Abstract
Background and aims
TACE/ADAM17 is a membrane bound metalloprotease, which cleaves substrates involved in immune and inflammatory responses and plays a role in coronary artery disease (CAD). We measured TACE and its substrates in CAD patients to identify potential biomarkers within this molecular pathway with potential for acute coronary syndrome (ACS) and major adverse cardiovascular events (MACE) prediction.
Methods
Blood samples were obtained from consecutive patients (n = 229) with coronary angiographic evidence of CAD admitted with ACS or electively. MACE were recorded after a median 3-year follow-up. Controls (n = 115) had a <10% CAD risk as per the HeartSCORE. TACE and TIMP3 protein and mRNA levels were measured by ELISA and RT-qPCR respectively. TACE substrates were measured using a multiplex proximity extension assay.
Results
TACE mRNA and cell protein levels (p < 0.01) and TACE substrates LDLR (p = 0.006), TRANCE (p = 0.045), LAG-3 (p < 0.001) and ACE2 (p < 0.001) plasma levels were significantly higher in CAD patients versus controls. TACE inhibitor TIMP3 mRNA levels were significantly lower in CAD patients and tended to be lower in the ACS population (p < 0.05). TACE substrates TNFR1 (OR:3.237,CI:1.514–6.923,p = 0.002), HB-EGF (OR:0.484,CI:0.288–0.813,p = 0.006) and Ep-CAM (OR:0.555,CI:0.327–0.829,p = 0.004) accurately classified ACS patients with HB-EGF and Ep-CAM levels being lower compared to electively admitted patients. TNFR1 (OR:2.317,CI:1.377–3.898,p = 0.002) and TNFR2 (OR:1.902,CI:1.072–3.373,p = 0.028) were significantly higher on admission in those patients who developed MACE within 3 years.
Conclusions
We demonstrate a possible role of TACE substrates LAG-3, HB-EGF and Ep-CAM in atherosclerotic plaque development and stability. We also underline the importance of measuring TNFR1 and TNFR2 earlier than previously appreciated for MACE prediction. We report an important role of TIMP3 in regulating TACE levels.
Keywords: Coronary artery disease, Tumour necrosis factor alpha converting enzyme TACE/ADAM17, Major adverse cardiovascular events, Acute coronary syndrome
Highlights
-
•
There is a clinical need to identify biomarkers for ACS and follow-up MACE diagnosis.
-
•
TACE/ADAM17 is a metalloproteinase known to cleave inflammatory mediators which play a role in CAD development.
-
•
TACE substrate LAG-3 might have a novel role in CAD which warrants further exploration.
-
•
TACE substrates HB-EGF and Ep-CAM were reduced in ACS patients suggesting a possible protective role in atherosclerosis.
Introduction
Cardiovascular events, such as acute coronary syndrome (ACS) and ischemic stroke, remain the leading cause of mortality worldwide despite apparently optimal clinical and medical management [1]. The remaining high incidence of cardiovascular events is attributed to several underlying mechanisms including residual inflammation [2]. Biomarkers of inflammation such as C reactive protein (CRP) [3] and IL-6 [4] have been shown to associate with cardiovascular events, however, they lack specificity [5]. Novel biomarkers or biomarker panels are needed to stratify individuals at high risk of major adverse cardiovascular events (MACE).
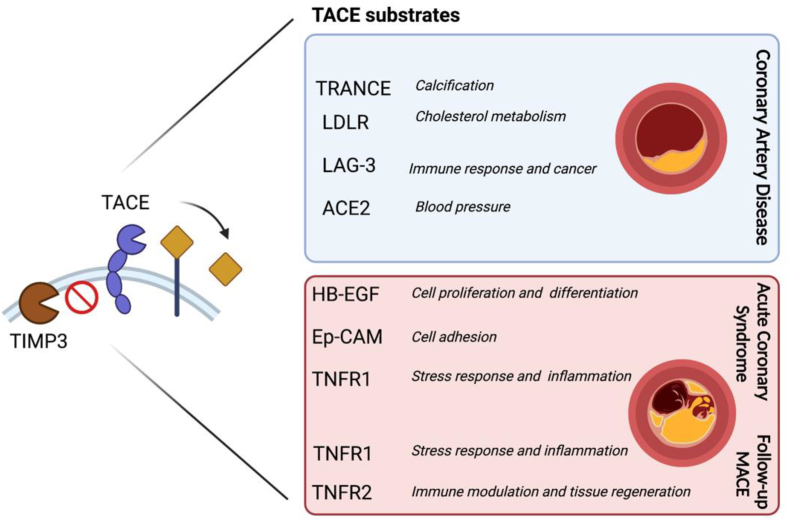
Tumour necrosis factor alpha converting enzyme (TACE/ADAM17) is a member of the A disintegrin and metalloproteinase (ADAM) family of ectodomain shedding proteinases [6]. TACE regulates several biological processes by cleaving cytokines, receptors, adhesion molecules and growth factors involved in inflammation, the immune response and cancer [7]. Many studies have highlighted the role of TACE in cardiovascular disease (CVD) (8) where increased TACE levels have been reported in hypertension [8], ACS complications, such as heart failure and arrhythmias [9,10], and atherosclerotic ischemic stroke [11]. Not only has TACE been directly associated with CVD, but many of the TACE substrates, such as tumour necrosis factor alpha (TNFα) and the receptors (TNFR1 and TNFR2) [12] as well as angiotensin converting enzyme 2 (ACE2) [13] are known to play major roles in CVD complications [14]. Nevertheless, it is still unknown which TACE substrates may be useful as biomarkers to identify patients at higher risk of cardiovascular events which may shed the light on unexplored biological pathways linking these substrates to CVD development. In this study, levels of TACE, TACE substrates and TACE inhibitor Tissue metalloprotease inhibitor 3 (TIMP3) were measured in coronary artery disease (CAD) patients and controls to observe associations with CAD presence, ACS occurrence and follow-up MACE (Fig. 1).
Fig. 1.
TACE/ADAM17 substrates in ACS and follow-up MACE. Levels of TACE/ADAM17 substrates TRANCE, LDLR, LAG-3 and ACE2 were significantly higher in CAD patients compared to controls. Levels of HB-EGF, Ep-CAM, TNFR1 were able to accurately classify ACS patients and levels of TNFR1 and TNFR2 associated with MACE upon follow-up. TACE inhibitor, TIMP3 mRNA levels were lower in CAD patients and tended to be lower in ACS compared to elective CAD patients.
Materials and methods
Participant recruitment
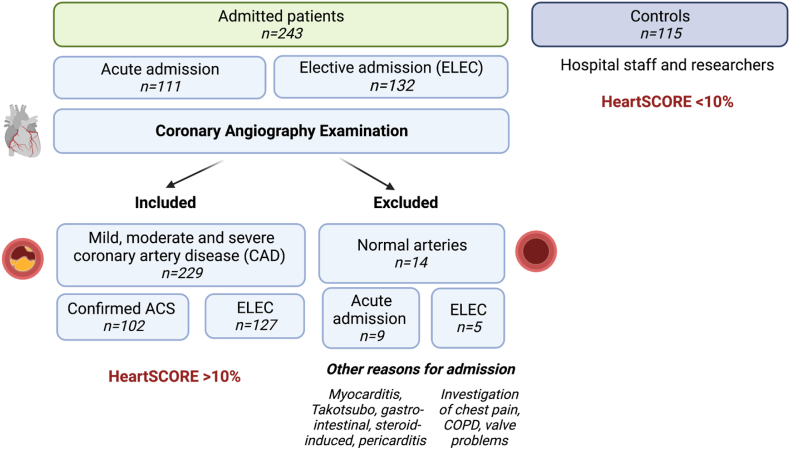
Patients were recruited from the catheterisation laboratory at Altnagelvin Hospital, L.Derry, UK over a two-year period (from 2015 until 2017) (see Fig. 2). A total of 243 patients undergoing coronary angiography of their coronary arteries were recruited either via an acute or elective admissions. A total of 229 patients with mild, moderate and severe atherosclerosis were included in the study where 102 had a confirmed diagnosis of acute coronary syndrome (ACS) and 127 were electively admitted for examination of their coronary arteries (ELEC) (Fig. 2). The remaining (n = 11) patients were excluded as their chest pain was not due to underlying atherosclerosis but conditions such as myocarditis, Takotsubo syndrome or gastro-intestinal problems (Fig. 2). The control cohort (n = 115) was recruited among hospital staff and researchers (mean age 46 years old). The control cohort did not undergo coronary angiography as this invasive procedure is not routinely performed in the general population and is only recommended by the physician in the presence of clinical symptoms (such as chest pain, following an ACS or in the presence of risk factors such as diabetes). Controls had a 10-year fatal CVD risk <10% which was calculated according to the “European low risk Systematic COronary Risk Evaluation (HeartSCORE)” (https://www.heartscore.org/en_GB) using the participants’ risk factors (age, sex, smoking status, systolic blood pressure and total cholesterol levels). During a median follow-up period of 3 years, major adverse cardiovascular events (MACE) were documented after consulting the patient electronic care records 3 years after admission (Northern Ireland Electronic Care Record (NIECR), NHS, UK). These electronic records document MACE which were serious enough for the patient to be admitted to the hospital or seek medical consultation. MACE included acute coronary syndrome (unstable angina, non-ST elevated myocardial infarction or ST elevated myocardial infarction), stent re-stenosis, persistent angina, stroke, transient ischemic attack, all cause deaths and deaths from cardiac origins, deep vein thrombosis, arrythmias, heart failure (including left ventricular impairment), admissions for percutaneous coronary intervention and bypass surgery.
Fig. 2.
Patient recruitment workflow.
The study was conducted in accordance with the declaration of Helsinki. The research protocol was approved by the Office for Research Ethics Committees Northern Ireland (ORECNI) (Ref:14/NI/0068). Each participant gave informed written consent and provided a blood sample and answered a health and lifestyle structured questionnaire. Demographic information as well as previous medical and clinical history were collected. Systolic and diastolic blood pressure at the time of recruitment was recorded and C reactive protein (CRP) were measured on the day. Fasting blood samples were taken and dispensed into EDTA-tubes, after discarding the first 5 ml of blood to avoid unnecessary biological activation. Tubes were immediately cooled on ice and centrifuged (within 4 h of collection) at 2000 RCF for 15 min. Plasma and buffy coat were aliquoted and frozen at −80 °C until further analysis.
Measurement of TACE and TIMP3 plasma protein and mRNA levels
TACE plasma (soluble) and cellular protein levels were measured by ELISA (Sigma TACE ELISA kit Sigma RAB0003) following the manufacturer's instructions. For the cellular protein levels, total proteins were extracted from a buffy coat preparation containing the peripheral blood mononuclear cells and TACE was measured by ELISA and normalised to total protein levels in each sample. The intra-assay CV was 11.91%. Plasma TIMP3 protein levels were measured by ELISA (Abcam TIMP3 (MIG-5)-Human-ab119608). The intra-assay CV was 5.49%. TACE and TIMP3 mRNA levels were measured from patient buffy coat which was preserved in RNAlater® Stabilization Solution (AM7020–ThermoFisher Scientific) at −80 °C. mRNA was extracted using the RiboPure™ RNA-Purification-Kit (AM1924-Ambion). Quantitative real time PCR was performed using the Roche Light cycler®480II and the LightCycler®480 probe Master reagents (04707494001-Roche) and probes (GAPDH-05532957001–Assay-ID-141139, Roche; ADAM17/TACE-05532957001–Assay-ID-136022, Roche; TIMP3 05532957001–Assay-ID-101221-Roche) after converting RNA into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (04379012001-Roche). TACE and TIMP3 mRNA expression levels were normalised to GAPDH and 2∧(-ΔcT) was calculated for each sample which represents the fold increase in gene expression of the target mRNA compared to the control group. The inter-assay CVs were 1.01% for GAPDH, 2.89% for TACE and 1.31% for TIMP3.
Measurement of TACE substrates protein levels in the plasma
A full list of TACE substrates was identified by consulting the literature [15,[16], [17], [18]] and by identifying TACE substrates that overlapped with the following proprietary Olink Bioscience (Uppsala, Sweden) panels: CVDII, CVDIII, immune response and inflammation. The list of TACE substrates which were evaluated in this study were: Interleukin-1 receptor type 1 (IL1RT), Interleukin-6 receptor subunit alpha (IL-6R), Tumour necrosis factor receptor 1 (TNFR1), Tumour necrosis factor receptor 2 (TNFR2), Low-density lipoprotein receptor (LDLR), Sortilin (SORT1), Tumour necrosis factor alpha (TNFα), Tumor necrosis factor-related activation-induced cytokine (TRANCE), Macrophage colony stimulating factor 1 (CSF-1), Lymphocyte activation gene protein 3 (LAG-3), Transforming growth factor alpha (TGFα), Heparin-binding EGF-like growth factor (HB-EGF), Amphiregulin (AREG), Fms Related Tyrosine Kinase 3 Ligand (FLT-3L), Activated leukocyte cell adhesion molecule (ALCAM), Junctional adhesion molecule A (JAM-A), Epithelial cell adhesion molecule (Ep-CAM), Angiotensin converting enzyme 2 (ACE-2). Plasma protein levels were measured by Proseek Multiplex proximity extension assay (Olink Bioscience, Uppsala, Sweden) which utilises two steps. In the first, two specific antibodies bind the target protein, bringing two single stranded DNA tags into close proximity. In step two, the double stranded tag was cleaved and amplified by qPCR. Relative expression values (normalised protein expression, NPX) were on a log2 scale and are derived from the qPCR step Ct values. One NPX difference corresponds to 2-fold in protein concentration.
Statistical methods
Data analysis was performed using IBM® SPSS version 28.0 software (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± SD, if approximately normally distributed, or as median (1st and 3rd quartile) otherwise; categorical variables were expressed as number; percentages. Analysis of Covariance (ANCOVA) was used to compare the levels of TACE and TACE substrates between CAD and the control group while controlling for confounding factors (age, gender, body mass index (BMI) and cholesterol levels) which were statistically associated with the dependant variable in question (TACE or TACE substrates) as per [19] and adjusted for multiple comparisons (FDR set at 0.05). Multivariable logistic regression was applied to compare levels of TACE substrates between ACS and electively admitted (denoted ELEC) CAD patients while controlling for creatinine levels, smoking status, history of previous myocardial infarction, stent or bypass and previous use of CAD medication (anti-hypertensive therapy, anti-cholesterol therapy, anti-platelet therapy) which were significantly different between ACS and elective patients. Multivariable Cox regression analysis was performed to evaluate the MACE predictive ability of TACE substrates, while controlling for age, creatinine levels, cholesterol levels, history of heart failure, history of previous myocardial infarction, stent or bypass and ACS status which were significantly different between ACS and elective patients. The Mann-Whitney test (non-parametric test) was used to compare TIMP3 mRNA expression levels between the CAD and the control groups and the ACS and ELEC groups. Kaplan-Meier curves were generated to describe the MACE-free survival of the patients within this study and stratified according to the median plasma levels of TNFR1 and TNFR2. Statistical significance was defined as values of p < 0.05 (two-tailed).
Results
A total of 229 CAD patients and 115 controls were recruited consecutively over a period of two years (Fig. 2 and Table 1). Out of the recruited CAD patients, 55.4% (n = 127) were admitted after an ACS and 44.4% (n = 102) were electively admitted for coronary angiography. After a median follow-up period of 3-years, MACE occurred in 35.5% (n = 80) of the CAD patients (Fig. 2). CAD participants were older than the controls, were mostly men and had significant CAD risk factors as expected (Table 1).
Table 1.
Population demographics.
| CAD Risk Factors | Controls | CAD |
p-value |
|---|---|---|---|
| (t-test) | |||
| Number of participants | 115 | 229 | |
| Age (years) | 46.2 ± 12.3 | 65.3 ± 10.7 | <0.0001 |
| Male (n; %) | 34; 29.6 | 170; 78.6 | <0.0001 |
| Weight (Kg) | 76.7 ± 16.6 | 86.1 ± 19.5 | <0.0001 |
| Height (cm) | 167.2 ± 9.3 | 171 ± 9.5 | 0.0005 |
| BMI (Kg/m2) | 27.5 ± 5.9 | 29.1 ± 6.4 | 0.0188 |
| Systolic BP (mmHg) | 124 ± 20 | 131 ± 24 | 0.0111 |
| Diastolic BP (mmHg) | 76 ± 14 | 72 ± 12 | 0.0142 |
| Total Cholesterol (mmol/L) | 5.0 ± 1.0 | 4.2 ± 1.3 | <0.0001 |
| LDL Cholesterol (mmol/L) | 2.9 ± 0.9 | 2.3 ± 1.0 | <0.0001 |
| HDL Cholesterol (mmol/L) | 1.6 ± 0.4 | 1.2 ± 0.5 | <0.0001 |
| TG (mmol/L) | 1.1 ± 0.6 | 1.8 ± 2.1 | <0.0001 |
| CRP (mg/L) |
2.5 ± 3.4 |
12.0 ± 31.2 |
0.0020 |
| n; % | p-value | ||
|
(Chi 2) | |||
| Hypertension | 15; 13.0 | 128; 55.9 | <0.0001 |
| Diabetes | 0; 0 | 44; 19.2 | N/A |
| Dyslipidaemia | 57; 49.6 | 134; 58.5 | 0.1157 |
| GFR <60 ml/min | 3; 2.7 | 45; 20.0 | <0.0001 |
| Arthritis | 6; 5.2 | 35; 15.3 | 0.0065 |
| Depression | 11; 9.6 | 43; 18.8 | 0.0267 |
| Employed | 104; 92.9 | 66; 33.3 | <0.0001 |
| Current smokers | 5; 4.4 | 42; 19.3 | 0.0002 |
| Ex-smokers | 45; 39.1 | 110; 50.4 | <0.0001 |
| Medications | % | % | |
| Statins | 8.69 | 73.45 | <0.0001 |
| Anti-hypertensive therapy | 11.30 | 75.78 | <0.0001 |
| Anti-platelet therapy | 6.08 | 67.84 | <0.0001 |
| Anti-angina therapy | 0.87 | 38.77 | <0.0001 |
| Diuretics | 2.60 | 24.67 | <0.0001 |
BMI, body mass index; BP: blood pressure; CAD, coronary artery disease, CRP, C-reactive protein; GFR, Glomerular filtration rate; HDL: high density lipoprotein; LDL, low density lipoprotein; TG, triglycerides.
TACE mRNA and TACE substrates plasma levels (LDLR, TRANCE, LAG-3 and ACE2) are significantly higher in CAD participants compared to controls.
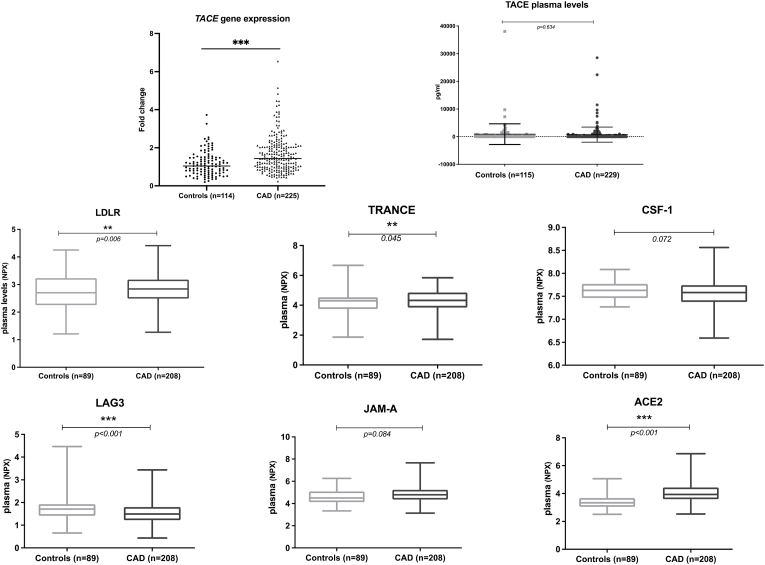
TACE mRNA levels were significantly higher in CAD patients compared to controls after adjusting for age, gender, BMI and cholesterol levels (1.65 ± 0.96 vs 1.16 ± 0.63; p < 0.001). However, this was not observed with TACE plasma levels which were not significantly different between CAD and controls (p = 0.634). Of note, TACE plasma levels were undetectable in 48.25% of the participants and this may be attributed to a low release of TACE protein into the plasma which was below the limit of the detection of the kit used (RAB0003 SIGMA) in some participants. When TACE protein levels were transformed into a detectable/non-detectable categorical variable, it is of note that a TACE plasma levels were detectable in a significantly higher percentage of controls compared to CAD patients (60.80% vs 47.16%, p < 0.05). TACE cellular protein levels measured in the buffy coat (peripheral blood mononuclear cells, PBMCs) of a subset of CAD patients were significantly higher in CAD (n = 80) compared to controls (n = 36) (383.90 ± 259.63 pg/ml vs. 221.92 ± 78.78 pg/ml; p < 0.01). These results, as well as previous reports [20], might indicate that, in CAD, TACE protein tends to accumulate in the cell and is less likely to be shed from the cell surface into the plasma.
TACE substrates levels were measured using the Olink platform (panel CVDII, CVDIII, inflammation and immune response panel). TACE substrates are known to be involved in the immune system, organ development, cell differentiation and cell adhesion, and include TNFR1, TNFR2, LDLR, IL6-R, TNFα, TGFα and ACE2 (refer to list in Table 2) among others. Plasma levels of LDLR (p = 0.006), TRANCE (p = 0.045), LAG-3 (p < 0.001) and ACE2 (p < 0.001) were significantly higher in CAD compared to controls after adjusting for age, gender, BMI and cholesterol levels (Fig. 3). In patients with CAD, this translates into an upregulation of pathways associated with cholesterol metabolism (LDLR), calcification (TRANCE) and blood pressure control (ACE2), which is already known [[21], [22], [23]], but highlights an unexplored role for LAG-3, known to be associated with the immune response, cancer and HDL-C levels, in CAD [24,25]. It is of note that there were no significant differences in ACE2 plasma levels between CAD patients on ACE inhibitors (38.64%) and those who were not (p = 0.091) as corroborated previously [26]. Moreover, there were no statistical difference in LDLR plasma levels in CAD patients on statins (73.45%) compared to those who were not (p = 0.292).
Table 2.
TACE and TACE substrates levels in CAD patients and controls.
| TACE | Controls (n = 114) |
CAD (n = 225) |
p valuea | |||
|---|---|---|---|---|---|---|
| Median, 1st, 3rd quartile | Median 1st, 3rd quartile | |||||
| TACE mRNA expression levels/GAPDH (fold change norm to Controls) | 1.04, 0.68, 1.47 | 1.43, 0.96, 2.07 | <0.001 | |||
| TACE plasma levels (pg/ml) | 128.8, 0, 404.9 | 0, 0, 351.07 | 0.634 | |||
| TACE cell protein levels (pg/ml) (n = 36/80) |
222.1, 153.2, 208.9 |
301.5240.32, 443.05 |
<0.001 |
|||
| TACE substrates | ||||||
| Protein | Olink panel | Controls (n = 89) | CAD (n = 208) | p valuea | ||
| Immune system |
Mean (NPX) |
SD |
Mean (NPX) |
SD |
||
| IL1R2 | CVDII | 4.28 | 0.31 | 4.33 | 0.36 | 0.218 |
| IL-6R | CVDIII | 10.77 | 0.34 | 10.84 | 0.40 | 0.926 |
| TNFR1 | CVDIII | 5.31 | 0.33 | 5.79 | 0.63 | 0.356 |
| TNFR2 | CVDIII | 3.71 | 0.31 | 4.05 | 0.55 | 0.928 |
| LDLR | CVDIII | 2.73 | 0.66 | 2.85 | 0.56 | 0.006 |
| SORT1 | CVDII | 7.63 | 0.26 | 7.75 | 0.32 | 0.249 |
| TNFα | Inflammation | 0.05 | 0.33 | 0.04 | 0.38 | 0.553 |
| TRANCE | Inflammation | 4.17 | 0.71 | 4.30 | 0.72 | 0.045 |
| CSF-1 | Inflammation | 7.63 | 0.20 | 7.57 | 0.29 | 0.072 |
| LAG-3 | Immune | 1.71 | 0.50 | 1.54 | 0.45 | <0.001 |
| Development and differentiation | ||||||
| TGFα | Inflammation | 2.92 | 0.29 | 3.00 | 0.52 | 0.564 |
| HB-EGF | CVDII | 4.99 | 0.63 | 4.96 | 0.72 | 0.869 |
| AREG | Immune | 1.12 | 0.55 | 1.15 | 0.59 | 0.378 |
| FLT-3L | Inflammation | 8.63 | 0.47 | 8.72 | 0.52 | 0.881 |
| Cell adhesion | ||||||
| ALCAM | CVDIII | 4.47 | 0.23 | 4.51 | 0.26 | 0.815 |
| JAM-A | CVDIII | 4.60 | 0.59 | 4.86 | 0.76 | 0.084 |
| Ep-CAM | CVDIII | 4.89 | 1.03 | 4.78 | 0.95 | 0.756 |
| Others | ||||||
| ACE-2 | CVDII | 3.44 | 0.56 | 4.10 | 0.70 | <0.001 |
Plasma levels of TACE substrates TNFR1, HB-EGF and Ep-CAM are significantly different in ACS compared to elective CAD patients.
p value after FDR correction and adjusted for age, gender, BMI and cholesterol levels between the two groups.
Fig. 3.
Levels of TACE and TACE substrates in CAD patients and controls. Plasma levels of LDLR, TRANCE, CSF-1, LAG-3, JAM-A, and ACE2 were significantly higher in CAD compared to controls after adjusting for age, gender, BMI and cholesterol levels.
ACS patients had significantly higher levels of creatinine (100.83 ± 77.00 vs 84.39 ± 20.64 μmol/L; p = 0.032) and were more likely to be smokers (27.0% vs 11.1%; p = 0.004) compared to elective CAD patients. ACS patients were less likely to be on medication or have a previous history of myocardial infarction (MI), stent or bypass (Table 3).
Table 3.
Demographics of ACS and elective CAD patients.
| ELEC (n = 114) |
ACS (n = 105) |
p value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 64.96 | 10.11 | 65.99 | 10.98 | 0.469 |
| BMI (Kg/m2) | 29.38 | 7.25 | 29.19 | 5.40 | 0.838 |
| Systolic BP | 131.87 | 21.50 | 128.93 | 25.41 | 0.402 |
| Cholesterol (mmol/L) | 4.11 | 1.31 | 4.32 | 1.33 | 0.278 |
| Creatinine (umol/L) | 84.38 | 20.64 | 100.83 | 77.00 | 0.032 |
| LDL cholesterol (mmol/L) | 2.27 | 0.96 | 2.36 | 1.03 | 0.545 |
| HDL cholesterol (mmol/L) | 1.18 | 0.30 | 1.21 | 0.53 | 0.550 |
| Triglycerides (mmol/L) |
1.65 |
1.16 |
1.96 |
2.80 |
0.309 |
| % |
% |
||||
| Male | 75.0 | 83.3 | 0.135 | ||
| Diabetic | 19.3 | 18.1 | 0.864 | ||
| Smoking | 11.1 | 27.0 | 0.004 | ||
| HF | 13.2 | 17.1 | 0.453 | ||
| Previous CAD meds | 83.3 | 70.2 | 0.024 | ||
| Previous (MI, Stent, bypass) | 53.5 | 35.2 | 0.010 | ||
ACS: Acute coronary syndrome; BMI, body mass index; BP: blood pressure; CAD: Coronary artery disease; ELEC: Elective CAD patients; HF: Heart Failure; HDL: high density lipoprotein; LDL, low density lipoprotein; TG, triglycerides.
Multivariable regression analysis of NPX values showed that TNFR1 (OR: 3.237, CI:1.514–6.923, p = 0.002), HB-EGF (OR: 0.484, CI:0.288–0.813, p = 0.006), Ep-CAM (OR: 0.555, CI: 0.327–0.829, p = 0.004) plasma levels were each independently associated with ACS after adjustment for creatinine, smoking status, previous MI, stent or bypass and previous CAD medications (Table 4). While TNFR1 plasma levels were significantly higher in ACS patients (5.63 ± 0.52 vs 5.98 ± 0.71, p = 0.002), Hb-EGF (5.15 ± 0.76 vs 4.78 ± 0.62, p = 0.006) and Ep-CAM (4.94 ± 0.96 vs 4.54 ± 0.86, p = 0.004) plasma levels were significantly lower in ACS patients compared to patients admitted electively with CAD history. It is of note that TNFR1, HB-EGF and Ep-CAM were not significantly different between patients admitted with unstable angina, NSTEMI or STEMI within the ACS cohort. TACE mRNA levels were not significantly different between ACS and ELC (p = 0.363) nor were TACE plasma (p = 0.539) or cellular protein levels (p = 0.249). Despite TACE being the major protease responsible for HB-EGF and Ep-CAM shedding, other MMPs (such as MMP3) can also participate in HB-EGF processing [27,28]. Similarly, Ep-CAM requires further processing by another protease (BACE1) [29]. Nevertheless, HB-EGF and Ep-CAM plasma levels were significantly decreased in the ACS cohort regardless of the extent of their processing by TACE or other proteases.
Table 4.
Logistic regression analysis. Multivariable regression analysis of NPX values demonstrates that TNFR1, HB-EGF and Ep-CAM plasma levels were each independently associated with ACS after adjustment for creatinine, smoking status, previous MI, stent or bypass and previous CAD medications for each variable separately.
| ELEC (n = 104) |
ACS (n = 95) |
Exp(B) | p valuea | 95% CI for EXP(B) |
||||
|---|---|---|---|---|---|---|---|---|
| mean (NPX) | Sd | mean (NPX) | Sd | Lower | Upper | |||
| TNFR1 | 5.63 | 0.52 | 5.98 | 0.71 | 3.24 | 0.002 | 1.51 | 6.92 |
| HB-EGF | 5.16 | 0.76 | 4.77 | 0.63 | 0.48 | 0.006 | 0.29 | 0.81 |
| Ep-CAM | 4.95 | 0.96 | 4.54 | 0.86 | 0.56 | 0.004 | 0.37 | 0.83 |
ACS: Acute coronary syndrome; ELEC: Elective CAD patients, NPX: Normalised protein expression.
TIMP3 is downregulated in CAD and tends to be lower in patients admitted with ACS compared to those admitted electively.
Each of TNFR1, HB-EGF and EpCAM were individually adjusted for creatinine levels, smoking, previous MI, stent or bypass and previous CAD medsRef category is ELEC.
It is of note that in CAD patients where TACE plasma levels were detected, no significant differences were observed between ACS and ELEC patients (p = 0.874) nor between CAD patients who developed follow-up MACE compared to those who did not (p = 0.860) increasing the relevance of further investigating the significance and origin of TACE plasma levels.
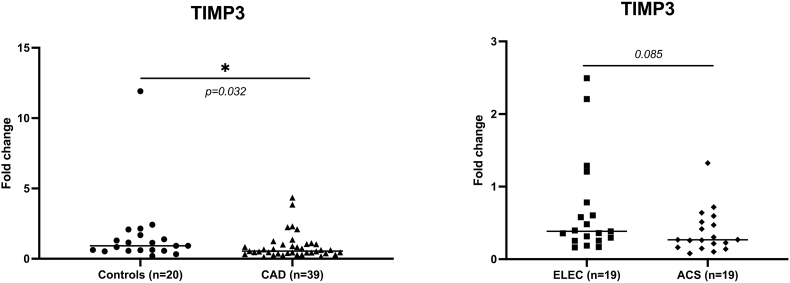
We investigated one of the possible mechanisms for TACE upregulation in a subgroup CAD patients (n = 39) and controls (n = 20) with no significant differences in terms of baseline characteristics. TACE is known to be inhibited by Tissue metalloprotease inhibitor 3 (TIMP3) [30]. Messenger RNA expression levels of TIMP3 were decreased in the peripheral blood in CAD patients compared to controls (0.90 ± 0.92 vs 1.60 ± 2.51; p < 0.05). TIMP3 also tended to be downregulated in CAD patients admitted with an ACS compared to electively admitted patients (0.65 ± 0.52 vs 1.17 ± 1.17; p = 0.085) (Fig. 4). Nevertheless, plasma TIMP3 protein levels were not statistically different between CAD patients and controls (p = 0.486). TIMP3 plasma and mRNA levels did not predict MACE in CAD patients. However, TIMP3 cellular protein levels measured in the buffy coat (PBMCs) of a subset of CAD participants tended to be lower in CAD patients (n = 26) vs controls (n = 15) (1267.45 ± 1070.10 vs 1766.48 ± 1285.97 pg/ml; p = 0.189).
Fig. 4.
TIMP3 mRNA expression levels is lower in CAD patients and tends to be lower in those admitted with ACS.
Plasma levels of TACE substrates TNFR1 and TNFR2 are predictive of MACE
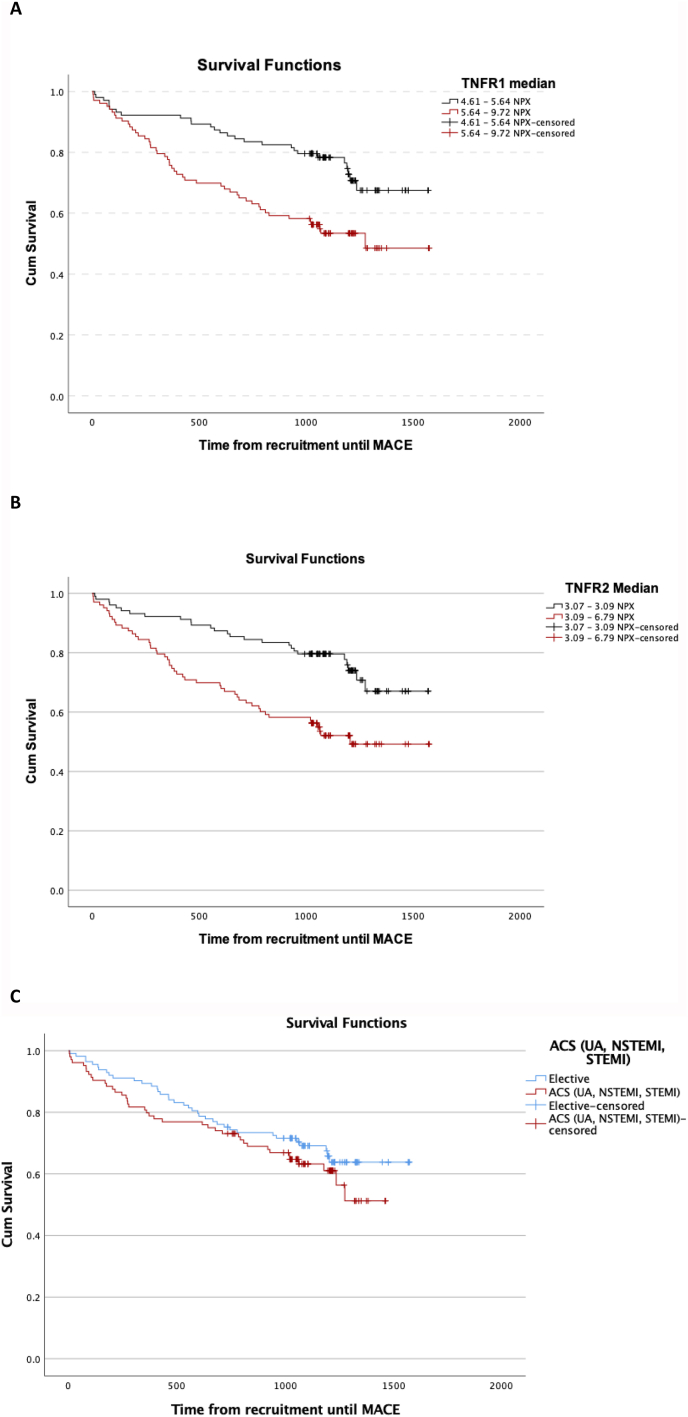
Multivariable Cox proportional hazard regression analysis identified that out of the measured TACE substrates, TNFR1 (OR: 2.317, CI: 1.377–3.898, p = 0.002) and TNFR2 (OR: 1.902, CI:1.072–3.373, p = 0.028) plasma levels were independently associated with MACE in CAD patients after adjustment for age, creatinine levels, cholesterol levels, heart failure, previous MI, stent or bypass and ACS status (Table 5 and Fig. 5A and B). It is of note that TACE protein and mRNA levels did not associate with MACE. There was no significant difference in the survival distributions between ACS and elective patients estimated by the log rank test (p = 0.267) (Fig. 5C).
Table 5.
Multivariable Cox proportional hazard regression analysis.
| Hazard Ratios | p value | 95.0% CI for Exp(B) |
||
|---|---|---|---|---|
| Lower | Upper | |||
| TNFR1 (NPX) | 2.65 | 0.002 | 1.447 | 4.86 |
| Age | 1.01 | 0.345 | 0.99 | 1.04 |
| Gender | 0.73 | 0.379 | 0.36 | 1.48 |
| Creatinine (umol/L) | 1.00 | 0.196 | 0.99 | 1.00 |
| Cholesterol (mmol/L) | 0.96 | 0.731 | 0.78 | 1.19 |
| HF | 1.60 | 0.134 | 0.87 | 2.92 |
| Previous MACE | 1.35 | 0.263 | 0.80 | 2.30 |
| ACS/ELEC | 0.90 | 0.715 | 0.53 | 1.55 |
| TNFR2 (NPX) | 1.97 | 0.031 | 1.06 | 3.66 |
| Age | 1.01 | 0.272 | 0.99 | 1.04 |
| Gender | 0.90 | 0.769 | 0.46 | 1.78 |
| Creatinine (umol/L) | 1.00 | 0.960 | 1.00 | 1.00 |
| Cholesterol (mmol/L) | 0.95 | 0.651 | 0.77 | 1.18 |
| HF | 1.80 | 0.058 | 0.98 | 3.26 |
| Previous MACE | 1.34 | 0.269 | 0.79 | 2.27 |
| ACS/ELEC | 1.15 | 0.590 | 0.69 | 1.93 |
ACS: Acute coronary syndrome; ELEC: Elective CAD patients, HF: Heart failure; MACE: Major adverse cardiovascular events; NPX: Normalised protein expression.
Fig. 5.
Kaplan-Meier Survival Curves. Survival function representing follow-up MACE in CAD patients stratified according to median plasma TNFR1 levels (A), TNFR2 levels (B) and ACS and elective status (C) In all graphs, the label “censored” on the graph indicates a truncated survival time.
Discussion
Currently, no available blood test can accurately predict the risk of a cardiovascular event in the general population [31]. To estimate a person's risk of cardiovascular death within 10-years, physicians rely on risk scoring charts which hold many limitations since the majority of people who experience an event are classified as low/medium risk [32]. Additionally, those that are risk-managed for their underlying CAD still experience cardiovascular events. There is a clear need therefore to improve event prediction for patients with CAD by optimising the methods of primary and secondary prevention. Blood biomarkers offer promise in this field of research and given the importance and contribution of inflammation to CAD [33], research into inflammatory biomarkers is warranted. Overall, this study demonstrated that TACE, a member of the ADAM protein family of disintegrins and metalloproteases, and a number of its substrates, which are heavily involved in regulating inflammatory processes, were upregulated in CAD. We also identified TNFR1, HB-EGF and Ep-CAM as potential biomarkers for ACS and corroborated the association of TNFR1 and TNFR2 with MACE. Interestingly, we also found that decreased levels of TIMP3, an inhibitor of TACE, was associated with in CAD and potentially ACS (Fig. 1).
First, our study replicated previous findings of TACE upregulation in patients with cardiovascular events [[9], [10], [11],34]. Higher TACE mRNA expression levels however did not translate to increased circulating TACE plasma levels. This is potentially due to the limit of detection of the assay used combined to a higher cellular protein retention of TACE on the peripheral blood mononuclear cells (PBMCs) observed in CAD patients compared to the controls. The mechanism of TACE release into the plasma requires additional investigation and previous studies, which have detected TACE protein on microparticles [35,36] and in the plasma of patients with inflammatory conditions including ANCA-associated vasculitis [[36], [37], [38]], can be used as a starting point for further exploration.
Since TACE is involved in several biological processes, it is challenging to attribute its increased mRNA expression and cellular protein levels to specific processes and pathways. An alternative way of observing the consequences of TACE activation is by complementing its measurement with that of its substrates, and in particular those of relevance to CAD complications [15]. After compiling a list of TACE substrates from the literature and overlapping it with those available within four Olink panels (Uppsala, Sweden), we sought to identify the substrates of interest with respect to their association with ACS and follow-up MACE. Among the measured TACE substrates, several upregulated proteins reflect pathways involving cholesterol metabolism (LDLR), calcification (TRANCE) and blood pressure control (ACE2) in patients with CAD which is already established [[21], [22], [23]]. Our investigation highlighted however an unexplored role of LAG-3, known to be associated with immune response, cancer and the regulation of HDL-C levels [24], in CAD. It has been recently shown that inhibiting proprotein convertase subtilisin/kexin 9 (PCSK9), an important regulator of LDLR and the lipid metabolism, enhanced cancer therapy [39]. This highlights the need to further explore associations between cancer associated proteins, such as LAG-3, and lipid pathways.
Tissue metalloprotease inhibitor 3 (TIMP3), the only known TACE endogenous inhibitor [40], associates with TACE dimer structures to inhibit its proteolytic activity [41]. We investigated one of the possible mechanisms explaining the observed increased TACE mRNA and cellular protein levels in CAD patients by measuring TIMP3 mRNA levels in PBMCs. Our results showed that, in contrast to the increase in TACE mRNA and cellular protein levels in CAD patients, TIMP3 mRNA levels were decreased compared to the control group. This suggests that, in CAD individuals, TIMP3 downregulation may not provide enough inhibitory effect on TACE leading to an increased protease activity and a potential higher risk of cardiovascular events. Previous studies reported that TIMP3 gene expression was downregulated in circulating human monocytes in people at high risk of diabetes [42] and was higher in stable and low in vulnerable atherosclerotic plaques [43] implying a particular TIMP3 down regulation in a high inflammatory state [44]. This is also shown in our present study where TIMP3 tended to be particularly downregulated after an ACS compared to electively admitted CAD patients. It has been previously demonstrated that TACE expression is increased during the early phases of an in vitro model of acute Crohn's disease, whereas TIMP3 increased in the later phases of the disease [45] suggesting that maintaining a balance between TACE and TIMP3 during acute phases of inflammation might be crucial to reduce adverse outcomes. From a clinical application perspective, recent reports have highlighted the challenges in inhibiting TACE directly [46]. Therefore, alternative strategies need to be explored [47,48] where TIMP3 administration might be a potential treatment for cardiac complications, such as heart failure [49], following ACS. Additionally, the exploration of TACE and TIMP3 levels at the site of the atherosclerotic plaque might also be of relevance to link the mechanisms taking place at site of the disease with those observed systemically.
Plasma levels of TNFR1, one of the most studied TACE substrates, were increased in ACS patients. This is a similar finding to previous reports associating increased TNFR1 levels to infarct size and ventricular dysfunction after myocardial infarction [50]. TNFR1 levels have also been linked to heart failure and increased levels following ACS associate with adverse outcomes such as left ventricular remodelling following myocardial infarction [51]. We also found that the plasma levels of both TNF-α receptors, TNFR1 and TNFR2 were associated with MACE over a 3-year follow-up period. Our results corroborate previous findings from the CLARICOR study where higher TNFR1 and TNFR2 plasma levels were also associated with cardiovascular events and mortality in CAD patients during a 10-year follow-up [52]. Here, we show that the association between TNFR1 and TNFR2 plasma levels with MACE was strong enough to be observed during a 3-year median follow-up. Therefore, measuring TNFR1 and TNFR2 plasma levels may be beneficial to predict short term MACE in CAD patients.
When we looked specifically at the ACS patients, we noted significantly lower levels of two TACE substrates; heparin binding EGF (HB-EGF) and epithelial cell adhesion molecule (EpCAM), as compared with the elective patients. To the best of our knowledge, these markers have not been previously reported in the literature. HB-EGF is produced by activated macrophages and endothelial cells and stimulates smooth muscle cells to proliferate and produce extracellular matrix [53]. Higher plasma levels of HB-EGF have been previously linked to a stable plaque phenotype and a reduced incidence of coronary events [54]. Local overexpression of HB-EGF is also associated with increased cardiac remodelling following MI [55]. Therefore, patients admitted with ACS might fail to present sufficient systemic HB-EGF levels to stabilise the atherosclerotic plaque. We also show that plasma levels of EpCAM, a tumour marker of epithelial origins [56], were decreased in ACS patients in our study. A recent study associated low EpCAM levels with microvascular obstruction and larger infarct size [57], highlighting the need to design future studies to understand the protective mechanisms of Ep-CAM in CAD especially in the light of recent research of overlapping pathways between cancer and cardiovascular disease [58,59].
As a limitation within the study, patients with CAD were a heterogenous population and presented with several degrees of disease severity. In addition, the ACS group included patients admitted with unstable angina, NSTEMI and with STEMI (n = 6) also representing a heterogenous group. Nevertheless, the proposed biomarkers were unable to distinguish between the subtypes of ACS. It is also impossible to exclude that the control population did not have minor atherosclerosis. However, it is not within routine practice to screen for atherosclerosis in the general population without any risk factors or clinical symptoms and biomarkers, such as those proposed in the present study, can aid in patient referral for invasive coronary angiography. Moreover, the control group had a low incidence of comorbidities (such as hypertension and diabetes) compared to CAD patients which might influence the biomarker levels independently.
In summary, our study highlights the important role of TACE and its substrates in CAD development and a possible modulatory function of TIMP3 on TACE levels. Most importantly, we describe a novel implication of LAG-3 in CAD and report reduced levels of HB-EGF and Ep-CAM in ACS patients highlighting a potential protective role in atherosclerotic plaque stability which requires further exploration. We also underline the importance of measuring TNFR1 and TNFR2 earlier than previously appreciated to predict MACE.
Financial Support
This work was supported by grant of £11.5 M awarded to A. J. B. from the European Union Regional Development Fund European Union Sustainable Competitiveness Programme for Northern Ireland; Northern Ireland Public Health Agency (Health and Social Care Research and Development Division) and the University of Ulster and the Department of Employment and Learning in Northern Ireland.
Author contributions
Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Data statement
The data used in this study is available upon request for collaboration projects.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Mr Mark Gibson Research Nurse for his help with patient recruitment.
References
- 1.Hammer Y., Iakobishvili Z., Hasdai D., Goldenberg I., Shlomo N., Einhorn M., et al. Guideline-recommended therapies and clinical outcomes according to the risk for recurrent cardiovascular events after an acute coronary syndrome. J Am Heart Assoc. 2018 Sep 18;7(18) doi: 10.1161/JAHA.118.009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soehnlein O., Libby P. Targeting inflammation in atherosclerosis — from experimental insights to the clinic. Nat Rev Drug Discov. 2021 Aug;20(8):589–610. doi: 10.1038/s41573-021-00198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P., Ridker P.M. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004 Mar 22;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Batra G., Ghukasyan Lakic T., Lindbäck J., Held C., White H.D., Stewart R.A.H., et al. Interleukin 6 and cardiovascular outcomes in patients with chronic kidney disease and chronic coronary syndrome. 2021 Aug 25. JAMA Cardiol [Internet] [DOI] [PMC free article] [PubMed]
- 5.Kanikowska D., Pyda M., Korybalska K., Grajek S., Lesiak M., Bręborowicz A., et al. Age-related limitations of interleukin-6 in predicting early mortality in acute ST-elevation myocardial infarction. Immun Ageing. 2014 Dec 4;11(1):23. doi: 10.1186/s12979-014-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zunke F., Rose-John S. The shedding protease ADAM17: physiology and pathophysiology. Biochim Biophys Acta Mol Cell Res. 2017 Nov;1864(11 Pt B):2059–2070. doi: 10.1016/j.bbamcr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Moss M.L., Minond D. Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediat Inflamm. 2017 doi: 10.1155/2017/9673537. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5688260/ Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takayanagi T., Forrester S.J., Kawai T., Obama T., Tsuji T., Elliott K.J., et al. Vascular ADAM17 as a novel therapeutic target in mediating cardiovascular hypertrophy and perivascular fibrosis induced by angiotensin IINovelty and significance. Hypertension. 2016 Oct 1;68(4):949–955. doi: 10.1161/HYPERTENSIONAHA.116.07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akatsu T., Nakamura M., Satoh M., Hiramori K. Increased mRNA expression of tumour necrosis factor-alpha and its converting enzyme in circulating leucocytes of patients with acute myocardial infarction. Clin Sci Lond Engl. 1979;105(1):39–44. doi: 10.1042/CS20020367. 2003 Jul. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda Y., Satoh M., Nakamura M., Akatsu T., Hiramori K. Activated tumour necrosis factor-alpha shedding process is associated with in-hospital complication in patients with acute myocardial infarction. Clin Sci Lond Engl. 1979;108(4):339–347. doi: 10.1042/CS20040229. 2005 Apr. [DOI] [PubMed] [Google Scholar]
- 11.yan Ling J., Shen L., Liu Q., Xue S., Ma W., Wu H., et al. Changes in platelet GPIbα and ADAM17 during the acute stage of atherosclerotic ischemic stroke among Chinese. J Huazhong Univ Sci Technol Med Sci. 2013 Jun;33(3):438–442. doi: 10.1007/s11596-013-1138-3. Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. [DOI] [PubMed] [Google Scholar]
- 12.Rolski F., Błyszczuk P. Complexity of TNF-α signaling in heart disease. J Clin Med. 2020 Oct 12;9(10):3267. doi: 10.3390/jcm9103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramchand J., Burrell L.M. Circulating ACE2: a novel biomarker of cardiovascular risk. Lancet. 2020 Oct 3;396(10256):937–939. doi: 10.1016/S0140-6736(20)32011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemaly M., McGilligan V., Gibson M., Clauss M., Watterson S., Alexander H.D., et al. https://www.sciencedirect.com/science/article/pii/S1875213617301857 Role of tumour necrosis factor alpha converting enzyme (TACE/ADAM17) and associated proteins in coronary artery disease and cardiac events. Arch Cardiovasc Dis [Internet]. [cited 2017 Nov 1]; Available from: [DOI] [PubMed]
- 15.Kawai T., Elliott K.J., Scalia R., Eguchi S. Contribution of ADAM17 and related ADAMs in cardiovascular diseases. Cell Mol Life Sci. 2021 May 1;78(9):4161–4187. doi: 10.1007/s00018-021-03779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zunke F., Rose-John S. The shedding protease ADAM17: physiology and pathophysiology. Biochim Biophys Acta BBA - Mol Cell Res. 2017 Nov 1;(11):1864. doi: 10.1016/j.bbamcr.2017.07.001. Part B):2059–70. [DOI] [PubMed] [Google Scholar]
- 17.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010 Apr;45(2):146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizza S., Copetti M., Cardellini M., Menghini R., Pecchioli C., Luzi A., et al. A score including ADAM17 substrates correlates to recurring cardiovascular event in subjects with atherosclerosis. Atherosclerosis. 2015 Apr;239(2):459–464. doi: 10.1016/j.atherosclerosis.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Kamangar F. Confounding variables in epidemiologic studies: basics and beyond. Arch Iran Med. 2012 Aug;15(8):508–516. [PubMed] [Google Scholar]
- 20.Lorenzen I., Lokau J., Korpys Y., Oldefest M., Flynn C.M., Künzel U., et al. Control of ADAM17 activity by regulation of its cellular localisation. Sci Rep. 2016 Oct 12;6(1) doi: 10.1038/srep35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tschiderer L., Klingenschmid G., Nagrani R., Willeit J., Laukkanen J.A., Schett G., et al. Osteoprotegerin and cardiovascular events in high-risk populations: meta-analysis of 19 prospective studies involving 27 450 participants. J Am Heart Assoc. 2018 Aug 21;7(16) doi: 10.1161/JAHA.118.009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi T., Hori M., Harada-Shiba M., Kataoka Y., Onozuka D., Nishimura K., et al. Patients with LDLR and PCSK9 gene variants experienced higher incidence of cardiovascular outcomes in heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2021 Feb 16;10(4) doi: 10.1161/JAHA.120.018263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libby P., Everett B.M. Novel antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol. 2019 Apr 1;39(4):538–545. doi: 10.1161/ATVBAHA.118.310958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez A. High HDL-cholesterol paradox: SCARB1-LAG3-HDL Axis. Curr Atherosclerosis Rep. 2021 Jan;23(1):5. doi: 10.1007/s11883-020-00902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden D., Kolmakova A., Sura S., Vella A.T., Manichaikul A., Wang X.Q., et al. 2016 Oct 20. https://insight.jci.org/articles/view/88628 Lymphocyte activation gene 3 and coronary artery disease. JCI Insight [Internet] [DOI] [PMC free article] [PubMed]
- 26.Zimmermann T., Walter J.E., Lopez-Ayala P., Strebel I., Amrein M., Koechlin M., et al. Influence of renin-angiotensin-aldosterone system inhibitors on plasma levels of angiotensin-converting enzyme 2. ESC Heart Fail. 2021;8(2):1717–1721. doi: 10.1002/ehf2.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dao D.T., Anez-Bustillos L., Adam R.M., Puder M., Bielenberg D.R. Heparin-binding epidermal growth factor–like growth factor as a critical mediator of Tissue repair and regeneration. Am J Pathol. 2018 Nov 1;188(11):2446–2456. doi: 10.1016/j.ajpath.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki M., Raab G., Moses M.A., Fernandez C.A., Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997 Dec 12;272(50):31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- 29.Tsaktanis T., Kremling H., Pavšič M., von Stackelberg R., Mack B., Fukumori A., et al. Cleavage and cell adhesion properties of human epithelial cell adhesion molecule (HEPCAM) J Biol Chem. 2015 Oct 2;290(40):24574–24591. doi: 10.1074/jbc.M115.662700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesaro A., Abakar-Mahamat A., Brest P., Lassalle S., Selva E., Filippi J., et al. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1332–G1343. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 31.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. 2018. https://www.frontiersin.org/article/10.3389/fimmu.2018.00754 Front Immunol [Internet] [DOI] [PMC free article] [PubMed]
- 32.Cooney M.T., Dudina A.L., Graham I.M. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009 Sep 29;54(14):1209–1227. doi: 10.1016/j.jacc.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017 Sep 21;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 34.Satoh M., Ishikawa Y., Itoh T., Minami Y., Takahashi Y., Nakamura M. The expression of TNF-alpha converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur J Clin Invest. 2008 Feb;38(2):97–105. doi: 10.1111/j.1365-2362.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 35.Canault M., Leroyer A.S., Peiretti F., Lesèche G., Tedgui A., Bonardo B., et al. Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am J Pathol. 2007 Nov;171(5):1713–1723. doi: 10.2353/ajpath.2007.070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertram A., Lovric S., Engel A., Beese M., Wyss K., Hertel B., et al. Circulating ADAM17 level reflects disease activity in proteinase-3 ANCA-associated vasculitis. J Am Soc Nephrol JASN. 2015 Nov;26(11):2860–2870. doi: 10.1681/ASN.2014050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Q., Hampel H., Blennow K., Lista S., Levey A., Tang B., et al. Increased plasma TACE activity in subjects with mild cognitive impairment and patients with Alzheimer's disease. J Alzheimers Dis JAD. 2014;41(3):877–886. doi: 10.3233/JAD-140177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen J.E.V., Mkumbaye S.I., Vaaben A.V., Manjurano A., Lyimo E., Kavishe R.A., et al. 2016 Oct 27. Plasma Ang2 and ADAM17 levels are elevated during clinical malaria; Ang2 level correlates with severity and expression of EPCR-binding PfEMP1.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5082358/ Sci Rep [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X., Bao X., Hu M., Chang H., Jiao M., Cheng J., et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020 Dec;588(7839):693–698. doi: 10.1038/s41586-020-2911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black R.A. TIMP3 checks inflammation. Nat Genet. 2004 Sep;36(9):934–935. doi: 10.1038/ng0904-934. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y., Schlondorff J., Blobel C.P. Evidence for regulation of the tumor necrosis factor alpha-convertase (TACE) by protein-tyrosine phosphatase PTPH1. J Biol Chem. 2002 Nov 8;277(45):42463–42470. doi: 10.1074/jbc.M207459200. [DOI] [PubMed] [Google Scholar]
- 42.Cardellini M., Menghini R., Luzi A., Davato F., Cardolini I., D'Alfonso R., et al. Decreased IRS2 and TIMP3 expression in monocytes from offspring of type 2 diabetic patients is correlated with insulin resistance and increased intima-media thickness. Diabetes. 2011 Dec;60(12):3265–3270. doi: 10.2337/db11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller A., Krämer S.D., Meletta R., Beck K., Selivanova S.V., Rancic Z., et al. Gene expression levels of matrix metalloproteinases in human atherosclerotic plaques and evaluation of radiolabeled inhibitors as imaging agents for plaque vulnerability. Nucl Med Biol. 2014 Aug;41(7):562–569. doi: 10.1016/j.nucmedbio.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 44.Ma R., Gu B., Gu Y., Groome L.J., Wang Y. Down-regulation of TIMP3 leads to increase in TACE expression and TNFα production by placental trophoblast cells. Am J Reprod Immunol N Y N. 1989;71(5):427–433. doi: 10.1111/aji.12205. 2014 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cesaro A., Abakar-Mahamat A., Brest P., Lassalle S., Selva E., Filippi J., et al. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1332–G1343. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 46.Nicolaou A., Zhao Z., Northoff B.H., Sass K., Herbst A., Kohlmaier A., et al. Adam17 deficiency promotes atherosclerosis by enhanced TNFR2 signaling in mice. Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):247–257. doi: 10.1161/ATVBAHA.116.308682. [DOI] [PubMed] [Google Scholar]
- 47.Jia Y., Kong W. ADAM17: a molecular switch to control TNFR2 during atherogenesis in vivo. Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):176–178. doi: 10.1161/ATVBAHA.116.308840. [DOI] [PubMed] [Google Scholar]
- 48.van der Vorst E.P.C., Zhao Z., Rami M., Holdt L.M., Teupser D., Steffens S., et al. Contrasting effects of myeloid and endothelial ADAM17 on atherosclerosis development. Thromb Haemostasis. 2017 Feb 28;117(3):644–646. doi: 10.1160/TH16-09-0674. [DOI] [PubMed] [Google Scholar]
- 49.Martz L. 2014 Mar 6. Taking TIMP3 to heart.http://www.nature.com/scibx/journal/v7/n9/full/scibx.2014.246.html SciBX Sci-Bus Exch [Internet] [Google Scholar]
- 50.Nilsson L., Szymanowski A., Swahn E., Jonasson L. Soluble TNF receptors are associated with infarct size and ventricular dysfunction in ST-elevation myocardial infarction. PLoS One. 2013 Feb 6;8(2) doi: 10.1371/journal.pone.0055477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ping Z., Aiqun M., Jiwu L., Liang S. TNF receptor 1/2 predict heart failure risk in type 2 diabetes mellitus patients. Int Heart J. 2017 Apr 6;58(2):245–249. doi: 10.1536/ihj.16-236. [DOI] [PubMed] [Google Scholar]
- 52.Carlsson Axel C., Ruge Toralph, Kjøller Erik, Hilden Jørgen, Kolmos Hans Jørn, Sajadieh Ahmad, et al. 10-Year associations between tumor necrosis factor receptors 1 and 2 and cardiovascular events in patients with stable coronary heart disease: a CLARICOR (effect of clarithromycin on mortality and morbidity in patients with ischemic heart disease) trial substudy. J Am Heart Assoc. 7(9):e008299. [DOI] [PMC free article] [PubMed]
- 53.Reynolds C.M., Eguchi S., Frank G.D., Motley E.D. Signaling mechanisms of heparin-binding epidermal growth factor-like growth factor in vascular smooth muscle cells. Hypertension. 2002 Feb 1;39(2):525–529. doi: 10.1161/hy0202.103076. [DOI] [PubMed] [Google Scholar]
- 54.Sara Rattik, Maria Wigren, Harry Björkbacka, Fredrikson Gunilla Nordin. Bo Hedblad, Agneta Siegbahn, et al. High plasma levels of heparin-binding epidermal growth factor Are associated with a more stable plaque phenotype and reduced incidence of coronary events. Arterioscler Thromb Vasc Biol. 2015 Jan 1;35(1):222–228. doi: 10.1161/ATVBAHA.114.304369. [DOI] [PubMed] [Google Scholar]
- 55.Ushikoshi H., Takahashi T., Chen X., Khai N.C., Esaki M., Goto K., et al. Local overexpression of HB-EGF exacerbates remodeling following myocardial infarction by activating noncardiomyocytes. Lab Investig J Tech Methods Pathol. 2005 Jul;85(7):862–873. doi: 10.1038/labinvest.3700282. [DOI] [PubMed] [Google Scholar]
- 56.Huang L., Yang Y., Yang F., Liu S., Zhu Z., Lei Z., et al. Functions of EpCAM in physiological processes and diseases (Review) Int J Mol Med. 2018 Oct;42(4):1771–1785. doi: 10.3892/ijmm.2018.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ríos-Navarro C., Gavara J., Núñez J., Revuelta-López E., Monmeneu J.V., López-Lereu M.P., et al. EpCAM and microvascular obstruction in patients with STEMI: a cardiac magnetic resonance study. Rev Esp Cardiol Engl Ed. 2021;75(5):384–391. doi: 10.1016/j.rec.2021.04.006. https://www.sciencedirect.com/science/article/pii/S1885585721001353 [Internet] [DOI] [PubMed] [Google Scholar]
- 58.Libby P., Sidlow R., Lin A.E., Gupta D., Jones L.W., Moslehi J., et al. Clonal hematopoiesis at crossroads of aging, cardiovascular diseases, and cancer: JACC topic of the week. J Am Coll Cardiol. 2019 Jul 30;74(4):567–577. doi: 10.1016/j.jacc.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alam N., Wright A.K., Ashcroft D.M., Renehan A.G. Cancer and cardiovascular disease. Lancet. 2020 Jun 20;395(10241):1903–1904. doi: 10.1016/S0140-6736(20)30222-1. [DOI] [PubMed] [Google Scholar]