Abstract
Background
Myocardial injury in patients with COVID-19 and suspected cardiac involvement is not well understood.
Objectives
The purpose of this study was to characterize myocardial injury in a multicenter cohort of patients with COVID-19 and suspected cardiac involvement referred for cardiac magnetic resonance (CMR).
Methods
This retrospective study consisted of 1,047 patients from 18 international sites with polymerase chain reaction–confirmed COVID-19 infection who underwent CMR. Myocardial injury was characterized as acute myocarditis, nonacute/nonischemic, acute ischemic, and nonacute/ischemic patterns on CMR.
Results
In this cohort, 20.9% of patients had nonischemic injury patterns (acute myocarditis: 7.9%; nonacute/nonischemic: 13.0%), and 6.7% of patients had ischemic injury patterns (acute ischemic: 1.9%; nonacute/ischemic: 4.8%). In a univariate analysis, variables associated with acute myocarditis patterns included chest discomfort (OR: 2.00; 95% CI: 1.17-3.40, P = 0.01), abnormal electrocardiogram (ECG) (OR: 1.90; 95% CI: 1.12-3.23; P = 0.02), natriuretic peptide elevation (OR: 2.99; 95% CI: 1.60-5.58; P = 0.0006), and troponin elevation (OR: 4.21; 95% CI: 2.41-7.36; P < 0.0001). Variables associated with acute ischemic patterns included chest discomfort (OR: 3.14; 95% CI: 1.04-9.49; P = 0.04), abnormal ECG (OR: 4.06; 95% CI: 1.10-14.92; P = 0.04), known coronary disease (OR: 33.30; 95% CI: 4.04-274.53; P = 0.001), hospitalization (OR: 4.98; 95% CI: 1.55-16.05; P = 0.007), natriuretic peptide elevation (OR: 4.19; 95% CI: 1.30-13.51; P = 0.02), and troponin elevation (OR: 25.27; 95% CI: 5.55-115.03; P < 0.0001). In a multivariate analysis, troponin elevation was strongly associated with acute myocarditis patterns (OR: 4.98; 95% CI: 1.76-14.05; P = 0.003).
Conclusions
In this multicenter study of patients with COVID-19 with clinical suspicion for cardiac involvement referred for CMR, nonischemic and ischemic patterns were frequent when cardiac symptoms, ECG abnormalities, and cardiac biomarker elevations were present.
Key Words: cardiac magnetic resonance (CMR), COVID-2019, myocardial injury
Central Illustration
Since December 2019, the COVID-19 pandemic has spread rapidly across the world, with more than 600 million cases and 6.4 million deaths reported as of August 2022.1 Although it is well known that COVID-19 results in significant pulmonary disease, several studies have shown that infection with SARS-CoV-2 is also associated with myocardial injury.2, 3, 4, 5 Prior studies have reported heterogeneous cardiac manifestations, including myocarditis,6, 7, 8, 9, 10 myocardial infarction (MI),11, 12, 13 arrhythmias,2 , 14 , 15 stress cardiomyopathy,7 , 16 , 17 and cardiogenic shock.18 Although the precise pathophysiological mechanisms of myocardial injury remain unclear,19 it is well established that myocardial injury in patients with COVID-19 is associated with increased morbidity.20 , 21 It is therefore crucial to better understand the etiologies of myocardial injury in patients with COVID-19, to provide optimal treatment and monitoring for patients.
Cardiac magnetic resonance (CMR) is a powerful imaging technique for the assessment of cardiac function, morphology, and patterns of myocardial injury. CMR is especially helpful in differentiating between nonischemic and ischemic patterns, through late gadolinium enhancement (LGE) and tissue mapping sequences.22 , 23 Previous studies examining CMR findings in patients with COVID-19 detected abnormalities consistent with myocardial inflammation and infarction, but these studies have been limited by small sample sizes and heterogeneous populations by selection and referral biases.24, 25, 26, 27
Therefore, the objectives of this retrospective, multicenter study were to characterize myocardial injury by CMR in a large cohort of patients with COVID-19 to understand differences in CMR characteristics of myocardial injury in: 1) hospitalized vs ambulatory patients; 2) patients with and without known coronary artery disease (CAD); and 3) patients with and without laboratory evidence of myocardial injury, and investigate factors associated with acute myocarditis and acute ischemic patterns.
Methods
Study population
We identified patients with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection referred for CMR at 18 international sites from the United States, Poland, United Kingdom, and Germany (n = 1,051) (Supplemental Table 1). This cohort included all 6 Society for Cardiovascular Magnetic Resonance COVID-19 Registry sites, and 3 general Society for Cardiovascular Magnetic Resonance Registry sites. Each site was requested to contribute at least 20 patients. Athletes undergoing screening CMR as part of their return to play evaluation were excluded. Patients with pacemakers or defibrillators and patients who underwent CMR more than 30 days before their confirmatory SARS-CoV-2 test were excluded from the database (n = 4). This study received approval from each site’s institutional review board for retrospective review of patient data and waiver of consent.
Data collection
The data coordinating center (DCC) for this study was the University of Pennsylvania. Every site was required to attend a virtual information session, where instructions for collecting the required data were provided. The following clinical data were requested: demographics, time between PCR-confirmed diagnosis of COVID-19 and CMR, presenting symptoms, comorbidities, medications before COVID-19 infection, treatments received for COVID-19, peak values for laboratory studies (including troponin and natriuretic peptides) at the time of COVID-19 presentation or CMR and whether these biomarkers were elevated based on locally defined thresholds, electrocardiogram (ECG) and echocardiogram findings before CMR, and known coronary anatomy (evaluated by coronary angiography or coronary computed tomography angiography) before CMR. The following CMR data were collected: indications for CMR, biventricular function and volumes, LGE presence and pattern, and tissue mapping characteristics. Sites were asked to characterize patterns of myocardial injury as nonischemic (acute myocarditis, nonacute nonischemic, or possible nonischemic) or ischemic (acute ischemic, nonacute ischemic, or possible ischemic). Acute myocarditis patterns required satisfying both of the main Modified Lake Louise criteria for nonischemic myocardial inflammation (1 T1-based criterion [elevated T1 mapping values or LGE] and 1 T2-based criterion [elevated T2 mapping values or abnormal T2-weighted signal]).28 Patients with clinically suspected acute myocarditis (referred for CMR due to chest pain and an elevated troponin, or an elevated troponin alone) and nonstrict CMR criteria for an acute myocarditis pattern (either 1 T1-based or 1 T2-based criterion), were designated as meeting clinical criteria for acute myocarditis and nonstrict CMR criteria for an acute myocarditis pattern. Nonacute nonischemic patterns were defined as abnormal T1 mapping values or presence of LGE in a nonischemic pattern, with normal T2 mapping values, normal T2-weighted short-tau inversion recovery (T2STIR), or clinical history suggesting a nonacute process. Diagnosis of ischemic patterns required subendocardial or transmural delayed enhancement in a vascular distribution. Acute ischemic injury patterns were defined by clinical history, elevated T2 mapping values, or abnormal T2STIR. Nonacute ischemic injury patterns were defined as an ischemic LGE pattern without clinical history, elevated T2 mapping values, or abnormal T2STIR suggesting an acute clinical process. Patients with nonischemic or ischemic injury patterns in which acuity could not be established were designated as “possible nonischemic pattern” or “possible ischemic pattern.”
Data organization
A deidentified datasheet was sent to the DCC by each participating site and was assessed for inconsistencies or incomplete data. Queries for clarification were sent to each site’s coordinators by the DCC. Datasheets were subsequently compiled and evaluated for incongruous data: 1) if the ejection fraction (EF) field was incomplete, it was calculated from the provided end-systolic and end-diastolic volumes; and 2) patients who had PCR testing after the incident CMR or had no CMR functional parameters were excluded from analysis. Characterization of myocardial injury patterns were checked to ensure that they satisfied the previously described criteria, and queries for clarification were answered by each site’s coordinators.
Statistical analysis
Continuous data are shown as mean ± SD for normally distributed variables and analyzed using the nonpaired Student’s t-test or analysis of variance. Categorical variables are shown as total counts with percentages and analyzed using the chi-square test or Fisher’s exact test. Statistical significance was defined as P < 0.05.
Logistic regression was performed to assess the relationship between various parameters and acute myocarditis/acute ischemic patterns. In univariate models, the factors included in the analysis were age, sex, troponin elevation, natriuretic peptide elevation, ECG/echocardiogram abnormalities, symptoms (chest discomfort, palpitations, dyspnea, fatigue) at the time of CMR, hospitalization status, intubation status, and known CAD (obstructive or nonobstructive CAD). An abnormal ECG was defined as the presence of sinus tachycardia, atrial arrhythmias, ventricular arrhythmias, and/or ST-segment abnormalities. An abnormal echocardiogram was defined as the presence of left ventricular (LV) systolic/diastolic dysfunction, right ventricular (RV) dysfunction, pericardial effusion, and/or valvular abnormalities. Any factor with a P ≤ 0.10 was included in the multivariate analysis, along with age and sex. All analyses were performed with SAS statistical software (version 9.4, SAS Institute).
Results
Characteristics of the overall cohort
The final study cohort consisted of 1,047 patients who underwent CMR after a PCR-confirmed COVID-19 diagnosis (mean age 47.4 ± 16.5 years, 47.5% women, 75.8% White, 14.9% Black). Baseline clinical characteristics of the entire cohort are shown in Table 1 . Comorbidities were most notable for hypertension (32.6%), hyperlipidemia (27.4%), diabetes (15.0%), preexisting cardiomyopathies (10.5%), and preexisting respiratory conditions (20.5%). Nearly all patients (1,046 of 1,047, 99.9%) underwent CMR after a positive COVID-19 result. One patient underwent CMR 3 days before their positive COVID-19 result, because of high suspicion for COVID-19 and myocardial involvement.
Table 1.
Clinical Characteristics and CMR Findings of the Entire Cohort
| N | Mean ± SD or n (%) | |
|---|---|---|
| Age, y | 1,047 | 47.4 ± 16.5 |
| Female | 1,047 | 497 (47.5) |
| Race | 913 | |
| White | 692 (75.8) | |
| Black | 136 (14.9) | |
| Asian | 41 (4.5) | |
| Multiracial | 5 (0.6) | |
| Other | 39 (4.3) | |
| Hispanic | 807 | 51 (6.3) |
| Hospitalized | 1,040 | 377 (36.3) |
| Intubated | 1,034 | 55 (5.3) |
| Presenting symptoms | ||
| Dyspnea | 846 | 468 (55.3) |
| Chest discomfort | 820 | 237 (28.9) |
| Palpitations | 754 | 92 (12.2) |
| Syncope | 801 | 28 (3.5) |
| Shock | 802 | 24 (3.0) |
| Fevers | 782 | 394 (50.4) |
| Comorbidities | ||
| Hypertension | 988 | 322 (32.6) |
| Hyperlipidemia | 965 | 264 (27.4) |
| Diabetes | 977 | 147 (15.0) |
| Smoking | 821 | |
| Current | 46 (5.6) | |
| Former | 129 (15.7) | |
| Previously known at least moderate CAD | 982 | 62 (6.3) |
| Previous myocardial infarction | 979 | 32 (3.3) |
| Known coronary anatomy | 364 | |
| No apparent CAD | 270 (74.2) | |
| Obstructive disease | 45 (12.4) | |
| Nonobstructive disease | 49 (13.5) | |
| Pre-existing cardiomyopathy | 961 | |
| Dilated cardiomyopathy | 46 (4.8) | |
| Hypertrophic cardiomyopathy | 7 (0.7) | |
| Cardiac amyloidosis | 1 (0.1) | |
| Cardiac sarcoidosis | 2 (0.2) | |
| Prior myocarditis | 4 (0.4) | |
| Other | 41 (4.3) | |
| Pre-existing respiratory condition | 952 | |
| COPD/emphysema | 37 (3.9) | |
| Asthma | 100 (10.5) | |
| Interstitial lung disease | 1 (0.1) | |
| Obstructive sleep apnea | 42 (4.4) | |
| Other | 15 (1.6) | |
| Pre-COVID-19 medications | ||
| Diuretics | 945 | 95 (10.1) |
| Beta blockers | 970 | 214 (22.1) |
| ACEI/ARB | 968 | 200 (20.7) |
| Statins or other cholesterol medications | 971 | 224 (23.1) |
| Antiplatelet agents | 966 | 111 (11.5) |
| Anticoagulation | 967 | 71 (7.3) |
| Diabetes medications | 946 | 123 (13.0) |
| Treatment for COVID-19 | ||
| Steroids | 887 | 200 (22.6) |
| Remdesivir | 893 | 95 (10.6) |
| Monoclonal antibodies | 892 | 42 (4.7) |
| Therapeutic anticoagulation | 898 | 80 (8.9) |
| Antiplatelet agents | 897 | 37 (4.1) |
| Laboratory studies | ||
| Troponin elevation | 709 | 191 (26.9) |
| NT-proBNP or BNP elevation | 584 | 193 (33.0) |
| C-reactive protein elevation | 595 | 315 (52.9) |
| Erythrocyte sedimentation rate elevation | 153 | 64 (41.8) |
| Lactate dehydrogenase elevation | 245 | 157 (64.1) |
| D-dimer elevation | 543 | 272 (50.1) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; CAD = coronary artery disease; CMR = cardiac magnetic resonance; COPD = chronic obstructive pulmonary disease; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
In this cohort, 377 of 1,040 (36.3%) patients were hospitalized for COVID-19, including 55 (14.6%) who were intubated. The most common presenting symptoms were dyspnea (55.3%), fever (50.4%), and chest discomfort (28.9%). Laboratory findings were notable for troponin elevation in 191 of 709 patients (26.9%) and elevated natriuretic peptides (N-terminal pro–B-type natriuretic peptide [NT-proBNP] or B-type natriuretic peptide [BNP]) in 193 of 584 patients (33.0%).
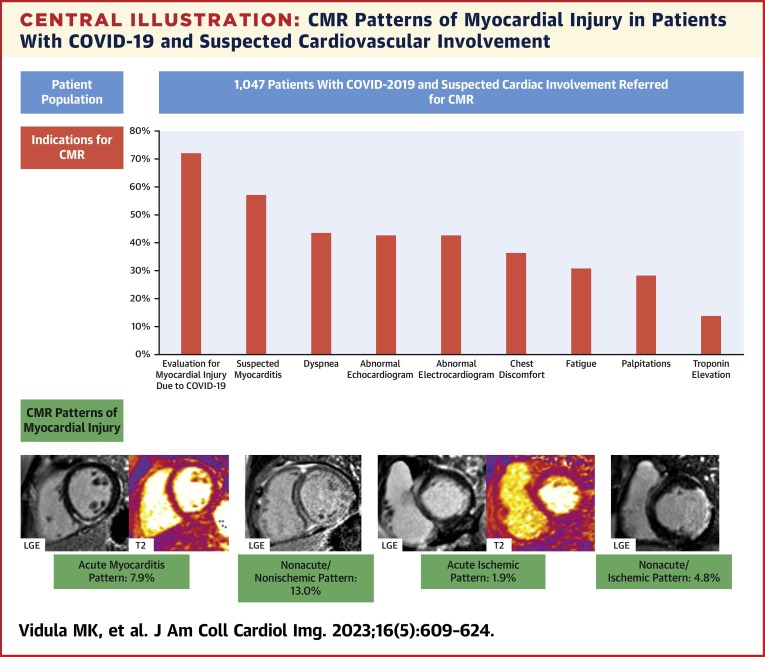
The most common indications for CMR were to evaluate for myocardial injury due to COVID-19 (72.1%) or suspected myocarditis (57.1%), further investigate symptoms (dyspnea [43.5%], chest discomfort [36.4%], fatigue [30.8%], and palpitations [28.4%]), assess abnormal findings on ECG (42.7%) and echocardiography (42.8%), and identify the etiology for a troponin elevation (13.8%) (Central Illustration ). The mean time from a PCR-confirmed COVID-19 test to CMR was 131.9 ± 94.6 days.
Central Illustration.
CMR Patterns of Myocardial Injury in Patients With COVID-19 and Suspected Cardiovascular Involvement
In this retrospective study of 1,047 patients from 18 international sites with polymerase chain reaction–confirmed COVID-19 infection who underwent cardiac magnetic resonance (CMR) imaging, CMR was performed for a variety of indications (graph). Representative images of the main nonischemic and ischemic patterns of myocardial injury are shown. LGE = late gadolinium enhancement.
Most CMRs were performed clinically (940 of 1,047; 89.8%), and the remaining were performed for research (107 of 1,047, 10.2%). Only 47 patients (4.5%) did not have a clear indication for CMR, of which 22 were clinical studies and 25 were research studies. Among patients without a clinical indication for CMR, there was 1 patient with an acute myocarditis pattern, and no patients with acute ischemic patterns (Supplemental Table 2). Similarly, myocardial injury patterns were rare in the research group, and no patients demonstrated acute myocarditis or acute ischemic patterns (Supplemental Table 2).
CMR findings for the entire cohort are shown in Table 2 . The mean LVEF was 56.6% ± 11.1% and mean RVEF was 54.1% ± 9.4%. The mean LV end-diastolic volume index was 84.2 ± 24.0 mL/m2 and the mean RV end-diastolic volume index was 82.3 ± 21.4 mL/m2. LGE sequences were performed in 1,039 patients (99.2%) and LGE was present in 403 patients (38.8%), most commonly with midmyocardial (22.4%) and subepicardial (10.0%) patterns. In our cohort, 912 patients (87.1%) had either T2 mapping (n = 756) or T2STIR (n = 480).
Table 2.
CMR Characteristics of the Entire Cohort
| N | Mean ± SD or n (%) | |
|---|---|---|
| Clinical presentations | ||
| Chest discomfort | 928 | 338 (36.4) |
| Palpitations | 892 | 253 (28.4) |
| Dyspnea | 932 | 405 (43.5) |
| Fatigue | 832 | 256 (30.8) |
| Troponin elevation | 842 | 116 (13.8) |
| Electrocardiogram findings | 674 | |
| Sinus tachycardia | 82 (12.2) | |
| Atrial arrhythmias | 53 (7.9) | |
| Ventricular arrhythmias | 57 (8.5) | |
| ST-segment abnormalities | 96 (14.2) | |
| Echocardiogram findings | 615 | |
| Left ventricular systolic dysfunction | 173 (28.1) | |
| Left ventricular diastolic dysfunction | 32 (5.2) | |
| Right ventricular dysfunction | 20 (3.3) | |
| Pericardial effusion | 22 (3.6) | |
| Valvular abnormalities | 16 (2.6) | |
| Evaluation for myocardial injury due to COVID-19 | 924 | 666 (72.1) |
| Suspected myocarditis | 766 | 437 (57.1) |
| Vital signs before CMR | ||
| Systolic blood pressure, mm Hg | 791 | 127.6 ± 17.4 |
| Diastolic blood pressure, mm Hg | 791 | 76.0 ± 11.1 |
| Heart rate, beats/min | 930 | 76.8 ± 15.3 |
| CMR findings | ||
| Days to CMR | 1,047 | 131.9 ± 94.6 |
| Left ventricular ejection fraction, % | 1,039 | 56.6 ± 11.1 |
| Right ventricular ejection fraction, % | 1,018 | 54.1 ± 9.5 |
| Left ventricular end-diastolic volume, mL | 1,041 | 165.5 ± 54.8 |
| Left ventricular end-diastolic volume index, mL/m2 | 1,039 | 84.2 ± 24.0 |
| Left ventricular end-systolic volume, mL | 1,039 | 75.2 ± 45.6 |
| Left ventricular end-systolic volume index, mL/m2 | 1,037 | 38.0 ± 21.4 |
| Right ventricular end-diastolic volume, mL | 1,018 | 162.0 ± 49.5 |
| Right ventricular end-diastolic volume index, mL/m2 | 1,017 | 82.3 ± 21.4 |
| Right ventricular end-systolic volume, mL | 1,018 | 76.0 ± 34.6 |
| Right ventricular end-systolic volume index, mL/m2 | 1,017 | 38.5 ± 15.6 |
| Left ventricular mass, g | 908 | 110.8 ± 38.4 |
| Left ventricular mass index, g/m2 | 907 | 56.5 ± 18.0 |
| LGE present | 1,039 | 403 (38.8) |
| LGE type | 1,039 | |
| Subepicardial | 104 (10.0) | |
| Midmyocardial | 233 (22.4) | |
| Subendocardial | 66 (6.4) | |
| Extracellular volume, % | 728 | 27.5 ± 6.1 |
| CMR patterns of myocardial injury (overall) | 1,047 | |
| Acute myocarditis pattern | 83 (7.9) | |
| Clinical criteria for acute myocarditis and nonstrict CMR criteria for acute myocarditis pattern | 15 (1.4) | |
| Nonacute nonischemic pattern | 136 (13.0) | |
| Possible nonischemic pattern | 75 (7.2) | |
| Acute ischemic pattern | 20 (1.9) | |
| Nonacute ischemic pattern | 50 (4.8) | |
| Possible ischemic pattern | 6 (0.6) | |
| CMR patterns of myocardial injury (single diagnoses) | 1,047 | |
| Acute myocarditis pattern | 76 (7.3) | |
| Clinical criteria for acute myocarditis and nonstrict CMR criteria for acute myocarditis | 15 (1.4) | |
| Nonacute nonischemic pattern | 131 (12.5) | |
| Possible nonischemic pattern | 68 (6.5) | |
| Acute ischemic pattern | 15 (1.4) | |
| Nonacute ischemic pattern | 40 (3.8) | |
| Possible ischemic pattern | 2 (0.2) | |
| CMR patterns of myocardial injury (dual diagnoses) | 1,047 | |
| Acute myocarditis pattern + acute ischemic pattern | 2 (0.2) | |
| Acute myocarditis pattern + nonacute ischemic pattern | 3 (0.3) | |
| Nonacute nonischemic pattern + acute ischemic pattern | 1 (0.1) | |
| Nonacute nonischemic pattern + nonacute ischemic pattern | 4 (0.4) |
CMR = cardiac magnetic resonance; LGE = late gadolinium enhancement.
Overall, 309 patients (29.5%) had nonischemic patterns of myocardial injury, of which 83 patients (7.9%) had an acute myocarditis pattern, 15 (1.4%) met clinical criteria for acute myocarditis and nonstrict CMR criteria for an acute myocarditis pattern, 136 (13.0%) had a nonacute nonischemic pattern, and 75 (7.2%) had a possible nonischemic pattern. All 83 patients with acute myocarditis patterns fulfilled at least 1 T1 criterion (71 [85.5%] with LGE, 52 [62.7%] with elevated T1 mapping values) and 1 T2 criterion (75 [90.4%] with elevated T2 mapping values, 22 [26.5%] with abnormal T2STIR). A total of 76 patients (7.3%) had ischemic patterns of injury, of whom 20 (1.9%) had an acute ischemic pattern, 50 (4.8%) had a nonacute ischemic pattern, and 6 (0.6%) had a possible ischemic pattern. Representative images of CMRs with acute myocarditis and acute ischemic patterns are shown in Figures 1 and 2 , respectively. Various nonischemic LGE patterns are shown in Figure 3 .
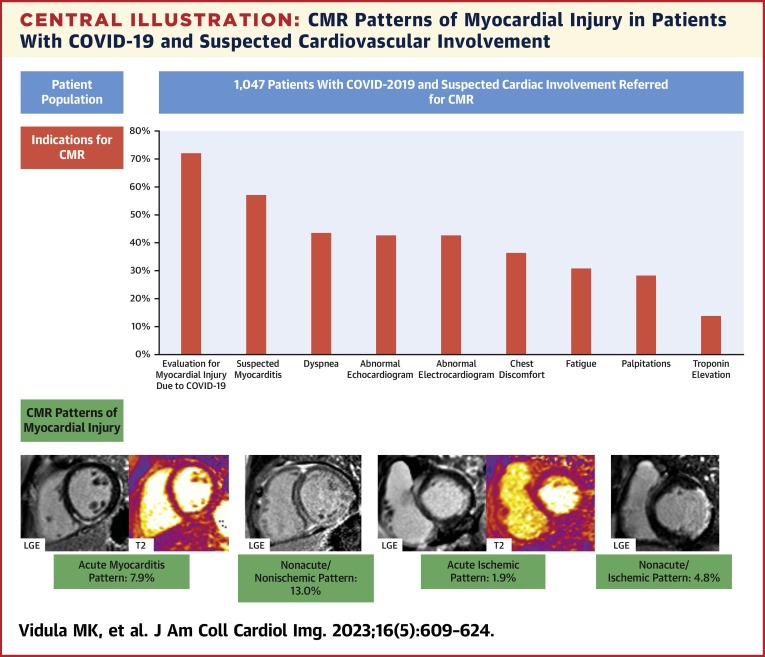
Figure 1.
Representative Images on CMR of Patients With Acute Myocarditis and Nonacute/Nonischemic Patterns
(A) Representative cardiac magnetic resonance (CMR) images of a patient with acute myocarditis pattern in the setting of a recent COVID-19 infection, demonstrating subepicardial late gadolinium enhancement (LGE) in the mid inferior and inferoseptal walls (left, white arrow), with corresponding elevation in native T2 times (middle, white arrow) and native T1 times (right, white arrow) in the mid inferior wall. (B) Representative CMR images of a patient presenting after a prolonged, complicated hospitalization with COVID-19, found to have CMR evidence of nonacute/nonischemic injury, with extensive midmyocardial LGE in the septum (left, white arrow) and subepicardial LGE in the inferior and inferolateral walls on the short axis view (left, red arrow). Midmyocardial LGE in the septum is again noted on the 4-chamber view (middle, white arrow), and subepicardial LGE in the inferior wall is noted on the 2-chamber view (right, white arrow).
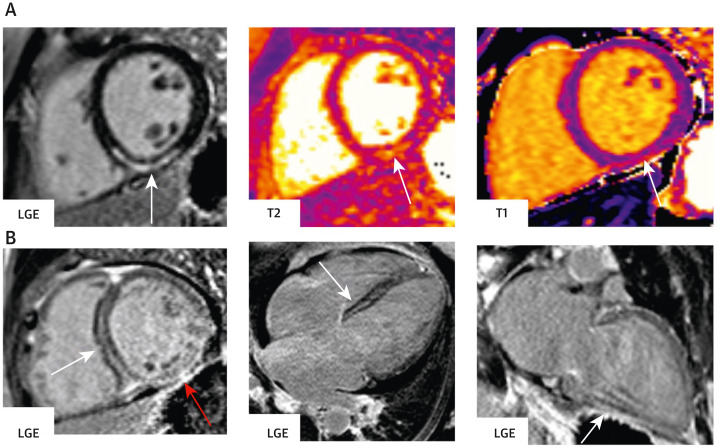
Figure 2.
Representative Images on CMR of Patients With Ischemic Patterns in the Setting of Acute and Prior COVID-19 Infections
(A) Representative CMR images of a patient presenting with an acute myocardial infarction (MI) in the setting of an active COVID-19 infection, demonstrating subendocardial LGE in the basal inferolateral wall on the short axis view (left, arrow), with corresponding elevation in native T2 times (middle, arrow). Coronary angiography (right) revealed a 99% proximal left circumflex occlusion (arrow). (B) Representative CMR images of a patient presenting 7 months after a COVID-19 infection that was complicated by a medically managed ST-segment elevation MI, with dyspnea and a reduced left ventricular ejection fraction. CMR showed evidence of a large prior infarct in the circumflex territory. There is transmural delayed enhancement in nearly the entire lateral wall, seen on the short axis view (left, arrow) and 3-chamber view (middle, arrow). Coronary angiography revealed an 80% stenosis in the mid circumflex (right, arrow), with diffuse distal disease. Abbreviations as in Figure 1.
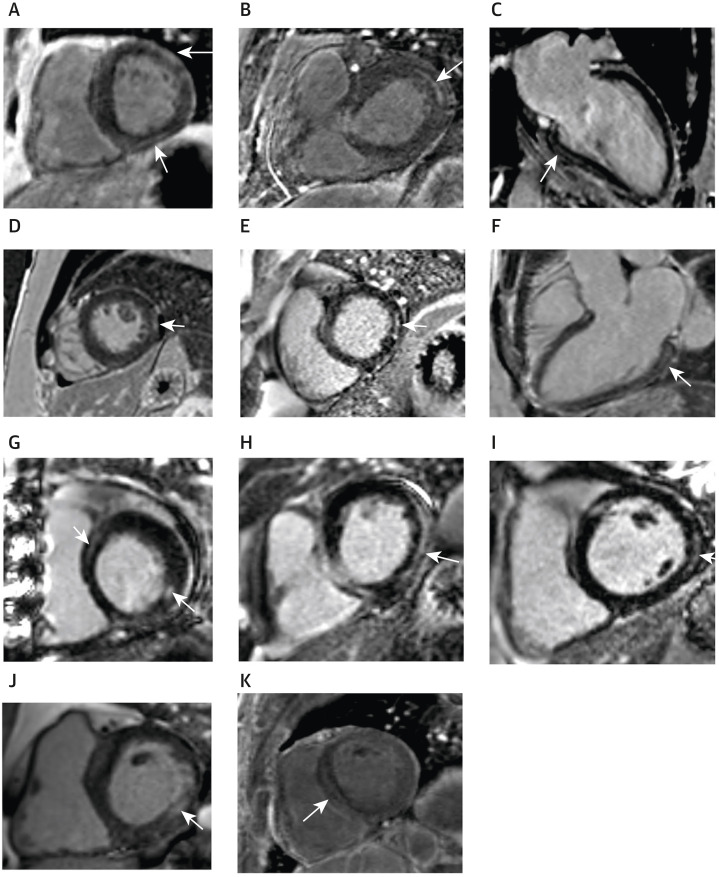
Figure 3.
Representative Images of LGE in Patients With Nonischemic Injury Patterns
(A) Patient with patchy subepicardial late gadolinium enhancement (LGE) in the basal anterolateral wall and midmyocardial LGE in the basal inferior wall (arrows), with corresponding elevated T1 and T2 mapping values in those regions, consistent with acute inflammation. (B) Patient with subepicardial LGE in the basal anterolateral wall (arrow), with corresponding elevated T1 and T2 mapping values in that region, consistent with acute inflammation. (C) Patient with subepicardial LGE involving the basal to mid inferior wall (arrow), with normal T2 mapping values in that region, consistent with nonacute nonischemic injury. (D) Patient with a small focus of subepicardial LGE in the mid inferolateral wall (arrow), with normal T2 mapping values in that region, consistent with nonacute nonischemic injury. (E) Patient with a small focus of midmyocardial LGE in the basal inferolateral wall (arrow), with normal T2 mapping values in that region, consistent with nonacute nonischemic injury. (F) Patient with midmyocardial LGE in the basal inferolateral wall (arrow), with elevated T1 and T2 mapping values in that region, consistent with acute inflammation. (G) Patient with midmyocardial LGE in the basal anteroseptum and basal inferolateral wall (arrows), with elevated T1 and T2 mapping values in those regions, consistent with acute inflammation. (H) Patient with subepicardial LGE involving the basal inferolateral wall and inferior wall (arrow), with normal T2 mapping values in that region, consistent with nonacute nonischemic injury. (I) Patient with subepicardial LGE in the basal inferolateral wall (arrow), with elevated T1 and T2 mapping values in those regions, consistent with acute inflammation. (J) Patient with midmyocardial LGE involving the basal inferolateral wall (arrow), with normal T2 mapping values in that region, consistent with nonacute nonischemic injury. (K) Patient with midmyocardial LGE involving the basal to mid septum (arrow), with normal T2 mapping values in that region, consistent with nonacute nonischemic injury.
In the overall cohort, 87 of 1,039 patients (8.4%) had midmyocardial or subepicardial LGE at the RV insertion point, of whom 15 were characterized with acute myocarditis patterns (with corresponding elevated T2 mapping values or abnormal T2STIR in that region), 20 with nonacute nonischemic patterns, 15 with possible nonischemic patterns, and 37 with no myocarditis patterns. Furthermore, the presence of any pattern of LGE was associated with increased biventricular volume (except for RV end-diastolic volume index) and reduced biventricular function (Supplemental Table 3). Nine patients had pericardial LGE, and 22 patients had pericardial effusions on echocardiography. The location of LGE in patients with a single diagnosis of acute myocarditis patterns is shown in Supplemental Table 4, and most patients had subepicardial and midmyocardial involvement of the inferolateral, inferior, and inferoseptal walls.
Characteristics of ambulatory and hospitalized patients
Clinical and CMR characteristics of ambulatory (n = 663) and hospitalized (n = 377) patients are shown in Table 3 . Hospitalized patients were older (53.1 ± 16.2 years vs 44.1 ± 15.7 years, P < 0.0001) and had a higher burden of comorbidities (Supplemental Table 5).
Table 3.
Clinical and CMR Characteristics of the Ambulatory and Hospitalized Cohorts
| Ambulatory (n = 663) |
Hospitalized (n = 377) |
P Value | |
|---|---|---|---|
| Age, y | 44.1 ± 15.7 | 53.1 ± 16.2 | <0.0001 |
| Female | 351 (52.9) | 144 (38.2) | <0.0001 |
| Race | <0.0001 | ||
| White | 469 (82.3) | 218 (64.5) | |
| Black | 65 (11.4) | 71 (21.0) | |
| Asian | 14 (2.5) | 27 (8.0) | |
| Multiracial | 3 (0.5) | 2 (0.6) | |
| Other | 19 (3.3) | 20 (5.9) | |
| Hispanic | 28 (5.3) | 23 (8.3) | 0.13 |
| CMR findings | |||
| Days to CMR | 133.6 ± 90.1 | 128.1 ± 102.0 | 0.39 |
| LV ejection fraction, % | 58.3 ± 9.5 | 53.7 ± 13.0 | <0.0001 |
| RV ejection fraction, % | 54.9 ± 8.3 | 52.5 ± 11.0 | 0.0003 |
| LV end-diastolic volume, mL | 162.7 ± 51.9 | 170.3 ± 59.5 | 0.039 |
| LV end-diastolic volume index, mL/m2 | 83.6 ± 21.9 | 85.0 ± 27.1 | 0.40 |
| LV end-systolic volume, mL | 70.3 ± 38.3 | 83.7 ± 55.3 | <0.0001 |
| LV end-systolic volume index, mL/m2 | 36.0 ± 17.6 | 41.6 ± 26.5 | 0.0003 |
| RV end-diastolic volume, mL | 161.0 ± 48.0 | 164.2 ± 52.1 | 0.33 |
| RV end-diastolic volume index, mL/m2 | 82.7 ± 20.9 | 81.7 ± 22.2 | 0.49 |
| RV end-systolic volume, mL | 73.6 ± 29.2 | 80.6 ± 42.3 | 0.005 |
| RV end-systolic volume index, mL/m2 | 37.7 ± 13.4 | 39.9 ± 18.9 | 0.045 |
| LV mass, g | 106.8 ± 35.6 | 117.2 ± 41.8 | 0.0002 |
| LV mass index, g/m2 | 54.8 ± 14.2 | 59.1 ± 22.7 | 0.002 |
| LGE presence | 227 (34.5) | 172 (46.0) | 0.0003 |
| LGE type | <0.0001 | ||
| None | 431 (65.5) | 202 (54.0) | |
| Subepicardial | 68 (10.3) | 35 (9.4) | |
| Midmyocardial | 138 (21.0) | 93 (24.9) | |
| Subendocardial | 21 (3.2) | 44 (11.8) | |
| Extracellular volume, % | 26.8 ± 5.5 | 28.8 ± 6.9 | <0.0001 |
| CMR patterns of myocardial injury | |||
| Nonischemic patterns | 0.29 | ||
| None | 475 (71.6) | 266 (70.6) | |
| Acute myocarditis pattern | 49 (7.4) | 32 (8.5) | |
| Nonacute nonischemic pattern | 93 (14.0) | 43 (11.4) | |
| Possible nonischemic pattern | 46 (6.9) | 36 (9.5) | |
| Ischemic patterns | <0.0001 | ||
| None | 638 (96.2) | 326 (86.5) | |
| Acute ischemic pattern | 6 (0.9) | 13 (3.4) | |
| Nonacute ischemic pattern | 16 (2.4) | 34 (9.0) | |
| Possible ischemic pattern | 3 (0.5) | 4 (1.1) |
Values are mean ± SD or n (%).
LV = left ventricle; RV = right ventricle; other abbreviations as in Table 2.
Biventricular function, as measured by CMR, was lower in the hospitalized cohort (mean LVEF: 53.7% ± 13.0% vs 58.3% ± 9.5%, P < 0.0001; mean RVEF: 52.5% ± 11.0% vs 54.9% ± 8.3%, P = 0.0003), but indexed biventricular end-diastolic volumes were similar in both cohorts. LGE was more common in the hospitalized group (46.0% vs 34.5%, P = 0.0003), with predominantly midmyocardial LGE in both groups but a higher percentage of subendocardial LGE in the hospitalized group.
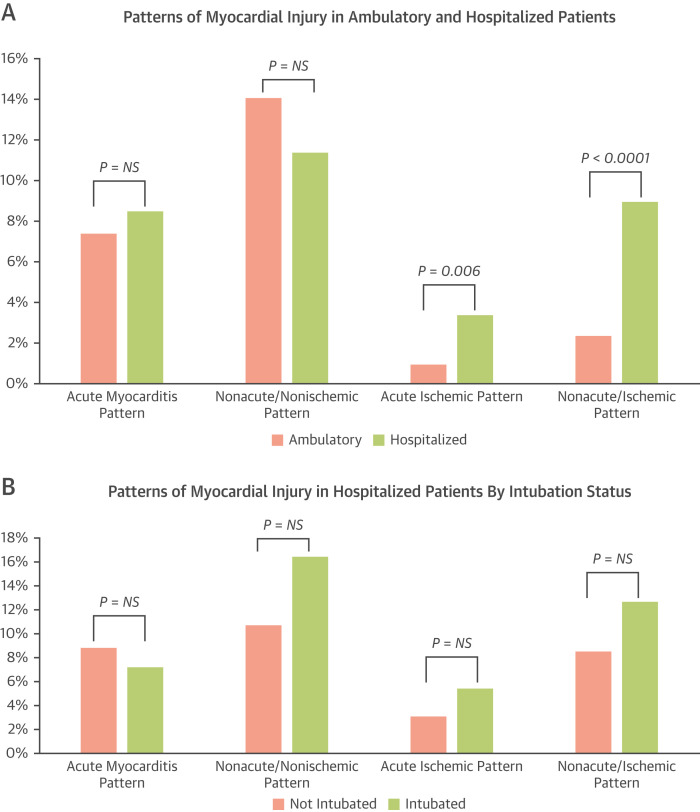
Nonischemic patterns of injury were more frequent than ischemic patterns in both cohorts. There was no difference in the frequency of acute myocarditis patterns between the hospitalized and ambulatory patients. However, ischemic patterns were more frequent in the hospitalized cohort than in the ambulatory cohort (Table 3, Figure 4A ).
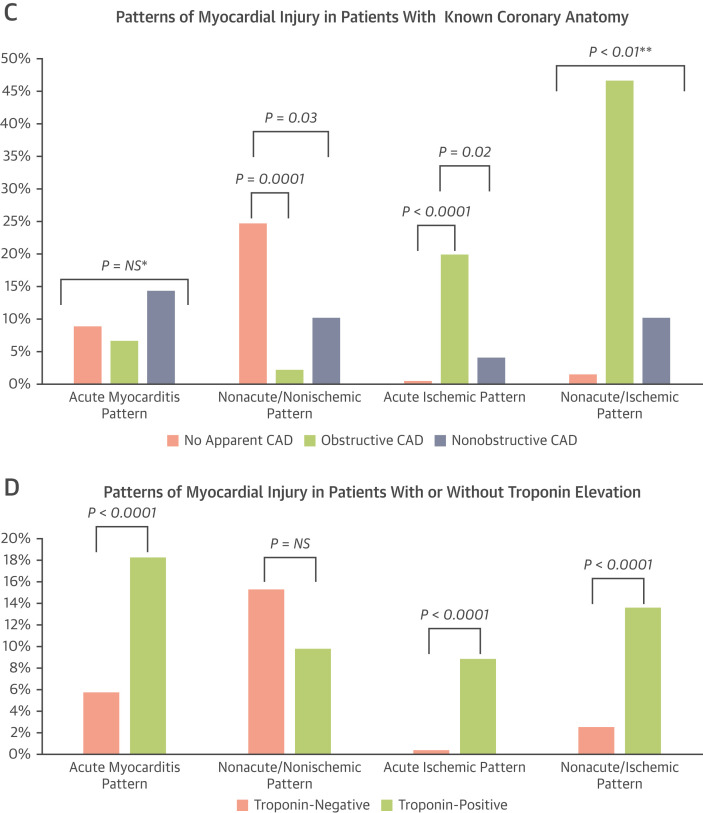
Figure 4.
Patterns of Myocardial Injury in Various Patient Cohorts
(A) Ambulatory and hospitalized patients. (B) Hospitalized patients by intubation status. (C) Patients with known coronary anatomy. (D) Patients with or without troponin elevation. CAD = coronary artery disease; NS = not significant. ∗All pairwise comparisons are not significant. ∗∗All pairwise comparisons are significant (P < 0.01).
Clinical and CMR characteristics of hospitalized patients by intubation status (n = 55) are shown in Supplemental Table 6. Intubated patients had a lower LVEF (50.7% ± 12.4% vs 54.6% ± 12.8%, P = 0.04), similar RV function and biventricular volumes, and a similar prevalence and pattern of LGE. Nonischemic patterns and ischemic patterns were not significantly different between the 2 groups (Figure 4B).
Characteristics of patients stratified by known CAD
In this study cohort, coronary anatomy was known in 364 patients. Of these patients, 270 (74.2%) did not have apparent CAD, 45 (12.4%) had obstructive CAD, and 49 (13.5%) had nonobstructive CAD. Clinical and CMR characteristics of this study population, stratified by coronary anatomy, are shown in Supplemental Table 7.
Nonacute nonischemic patterns of injury were more common in patients with no apparent CAD than in patients with either obstructive or nonobstructive CAD. Acute ischemic patterns were more common in patients with obstructive CAD than in patients with either nonobstructive CAD or no apparent CAD. Nonacute ischemic patterns were significantly more common in patients with obstructive CAD than in patients with nonobstructive CAD or no apparent CAD, and were also more common in patients with nonobstructive CAD than in patients with no apparent CAD (Figure 4C).
Characteristics of patients with and without troponin elevation at the time of COVID-19 infection or CMR
Clinical and CMR characteristics of patients with (n = 191) and without (n = 518) troponin elevation at the time of COVID-19 presentation or CMR are shown in Table 4 .
Table 4.
Clinical and CMR Characteristics of Troponin-Negative and Troponin-Positive Patients
| Troponin-Negative (n = 518) | Troponin-Positive (n = 191) | P Value | |
|---|---|---|---|
| Age, y | 46.6 ± 16.2 | 51.3 ± 18.5 | 0.002 |
| Female | 259 (50.0) | 62 (32.5) | <0.0001 |
| Race | <0.0001 | ||
| White | 372 (78.5) | 88 (54.0) | |
| Black | 63 (13.3) | 47 (28.8) | |
| Asian | 22 (4.6) | 11 (6.7) | |
| Multiracial | 1 (0.2) | 3 (1.8) | |
| Other | 16 (3.4) | 14 (8.6) | |
| Hispanic | 23 (5.4) | 14 (9.8) | 0.08 |
| CMR findings | |||
| Days to CMR | 130.7 ± 86.6 | 109.8 ± 109.2 | 0.02 |
| LV ejection fraction, % | 58.0 ± 9.4 | 50.3 ± 14.1 | <0.0001 |
| RV ejection fraction, % | 53.9 ± 8.7 | 50.8 ± 12.0 | 0.002 |
| LV end-diastolic volume, mL | 160.6 ± 48.4 | 182.0 ± 67.5 | <0.0001 |
| LV end-diastolic volume index, mL/m2 | 81.7 ± 20.2 | 90.7 ± 29.9 | 0.0002 |
| LV end-systolic volume, mL | 69.9 ± 37.6 | 95.8 ± 61.4 | <0.0001 |
| LV end-systolic volume index, mL/m2 | 35.4 ± 16.9 | 47.5 ± 29.4 | <0.0001 |
| RV end-diastolic volume, mL | 159.9 ± 45.0 | 172.4 ± 58.6 | 0.009 |
| RV end-diastolic volume index, mL/m2 | 81.3 ± 19.0 | 85.6 ± 24.9 | 0.03 |
| RV end-systolic volume, mL | 74.8 ± 29.6 | 87.8 ± 48.1 | 0.0007 |
| RV end-systolic volume index, mL/m2 | 37.9 ± 13.0 | 43.5 ± 21.6 | 0.001 |
| LV mass, g | 106.8 ± 33.8 | 124.6 ± 46.0 | <0.0001 |
| LV mass index, g/m2 | 54.2 ± 13.4 | 63.9 ± 27.6 | <0.0001 |
| LGE present | 172 (33.4) | 117 (61.9) | <0.0001 |
| LGE type | <0.0001 | ||
| None | 343 (66.6) | 73 (38.1) | |
| Subepicardial | 57 (11.1) | 23 (12.2) | |
| Midmyocardial | 102 (19.8) | 53 (28.0) | |
| Subendocardial | 13 (2.5) | 41 (21.7) | |
| Extracellular volume, % | 27.3 ± 5.5 | 29.9 ± 7.4 | 0.0005 |
| CMR patterns of myocardial injury | |||
| Nonischemic patterns | <0.0001 | ||
| None | 371 (71.6) | 119 (62.3) | |
| Acute myocarditis pattern | 30 (5.8) | 35 (18.3) | |
| Nonacute nonischemic pattern | 79 (15.3) | 19 (9.9) | |
| Possible nonischemic pattern | 38 (7.3) | 18 (9.4) | |
| Ischemic patterns | <0.0001 | ||
| None | 503 (97.1) | 143 (74.9) | |
| Acute ischemic pattern | 2 (0.4) | 17 (8.9) | |
| Nonacute ischemic pattern | 13 (2.5) | 26 (13.6) | |
| Possible ischemic pattern | 0 (0) | 5 (2.6) |
Biventricular function on CMR was lower in troponin-positive patients compared with troponin-negative patients (LVEF: 50.3% ± 14.1% vs 58.0% ± 9.4%, P < 0.0001; RVEF: 50.8% ± 12.0% vs 53.9% ± 8.7%, P = 0.002), and indexed biventricular volumes were larger (LV end-diastolic volume index: 90.7 ± 29.9 mL/m2 vs 81.7 ± 20.2 mL/m2, P = 0.0002; RV end-diastolic volume index: 85.6 ± 24.9 mL/m2 vs 81.3 ± 19.0 mL/m2, P = 0.03). LGE was significantly more prevalent in the troponin-positive cohort (61.9% vs 33.4%, P < 0.0001).
Compared with the troponin-negative cohort, the troponin-positive cohort was more likely to have evidence of acute myocarditis patterns (18.3% vs 5.8%, P < 0.0001), acute ischemic patterns (8.9% vs 0.4%, P < 0.0001), and nonacute ischemic patterns (13.6% vs 2.5%, P < 0.0001) (Figure 4D).
Association of various factors with patterns of injury
In a univariate analysis, variables associated with acute myocarditis patterns included chest discomfort (OR: 2.00; 95% CI: 1.17-3.40, P = 0.01), abnormal ECG (OR: 1.90; 95% CI: 1.12-3.23; P = 0.02), natriuretic peptide elevation (OR: 2.99; 95% CI: 1.60-5.58; P = 0.0006), and troponin elevation (OR: 4.21; 95% CI: 2.41-7.36; P < 0.0001). In a multivariate analysis, only troponin elevation was significantly associated with acute myocarditis patterns (OR: 4.98; 95% CI: 1.76-14.05; P = 0.003) (Supplemental Table 8).
Factors associated with acute ischemic patterns in a univariate analysis included chest discomfort (OR: 3.14; 95% CI: 1.04-9.49; P = 0.04), abnormal ECG (OR: 4.06; 95% CI: 1.10-14.92; P = 0.04), known coronary disease (OR: 33.30; 95% CI: 4.04-274.53; P = 0.001), hospitalization (OR: 4.98; 95% CI: 1.55-16.05; P = 0.007), natriuretic peptide elevation (OR: 4.19; 95% CI: 1.30-13.51; P = 0.02), and troponin elevation (OR: 25.27; 95% CI: 5.55-115.03; P < 0.0001) (Supplemental Table 8). Multivariate analysis was limited by the small number of patients, because of the exclusion of patients with missing data.
Discussion
This is a large, international, multicenter retrospective study of patients with PCR-confirmed COVID-19 and suspected cardiac involvement who underwent CMR. In this study, we characterized myocardial injury by CMR and identified differences in 3 comparison groups: 1) hospitalized vs ambulatory patients; 2) patients with and without known CAD; and 3) patients with and without troponin elevation. In the overall cohort, we found that patterns of myocardial injury were frequent, and were most commonly nonischemic. In addition, we found that nonischemic patterns of injury were detected at similar rates in hospitalized and ambulatory patients, but ischemic patterns were more common in the hospitalized cohort. Our findings also suggest that patients with known obstructive CAD were more likely to have ischemic patterns than patients with either nonobstructive disease or without apparent CAD. Furthermore, we found that both nonischemic and ischemic patterns were more frequently detected in patients with troponin elevation at the time of COVID-19 presentation or CMR. Finally, we identified several clinical factors associated with acute myocarditis and acute ischemic patterns on CMR. These findings further our understanding of the characteristics and patterns of myocardial injury in patients with COVID-19.
The overall cohort represents a large patient population from across the United States, Poland, the United Kingdom, and Germany, with heterogeneous comorbidities and COVID-19 manifestations. This cohort consists of a patient population with a high likelihood of cardiac involvement by COVID-19, as most patients (1,000 of 1,047; 95.5%) underwent CMR for further evaluation of cardiac symptoms, abnormalities on echocardiography or ECG, further investigation of an elevated troponin, or because of high clinical suspicion of myocardial involvement or myocarditis. This study cannot determine the overall prevalence of specific types of myocardial injury in all patients with COVID-19. It remains unclear whether the abnormalities observed can be attributed to COVID-19, because we do not have CMR data for these patients before their diagnosis with COVID-19. In addition, questions remain regarding the prognostic significance of these findings, for which long-term follow-up data will be required.
In our analysis of patients hospitalized with COVID-19, the rates of nonischemic patterns of injury, and specifically acute myocarditis patterns, were similar when compared with patients who were treated in the ambulatory setting, which is concordant with a prior study.24 However, hospitalized patients were significantly more likely to have CMR evidence of ischemic injury, including acute ischemic injury patterns. The differences in these patterns of injury may be related to the burden of comorbidities in the hospitalized and ambulatory cohorts. Similar to prior studies,14 , 29 the hospitalized patients in our analysis had a high burden of comorbidities and cardiovascular risk factors, which likely increased their risk for experiencing an acute or prior MI. Furthermore, MI is a serious event often requiring hospitalization, which may also explain the higher rate of ischemic injury in the hospitalized cohort. Interestingly, rates of acute myocarditis patterns were similar in both groups, suggesting that baseline comorbidities are not significant risk factors for developing myocardial inflammation. It is also possible that the patients who were hospitalized had reduced cardiac reserve to tolerate nonischemic injury because of their higher burden of comorbidities. In addition, the similar rates of acute myocarditis patterns or nonacute nonischemic patterns in hospitalized patients who were intubated and those who were not intubated suggests that severity of pulmonary involvement of COVID-19 is unlikely to be a risk factor for myocardial inflammation. Indeed, hospitalization status and intubation status were not factors that were significantly associated with acute myocarditis patterns in our regression analyses. It is important to note that compared with a prior study by Puntmann et al,24 our study found lower incidence of nonischemic injury, possibly because of the significantly larger sample size in this study, referral bias for CMR, reference standards of mapping, and differences in evaluation of nonischemic injury.
When stratified by known coronary disease, ischemic injury patterns were significantly more common in patients with known obstructive disease, but these ischemic patterns also occurred in patients with nonobstructive disease and patients without apparent CAD. Although patients with obstructive CAD are known to be at higher risk for type I and type II MIs, patients with nonobstructive CAD or without apparent CAD may experience acute coronary syndromes through a variety of previously proposed mechanisms, such as development of a proinflammatory state resulting in plaque rupture and thrombus formation, direct viral injury of the endothelium, thromboembolism, or coronary spasm.30 Nonischemic patterns of injury were more common in patients without apparent CAD, further suggesting that known cardiovascular disease or risk factors may not elevate the risk of developing myocardial inflammation in the setting of COVID-19.
We also stratified patients by troponin elevation and found that both nonischemic and ischemic patterns of injury were frequent in this cohort. Nonischemic injury was present in 37.7% of patients with elevated troponin levels, and ischemic injury was present in 25.1%. These rates are slightly higher than those reported in a prior smaller study of 148 troponin-positive patients, which found myocarditis-like injury in 26% of patients and MI in 19%.31 In addition, a proportion of patients without troponin elevation were diagnosed with acute myocarditis patterns (5.8%), suggesting that biomarker elevation may not always be present or identified in patients with CMR evidence of acute nonischemic myocardial inflammation. Understanding the prognosis of these patients will be critical to guide further therapy and monitoring.
Finally, we identified several clinical variables associated with findings of acute myocarditis patterns and acute ischemic patterns on CMR, which may help guide clinicians in deciding which patients may benefit from further investigation with CMR. Based on this analysis, CMR may identify acute myocarditis or acute ischemic patterns in patients with certain cardiac symptoms, specifically chest discomfort or abnormal ECG findings, and in patients with troponin elevation or natriuretic peptide elevation. On the other hand, CMR may not be as helpful if there is no or low concern for cardiac involvement, as seen by the very low yield of injury patterns in the research cohort and in the group of patients without definite clinical suspicion for cardiac involvement.
Our study should be considered in the context of its strengths and limitations. The strengths of this study include the large sample size and the inclusion of patients who underwent CMR for high suspicion of myocardial involvement by COVID-19. There are also limitations. First, in this retrospective study of a selected cohort of patients, we are unable to define the prevalence of myocardial injury in patients with COVID-19 because of the referral bias of the included population. However, we provide characterization of myocardial injury in patients with high likelihood of cardiac involvement, which can help inform clinical practice. Second, a proportion of these CMR findings may have been preexisting or unrelated to COVID-19 infection, and a causal relationship to COVID-19 cannot be established. Third, study center differences were not evaluated. Fourth, for this retrospective analysis, we did not standardize CMR imaging protocols for the detection of myocardial injury (such as uniform performance of tissue mapping sequences vs T2STIR sequences), and we relied on each site’s own expertise to determine the patterns of myocardial injury and diagnose myocarditis based on the Modified Lake Louise criteria. Although the CMR findings and diagnoses were not adjudicated, this is a real-world situation with expert CMR physicians making clinical diagnoses based on their assessment of the CMR images that were obtained locally. Furthermore, in this real-world study, there was significant variation in the time from COVID diagnosis to CMR, likely due to a variety of factors including acuity of illness, scanner availability, and ability to undergo CMR, thereby potentially limiting the ability to detect acute edema. In addition, CMR scans were not systematically reviewed for pericardial effusion, therefore limiting this study’s contributions to the understanding of pericardial abnormalities in COVID-19. Finally, sparse outcomes were reported and therefore the prognostic value of the CMR findings could not be reported. Future studies should focus on the prognostic value of myocardial injury in patients with COVID-19.
Conclusions
In this large, international, multicenter CMR study, we characterized myocardial injury in patients with PCR-confirmed COVID-19 and suspicion for cardiac involvement. We found that nonischemic and ischemic injury patterns are frequent in this patient cohort across various patient subgroups, and identified clinical variables associated with acute myocarditis and acute ischemic patterns. These findings further our understanding of the characteristics and patterns of myocardial injury in patients diagnosed with COVID-19, and future studies are required to understand the prognostic significance of these findings.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Nonischemic and ischemic patterns of injury are frequently identified on CMR in patients with COVID-19 and suspected cardiac involvement, especially in patients with cardiac symptoms, ECG abnormalities, and biomarker elevations.
TRANSLATIONAL OUTLOOK: Patients with COVID-19 with clinical findings including cardiac symptoms, ECG abnormalities, and biomarker elevations are at higher risk of having CMR findings consistent with acute myocarditis or acute infarction. Further studies with longer-term follow-up are required to identify the prognostic significance of the nonischemic and ischemic patterns of injury identified in this cohort of patients with COVID-19.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Eike Nagel, MD, served as the Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.COVID-19 Map Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
- 2.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y., Chen T., Mui D., et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106(15):1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried J.A., Ramasubbu K., Bhatt R., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42(2) doi: 10.1093/eurheartj/ehaa190. 206–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia S., Dehghani P., Grines C., et al. Initial findings from the North American COVID-19 Myocardial Infarction Registry. J Am Coll Cardiol. 2021;77(16):1994–2003. doi: 10.1016/j.jacc.2021.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothstein E.S., Welch T.D., Andrus B.W., Jayne J.E. Management of a patient presenting with anterior STEMI with concomitant COVID -19 infection early in the course of the U.S. pandemic. Catheter Cardiovasc Interv. 2021;97(3):E333–E338. doi: 10.1002/ccd.28967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salido-Tahoces L., Sánchez-Recalde A., Pardo-Sanz A., Zamorano Gómez J.L. Unusual presentation of acute coronary syndrome in a patient with SARS-CoV-2 infection. Eur Heart J Cardiovasc Imaging. 2020;21(9) doi: 10.1093/ehjci/jeaa147. 1053–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y., Tu L., Zhu P., et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer P., Degrauwe S., Van Delden C., Ghadri J.R., Templin C. Typical Takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41(19) doi: 10.1093/eurheartj/ehaa306. 1860–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt A.S., Adler E.D., Albert N.M., et al. Coronavirus disease-2019 and heart failure: a scientific statement from the Heart Failure Society of America. J Card Fail. 2022;28(1):93–112. doi: 10.1016/j.cardfail.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smilowitz N.R., Jethani N., Chen J., et al. Myocardial injury in adults hospitalized with COVID-19. Circulation. 2020;142(24):2393–2395. doi: 10.1161/CIRCULATIONAHA.120.050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agewall S., Beltrame J.F., Reynolds H.R., et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38(3):143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 23.Cosyns B., Lochy S., Luchian M.L., et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21(7):709–714. doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L., Zhao P., Tang D., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salerno M., Kwong R.Y. CMR in the era of COVID-19. J Am Coll Cardiol Img. 2020;13(11):2340–2342. doi: 10.1016/j.jcmg.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen S.E., Friedrich M.G., Leiner T., et al. Cardiovascular magnetic resonance for patients with COVID-19. J Am Coll Cardiol Img. 2022;15(4):685–699. doi: 10.1016/j.jcmg.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 29.Inciardi R.M., Adamo M., Lupi L., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotecha T., Knight D.S., Razvi Y., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab075. (ehab075). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.