Abstract
Objectives:
The objective of this study was to determine prevalance, severity, course, and long-term findings of smell and taste disorders of coronavirus disease 2019 (COVID-19) patients in mild-moderate and severe-critical disease spectrum.
Methods:
All adult patients conducted in our hospital with positive severe acute respiratory syndrome coronavirus 2 between March and April 2020 were surveyed during disease course and those with olfactory and taste loss were re-surveyed to determine the course and progress of these symptoms after at least 12-month follow-up. Demographic features and clinical findings were evaluated as well as disease severity.
Results:
A total of 77 patients with smell and/or taste loss symptoms were included in the study. At diagnosis, 58 (75.3%) patients had loss of smell and 75 (97.4%) had loss of taste. After a follow-up period of 12–14 months, 12 (15.6%) patients had ongoing loss of smell and six (8%) patients had ongoing loss of taste. Three (3.9%) patients complained of cacosmia and 5 (6.5%) complained of parosmia at follow-up. The presence of ongoing visual analog scale scores of smell and/or taste was not statistically significant between male and female patients. When presence and severity of symptoms were compared by disease severity, no statistically significant difference was found.
Conclusion:
Smell and taste loss seem to be among the presenting symptoms of COVID-19. The prognosis and the treatment of the smell loss and taste loss in COVID-19 patients remains unclear. To improve and accelerate recovery, the pathophysiology and the treatment options must be validated.
Keywords: COVID-19, olfaction, smell, taste
In Wuhan at the end of 2019 on February 11, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was defined and the disease was officially named as coronavirus disease 2019 (COVID-19) by 2020 World Health Organization (WHO).[1,2] First reports from China described the disease as a lower respiratory tract infection with common symptoms such as fever, cough, and shortness of breath. Upper respiratory tract symptoms such as sore throat or rhinorrhea were reported as uncommon symptoms in these first reports.[3–8] Later, studies revealed higher incidence of the upper respiratory symptoms andamong them smell and taste loss has become prominent futures of the disease lately.[9–11] In some reports, even the only symptom of COVID-19 infection was reported to be anosmia. Many reports confirmed the importance of this symptom affecting most of the COVID-19 patients.[12,13]
The frequency of the anosmia in COVID-19 patients and the severity of this symptom was conducted in several studies. A clinical trial was conducted in our clinic among 223 COVID-19 patients. As a result 71 patients had complain of smell loss and 77 patients had taste loss that are defined as disease symptoms.[14–16] Furthermore, in a prospective study, among 88 COVID-19 anosmic patients, after the onset of the disease 79.5% of patients had recovered in the first 2 months.[17] The value of smell and taste loss as a symptom of COVID-19 was highlightened by all these reports.
The prognosis and the treatment of the anosmia and taste loss symptoms for COVID-19 patients remains unclear. The effect of variant forms of the virus on smell and taste is another issue to be solved. The present study was carried out to provide information about the long-term findings and recovery process and prognosis of smell and taste loss of the non-variant COVID-19 patients with present symptoms during the disease.
Methods
After the research plan was approved by the Local Institutional Ethics Committee (Date: April 27, 2021, Number: 3242), the study was conducted at a tertiary care hospital. We assessed patients who had positive SARSCoV-2 RNA detected by reverse transcription-polymerase chain reaction (RT-PCR). A total of 77 patients with smell and/or taste loss symptoms (36 males and 41 females) were included in the present study. Written informed consents were obtained from all participants.
Patient Selection and Classification
Nasopharyngeal and oropharyngeal swab specimens obtained from the suspicious patients were tested for the presence of SARS-CoV-2 RNA by Nucleic Acid Amplification Tests (RT-PCR). All COVID-19 patients were diagnosed, classified, and managed according to guidelines published by the Ministry of Health of Turkey Study Board.[18] Adult patients (age >18 years) with smell and taste loss symptoms after initial diagnoses of COVID-19 on date between March and April, 2020 during early pandemic period in our country were searched from the hospital data base. The patients with smell and taste loss were identified and after a follow-up of at least 12 months, these symptoms were again re-evaluated, and the course of the symptoms were noted.
Patients who refused to participate in the study, those who could not be reached, or unable to complete to survey and those under the age of 18 were excluded from the study. Figure 1 provides the diagram for patient selection and exclusion as well as the clinical distribution of included patients.
Figure 1.
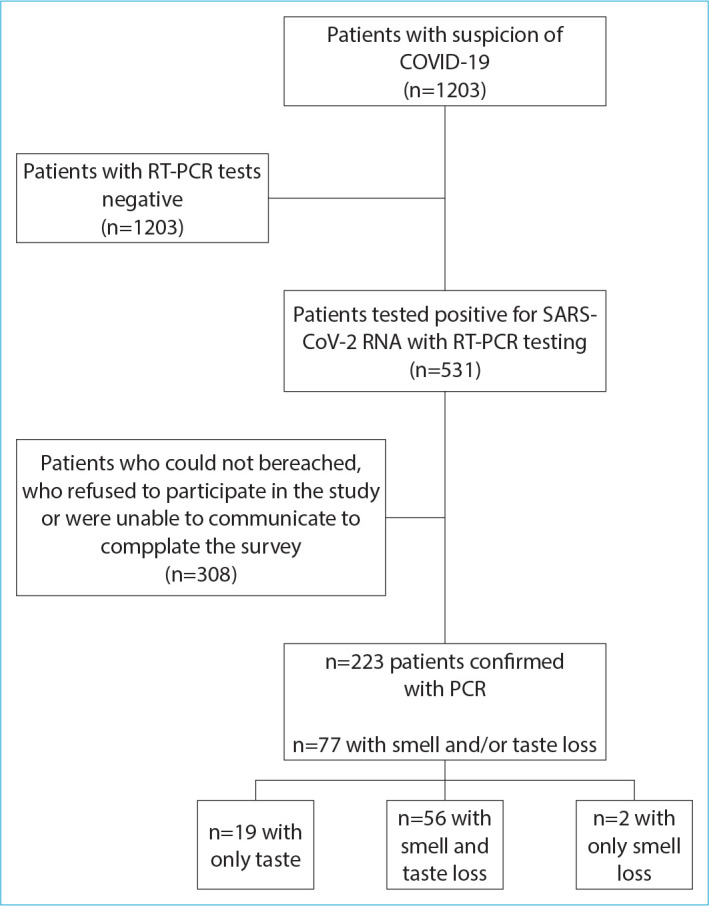
The diagram for patient recruitment and exclusion and clinical distribution of included patients. RT-PCR: Reverse transcription-polymerase chain reaction, SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Following the clinical mangement guidance provided both the National Ministry of Health and WHO, the patients were classifed into groups according to disease severity during the initial diagnosis (Table 1).[18,19] Patients with mild and moderate disease were combined together as mild–moderate group and the ones in critical and severe groups were grouped as critical-severe group.
Table 1.
Classification criteria for disease severity
| Mild disease | Mild symptoms, but no signs suggestive of pneumonia, and SpO2 >93% on room air, and negative chest imaging findings suggestive of viral pneumonia |
| Moderate disease | With clinical signs of mild pneumonia, (SpO2 ≥90% on room air, respiratory rate <30 breaths/min) or with chest imaging findings suggestive of viral pneumonia |
| Severe disease | With severe pneumonia, (SpO2< 90% on room air, respiratory rate >30 breaths/min, or severe respiratory distress) but not necessitating intensive care |
| Critical Disease | Necessitating intensive care unit |
SpO2: Oxygen saturation.
Survey Design
A questionnaire for demographical features, reported general, and otorhinolaryngologic symptoms of COVID-19 had been done during the initial diagnosis. The patients with smell and taste loss symptoms were included in the present study and re-evaluated after at least 12 months follow-up period.
Patient Interviews
All patients signed the informed consents forms. After the initial diagnosis of COVID-19, authors conducted interviews and filled the survey with the patients participating in our study. Due to the high risk of contamination (since there had been no vaccines, etc) the objective smell tests were not performed, visual analog scale (VAS) was used for assesing the symptom severity; a subjective scale of 0–10, with 0 denoting “no symptoms” and 10 “severe symptoms.” The patients with smell and/or taste loss were noted and re-assessed after at least 12-month follow-up. These patients were examined in our outpatient clinic and a second survey was filled. The VAS score of symptom severity of smell loss and taste loss was noted on the surveys. The duration of symptoms, present findings, ongoing symptoms, and the treatment models if used was questioned. The initial VAS scores and the present VAS scores of symptoms were compared. The changes in the smell and taste perception such as parosmia and cacosmia were also investigated and noted.
In the present study, if there is a relationship between the smell and taste loss symptoms and gender difference was evaluated. Also the relationship between the symptoms and disease severrity was evaluated.
Statistical Analysis
All statistical analyses were conducted by SPSS (Version 22.0, Chicago, IL, USA). Categorial variables were reported as numbers and percentages and Chi-square test was used to compare with Fisher’s exact test as needed. Numerical variables were expressed as means and standard deviation when normality criteria were met, otherwise as median and interquartile ranges (IQR). Mann–Whitney U-test was used to compare numerical variables when normality criteria were not met. Statistical significance level was taken as p<0.05.
Results
A total of 77 patients had complaints of either loss of smell, loss of taste or both at presentation. Two patients had only loss of smell, 19 had only loss of taste, and the remaining 56 had both. Mean age was 42 (±14.6) year old. Thirty-six (46.8%) were male and 41 (53.2%) were female. Sixty-eight (88.3%) patients had mild-moderate and 9 (11.7%) patients had severe-critical COVID disease.
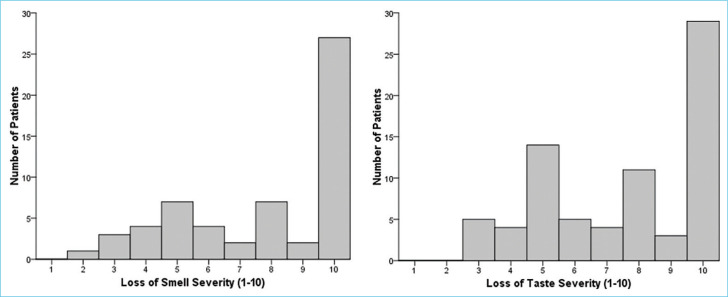
At diagnosis, 58 (75.3%) patients had loss of smell and 75 (97.4%) had loss of taste. Median VAS scores for loss of smell and loss of taste were 9 (IQR: 5–10) and 8 (IQR: 5–10), respectively. Figure 2 shows histograms of VAS scores at diagnosis for loss of smell and taste.
Figure 2.
Histograms of VAS scores at diagnosis for loss of smell and taste. VAS: Visual analog scale.
For follow-ups, 7 (9.1%) patients were questioned 12 months after the diagnosis; 60 (77.9%) patients, after 13 months and 10 (13%) patients, after 14 months.
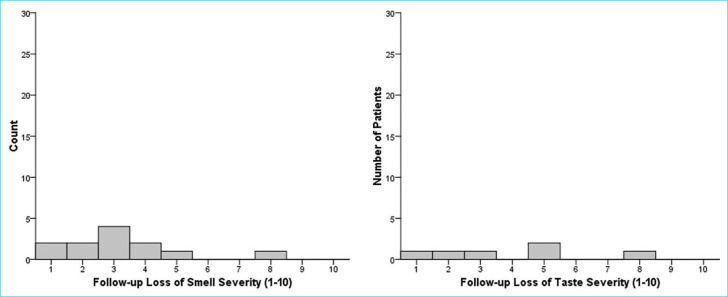
Of 58 patients with loss of smell at diagnosis, 12 (20.7%) had ongoing loss of smell at follow-up with a median VAS score of 3 (IQR: 2–4). Of 75 patients with loss of taste, six (8%) patients had ongoing loss of taste at follow-up with a median VAS score of 4 (IQR: 1.5–5.75). Figure 3 shows histograms of follow-up VAS scores. Three (3.9%) patients complained of cacosmia and 5 (6.5%) complained of parosmia at follow-up. Only 6 (7.8%) patients used some type of treatment (four patients had used nasal steroid, two patients had applied saline nasal irrigation and smell training tecknique) for smell loss.
Figure 3.
Histograms of follow-up VAS scores. VAS: Visual analog scale.
The presence of ongoing smell and/or taste loss was not statistically significantly different between male and female patients. VAS scores also did not differ significantly (Table 2). When presence and severity of symptoms were compared by disease severity, no statistically significant difference was found (Table 3).
Table 2.
Presence of ongoing complaints and severity scores by sex
| Male | Female | p | |||
|---|---|---|---|---|---|
| Median or n | IQR or % | Median or n | IQR or % | ||
| Ongoing loss of smell present, % | 6 | 25 | 6 | 17.6 | 0.52 |
| Ongoing loss of taste present, % | 4 | 11.4 | 2 | 5 | 0.41 |
| Loss of smell severity | 3 | 1.75–4.25 | 3 | 1.75–5 | 0.93 |
| Loss of taste severity | 4 | 2.25–5 | 4 | 0–8 | 1 |
IQR: Interquartile ranges.
Table 3.
Presence of ongoing complaints and severity scores by disease severity
| Mild disease | Severe or critical disease | p | |||
|---|---|---|---|---|---|
| Median or n | IQR or % | Median or n | IQR or % | ||
| Ongoing loss of smell present, % | 11 | 20.4 | 1 | 25 | 1 |
| Ongoing loss of taste present, % | 6 | 9.1 | 0 | 0 | 1 |
| Loss of smell severity | 3 | 2–4 | 4 | N/A | 0.37 |
| Loss of taste severity | 4 | 1.5–5.75 | N/A | N/A | N/A |
IQR: Interquartile ranges.
Discussion
Smell and/or taste loss significantly impacts quality of life. Recently, there have been many reports evaluating the smell loss and taste disorders of patients with COVID-19. Most of these reports include self report surveys from different countries.[20–23] Many authorities have pointed out that loss of smell and taste may be a strong predictor and finding of COVID-19.[13,24] Moreover, the exact proportion of COVID-19 patients experiencing true smell and taste disorder is unknown, because the patients were aware of the smell loss if only this symptom was prominent and noticable.[25,26] This study evaluated the long-term course, duration, and progress of the smell and taste loss symptoms of COVID-19 patients.
The pathophysiology for the smell and/or taste loss due to COVID-19 is not entirely clear.[27] In a mouse model, it has been shown that anterograde propagation of the virus to central nervous system through the olfactory bulb was prevented by the apoptosis of the infected olfactory neurons.[28] In terms of the etiology of olfactory dysfunction, several hypothesis such as inflammation and obstruction of the olfactory cleft or damage to olfactory bulb were proposed for etiology.[29–32] Moreover, it was reported that parosmia was associated with disordered regrowthof neurons and a predominanceof immature cells.[33] In the present study 5 (6.5%) patients complaint about parosmia, 3 (3.9%) patients complaint about cacosmia still after 12 months follow-up. These symptoms seem to be a significant public health issue as pandemic progresses and time passes. Furthermore, there is no standart treatment protocol for these symtomps.
In a prospective clinical series, among the 88 anosmic patients with COVID-19, 79.5% of the patients recovered in the first 2 months following the onset of the disease.[17] On the other hand, some reports revelaed that true prevelance of olfactory dysfuntion was underestimated in cases of self reporting.[11,34] In a clinical trial conducted in our clinic, among 223 patients, in total, 31.8% (n=71) of the patients were with smell dysfuntion, while 34.5% (n=77) of the patients were with taste loss. After comparing mild-moderate to severe-critical forms of COVID-19 infection reveals that the patients with mild-moderate symptoms were representing 36.5% (65 patients among 178) smell loss and 36.5% (65 patients among 178) taste loss. Among the severe-critical form of disease, smell loss was representing 13.3% (six patients among 45) and taste loss 12% (12 patients among 45).[14] In the present study, 75 of 223 COVID-19 patients were with taste loss complaint at the onset of COVID-19. Seventy (93.3%) patients were recovered and 5 (6.6%) patients were still complian taste loss after 12 months follow-up that affects the quality of life.
We found out that the presence of ongoing loss of taste or smell was not statistically different between male and female patients. Besides, VAS scores also did not differ significantly between the sexes. The severity of smell and taste loss in terms of VAS scores did not differ significantly between sexs in our study. These findings were consistent with the findings of an other study carried out to determine olfactory dysfunction in COVID-19 patients. There was no difference in terms of sex between female and male patientsin that study as well.[11]
On the other hand, there is no certain evidence if there is a difference between the initial form and the variant forms of COVID-19 impact on smell and taste functions. Our study only shows the long-term effect of initial form of COVID-19 since there has not been reported variant cases during the initial period of the studied group.
When presence and severity of symptoms were compared by disease severity, no statistically significant difference was found (Table 3).
In animal models, sytemic corticosteroids were shown to prevent regrowth and to reduce scar formation.[35] We found that only four patients who had recovered from smell loss had used nasal corticosteroid for 2 months. However, the number of the patients is insufficient to make a decision for this treatment model. The treatment options, durations, and doses were still not clear and yet not determined. Smell training techniques could also be an other treatment option. However, there is no standardisation for this treatment in terms of duration of the treatment as well as the frequency.
The taste disorder is also an other problem. If it is a taste disorder only or if it is a perception disorder due to smell dysfunction is still remains unclear.
Smell and taste dysfunction impacts on the quality of life, therefore ongoing evaluation of symptoms, and establishing the factors determining the severity of smell and taste loss and to improve and accelerate the recovery must be established. The treatment strategies and options must be carried out in clinical trials and we must evaluate whether preventative actions are possible. As the COVID pandemic comes to a more stabile level, the long-term effect of the disease and ongoing symptoms that impacts quality of life becomes more challenging.
Strengths and Limitations
The present study has both strenghts and limitations to note. As for strength of the study, long-term follow-up COVID-19 patients was available. Thus, whether the smell and/or taste loss are transient, long lasting or permenant could have been determined. Information as to permanency or changes in perception is of considerable significant. The limitations of the present study were; the exact proportion of COVID-19 patients presenting true smell and taste disorder is unknown. Furthermore, only the participants confirmed with positive PCR results were included in the present study that there were patients who were not included that clinically presented and treated like COVID-19 but with negative PCR results. An other limitation is multivariate or regression analyses were not conducted in the study. Our study is dependent on self-reports, due to the high risk of contamination of COVID-19, quantitive smell and taste tests could not be used. Finally, as only the pediatric patients were not included in the study, the results should only be interpreted for the adult population.
Conclusion
Understanding and evaluation the presence and the course of smell and taste dysfunction in COVID-19 patients is important. The present study provides valuable information about the smell and taste loss symptoms of a cohort of patients with COVID-19. The report also gives information about long-term results and the course of these symptoms. Its findings strongly suggest that smell loss and taste loss could bepersistant in some percent. In light of the current findings, smell and taste loss seems to be a clinical symptom of COVID-19.
To improve and accelerate recovery, the pathophysiology and the treatment options must be validated and special therapies should be initiated. We need to establih the factors determining the severity and the prognosis of symptoms. To prevent the possibility of persistance of the smell and taste loss and to determine the predisposing factors and treatment modalities, further controlled, multi-center studies must be carried out.
Disclosures
Ethics Committee Approval:
The study was approved by the Şişli Hamidiye Etfal Training and Research Hospital Ethics Committee Ethics Committee (date: 27/04/2021, no: 3242).
Peer-review:
Externally peer-reviewed.
Conflict of Interest:
None declared.
Authorship Contributions
Concept – B.T., S.K.D., S.T.; Design – B.T., A.A., E.S., S.K.D., S.T.; Supervision – B.T., S.K.D., S.T.; Materials – B.T., A.A., E.S., S.K.D., S.T; Data collection &/or processing – A.A., E.S., S.T.; Analysis and/or interpretation – B.T., S.K.D., S.T.; Literature search – B.T., A.A., E.S., S.K.D., S.T; Writing – B.T., S.K.D., S.T.; Critical review – B.T., S.T.
References
- 1.Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27:taaa008. doi: 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol. 2020;92:612–7. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-ınfected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020;99:569–76. doi: 10.1177/0145561320920762. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19) doi: 10.1016/j.tmaid.2020.101623. Electronic address: https://www.lancovid.org Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58:295–8. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 10.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42:1560–9. doi: 10.1002/hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–50. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19. Ann Intern Med. 2020;173:672–5. doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salepci E, Turk B, Ozcan SN, Bektas ME, Aybal A, Dokmetas I, et al. Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur Arch Otorhinolaryngol. 2021;278:525–35. doi: 10.1007/s00405-020-06284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Plzak J, Hans S, et al. Psychophysical olfactory tests and detection of COVID-19 in patients with sudden onset olfactory dysfunction: a prospective study. Ear Nose Throat J. 2020;99:579–83. doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- 16.Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42:1583–90. doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. 2020;134:703–9. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turkish Ministry of Health Scientific Board COVID19 (SARS-CoV-2 Infection) Guide. 2020. Available at https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf Accessed Dec 7 2022.
- 19.World Health Organization COVID-19 Clinical management: living guidance. 2021. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 Accessed Dec 7 2022.
- 20.Lüers JC, Klußmann JP, Guntinas-Lichius O. The COVID-19 pandemic and otolaryngology: What it comes down to? [Article in German] Laryngorhinootologie. 2020;99:287–91. doi: 10.1055/a-1095-2344. [DOI] [PubMed] [Google Scholar]
- 21.Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–40. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagheri SH, Asghari A, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran. 2020;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–90. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Academy of Otolaryngology-Head and Neck Surgery Foundation Hyposmia and anosmia. ENT Health. 2020. Available at: https://www.enthealth.org/conditions/hyposmia-and-anosmia/ Accessed Dec 8, 2022.
- 25.Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- 26.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–44. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 27.Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519–28. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 28.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–87. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272–7. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–75. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graziadei PP, Karlan MS, Graziadei GA, Bernstein JJ. Neurogenesis of sensory neurons in the primate olfactory system after section of the fila olfactoria. Brain Res. 1980;186:289–300. doi: 10.1016/0006-8993(80)90976-2. [DOI] [PubMed] [Google Scholar]
- 32.Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- 33.Leopold DA, Loehrl TA, Schwob JE. Long-term follow-up of surgically treated phantosmia. Arch Otolaryngol Head Neck Surg. 2002;128:642–7. doi: 10.1001/archotol.128.6.642. [DOI] [PubMed] [Google Scholar]
- 34.Hornuss D, Lange B, Schröter N, Rieg S, Kern WV, Wagner D. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020;26:1426–7. doi: 10.1016/j.cmi.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian J, Pinto JM, Xin Y, Zhang H, Li L, Sun Z, et al. Dexamethasone affects mouse olfactory mucosa gene expression and attenuates genes related to neurite outgrowth. Int Forum Allergy Rhinol. 2015;5:907–18. doi: 10.1002/alr.21586. [DOI] [PubMed] [Google Scholar]