 10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 210.Miura T., Miyashita Y., Soga Y., et al. Drug-eluting versus bare-metal stent implantation with or without cilostazol in the treatment of the superficial femoral artery. Circ Cardiovasc Interv. 08 2018;11(8) doi: 10.1161/CIRCINTERVENTIONS.118.006564. [DOI] [PubMed] [Google Scholar]
- 211.Vemulapalli S., Parikh K., Coeytaux R., et al. Systematic review and meta-analysis of endovascular and surgical revascularization for patients with chronic lower extremity venous insufficiency and varicose veins. Am Heart J. 02 2018;196:131–143. doi: 10.1016/j.ahj.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 212.Jones W.S., Baumgartner I., Hiatt W.R., et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. Jan 2017;135(3):241–250. doi: 10.1161/CIRCULATIONAHA.116.025880. [DOI] [PubMed] [Google Scholar]
- 213.Hess C.N., Norgren L., Ansel G.M., et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: a TASC (InterSociety consensus for the management of peripheral artery disease) initiative. Circulation. Jun 2017;135(25):2534–2555. doi: 10.1161/CIRCULATIONAHA.117.024469. [DOI] [PubMed] [Google Scholar]
- 214.Schillinger M., Minar E. Restenosis after percutaneous angioplasty: the role of vascular inflammation. Vasc Health Risk Manag. 2005;1(1):73–78. doi: 10.2147/vhrm.1.1.73.58932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schillinger M., Exner M., Mlekusch W., et al. Balloon angioplasty and stent implantation induce a vascular inflammatory reaction. J Endovasc Ther. Feb 2002;9(1):59–66. doi: 10.1177/152660280200900111. [DOI] [PubMed] [Google Scholar]
- 216.Schillinger M., Exner M., Mlekusch W., et al. Inflammatory response to stent implantation: differences in femoropopliteal, iliac, and carotid arteries. Radiology. Aug 2002;224(2):529–535. doi: 10.1148/radiol.2241011253. [DOI] [PubMed] [Google Scholar]
- 217.Varela C., Acin F., De Haro J., March J., Florez A., Lopez-Quintana A. Influence of surgical or endovascular distal revascularization of the lower limbs on ischemic ulcer healing. J Cardiovasc Surg (Torino) Jun 2011;52(3):381–389. [PubMed] [Google Scholar]
- 218.White C.J., Gray W.A. Endovascular therapies for peripheral arterial disease: an evidence-based review. Circulation. Nov 2007;116(19):2203–2215. doi: 10.1161/CIRCULATIONAHA.106.621391. [DOI] [PubMed] [Google Scholar]
- 219.Biscetti F., Giovannini S., Straface G., et al. RANK/RANKL/OPG pathway: genetic association with history of ischemic stroke in Italian population. Eur Rev Med Pharmacol Sci. 11 2016;20(21):4574–4580. [PubMed] [Google Scholar]
- 220.Signorelli S.S., Katsiki N. Oxidative stress and inflammation: their role in the pathogenesis of peripheral artery disease with or without type 2 diabetes mellitus. Curr Vasc Pharmacol. 2018;16(6):547–554. doi: 10.2174/1570161115666170731165121. [DOI] [PubMed] [Google Scholar]
- 221.Pola R., Flex A., Ciaburri M., et al. Responsiveness to cholinesterase inhibitors in Alzheimer's disease: a possible role for the 192 Q/R polymorphism of the PON-1 gene. Neurosci Lett. Jul. 2005;382(3):338–341. doi: 10.1016/j.neulet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 222.Pola R., Flex A., Gaetani E., et al. Intercellular adhesion molecule-1 K469E gene polymorphism and Alzheimer's disease. Neurobiol Aging. 2003 Mar-Apr 2003;24(2):385–387. doi: 10.1016/s0197-4580(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 223.Pola R., Flex A., Gaetani E., Pola P., Bernabei R. The -174 G/C polymorphism of the interleukin-6 gene promoter and essential hypertension in an elderly Italian population. J Hum Hypertens. Sep. 2002;16(9):637–640. doi: 10.1038/sj.jhh.1001462. [DOI] [PubMed] [Google Scholar]
- 224.Pola R., Gaetani E., Flex A., et al. Lack of association between Alzheimer's disease and Gln-Arg 192 Q/R polymorphism of the PON-1 gene in an Italian population. Dement Geriatr Cognit Disord. 2003;15(2):88–91. doi: 10.1159/000067975. [DOI] [PubMed] [Google Scholar]
- 225.Giovannini S., Tinelli G., Biscetti F., et al. Serum high mobility group box-1 and osteoprotegerin levels are associated with peripheral arterial disease and critical limb ischemia in type 2 diabetic subjects. Cardiovasc Diabetol. 08 2017;16(1):99. doi: 10.1186/s12933-017-0581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Straface G., Biscetti F., Pitocco D., et al. Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRSF11B, on serum osteoprotegerin levels and carotid plaque vulnerability. Stroke. Nov 2011;42(11):3022–3028. doi: 10.1161/STROKEAHA.111.619288. [DOI] [PubMed] [Google Scholar]
- 227.Protogerou A.D., Zampeli E., Fragiadaki K., Stamatelopoulos K., Papamichael C., Sfikakis P.P. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. Dec 2011;219(2):734–736. doi: 10.1016/j.atherosclerosis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 228.Mugabo Y., Li L., Renier G. The connection between C-reactive protein (CRP) and diabetic vasculopathy. Focus on preclinical findings. Curr Diabetes Rev. Jan 2010;6(1):27–34. doi: 10.2174/157339910790442628. [DOI] [PubMed] [Google Scholar]
- 229.Biscetti F., Porreca C.F., Bertucci F., et al. TNFRSF11B gene polymorphisms increased risk of peripheral arterial occlusive disease and critical limb ischemia in patients with type 2 diabetes. Acta Diabetol. Dec 2014;51(6):1025–1032. doi: 10.1007/s00592-014-0664-1. [DOI] [PubMed] [Google Scholar]
- 230.Tousoulis D., Antoniades C., Stefanadis C. Assessing inflammatory status in cardiovascular disease. Heart. Aug 2007;93(8):1001–1007. doi: 10.1136/hrt.2006.088211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Stone P.A., Schlarb H., Campbell J.E., et al. C-reactive protein and brain natriuretic peptide as predictors of adverse events after lower extremity endovascular revascularization. J Vasc Surg. Sep 2014;60(3):652–660. doi: 10.1016/j.jvs.2014.03.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Biscetti F., Straface G., Bertoletti G., et al. Identification of a potential proinflammatory genetic profile influencing carotid plaque vulnerability. J Vasc Surg. Feb 2015;61(2):374–381. doi: 10.1016/j.jvs.2014.08.113. [DOI] [PubMed] [Google Scholar]
- 233.Schillinger M., Exner M., Mlekusch W., et al. Endovascular revascularization below the knee: 6-month results and predictive value of C-reactive protein level. Radiology. May 2003;227(2):419–425. doi: 10.1148/radiol.2272020137. [DOI] [PubMed] [Google Scholar]
- 234.Lin C.W., Hsu L.A., Chen C.C., et al. C-reactive protein as an outcome predictor for percutaneous transluminal angioplasty in diabetic patients with peripheral arterial disease and infected foot ulcers. Diabetes Res Clin Pract. Nov 2010;90(2):167–172. doi: 10.1016/j.diabres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 235.Biscetti F., Nardella E., Bonadia N., et al. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc Diabetol. 06 2019;18(1):74. doi: 10.1186/s12933-019-0880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Biscetti F., Rando M.M., Nardella E., et al. High mobility group box-1 and diabetes mellitus complications: state of the art and future perspectives. Int J Mol Sci. Dec 11 2019;(24):20. doi: 10.3390/ijms20246258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Domingueti C.P., Dusse L.M., Carvalho M., de Sousa L.P., Gomes K.B., Fernandes A.P. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabet Complicat. 2016 May-Jun 2016;30(4):738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 238.Augoulea A., Vrachnis N., Lambrinoudaki I., et al. Osteoprotegerin as a marker of atherosclerosis in diabetic patients. Internet J Endocrinol. 2013;2013:182060. doi: 10.1155/2013/182060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Aboyans V., Ricco J.B., Bartelink M.E.L., et al. ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European stroke organization (ESO)the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS) Eur Heart J. 2017;39(9):763–816. doi: 10.1093/eurheartj/ehx095. 03 01 2018. [DOI] [PubMed] [Google Scholar]
- 240.Kithcart A.P., Beckman J.A. ACC/AHA versus ESC guidelines for diagnosis and management of peripheral artery disease: JACC guideline comparison. J Am Coll Cardiol. 12 04 2018;72(22):2789–2801. doi: 10.1016/j.jacc.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 241.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 07 2019;58(1S):S1–S109. doi: 10.1016/j.ejvs.2019.05.006. e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2016;135(12):e726–e779. doi: 10.1161/CIR.0000000000000471. 03 21 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Itoga N.K., Minami H.R., Chelvakumar M., et al. Cost-effectiveness analysis of asymptomatic peripheral artery disease screening with the ABI test. Vasc Med. 04 2018;23(2):97–106. doi: 10.1177/1358863X17745371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brown R.J.L. Review of article: one simple claudication question as first step in peripheral artery disease (PAD) screening: a meta-analysis of the association with reduced ankle brachial index (ABI) in 27,945 subjects-Kieback AG, Espinola-Klein C, Lamina C et al., 2019. J Vasc Nurs. 09 2020;38(3):156–159. doi: 10.1016/j.jvn.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 245.Cannon C.P., McGuire D.K., Pratley R., et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV) Am Heart J. 12 2018;206:11–23. doi: 10.1016/j.ahj.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 246.Heyward J., Mansour O., Olson L., Singh S., Alexander G.C. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: a systematic review and meta-analysis. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Huang C.Y., Lee J.K. Sodium-glucose co-transporter-2 inhibitors and major adverse limb events: a trial-level meta-analysis including 51713individuals. Diabetes Obes Metabol. 12 2020;22(12):2348–2355. doi: 10.1111/dom.14159. [DOI] [PubMed] [Google Scholar]
- 248.Chang H.Y., Singh S., Mansour O., Baksh S., Alexander G.C. Association between sodium-glucose cotransporter 2 inhibitors and lower extremity amputation among patients with type 2 diabetes. JAMA Intern Med. 09 01 2018;178(9):1190–1198. doi: 10.1001/jamainternmed.2018.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Svanström H., Ueda P., Melbye M., et al. Use of liraglutide and risk of major cardiovascular events: a register-based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol. 02 2019;7(2):106–114. doi: 10.1016/S2213-8587(18)30320-6. [DOI] [PubMed] [Google Scholar]
- 250.Mentz R.J., Bethel M.A., Gustavson S., et al. Baseline characteristics of patients enrolled in the exenatide study of cardiovascular event lowering (EXSCEL) Am Heart J. May 2017;187:1–9. doi: 10.1016/j.ahj.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lugner M., Sattar N., Miftaraj M., et al. Cardiorenal and other diabetes related outcomes with SGLT-2 inhibitors compared to GLP-1 receptor agonists in type 2 diabetes: nationwide observational study. Cardiovasc Diabetol. 03 22 2021;20(1):67. doi: 10.1186/s12933-021-01258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Saely C.H., Sternbauer S., Vonbank A., et al. Type 2 diabetes mellitus is a strong predictor of LDL cholesterol target achievement in patients with peripheral artery disease. J Diabet Complicat. 11 2020;34(11):107692. doi: 10.1016/j.jdiacomp.2020.107692. [DOI] [PubMed] [Google Scholar]
- 253.Jagadish P.S., Kabra R. Stroke risk in atrial fibrillation: beyond the CHA. Curr Cardiol Rep. 07 2019;21(9):95. doi: 10.1007/s11886-019-1189-6. [DOI] [PubMed] [Google Scholar]
- 254.Zhu W., He W., Guo L., Wang X., Hong K. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. Sep 2015;38(9):555–561. doi: 10.1002/clc.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wardlaw J.M., Brazzelli M., Chappell F.M., et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology. Jul 2015;85(4):373–380. doi: 10.1212/WNL.0000000000001780. [DOI] [PMC free article] [PubMed] [Google Scholar]