Abstract
The role of endogenous gamma interferon (IFN-γ) in protective immunity against blood-stage Plasmodium chabaudi AS malaria was studied using IFN-γ gene knockout (GKO) and wild-type (WT) C57BL/6 mice. Following infection with 106 parasitized erythrocytes, GKO mice developed significantly higher parasitemia during acute infection than WT mice and had severe mortality. In infected GKO mice, production of interleukin 12 (IL-12) p70 and tumor necrosis factor alpha in vivo and IL-12 p70 in vitro by splenic macrophages was significantly reduced compared to that in WT mice and the enhanced nitric oxide (NO) production observed in infected WT mice was completely absent. WT and GKO mice had comparable numbers of total nucleated spleen cells and B220+ and Mac-1+ spleen cells both before and after infection. Infected WT mice, however, had significantly more F4/80+, NK1.1+, and F4/80+Ia+ spleen cells than infected GKO mice; male WT had more CD3+ cells than male GKO mice. In comparison with those from WT mice, splenocytes from infected GKO mice had significantly higher proliferation in vitro in response to parasite antigen or concanavalin A stimulation and produced significantly higher levels of IL-10 in response to parasite antigen. Infected WT mice produced more parasite-specific immunoglobulin M (IgM), IgG2a, and IgG3 and less IgG1 than GKO mice. Significant gender differences in both GKO and WT mice in peak parasitemia levels, mortality, phenotypes of spleen cells, and proliferation of and cytokine production by splenocytes in vitro were apparent during infection. These results thus provide unequivocal evidence for the central role of endogenous IFN-γ in the development of protective immunity against blood-stage P. chabaudi AS.
Studies of experimental murine models as well as humans suggest an important role for gamma interferon (IFN-γ) in protective immune responses to blood-stage malaria (19, 22). Treatment of mice with exogenous IFN-γ delays the onset of parasitemia and decreases the number of infected erythrocytes during Plasmodium chabaudi adami infection (4). Shear and her colleagues (31) demonstrated that daily treatment with recombinant IFN-γ resulted in a less severe course of infection and increased survival in mice infected with the lethal strain of Plasmodium yoelii 17x. Furthermore, these investigators found a correlation between the timing and level of IFN-γ production in vitro by spleen cells and the outcome of infection with lethal versus nonlethal strains of P. yoelii 17x. These observations were confirmed in a recent study demonstrating that endogenous levels of IFN-γ in the spleen during blood-stage malaria infection differ between nonlethal and lethal Plasmodium species at 24 h after infection (5). Studies in our laboratory of resistant C57BL/6 and susceptible A/J mice demonstrated a correlation between the level of resistance to blood-stage P. chabaudi AS infection and IFN-γ mRNA expression and protein production by spleen cells (15, 27, 34). In addition, treatment of P. chabaudi AS-infected C57BL/6 mice with neutralizing monoclonal antibodies (MAbs) to IFN-γ exacerbates the course of infection, but there is no effect on survival (21, 35).
Recent studies by van der Heyde et al. (42) using IFN-γ knockout (GKO) mice on the 129 background and Favre et al. (6) using IFN-γ receptor knockout (KO) mice on a mixed genetic background demonstrated a role for endogenous IFN-γ in the development of protective immunity to infection with P. chabaudi adami and P. chabaudi AS, respectively. In contrast, Tsuji et al. (41) failed to observe significant differences in parasitemia levels between IFN-γ receptor KO and wild-type (WT) mice on a mixed genetic background during blood-stage infection with P. chabaudi adami although protection induced by immunization with attenuated sporozoites against liver-stage P. yoelii 17XNL was impaired in the IFN-γ receptor KO mice. None of these studies, however, addressed the issue of the effects of background genes on the immune responses against blood-stage malaria in KO mice lacking IFN-γ responses.
The major cell types producing IFN-γ during blood-stage malaria are NK cells and T cells, primarily CD4+ Th cells. Studies of nude mice or NK cell-depleted mice demonstrated that early production of IFN-γ during infection with nonlethal P. yoelii is dependent on both NK and T cells (5). Using the model of P. chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice, we demonstrated that NK cells produce IFN-γ during early infection and that the ability of these cells to produce IFN-γ correlates with resistance (23). However, during the acute phase of P. chabaudi AS infection, just before peak parasitemia, CD4+ Th cells are the major source of IFN-γ (17, 21, 34). Taken together, these observations demonstrate that IFN-γ produced during innate as well as acquired immune responses plays a central role in protective immunity during blood-stage malaria.
This study was performed to determine the role of endogenous IFN-γ in the development of protective immunity against blood-stage P. chabaudi AS infection. We used GKO mice on the resistant C57BL/6 background to investigate the protective effect of this cytokine and, more importantly, to elucidate its immunoregulatory role in the development of protection against blood-stage infection with this parasite. We previously demonstrated that male mice are more susceptible to infection with this parasite than female mice (33). Since male GKO mice were also found to be more susceptible to infection than female GKO mice, we performed separate analyses of male and female GKO as well as WT mice. Our results demonstrate the pivotal role of endogenous IFN-γ in the development of protective immune responses and survival during blood-stage P. chabaudi AS infection. Furthermore, we identified important gender differences in host responses to this infection.
MATERIALS AND METHODS
Mice, parasite, and experimental infections.
Breeding pairs of GKO mice, provided by Genentech, Inc. (South San Francisco, Calif.), and backcrossed onto the C57BL/6 strain for eight generations, were a kind gift from F. P. Heinzel (Case Western Reserve University School of Medicine, Cleveland, Ohio) (12). GKO mice were bred in the animal facility of the Montreal General Hospital Research Institute under specific-pathogen-free conditions. Spleen cells from these mice stimulated in vitro with either concanavalin A (ConA) or parasite antigen failed to produce detectable levels of IFN-γ. WT control C57BL/6 mice were obtained from Charles River (St. Constant, Quebec, Canada) and maintained in the same facility. WT and GKO mice, 8 to 12 weeks old, were age and sex matched in all experiments. P. chabaudi AS was maintained as previously described (26). Infections were initiated by intraperitoneal injection of 106 P. chabaudi AS parasitized erythrocytes (PRBC). Parasitemia and mortality were monitored daily as previously described (26). Mice were sacrificed at various times, and blood was obtained by cardiac puncture, allowed to clot for 30 min at 4°C, and centrifuged at 3,000 × g for 3 min. Sera were collected and stored at 4°C for measurement of interleukin 12 (IL-12) p70 or at −20°C for determination of the levels of other cytokines.
Spleen cell culture and proliferation assay.
Spleens from normal and infected mice were removed aseptically and pressed through a sterile fine-wire mesh with 10 ml of RPMI 1640 (Life Technologies, Burlington, Ontario, Canada) supplemented with 10% heat-inactivated fetal calf serum (Hyclone Laboratories, Logan, Utah), 25 mM HEPES (Life Technologies), 0.12% gentamicin (Schering, Montreal, Quebec, Canada), and 2 mM glutamine (Life Technologies). Cell suspensions were centrifuged at 350 × g for 10 min. Erythrocytes were lysed with 0.175 M NH4Cl, and the cells were washed twice in fresh medium. Membrane debris was removed by filtering the cell suspensions through sterile gauze. The viability of the cells was determined by trypan blue exclusion and was always >90%. Total cell counts were performed on individual samples, and differential counts were performed on cytospin preparations stained with Diff-Quik (American Scientific Products, McGaw Park, Ill.). For proliferation assays, spleen cells were adjusted to 2.5 × 106 cells/ml and aliquots of 0.1 ml were plated in triplicate in 96-well flat-bottom plates, stimulated with 106 washed PRBC/ml as the malaria parasite antigen, 5 μg of ConA (Calbiochem, La Jolla, Calif.)/ml, or medium as the control, and incubated for 72 h at 37°C in a humidified CO2 incubator. During the last 16 h of culture, 1 μCi of [3H]thymidine (specific activity, 6.7 Ci/mmol) was added to each well, the cells were harvested with an automatic cell harvester, and the incorporated radioactivity was measured in a liquid scintillation counter. For determination of cytokine production, spleen cells were adjusted to 5 × 106 cells/ml and aliquots of 1 ml were plated in triplicate in 24-well tissue culture plates in the presence or absence of PRBC, as described above, and incubated for 48 h at 37°C in a humidified CO2 incubator. Supernatants were collected, centrifuged at 350 × g for 5 min, and stored at −20°C until assayed for cytokine levels. For culture of splenic macrophages, the percentages of macrophages in spleen cells were determined on Diff-Quik-stained cytospin slides and the cell suspensions were adjusted to 106 macrophages/ml. Aliquots of 0.1 ml/well in triplicate were incubated in flat-bottom 96-well plates at 37°C for 2 h. Nonadherent cells were removed. Adherent cells, which were >95% macrophages based on morphology, phagocytosis of inert latex beads, and nonspecific esterase staining (28), were washed twice with warm medium and incubated with 0.2 ml of medium alone or medium containing 1 μg of Escherichia coli 0127:B8 lipopolysaccharide (LPS) (Difco, Detroit, Mich.)/ml. Supernatants were collected 20 h later and assayed for IL-12 p70 and nitric oxide (NO).
Cytokine ELISAs.
Cytokine levels in sera and spleen cell or macrophage supernatants were measured using two-site sandwich enzyme-linked immunosorbent assays (ELISAs) for IFN-γ, tumor necrosis factor alpha (TNF-α), and IL-12 p70 as previously described (27, 36). For IL-4, the capturing and detecting antibodies were BVD4-1D11 MAb and biotinylated BVD6-24G2 MAb, respectively. For IL-10, JES5.2A5 MAb (American Type Culture Collection, Manassas, Va.) and biotinylated SXC-1 MAb (PharMingen Canada, Mississauga, Ontario, Canada) were used as capturing and detecting antibodies, respectively. Standard curves for each cytokine were generated using recombinant cytokines (PharMingen Canada). Reactivity was revealed using ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Boehringer Mannheim, Laval, Quebec, Canada), and optical density (OD) values were read in a microplate reader at 405 nm with a reference wavelength of 492 nm.
Determination of nitrite (NO2−) and nitrate (NO3−) concentrations.
For NO, the concentration of NO2− in cell culture supernatants was measured by the Griess reaction (11). NO2− concentrations were calculated using NaNO2 as a standard. Serum NO levels were determined based on NO3− concentrations using a previously described method (14). NaNO3, diluted in serum from uninfected WT mice and dialyzed against phosphate-buffered saline (PBS) for 24 h, was used as a standard to calculate serum NO3− levels.
Flow cytometry.
Spleen cells were adjusted to 2 × 107 cells/ml in staining buffer (PBS with 1% bovine serum albumin and 0.2% sodium azide). Aliquots of 50 μl of cells were incubated with anti-mouse CD16/CD32 MAb (clone 2.4G2; PharMingen) to block FcR. Blocked spleen cells were then labeled with a fluorescein isothiocyanate (FITC)-conjugated MAb against mouse CD3 (clone 145-2C11; PharMingen), B220 (clone RA3-6B2; PharMingen), Mac-1 (clone MI/70; Serotec, Oxford, United Kingdom), macrophage activation marker F4/80 (clone CL:A3-1; Serotec), or a phycoerythrin (PE)-conjugated MAb to mouse NK1.1 (clone PK136; PharMingen). To determine Ia antigen expression on macrophages, two-color flow cytometry was performed by labeling with a PE-conjugated MAb against F4/80 followed, after washing with staining buffer, by staining with a FITC-conjugated MAb to I-Ab(Aαb) (clone AF6-120.1; PharMingen). Isotype-matched MAbs conjugated to either FITC or PE were used as negative controls for all experiments. Acquisition of cells and analysis of data were performed immediately after staining using a FACscan equipped with CellQuest software (Becton Dickinson, Mountain View, Calif.).
Serum P. chabaudi AS-specific antibody titers.
Levels of P. chabaudi AS-specific antibody isotypes in serum were determined by ELISA. P. chabaudi AS antigen was prepared as described previously (47). Immulon II plates (Dynatech, Chantilly, Va.) were coated with parasite antigen overnight at 4°C and subsequently blocked with 1% bovine serum albumin in PBS for 1 h. Individual serum samples were serially diluted twofold, and 50 μl of each dilution was added to each plate and incubated for 2 h at room temperature. After extensive washing, horseradish peroxidase-conjugated goat anti-mouse isotype antibodies (SBA, Birmingham, Ala.) were added and incubated at room temperature for another 2 h. Reactivity was visualized using ABTS substrate, and OD values were read in a microplate reader at 405 nm with a reference wavelength of 492 nm. Antibody isotype levels in serum are expressed as ELISA titers, the reciprocal of the lowest dilution that yields the background OD.
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). Statistical significance of differences in means between WT and GKO mice, between normal and infected mice, and between sexes was analyzed by Student's t test using Mystat (Systat, Evanston, Ill.). A P value of <0.05 was considered significant.
RESULTS
Course of P. chabaudi AS infection in WT and GKO mice.
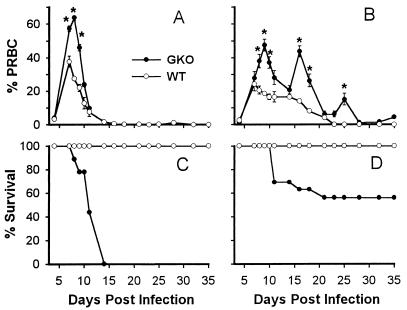
First, we examined the course of parasitemia and monitored mortality in WT and GKO mice on the resistant C57BL/6 background. WT mice, either male or female, developed primary parasitemia which peaked on day 7 postinfection (Fig. 1A and B). The peak parasitemia level in male WT mice (38% ± 3.4% PRBC) was significantly higher than that in female WT mice (24% ± 4.2%; P < 0.05). Parasitemia levels declined in female WT mice, and infection was cleared by day 23. Male WT mice also showed a reduction in parasitemia after the first peak and cleared the parasite by day 35 postinfection. All male and female WT mice survived primary infection (Fig. 1C and D). In comparison, GKO mice experienced a more severe course of P. chabaudi AS infection. Male GKO mice developed significantly higher parasitemia during days 7 to 9 postinfection than male WT mice (Fig. 1A), and 100% of male GKO mice died by day 14 after infection (Fig. 1C). Female GKO mice had significantly higher parasitemia between days 8 and 10 than female WT mice (Fig. 1B), and 40% died between days 12 and 20 postinfection (Fig. 1D). Surviving female GKO mice experienced two recrudescent parasitemias of 45 and 15% PRBC on days 16 and 25 postinfection, respectively. These results demonstrate that GKO mice are susceptible to P. chabaudi AS infection in terms of both increased parasitemia and decreased survival. Furthermore, male mice, either GKO or WT, developed significantly higher peak parasitemias than their female counterparts and male GKO mice suffered more severe mortality than female GKO mice.
FIG. 1.
Parasitemia and mortality of WT and GKO mice following infection with P. chabaudi AS. Male (A and C) and female (B and D) WT and GKO mice were infected intraperitoneally with 106 PRBC, and the course of parasitemia (A and B) was determined. Data are means ± SEM of six mice per group from one of three experiments. Cumulative survival (C and D) of infected WT and GKO mice was determined from 16 to 19 mice pooled from three experiments. ∗, statistically significant differences in mean parasitemia between WT and GKO mice (P < 0.05).
Serum cytokine levels in P. chabaudi AS-infected WT and GKO mice.
The immunoregulatory role of IFN-γ in the development of resistance to acute P. chabaudi AS infection was analyzed by determining levels of IL-12 p70, TNF-α, and NO in serum from WT and GKO mice during infection. Basal serum IL-12 p70 and NO levels in uninfected WT and GKO mice were not significantly different, while basal levels of TNF-α were significantly higher in uninfected WT mice (Table 1). Consistent with our previous findings (27), P. chabaudi AS infection in WT mice induced increased IL-12 p70 production in vivo, which peaked on day 2 postinfection (Table 1) and then declined thereafter (data not shown). GKO mice also had significant increases (P < 0.05 for both sexes) in serum IL-12 p70 on day 2 postinfection, but the levels were significantly lower than those observed in WT mice (P < 0.01 for both sexes). On day 7 postinfection, there were significant increases in serum TNF-α levels in WT (P < 0.01) as well as GKO (P < 0.05) mice compared to those in their uninfected controls. TNF-α levels were, however, significantly higher in WT mice than the levels detected in their GKO counterparts (P < 0.01 for both sexes). These observations suggest that optimum production of IL-12 p70 as well as TNF-α during blood-stage malaria is IFN-γ dependent. Infection with blood-stage P. chabaudi AS in WT mice resulted in significant and substantial increases in serum NO on day 7 postinfection. However, only basal levels of NO were detected in the sera of infected GKO mice, suggesting that NO production in vivo during blood-stage malaria is totally IFN-γ dependent. Serum IFN-γ levels increased significantly in both male and female WT mice following blood-stage P. chabaudi AS infection; in vivo IFN-γ production was significantly higher in female than in male WT mice (Table 1). No gender differences in serum IL-12 p70, TNF-α, or NO levels in WT and GKO mice were detected before or after infection.
TABLE 1.
Serum cytokine and NO levels in uninfected and P. chabaudi AS-infected WT and GKO micea
| Cytokine or NO and mouse type | Levelb in mice that were:
|
|||
|---|---|---|---|---|
| Uninfected
|
Infected
|
|||
| Male | Female | Male | Female | |
| IL-12p70 | ||||
| WT | 0.27 ± 0.04 | 0.27 ± 0.06 | 2.97 ± 0.3* | 3.32 ± 0.19* |
| GKO | 0.22 ± 0.03 | 0.16 ± 0.08 | 0.71 ± 0.20 | 0.78 ± 0.14 |
| IFN-γ | ||||
| WT | 1.3 ± 0.2 | 1.1 ± 0.2 | 32.3 ± 3.6 | 50.8 ± 4.3# |
| GKO | NDc | ND | ND | ND |
| TNF-α | ||||
| WT | 0.32 ± 0.05* | 0.31 ± 0.02* | 1.25 ± 0.10* | 1.01 ± 0.10* |
| GKO | 0.15 ± 0.02 | 0.22 ± 0.01 | 0.39 ± 0.03 | 0.40 ± 0.02 |
| NO | ||||
| WT | 9.8 ± 2.0 | 5.1 ± 1.3 | 59.6 ± 6.5* | 76.2 ± 5.8* |
| GKO | 9.9 ± 3.0 | 4.8 ± 0.9 | 11.1 ± 1.2 | 9.2 ± 1.6 |
Levels of IL-12 p70 in serum of infected mice were analyzed on day 2, and levels of IFN-γ, TNF-α, and NO were analyzed on day 7, following P. chabaudi AS infection. Data are means ± SEM for three to five mice per group. Results from one of three replicate experiments are shown.
Units are nanograms per milliliter for cytokines and micromolar for NO. ∗, P < 0.01 for WT versus GKO mice; #, P < 0.01 for WT female versus male mice.
ND, not detectable.
Spleen cell phenotypes in P. chabaudi AS-infected WT and GKO mice.
Next, we analyzed the numbers and phenotypes of leukocytes in the spleens of WT and GKO mice during P. chabaudi AS infection. There were no significant differences in the total numbers of nucleated spleen cells or in the numbers of T cells, B cells, macrophages, and NK cells between uninfected WT and GKO mice (Table 2). There were significant and similar increases in the total numbers of nucleated cells, B cells, and Mac-1+ cells in the spleens of infected mice compared to corresponding values for uninfected WT and GKO mice (P < 0.01). Among infected male mice, the number of CD3+ cells in WT mice was significantly higher than the number in GKO mice (P < 0.05). Compared to infected GKO mice, infected WT mice had significantly higher numbers of F4/80+ macrophages (P < 0.01 for male and P < 0.05 for female mice) and significantly higher numbers of NK 1.1+ cells (P < 0.01 for male and P < 0.05 for female mice) in their spleens. There were significant increases in the number of F4/80+Ia+ cells following P. chabaudi AS infection compared to corresponding values for uninfected WT (P < 0.01 for both sexes) and GKO (P < 0.05 for both sexes) mice (data not shown). The number of F4/80+Ia+ spleen cells from GKO mice was, however, significantly lower than that from WT mice (P < 0.05 for males and P < 0.01 for females). There were significantly higher numbers of F4/80+ macrophages in infected female WT mice than in male WT mice (P < 0.05) and higher numbers of F4/80+Ia+ spleen cells in uninfected and infected female WT mice than in their male counterparts (P < 0.05). These results suggest that, in the absence of IFN-γ, the recruitment and/or local proliferation of F4/80+ macrophages and NK 1.1+ cells is impaired during blood-stage malaria. These results also suggest that higher numbers of F4/80+ and F4/80+Ia+ cells in female WT mice may be linked to significantly lower their peak parasitemia levels being than those in WT male mice.
TABLE 2.
Spleen cellularity and phenotypes in uninfected and P. chabaudi AS-infected WT and GKO micea
| Cell type | No. of nucleated cells (107)/spleen (%) for mice that wereb:
|
|||
|---|---|---|---|---|
| Uninfected
| ||||
| WT
|
GKO
|
|||
| Male | Female | Male | Female | |
| Total | 8.0 ± 1.0 | 9.7 ± 1.0 | 6.6 ± 0.6 | 8.2 ± 0.9 |
| CD3+ | 2.80 ± 0.4 (31.8 ± 0.6) | 3.50 ± 0.3 (36.0 ± 1.3) | 2.10 ± 0.2 (31.5 ± 1.2) | 2.70 ± 0.3 (33.5 ± 1.5) |
| B220+ | 5.30 ± 0.7 (61.0 ± 1.1) | 5.50 ± 0.6 (57.0 ± 0.8) | 4.30 ± 0.4 (66.1 ± 0.5) | 4.80 ± 0.7 (57.9 ± 2.0) |
| Mac-1+ | 0.34 ± 0.03 (4.0 ± 0.2) | 0.44 ± 0.05 (4.5 ± 0.1) | 0.23 ± 0.03 (3.5 ± 0.3) | 0.37 ± 0.08 (4.5 ± 0.7) |
| F4/80+ | 0.28 ± 0.04 (3.2 ± 0.1) | 0.50 ± 0.04 (5.2 ± 0.3) | 0.21 ± 0.04 (3.1 ± 0.4) | 0.42 ± 0.07 (5.3 ± 0.5) |
| NK1.1 | 0.35 ± 0.05 (4.0 ± 0.1) | 0.37 ± 0.04 (3.8 ± 0.2) | 0.23 ± 0.04 (3.5 ± 0.2) | 0.36 ± 0.03 (4.4 ± 0.2) |
| No. of nucleated cells (107)/spleen (%) for mice that wereb:
| |||
|---|---|---|---|
| Infected
| |||
| WT
|
GKO
|
||
| Male | Female | Male | Female |
| 30.5 ± 2.2 | 31.6 ± 4.5 | 24.2 ± 2.9 | 28.2 ± 1.6 |
| 8.7 ± 0.3* (28.8 ± 1.1) | 10.6 ± 1.8 (33.2 ± 1.3) | 6.5 ± 0.6 (26.5 ± 1.8) | 9.5 ± 0.3 (33.9 ± 2.8) |
| 18.4 ± 2.8 (59.6 ± 4.9) | 19.7 ± 2.8 (62.4 ± 0.8) | 15.3 ± 1.7 (63.0 ± 3.5) | 14.1 ± 0.2 (50.4 ± 3.6) |
| 1.76 ± 0.3 (5.8 ± 0.9) | 1.81 ± 0.32 (5.7 ± 0.3) | 1.30 ± 0.30 (5.4 ± 0.5) | 1.43 ± 0.14 (4.9 ± 0.4) |
| 1.53 ± 0.14** (5.0 ± 0.3)* | 3.82 ± 0.35*# (12.4 ± 1.2)** | 0.53 ± 0.08 (2.3 ± 0.8) | 0.99 ± 0.14 (3.5 ± 0.6) |
| 0.84 ± 0.11** (2.7 ± 0.2) | 1.34 ± 0.29* (4.1 ± 0.4)* | 0.39 ± 0.04 (1.7 ± 0.3) | 0.66 ± 0.10 (2.3 ± 0.2) |
Percentages of spleen cell phenotypes from uninfected and day-7 P. chabaudi AS-infected mice were determined by fluorescence-activated cell sorter analysis. The number of cells of each phenotype was calculated from the total number of spleen cells and the percentage of cells with the phenotype (in parentheses). Data are means ± SEM of three individual mice per group.
∗, and ∗∗, P < 0.05 and P < 0.01, respectively, for WT versus GKO mice; #, P < 0.05 for female versus male mice.
In vitro proliferation and cytokine production by spleen cells from P. chabaudi AS-infected WT and GKO mice.
We also compared the in vitro function of spleen cells and splenic macrophages from WT and GKO mice. As shown in Table 3, the in vitro proliferation of spleen cells from uninfected mice was low in the presence of medium or PRBC and there were no significant differences between WT and GKO mice. In cultures stimulated with ConA, there was a significantly higher response in cells from uninfected GKO than in cells from WT mice (P < 0.01 for both sexes). In comparison to those of spleen cells from uninfected mice, the responses of spleen cells from P. chabaudi AS-infected WT or GKO mice to medium control or PRBC were significantly higher (P < 0.001). Compared to infected WT mice, infected GKO mice had significantly higher spontaneous proliferation in cultures containing medium (P < 0.01 for both sexes) and significantly higher responses to parasite antigen (P < 0.01 for both sexes). The responses of cells from infected mice of either genotype or gender to ConA were significantly lower than those of cells from their uninfected counterparts (P < 0.001), but infected GKO mice had significantly higher responses than infected WT mice (P < 0.01 for both sexes). Notable gender differences were observed in cultures stimulated with parasite antigen. Infected male WT mice had a significantly lower response to PRBC than their female counterparts (P < 0.05), while male GKO mice had significantly higher responses to PRBC than female GKO mice (P < 0.01).
TABLE 3.
Proliferative responses of spleen cells from uninfected and P. chabaudi AS-infected WT and GKO micea
| Mouse type | [3H]thymidine uptake (cpm) by spleen cells from mice that wereb:
|
|||||
|---|---|---|---|---|---|---|
| Uninfected
|
Infected
|
|||||
| Medium | PRBC | ConA | Medium | PRBC | ConA | |
| Female | ||||||
| WT | 396 ± 40 | 270 ± 24 | 10,995 ± 890 | 767 ± 33† | 1,456 ± 221†# | 3,331 ± 603† |
| GKO | 406 ± 58 | 221 ± 6 | 22,451 ± 1,229* | 1,743 ± 426*† | 3,298 ± 568*† | 7,767 ± 1,225*† |
| Male | ||||||
| WT | 250 ± 19 | 190 ± 6 | 8,691 ± 635 | 695 ± 74† | 837 ± 70† | 2,273 ± 241† |
| GKO | 301 ± 31 | 215 ± 8 | 19,261 ± 1,503* | 1,314 ± 125*† | 6,250 ± 474*†## | 7,599 ± 1,129*† |
Spleen cells from uninfected or day-7 P. chabaudi AS-infected WT and GKO mice were cultured with PRBC (106/ml), ConA (5 μg/ml), or medium as the control. [3H]thymidine uptake was measured, and data are means ± SEM for three mice per group.
†, P < 0.001 for uninfected versus infected mice; ∗, P < 0.01 for WT versus GKO mice; # and ##, P < 0.05 and P < 0.01, respectively, for male versus female mice.
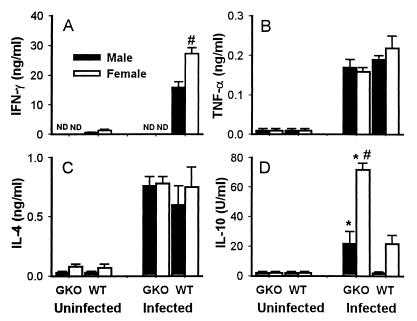
Spleen cells harvested from WT mice during acute P. chabaudi AS infection produced high levels of IFN-γ in vitro following stimulation with parasite antigen (Fig. 2A). Interestingly, PRBC-stimulated spleen cells from infected female WT mice produced significantly higher levels of IFN-γ than similarly stimulated cells from infected male WT mice (P < 0.05). As expected, there was no detectable IFN-γ in spleen cell supernatants from GKO mice stimulated with specific antigen regardless of infection. Production of the proinflammatory cytokine TNF-α was markedly increased in cultures of spleen cells from both infected WT and GKO mice stimulated with PRBC, and there were no significant differences between WT and GKO mice (Fig. 2B). As shown in Fig. 2C, spleen cells from infected WT and GKO mice produced comparable levels of IL-4 following in vitro stimulation with PRBC. However, PRBC-stimulated spleen cells from infected GKO mice produced significantly higher levels of IL-10 than their WT counterparts (P < 0.05 for both sexes) (Fig. 2D). Production of IL-10 and TNF-α was not detectable in nonstimulated, medium control cultures regardless of infection or the genotype of the mice. Spontaneous production of IFN-γ was detected only in spleen cell cultures from infected WT mice (male, 10.9 ± 1.0 ng/ml; female, 13.5 ± 0.3 ng/ml), but the difference was not significant. Supernatants from medium control cultures from infected WT and GKO mice had low levels of IL-4, and no significant difference between these two groups of mice was observed (data not shown). Gender differences similar to those in IFN-γ production were observed in IL-10 production. Spleen cells from infected female GKO mice, compared to their male counterparts, produced significantly higher levels of IL-10 in response to PRBC (P < 0.05). Together, these results demonstrate that, in the absence of IFN-γ, IL-10 production in response to parasite antigen is significantly increased in male and female GKO mice.
FIG. 2.
Cytokine production in vitro by spleen cells from uninfected and P. chabaudi AS-infected WT and GKO mice. Single cell suspensions were prepared from spleens recovered from uninfected and day-7-infected male and female WT and GKO mice and cultured for 48 h in the presence of PRBC (106/ml). Supernatants were collected, and the levels of IFN-γ (A), TNF-α (B), IL-4 (C), and IL-10 (D) were analyzed by ELISA. Data are means ± SEM of four or five individual mice from one of three experiments. ∗ and #, statistically significant differences (P < 0.05) between GKO and WT mice and between female and male mice of the same genotype, respectively; ND, not detectable.
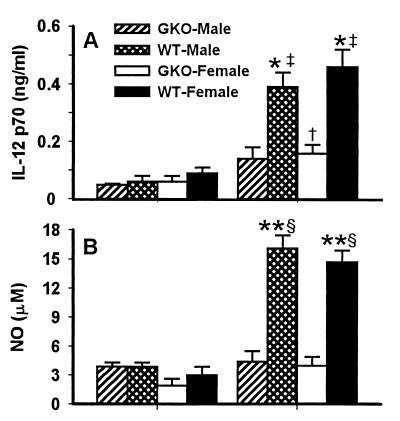
To determine if macrophage effector functions are impaired in GKO mice during acute P. chabaudi AS infection, splenic macrophages were analyzed for their ability to produce IL-12 p70 and NO in vitro in response to LPS. In contrast to the marked and significant increases in IL-12 p70 production by macrophages from infected mice compared to that by macrophages from uninfected WT mice (P < 0.01 for both sexes), IL-12 p70 production by macrophages from infected GKO mice was only modestly increased over basal levels (P < 0.05 for female mice only) and the levels were significantly lower than those in infected WT mice (P < 0.05 for both sexes) (Fig. 3A). In addition, macrophages from infected WT mice, compared to their uninfected counterparts, produced significantly higher levels of NO in vitro in response to LPS (P < 0.001) (Fig. 3B). Consistent with in vivo observations described above, splenic macrophages from infected GKO mice produced only basal levels of NO. No gender differences in production of IL-12 p70 or NO in vitro were observed.
FIG. 3.
Production of IL-12 p70 and NO in vitro by splenic macrophages from uninfected and P. chabaudi AS-infected WT and GKO mice. Splenic macrophages from uninfected and infected (day 2 for IL-12 p70 and day 7 for NO) mice were cultured for 20 h in the presence of 1 μg of LPS/ml. Supernatants were collected, and the levels of IL-12 p70 (A) and NO2− (B) were determined. Data are means ± SEM of three individual mice per group from one of two experiments. Statistically significant differences are indicated as follows: †, P < 0.05; ‡, P < 0.01; §, P < 0.001 (uninfected versus infected mice); ∗, P < 0.05; ∗∗, P < 0.01 (WT versus GKO mice).
Parasite-specific antibody responses in P. chabaudi AS-infected WT and GKO mice.
To investigate the effect of IFN-γ deficiency on malaria-specific antibody production, specific antibody isotype levels were determined in the sera of infected WT and GKO mice. Total parasite-specific immunoglobulin (Ig) levels among P. chabaudi AS-infected male and female WT mice and infected female GKO mice were similar (Table 4). However, marked differences in antibody isotypes between infected WT and GKO mice were evident. Infected female WT mice had significantly higher levels of parasite-specific IgM (P < 0.05), IgG2a (P < 0.01), and IgG3 (P < 0.005) than their GKO counterparts, while infected female GKO mice had significantly higher levels of IgG1 than their WT counterparts (P < 0.01). There were no significant differences between male and female WT mice. P. chabaudi AS-infected male GKO mice were unavailable for this analysis since 100% of these animals succumbed to infection.
TABLE 4.
Malaria-specific antibody isotype levels in serum of P. chabaudi AS infected WT and GKO micea
| Mouse type | Level of P. chabaudi AS-specific antibody isotypeb:
|
|||||
|---|---|---|---|---|---|---|
| Total Ig | IgM | IgG1 | IgG2a | IgG2b | IgG3 | |
| Female | ||||||
| WT | 1,610 ± 119 | 3,069 ± 578* | 113 ± 27 | 755 ± 84** | 72 ± 13 | 604 ± 92* |
| GKO | 1,611 ± 144 | 1,428 ± 169 | 612 ± 83** | 153 ± 29 | 91 ± 23 | 158 ± 77 |
| Malec | ||||||
| WT | 1,807 ± 165 | 2,228 ± 187 | 138 ± 21 | 665 ± 40 | 153 ± 34 | 624 ± 40 |
Parasite-specific antibody titers were determined by ELISA in serum collected 14 days following infection with P. chabaudi AS. Data are means ± SEM for four mice per group.
∗ and ∗∗, P < 0.05 and P < 0.01, respectively, for WT versus GKO mice of the same sex.
All male GKO mice died by day 14.
DISCUSSION
GKO mice on the resistant C57BL/6 background were used in the present study to directly assess the role of endogenous IFN-γ in the development of protective immunity against P. chabaudi AS infection. GKO mice, both male and female, developed significantly higher levels of parasitemia than WT mice during acute infection, and surviving female GKO mice had several prominent recrudescent parasitemias during the chronic stage of infection. The infection was lethal in 100% of male and 40% of female GKO mice. These results provide unequivocal evidence for the protective effect of this Th1 cytokine against blood-stage P. chabaudi AS infection. Unlike what was found for P. chabaudi AS-infected GKO mice, which had severe mortality, mortality was not observed in studies using neutralizing MAbs to block IFN-γ activity during infection in WT mice (21, 35) or in studies of GKO mice infected with P. yoelii 17XNL and P. chabaudi adami (42). This is likely due to the inability of neutralizing antibodies to completely block the activity of IFN-γ in vivo and, for P. yoelii and P. chabaudi adami infections, to differences in virulence among various rodent malaria species.
To elucidate the immunological mechanisms underlying the dramatic difference between WT and GKO mice, we analyzed the effects of IFN-γ deficiency on the in vivo and in vitro production of several key molecules known to modulate protective immunity against P. chabaudi AS infection. Early IL-12 production during infection with a number of intracellular pathogens, including protozoan parasites (8, 20), has been recognized as important for stimulating IFN-γ production by NK cells and inducing the differentiation of CD4+ Th0 cells to a Th1 phenotype (9, 40). Previously, we demonstrated that an early IL-12 response is critical for development of IFN-γ-dependent protection against P. chabaudi AS infection (27, 36). It is not known, however, if the early IL-12 response during P. chabaudi AS infection requires IFN-γ. The dependency of IL-12 production on IFN-γ varies with different intracellular pathogens (7, 8, 30). Results in the present study show that, although IL-12 production was increased in GKO mice following P. chabaudi AS infection, the level at day 2 postinfection was significantly lower than the response observed in WT mice. This observation suggests that production of optimum levels of IL-12 early during infection is dependent on IFN-γ, which is produced as early as 24 h after blood-stage malaria infection (5). Analysis of IL-12 production in vitro by splenic macrophages from infected WT and GKO mice further demonstrated the dependence of IL-12 production on IFN-γ. These observations indicate that there may be a positive-feedback loop between IL-12 and IFN-γ which is important for the host to rapidly mount IFN-γ-dependent protective immunity against P. chabaudi AS infection. This is supported by our earlier observation that recombinant IL-12 (rIL-12) treatment of susceptible A/J mice against P. chabaudi AS infection was most effective when treatment was started on the day of infection (36; unpublished observation). Furthermore, treatment with rIL-12 did not alter the lethal course of P. chabaudi AS infection in GKO mice (data not shown). Taken together, these results demonstrate that IFN-γ is required for optimum IL-12 production during early infection and for expression of IL-12-induced protection.
TNF-α is both protective and pathogenic during malaria infection in humans and in mice depending on the quantity, timing, and tissue site of its production as well as the malaria parasite species involved (10, 15, 37). Treatment of P. chabaudi AS-susceptible A/J mice with human recombinant TNF-α suppresses parasitemia and reduces mortality. Furthermore, a Th1-associated increase in TNF-α expression in the spleen correlates with resistance to this infection (15, 32). However, mice deficient in TNF-α (unpublished observation) or in the TNF-α p55 and p75 receptors (29) develop similar levels of peak parasitemia and clear P. chabaudi AS infection at the same time as WT control mice, suggesting that the absence of TNF-α activity does not impair protective Th1 responses against blood-stage malaria. Indeed, TNF-α receptor-deficient mice have normal serum IL-12 p70, IFN-γ, and NO levels during infection (29). TNF-α production was increased in vivo in both WT and GKO mice during infection, but serum TNF-α levels were significantly lower in GKO mice. These observations are consistent with findings in experimental models of sepsis in mice lacking either IFN-γ (12) or the IFN-γ receptor (16) and demonstrate that IFN-γ has an important role in up-regulating TNF-α production during malaria.
High levels of NO in the sera of P. chabaudi AS-infected WT mice and in supernatants of LPS-stimulated splenic macrophage from these mice were detected, but this response was completely abolished in infected GKO mice. These results demonstrate the critical role of IFN-γ in inducing NO production during blood-stage malaria and, consistent with our previous findings (14), suggest a correlation between NO production and resistance to P. chabaudi AS infection. Previously, we reported that treatment of P. chabaudi AS-infected mice with the selective inducible NO synthase (iNOS) inhibitor, aminoguanidine, results in high mortality but does not alter the course of parasitemia (14, 36). Based on these observations, we proposed that NO plays a role in protecting the host by regulating the immune response rather than direct parasite killing (14). Indeed, we showed previously that NO suppresses the in vitro proliferative responses of spleen cells from P. chabaudi AS-infected WT B6 mice to specific antigens and the mitogen ConA (1). The proliferation of spleen cells from infected WT mice in response to ConA and malaria antigen was significantly lower than the response of cells from infected GKO mice, and suppression was coincident with high levels of NO in spleen cell cultures from WT but not GKO mice (data not shown). Recent studies of P. chabaudi AS-infected mice treated with aminoguanidine demonstrated that NO also suppresses IFN-γ, TNF-α, and IL-2 production during acute infection (39). NO-mediated suppression of spleen cell proliferation and IFN-γ production has also been demonstrated in iNOS-deficient mice infected with Leishmania major (46) and Trypanosoma brucei rhodesiense (13). Together, these results suggest that NO production during P. chabaudi AS infection regulates the intensity and duration of Th1-associated immune responses and maintains the balance between the protective and pathologic effects of IFN-γ and TNF-α.
While increased mRNA expression and production of the Th1 cytokines IL-12, IFN-γ, and TNF-α by spleen cells correlate with resistance to P. chabaudi AS infection, the exquisite susceptibility of A/J mice to this parasite correlates with spleen cell production of Th2 cytokines (15, 23, 27, 34, 36). Interestingly, the absence of IFN-γ coincided with significantly increased production of the Th2 cytokine IL-10 by spleen cells from P. chabaudi AS-infected GKO compared to that by spleen cells from WT mice. We also investigated whether the abnormalities in systemic and in vitro IL-12 p70, TNF-α, NO, and IL-10 production in GKO mice were related to imbalances in splenic leukocyte populations. Fluorescence-activated cell sorter analysis of nucleated spleen cells showed that the recruitment and/or local proliferation of NK cells and F4/80+ macrophages expressing Ia antigen were deficient in infected male and female GKO mice compared to their WT counterparts. Studies of P. chabaudi AS-infected IL-10 deficient mice showed that increased mortality among these mice is accompanied by an enhanced Th1 IFN-γ response during acute infection which is retained in the chronic phase of infection (18). Th1 cytokine responses are also sustained during P. chabaudi AS infection in IL-4-deficient mice compared to WT littermates (44). We observed that IL-10 production was significantly higher in spleen cells from TNF-α receptor-deficient mice than in those from WT mice, but comparable levels of IFN-γ and IL-4 were produced by cells from the two genotypes (29). These observations suggest that there is coordinate and tight counterregulation by cytokines during blood-stage P. chabaudi AS infection. Cytokine-deficient mice should, therefore, be very useful in understanding the interactions of cytokines and their balance during blood-stage malaria.
The requirement for antibodies in the resolution of blood-stage P. chabaudi AS infection was clearly demonstrated in B-cell-depleted mice, which are unable to resolve P. chabaudi AS infection efficiently (38, 43, 45). Intact control mice, which control the infection and clear the parasites, initially mount a strong Th1-associated IgG2a response followed by a Th2-associated IgG1 response during the chronic stage of infection (38). The IgG fraction of immune sera from P. chabaudi AS-infected mice binds to the surfaces of PRBC and facilitates their phagocytosis by macrophages (24). We observed that female GKO mice produced significantly lower levels of parasite-specific IgM, IgG2a, and IgG3 but more IgG1 than their WT counterparts. This alteration in antibody isotypes may, in part, account for the persisting parasitemia in female GKO mice, the majority of which survive primary blood-stage P. chabaudi AS infection.
Gender differences among humans as well as experimental animals in resistance to parasitic diseases, including malaria, are apparent. We observed that male mice, either WT or GKO, developed higher peak parasitemias than their female counterparts, and 100% of male GKO mice succumbed to the infection. These results confirm and extend our previous observation of a gender difference in resistance to P. chabaudi AS infection (33). The increased susceptibility of male mice to this parasite is due to the immunosuppressive effects of testosterone, which is known to modulate the production of and response to cytokines (2, 3, 25). Here, we provide evidence of gender-associated immunologic differences in response to P. chabaudi AS infection. In comparison with infected female WT mice, male WT mice had significantly lower levels of serum IFN-γ and reduced in vitro production of this cytokine by spleen cells in response to PRBC. Significantly lower levels of IL-10 were also apparent in supernatants of spleen cells from male versus female GKO mice stimulated with parasite antigen or ConA (data not shown). A difference between male and female WT mice in the numbers of F4/80+Ia+ macrophages in the spleens of infected as well as uninfected mice was also apparent. Intriguingly, as observed here and previously, the gender difference in response to P. chabaudi AS infection is more prominent in cytokine- or cytokine receptor-deficient mice (18, 29). Further studies are required to understand the interactions between sex hormones and the immune response during blood-stage malaria.
In conclusion, the findings presented in this study unequivocally demonstrate the critical and central role of endogenous IFN-γ in regulating protective immune responses against blood-stage P. chabaudi AS infection. In the absence of IFN-γ, production of important counterregulatory molecules is dramatically altered. Production of protective Th1 mediators, including IL-12, TNF-α, and NO, is deficient, while there is increased production of the Th2 cytokine IL-10. Antibody production in GKO mice is also altered. Furthermore, this study highlights the additive effects of the immunosuppressive male sex hormone testosterone and the lack of IFN-γ, which render male GKO mice extremely susceptible to P. chabaudi AS.
ACKNOWLEDGMENTS
This work was supported by a grant from the Medical Research Council of Canada (MT14663) to M.M.S.
We gratefully acknowledge the technical assistance of Mi Fong Tam for breeding GKO mice, maintaining the parasite, and performing the infection studies. The secretarial assistance of Marlene Salhany is also gratefully appreciated.
REFERENCES
- 1.Ahavazi B C, Jacobs P, Stevenson M M. Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria. J Leukoc Biol. 1995;58:23–31. doi: 10.1002/jlb.58.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Benten W P M, Bettenhaeuser U, Wunderlich F, van Viliet E, Mossmann H. Testosterone-induced abrogation of self-healing of Plasmodium chabaudi malaria in B10 mice: mediation by spleen cells. Infect Immun. 1991;59:4486–4490. doi: 10.1128/iai.59.12.4486-4490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerinic M M, Konttinen Y, Grenerini S, Cutolo M. Neuropeptides and steroid hormones in arthritis. Curr Opin Rheumatol. 1998;10:220–235. doi: 10.1097/00002281-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Clark I A, Hunt N H, Butcher G A, Cowden W B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant IFN-γ or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987;139:3493–3496. [PubMed] [Google Scholar]
- 5.De Souza J B, Williamson K H, Otani T, Playfair J H L. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997;65:1593–1598. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favre N, Ryffel B, Bordmann G, Rudin W. The course of Plasmodium chabaudi chabaudi infections in interferon-gamma receptor deficient mice. Parasite Immunol. 1997;19:375–383. doi: 10.1046/j.1365-3024.1997.d01-227.x. [DOI] [PubMed] [Google Scholar]
- 7.Flesch I E A, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H E. Early interleukin-12 production by macrophages in response to mycobacterial infection depends on interferon-gamma and tumor necrosis factor-alpha. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flesch I E A, Kaufman S H E. Differential induction of IL-12 synthesis by Mycobacterium bovis BCG and Listeria monocytogenes. Res Immunol. 1995;146:520–526. doi: 10.1016/0923-2494(96)83026-4. [DOI] [PubMed] [Google Scholar]
- 9.Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. The interleukin-12/interleukin-12 receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 10.Grau G E, Fajardo L F, Piguet P F, Allet B, Lambert P H, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 11.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and 15nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Heinzel F P, Rerko R M, Ahmed F, Hujer A M. IFN-γ independent production of IL-12 during murine endotoxemia. J Immunol. 1996;157:4521–4528. [PubMed] [Google Scholar]
- 13.Herts C J, Mansfield J M. IFN-γ-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell Immunol. 1999;193:24–32. doi: 10.1006/cimm.1998.1429. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs P, Radzioch D, Stevenson M M. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J Immunol. 1995;155:5306–5313. [PubMed] [Google Scholar]
- 15.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamijo R, Le J, Shapiro D D, Havell E A, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon gamma receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langhorne J, Gillard S, Simon B, Slade S, Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1:416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 18.Linke A, Kühn R, Müller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 19.Luty A J F, Lell B, Schmidt-Ott R, Lehman L G, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Migot-Nabias F, Deloron P, Nussenzweig R S, Kremsner P G. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in African children. J Infect Dis. 1999;179:980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 20.Mattner F, Magram J, Ferrante J, Launois P, Padova K D, Behin R, Gately M K, Louis J A, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 21.Meding S J, Cheng S C, Simon-Haarhaus B, Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990;58:3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan K, Stevenson M M. Acquired immunity to asexual blood-stages of malaria. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis and protection. Washington, D.C.: ASM Press; 1998. pp. 467–493. [Google Scholar]
- 23.Mohan K, Moulin P, Stevenson M M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 24.Mota M M, Brown K N, Holder A A, Jarra W. Acute Plasmodium chabaudi chabaudi malaria infection induces antibodies which bind to the surfaces of parasitized erythrocytes and promote their phagocytosis by macrophages in vitro. Infect Immun. 1998;66:4080–4086. doi: 10.1128/iai.66.9.4080-4086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouihate A, Chen X, Pittman Q J. Interleukin-1 beta fever in rats: gender differences and estrous cycle influence. Am J Physiol. 1998;275:R1450–R1454. doi: 10.1152/ajpregu.1998.275.5.R1450. [DOI] [PubMed] [Google Scholar]
- 26.Podoba J E, Stevenson M M. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991;59:51–58. doi: 10.1128/iai.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sam H, Stevenson M M. In vivo IL-12 production and IL-12 receptors β1 and β2 mRNA expression in the spleen are differentially up-regulated in resistant B6 and susceptible A/J mice during early blood-stage Plasmodium chabaudi AS malaria. J Immunol. 1999;162:1582–1589. [PubMed] [Google Scholar]
- 28.Sam H, Stevenson M M. Early IL-12 p70, but not p40, production by splenic macrophages correlates with host resistance to blood-stage Plasmodium chabaudi AS malaria. Clin Exp Immunol. 1999;117:343–349. doi: 10.1046/j.1365-2249.1999.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sam H, Su Z, Stevenson M M. Deficiency in tumor necrosis factor alpha activity does not impair early protective Th1 responses against blood-stage malaria. Infect Immun. 1999;67:2660–2664. doi: 10.1128/iai.67.5.2660-2664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 31.Shear H L, Srinivasan R, Nolan T, Ng C. Role of IFN-γ in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol. 1989;143:2038–2044. [PubMed] [Google Scholar]
- 32.Stevenson M M, Ghadirian E. Human recombinant tumor necrosis factor alpha protects susceptible A/J mice against lethal Plasmodium chabaudi AS infection. Infect Immun. 1989;57:3936–3939. doi: 10.1128/iai.57.12.3936-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson M M, Lyanga J J, Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982;38:80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson M M, Tam M F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson M M, Tam M F, Belosevic M, van der Meide P H, Podoba J E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990;58:3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 37.Taverne J, Sheikh N, de Souza J B, Playfair J H, Probert L, Kollias G. Anaemia and resistance to malaria in transgenic mice expressing human tumor necrosis factor. Immunology. 1994;82:397–403. [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor-Robinson A W, Phillips R S. B cells are required for the switch from Th1- to Th2-regulated immune responses to Plasmodium chabaudi chabaudi infection. Infect Immun. 1994;62:2490–2498. doi: 10.1128/iai.62.6.2490-2498.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor-Robinson A W, Smith E D. A dichotomous role for nitric oxide in protection against blood stage malaria infection. Immunol Lett. 1999;67:1–9. doi: 10.1016/s0165-2478(98)00148-5. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri G. Interleukin 12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji M, Miyahira Y, Nussenzweig R S, Arguet M, Reichel M, Zavala F. Development of antimalaria immunity in mice lacking IFN-γ receptor. J Immunol. 1995;154:5338–5344. [PubMed] [Google Scholar]
- 42.van der Heyde H C, Pepper B, Batchelder J, Cigel F, Weidanz W P. The time course of selected malarial infections in cytokine-deficient mice. Exp Parasitol. 1997;85:206–213. doi: 10.1006/expr.1996.4132. [DOI] [PubMed] [Google Scholar]
- 43.von der Weid T, Langhorne J. Altered response of CD4+ T cell subsets to Plasmodium chabaudi chabaudi in B cell-deficient mice. Int Immunol. 1993;5:1343–1348. doi: 10.1093/intimm/5.10.1343. [DOI] [PubMed] [Google Scholar]
- 44.von der Weid T, Kopf M, Köhler G, Langhorne J. The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 45.von der Weid T, Honarvar N, Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 1996;156:2510–2516. [PubMed] [Google Scholar]
- 46.Wei X-Q, Charles I G, Smith A, Ure J, Fang G J, Huang F P, Xu D, Muller W, Mondada S, Liew F Y. Altered immune response in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 47.Yap G S, Stevenson M M. Differential requirements for an intact spleen in induction and expression of B-cell-dependent immunity to Plasmodium chabaudi AS. Infect Immun. 1994;62:4219–4225. doi: 10.1128/iai.62.10.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]