Abstract
OBJECTIVE
Post-operative adhesion is a common problem in abdominal surgery. Especially, foreign materials are strong stimulus for the development of adhesions. The aim of this study was to investigate whether drug release material coated prosthetic mesh decreases intra-abdominal adhesion formation or not.
METHODS
5-Fluorouracil (5-FU) releasing “chitosan gels” were loaded to polypropylene and polyglactin-910 grafts. Polypropylene, polyglactin-910 grafts, chitosan gel, and 5-FU-loaded polyglactin 910, polypropylene grafts were used to cover abdominal defects of rats which were created under sterile conditions (n=84). Each group was divided into two subgroups (n=6). Subgroups were sacrificed on the 7th and 30th days.
RESULTS
The 7th day macroscopic examinations were similar. Polypropylene group was most adhesive group on the 30th day. There were less adhesions in chitosan gel and 5-FU-loaded groups. Capsule and capsule margins showed no difference on both the 7th and 30th days. Polypropylene-5-FU group and polypropylene-chitosan gel group showed significantly less macroscopic adhesions than polypropylene control group. Furthermore, polyglactin-910-chitosan gel group was less adhesive than polypropylene control group.
CONCLUSION
This study showed that 5-FU decreases the adhesions but the dosage and release kinetics need further investigations.
Keywords: Adhesions, polyglactin 910-chitosan gel, polypropylene, 5-fluorouracil
Highlight key points
5-FU loaded on graft materials prevents capsule formation and adhesion formation.
Antiadhesive and antifibrotic effects of chitosan gel were observed.
No toxic effect of chitosan gel was observed.
Post-operative intra-abdominal adhesions represent a major unresolved and sometimes life-threatening problem that develops after 50–100% of all abdominal surgeries [1]. Intra-abdominal adhesions seriously complicate the surgical process and its aftermath. In their prospective study, Menzies and Ellis reported adhesion in 93% of the patients (n=210) undergoing laparotomy who had undergone abdominal surgery previously, while they detected adhesion in 10.4% of the patients in the control group of 114 patients who underwent their first surgery [1, 2]. This shows that post-operative adhesions are an almost inevitable process.
Similar to post-operative adhesions, incisional hernias are common surgical complications likely caused by the improper closure of the fascia and/or poor wound healing [3]. Factors that cause chronic or long-term post-operative intra-abdominal pressure to increase and affect wound healing contribute to the development of incisional hernias [3]. The incidence of incisional hernia is between 12.7% and 23% [4, 5]. One of the most frequently performed procedures in general surgery practice is incisional hernia repair and the most common use of synthetic meshes is abdominal (inguinal, incisional, etc.) hernia repairs. Tension-free closures with prosthetic materials in incisional hernia repair reduce the rate of recurrence [6, 7]. The use of prosthetic materials has become almost routine in hernia repair. Meshes derived from many different materials have been safely placed on the fascia for a very long time to strengthen the abdominal wall [8]. The ideal synthetic mesh would integrate into the abdominal wall, thereby strengthening the repaired area, but this strong integrating property becomes a real problem in hernias where it is impossible to join the fascial edges and laparoscopic repairs. Foreign bodies in contact with visceral surfaces are important factors in the formation of intra-abdominal adhesions [9]. Especially when implanted intraperitoneally, these materials cause adhesions between the intestines and the mesh [10]. These adhesions can cause serious complications such as intestinal obstruction or enterocutaneous fistula formation [11]. The number and activity of fibroblasts, and accordingly, excessive and irregular collagen deposition, are responsible for the development of the adhesions associated with intra-abdominal prosthetic materials and those that develop after many surgeries [12].
5-Fluorouracil (5-FU) has been the cornerstone of adjuvant therapy for colorectal cancer for a long time. Moreover, it has been used to prevent fibrosis in glaucoma surgery and adhesions in tendon repair [13–15]. Administering 5-FU when inflammation is intense and fibroblast activity is high keeps inflammation and fibroblast activity at bay for long enough to prevent excessive collagen synthesis and fibrosis development. Chitosan is a natural aminopolysaccharide formed by the deacetylation of chitin, the primary component of crustaceans’ shells. Its high biodegradability and low toxicity render chitosan suitable for biomedical and pharmacological applications. Chitosan is clinically relevant due to its hemostatic, anti-inflammatory, antimicrobial, and wound healing effects [16].
The inflammatory reaction against intra-abdominal prosthetic graft materials is a time-dependent, continuous foreign body response. Yet, therapeutic agents applied around the graft are effective only once and for a limited period of time and, therefore, are unable to suppress hyper-reactive fibroblasts for the entire course of the reaction. The slow release of an agent into the environment maintains its effect during the inflammatory process, fibroblast activity, and therefore collagen synthesis. In wound healing, inflammation and fibroblast activity are, ideally, suppressed for as long as possible to minimize adhesions. Using an experimental animal model of incisional hernia, this study aimed to investigate the effect of 5-FU-loaded chitosan gel-coated, prosthetic materials on intra-abdominal post-operative adhesions.
MATERIALS AND METHODS
Mesh Preparation
“Ethicon Vicryl Woven Mesh” brand polyglactin 910 mesh and “Ethicon Prolene Mesh” brand polypropylene mesh were cut in 2x2 cm dimensions. In our study, Chitosan H (molecular weight: 1400 kDa, deacetylation degree 80%; MGF Co. Ltd. – Japan) was used as a natural polymeric carrier system due to its cationic charge, non-toxicity, and biological compatibility. Chitosan gel was prepared at 2% (w/v) concentration. Chitosan was mixed with 0.1 M acetate buffer (pH: 4.5) and incubated overnight at room temperature. Chitosan gel obtained as a result of incubation was used to coat 4 cm2 meshes. The upper and lower layers of the meshes were covered with 0.5 mL chitosan gel using a mold. The coated meshes were frozen at -80 °C for 24 h. Then, the molds covered with chitosan gel were lyophilized by keeping them in the lyophilizer for 48 h. For this purpose, “Virtis Gardiner NY12525” brand lyophilizer device was used. After lyophilization, 5-FU (1 mg) was impregnated into the upper and lower layers of the mold. The prepared meshes were sterilized by exposing a total of 24 kgy gamma rays over 16 h in sterile Petri dishes at the Turkish Atomic Energy Agency facility.
Experimental Model and Surgical Protocol
With the approval of the Hacettepe University Experimental Animals Ethics Committee (no: 2004/21), 84 male Wistar albino rats weighing 200–250 g were housed under the standard conditions of the Hacettepe University Experimental Animals Unit. The animals were fed standard rat chow and water.
The protocol and experimental groups are summarized in Table 1. The prepared mesh materials were placed in a 2×2 cm musculofascial defect created on the anterior abdominal wall of the experimental animals under sterile conditions with general anesthesia (intramuscularly administered 90 mg/kg ketamine-10 mg/kg xylazine). The meshes were fixed to the anterior abdominal wall with 5.0 polypropylene sutures. The skin was closed with a 4.0 polypropylene suture and post-operative analgesia was provided. All procedures were performed by two surgeons (Balas and Erol). Half of the rats in each group were sacrificed on day 7 and a biopsy was taken from the anterior abdominal wall that included the prosthetic material. The remaining subjects were sacrificed on day 30, at which point the same samples were collected. Adhesions formed between the graft and the intra-abdominal organs were evaluated by a single-blinded surgeon (Akyollu) using the adhesion scoring method [17] (Table 2).
TABLE 1.
Experimental groups
| Groups | |
|---|---|
| 1 | Polypropylene control group. 12 animals |
| 2 | Polyglactin 910 control group. 12 animals |
| 3 | Chi gel group 12 animals |
| 4 | Polypropylene+Chi gel+ 5-FU group. 12 animals |
| 5 | Polyglactin 910+Chi gel+ 5-FU group 12 animals |
| 6 | Polypropylene+Chi gel group 12 animals |
| 7 | Polyglactin 910+Chi gel group 12 animals |
TABLE 2.
Adhesion scoring
| Degree of adhesion | |
|---|---|
| No adhesion | 0 |
| Single band adhesion (between organs or | |
| between abdominal wall and organs) | 1 |
| Two band adhesion (between organs or | |
| between abdominal wall and organs) | 2 |
| Adhesion with two or more bands (between organs | |
| or between the abdominal wall, organ or all intestines) | 3 |
| Adhesion of organs directly to the abdominal wall | |
| independent from number | 4 |
Histological Evaluation
Tissue samples were fixed at room temperature in 10% formalin prepared in phosphate buffer adjusted to pH 7.0. Sections were stained with hematoxylin-eosin and the modified Masson Trichrome method after which general tissue morphology, adhesion, and tissue-biomaterial response were evaluated. At least 10 sections from different levels in each tissue sample were analyzed. After histological findings were evaluated, they were evaluated for different structural categories. For this purpose, a semi-quantitative scoring system was used [18] (Table 3).
TABLE 3.
Histological scoring system includes the light microscopic features of the capsule and surrounding tissues surrounding the biomaterial
| Criteria | Scores | |||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 | |
| Capsule placement | Capsule on both sides | Capsule on the bottom | Capsule on the upper dermis side | No capsule |
| Structure of the capsule | Tight | Loose fibroadipose or loose adipose | Loose fibroelastic | No capsule |
| Cellular properties of the capsule | ||||
| Fibroblast thickness | More than 30 times | 30–10 times | 10–0 times | 0 times |
| Fibroblast contact surface | No | Yes | ||
| Degree of fibrosis | Severe | Moderate | Mild | None |
| Presence of acute/chronic inflammation | Chronic | Acute | ||
| Degree of inflammation | Severe (many lymphocytes, giant cells and plasma cells, microabscesses in addition to neutrophils) | Moderate (moderate lymphocytes, plasma cells, and neutrophils in addition to giant cells) | Mild (few giant cells lymphocytes and plasma cells) | None |
| Location of inflammatory cells | ||||
| Location of inflammatory cells | Outside and inside | Inside-outside | Outside | None |
| Contact surface of macrophages | No | Yes | ||
| Contact surface of giant cells | No | Yes | ||
| Contact surface of polymorphonuclear leukocytes | No | Yes | ||
| Contact surface of plasma cells | No | Yes | ||
| Presence of blood vessels | No | Yes | ||
| Tissues around the capsule | ||||
| Presence of acute/chronic inflammation | Chronic | Acute | ||
| Degree of inflammation | Severe (many lymphocytes, giant cells and plasma cells, microabscesses in addition to neutrophils) | Moderate (moderate lymphocytes, plasma cells, and neutrophils in addition to giant cells) | Mild (few giant cells lymphocytes and plasma cells) | None |
| Degree of fibrosis | Severe | Moderate | Mild | None |
| Macrophages | None | Yes | ||
| Giant cells | None | Yes | ||
| Polymorphonuclear leukocytes | None | Yes | ||
| Plasma cells | None | Yes | ||
| Presence of blood vessels | None | Yes | ||
Tissue samples were fixed in 2.5% glutaraldehyde in Sorenson’s phosphate buffer overnight at room temperature, washed in a buffer solution, and fixed for the 2nd time in phosphate-buffered 1% osmium tetroxide at 4 °C for 2 h. Tissue samples were dehydrated by passing through graded ethyl alcohol solutions and vacuum embedded in Araldite (Cy 212, Agar, Germany). After staining the sections with uranyl acetate-lead citrate, they were imaged using a Carl Zeiss transmission electron microscope (TEM) for subcellular examination of the tissue around the biomaterial.
Statistical Analysis
The continuous variables obtained from histological and macroscopic scoring were expressed as medians (min-max); categorical data were denoted as numbers and percentages. Normality analyses were performed using the Shapiro–Wilk goodness-of-fit test. The Kruskal–Wallis test (Mann–Whitney U-test with Bonferroni correction) was used to analyze the differences between groups as the continuous data were not normally distributed. The Wilcoxon ordered signs test was used for in. The categorical data were compared with the Chi-square test. All analyses were performed with IBM SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). Statistical significance was set as p<0.05 for the group analyses.
RESULTS
During the experiment, one rat in the polypropylene control group and two rats in the polyglactin 910 control group died on days 14, 15, and 17 for reasons deemed unrelated to the experiment, and, therefore, were excluded from the study. While there was no significant difference between the groups in terms of the median values of macroscopic adhesion scores on day 7 (p=0.195), there was a significant difference between the groups on day 30 (p=0.022). Adhesion scores [0.5 (0–1)] in the polypropylene+Chi gel+ 5-FU group were found to be statistically significantly lower than in the polypropylene control group [2 (1–3)] (p=0.020) (Table 4). The median values of day 30 adhesion scores were lower in the 5-FU added groups and the laparotomy Chi gel group compared with day 7 values. It was significantly lower in the polyglactin 910+Chi gel group (p=0.046), but not the polypropylene+Chi gel+5-FU and polypropylene+Chi gel groups, despite being very close to significance (p=0.063 and p=0.083, respectively).
TABLE 4.
Comparison of adhesion scores between and within groups
| n | 7th day [Median (min–max)] | 30th day [Median (min–max)] | p1 | |
|---|---|---|---|---|
| Polypropylene+Chi gel+5-FU | 6 | 1.5 (1–3) | 0.5 (0–1)a | 0.063* |
| Polypropylene+ Chi gel | 6 | 1.5 (1–2) | 1 (1–1) | 0.083* |
| Polyglactin910+Chi gel+5-FU | 6 | 1.5 (0–3) | 1 (0–2) | 0.334* |
| Polyglactin 910+ Chi gel | 6 | 1.5 (1–2) | 1 (0–1) | 0.046* |
| Laparotomy+Chi gel | 6 | 1.5 (0–3) | 0.5 (0–1) | 0.131* |
| Polypropylene | 5 | 1.5 (1–4) | 2 (1–3)a | 1.000* |
| Polyglactin 910 | 4 | 0.5 (0–1) | 1 (1–2) | 0.102* |
| p2=0.195** | p2=0.022** |
Min: Minimum; Max: Maximum; *: Wilcoxon sequential signs test; **: Kruskal–Wallis test (Mann–Whitney U-test with a: Bonferroni correction).
In general, the median values of the capsule scores on day 30 were lower than day 7 values in the 5-FU applied groups and in all control groups. The difference was significantly lower in polypropylene+Chi gel group (p=0.026), polyglactin 910+Chi gel group (p=0.038), laparotomy Chi gel group (p=0.027), and polyglactin 910 control group (p=0.041). The polypropylene+ Chi gel+5-FU and polyglactin 910+ Chi gel+5-FU groups were close to but did not reach statistical significance (p=0.068 and p=0.084, respectively). There was no significant difference between the groups on day 7 and on day 30 (p=0.124 and p=0.257, respectively) (Table 5).
TABLE 5.
Comparison of capsule scores between and within groups
| n | 7th day [Median (min–max)] | 30th day [Median (min–max)] | p1 | |
|---|---|---|---|---|
| Polypropylene+Chi gel+5-FU | 6 | 21 (20–23) | 20 (18–20) | 0.068* |
| Polypropylene+Chi gel | 6 | 22 (19–24) | 20 (18–21) | 0.026* |
| Polyglactin910+ Chi gel+5-FU | 6 | 21 (19–23) | 20 (18–21) | 0.084* |
| Polyglactin 910+ Chi gel | 6 | 21 (20–23) | 20 (19–21) | 0.038* |
| Laparotomy +Chi gel | 6 | 23 (20–24) | 20 (18–22) | 0.027* |
| Polypropylene | 5 | 24 (21–25) | 22 (20–23) | 0.041* |
| Polyglactin 910 | 4 | 21.5 (19–22) | 20 (20–22) | 0.655* |
| p2=0.124** | p2=0.257** |
Min: Minimum; Max: Maximum; *: Wilcoxon sequential signs test; **: Kruskal–Wallis test (Mann–Whitney U-test with a Bonferroni correction).
The median values of the capsule surroundings scores on day 30 were lower than day 7 values in the treated groups and all of the control groups. The difference was in the polypropylene+ Chi gel+5-FU group (p=0.023), the polypropylene+ Chi gel group (p=0.025), the polyglactin 910+ Chi gel group (p=0.020), and the laparotomy+ Chi gel control group (p=0.020) was found to be significantly lower, and in the polyglactin 910 control group, although it was not significant, it was very close to significance (p=0.059). There was no significant difference between the groups on day 7 and day 30 (p=0.099 and p=0.218), respectively (Table 6, Fig. 1).
TABLE 6.
Comparison of capsule surroundings scores between and within groups
| n | 7th day [Median (min–max)] | 30th day [Median (min–max)] | p1 | |
|---|---|---|---|---|
| Polypropylene+Chi gel+5-FU | 6 | 10 (9–10) | 8.5 (7–9) | 0.023* |
| Polypropylene+Chi gel | 6 | 9.5 (8–11) | 8.5 (8–10) | 0.025* |
| Polyglactin 910 + Chi gel+5-FU | 6 | 9 (7–10) | 8.5 (8–9) | 0.480* |
| Polyglactin 910 Chi gel | 6 | 9 (9–10) | 8 (7–9) | 0.020* |
| Laparotomy + Chi gel | 6 | 10 (10–11) | 9 (9–10) | 0.020* |
| Polypropylene | 5 | 10 (9–12) | 9 (8–11) | 0.157* |
| Polyglactin 910 | 4 | 9.5 (9–11) | 8.5 (8–10) | 0.059* |
| P2=0.099** | P2=0.218** |
Min: Minimum; Max: Maximum; *: Wilcoxon sequential signs test; **: Kruskal–Wallis test (Mann–Whitney U-test with a Bonferroni correction).
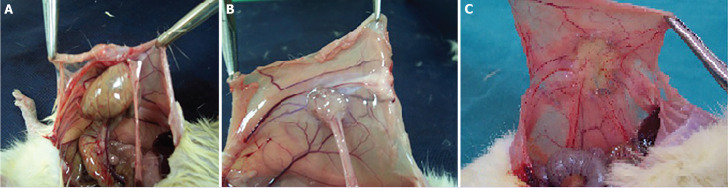
FIGURE 1.
(A) Polypropylene 30 days Grade 4 adhesion, (B) polyglactin 910 7th day Grade 1 adhesion, and (C) polyglactin 910 30 days Grade 0 adhesion.
Light Microscopy Findings
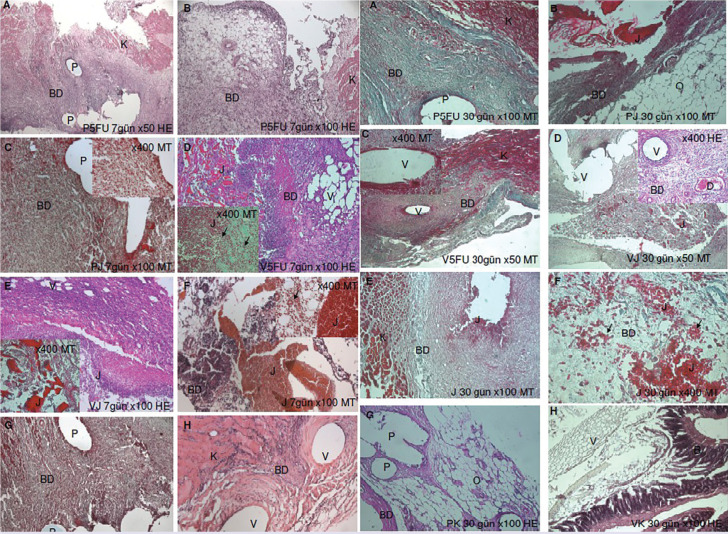
In all of the groups, the restructuring process of the abdominal wall in the defect area; various stages of repair were observed, accompanied by the integration of the biomaterials with the tissue. During the follow-up period, we observed that the defect area was filled with fibrous tissue in various amounts in all groups (Fig. 2). Comparing the drug-treated groups with their untreated counterparts revealed that the tissue around the capsule was remodeled faster in these groups. On day 7, the periphery of the polypropylene or polyglactin 910 mesh material was observed as surrounded by a medium thickness fibrous capsule. In this period, the thickness of the capsule was observed to be slightly reduced in the drug-treated groups than in the non-administered groups. Subacute inflammation findings were observed on day 7. In this period, macrophages, lymphocytes, polymorphonuclear leukocytes, giant cells, and vessels were observed around the meshes. In the groups with polypropylene and polyglactin 910 but no gel, the connective tissue forming the capsule was relatively richer in collagen fibrils with fewer cells and vessels. At this point, the capsule was thicker on the outside than on the side facing the peritoneum (Fig. 2).
FIGURE 2.
Light microscopy images of the 7th and 30th days.
HE: Hematoxylin and eosin; MT: Mason trichrome; P: Polyprolene; V: Polyglactin; J: Chitosan gel; BD: Connective tissue; O: Omentum; K: Abdominal muscle.
On day 30, subacute inflammation was replaced by chronic inflammation findings. The inflammatory cells began to decrease and were increasingly replaced by fibroblasts and collagen fibrils, while lymphocytes and phagocytic cells continued to colocalize with the vessels (Fig. 2). On day 30, drug-treated and untreated polypropylene and polyglactin 910 meshes were observed in close association with the surrounding tissue. However, the progression of connective tissue cells into the polymers was still not evident (Fig. 2). Only in the areas where the chitosan gel had dissolved, the separated chitosan particles mixed with the connective tissue cells and localized to the fibroblasts and macrophages (Fig. 2). During the following period, the polyglactin 910 and polypropylene meshes continued to exist in the tissue, surrounded by fibrous capsules of varying thickness, without degrading as expected. The fibrous capsule was observed as a vascular-rich structure mostly consisting of spindle-shaped fibroblasts and phagocytic cells surrounding the meshes. The capsule in the polypropylene and Vicryl control groups was relatively more fibrous and poor in vessels compared with the other groups. In the gel group, tissue remodeling around the capsule was slightly slower (Fig. 2). No necrotic foci were found at any stage. On day 30, the thickness of the fibrous tissue surrounding the polymers decreased in the drug-treated groups. The capsule structures surrounding the drug-loaded and non-drug-loaded gel meshes were surrounded by fibroelastic loose adipose connective tissues. The surrounding tissue in the drug-free groups was tighter and fibrous than the drug-treated groups. The surrounding connective tissue was attached to the peritoneal sheaths of the abdominal muscles externally and the omental components internally. Based on all these findings, the applied polymer meshes were considered to be moderately compatible with the tissue, especially when combined with drug-loaded gels. The structures participating in the adhesion were generally detected as omentum and sometimes intestines in histological sections (Fig. 2).
Electron Microscopy Findings
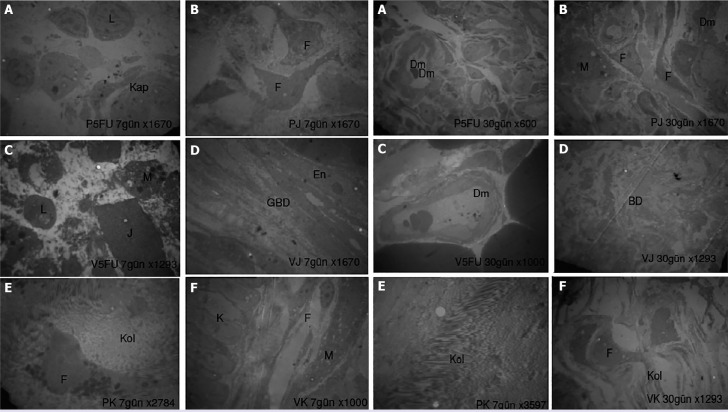
The connective tissues surrounding the biomaterials were examined at the structural level using TEM level. On day 7, in the polypropylene +Chi gel +5FU and polyglactin 910+chigel+5FU groups, vessels were restructured in the connective tissue rich in cells forming the capsule (Fig. 3). On day 30, collagen production accelerated in all groups, and accordingly, the surrounding tissue gained a highly fibrous structure (Fig. 3).
FIGURE 3.
Electron microscopic images of the 7th and 30th days.
Uranyl acetate lead citrate, Cap: Capillary; En: Endothelial cell; Dm: Vessel; F: Fibroblast; L: Lymphocyte; M: Macrophage; GBD: Loose connective tissue; PK: Polypropylene control; VK: Polyglactin 910 control; J: Chitosan gel; P5FU: Polypropylene 5-fluorouracil; V5FU: Polyglactin 910 5-fluorouracil; PJ: Polypropylene gel; VJ: Polyglactin 910 gel. Uranyl acetate lead citrate.
DISCUSSION
Incisional hernia repair is one of the most common complication surgeries in daily surgical practice. Dietz et al. [19], in their meta-analysis with incisional hernia repair, stated that incisional hernia develops at a rate of 4–10% depending on the type of primary surgery, in the same study, they stated that these rates would be higher when the follow-up period was extended, and because many patients were asymptomatic or attributed their symptoms to previous surgeries, they did not realize the development of hernia.
The basic principle of hernia surgery is to return the herniated organs and tissues to their place, then to close the abdominal wall area where the hernia develops and to strengthen it simultaneously. Repair is possible by primary suturing the defect or strengthening with synthetic materials. To increase the effectiveness of surgical treatment, primary repair has been replaced by synthetic meshes with the evolution of treatment methods. In their meta-analysis, Lopez et al. [20] stated that the use of meshes in incisional hernia repair reduced the recurrence rate by 61%.
Non-toxicity, mechanical durability, chemical stability, and full integration into the tissue to be strengthened are the ideal properties of the graft.
In endoscopic and robotic methods and intra-abdominal applications, foreign body reactions develop between the mesh and intra-abdominal organs, resulting in severe adhesions, fistulas, and perforations [11]. For this purpose, there are many efforts to develop meshes for use in intra-abdominal repairs. This study was planned to control the foreign body reaction, capsule, and adhesion formation caused by the graft materials used in the experimental incisional hernia model.
The foreign body reaction begins with the contact of the material with the tissue. With the placement of the material, bleeding and edema develop in the area. In edema fluid, plasma proteins, blood components, albumin, fibrinogen, fibronectin, vitronectin complement, and other immunomodulatory proteins interact with the surface of the body. This interaction activates neutrophils. After cytokines are released from neutrophils, monocytes and macrophages migrate to the region. The process is guided by pro- and anti-inflammatory cytokines released into the environment. Fibroblasts are then activated. Tissue is regulated by releasing collagen and extracellular matrix from fibroblasts. If collagen secretion is uncontrolled, the healing process progresses to capsule formation and the foreign body is surrounded by the tissue and a capsule is formed [21]. This process may render the implanted material ineffective. The benefit of Prolene graft in hernia surgery is its ability to merge with the surgically repaired tissues over time, and its protective and reinforcing feature created by fibrosis caused by inflammation in the repaired defect area. While the adhesion and inflammation created on the tissue surface where the graft is fixed, is a desired, and expected effect that contributes to its healing mechanism, the adhesions it creates in the peritoneal region pose a risk.
There are many studies in the literature that use 5-FU, an antimetabolite, to prevent adhesion development, capsule formation, and hypertrophic scarring. Duci et al. [22] showed that a single application of topical 5-FU is effective in preventing adhesions that develop after tendon repair. In their systematic review, Nazifi et al. [15] supervised the use of 5-FU for the prevention of adhesions developing after tendon repairs and found that 5-FU application after tendon repair has a positive effect on adhesion development. In only one of the studies examined, 5-FU administration was performed with a slow release model. In their prospective clinical studies, Nuseir et al. [23] applied topical 5-FU to patients who underwent inferior turbinoplasty at once, and they found that intranasal adhesions developed much less in the group that was administered 5-FU. In the treatment of keloid, the application of 5-FU is one of the standard methods. Fitzpatrick et al. [24] in their study in which they presented their 9-year experience, they stated that 5-FU application is effective in the treatment of keloid, but it requires sequential applications. Park et al. [25] tried experimental keloid treatment with microneedles by loading chitosan nanoparticles with 5-FU. They reported that 5-FU-loaded nanoparticles reduced the proliferation of human keloid fibroblast cells by 41%. They stated that TGF-β decreased independently of the chitosan concentration, while 5-FU-loaded chitosan particles increased this decrease significantly. Wu et al. [26] loaded 5-FU and mitomycin on chitosan membranes to determine the efficacy and safety of both drugs in glaucoma infiltration surgery. They found that mitomycin and 5-FU carrier membranes were effective against the fibrotic process that developed after glaucoma infiltration surgery. In their experimental study, Canter et al. [27] controlled the effect of slow release 5–FU on capsule formation surrounding silicone breast implants by coating the silicon pieces with gelatin loaded with 5-FU. They found that the rate of capsule development in the drug-loaded group was the lowest.
In our study, we checked the inhibitory effectiveness of 5-FU in capsule development and adhesion formation at the fibroblast proliferation stage. In accordance with the above-mentioned literature for the immediate release or long-acting drug, we have added it to a delivery system model. One of the important limitations of our study is that the release time of 5-FU added on the gels was not determined. Therefore, no comment can be made on the duration of action of 5-FU.
In this experimental incisional hernia model, we used chitosan gel as the covering material of the grafts we used for repair. In their experimental intra-abdominal adhesion model, Ren et al. [28] controlled adhesion levels by covering incisions into the rabbit uterus with chitosan and obtained lower levels of adhesion, interorgan bands, and adhesion type. Li et al. [29] inspected electrospun fiber membranes as an adhesion barrier in their experimental intra-abdominal adhesion model. They used polyethylene glycol-poly-β-hydroxybutyrate valerate, polylactic acid, and chitosan-polyethylene glycol, and reported that they significantly reduced the amount and degree of adhesion in all three electrospun membranes.
In their experimental studies investigating the effects of chitosan-dextran gel on peritoneal adhesions, Lauder et al. [30] showed that gel application after bowel resection reduces the adhesions that develop after the first surgery and prevents new developments in their application after the adhesiolysis performed with a laparoscopic control. They found that chitosan gel has no negative effect on the healing of anastomosis.
In their randomized controlled experimental study, Jayanth et al. [31] compared chitosan-covered polypropylene mesh and Proceed™ a composite graft, in an incisional hernia model, they created in rabbits. They evaluated both meshes in terms of macroscopic adhesion, inflammation, and fibrosis to find that all parameters were similar. They attributed the adhesions evaluated at week 12 being higher in the chitosan group to the decomposition of the chitosan coating and the ensuing exposure of the Prolene mesh. In our study, chitosan gel showed efficacy both as a drug carrier and as an anti-adhesive barrier. Although there was no significant difference between the groups in the macroscopic values on day 7, the macroscopic adhesion levels of the drug-treated and drug-free gel-coated groups were found to be lower grades. In day 30 macroscopic evaluation, a significant difference was found in the polypropylene + Chi gel + 5 FU group. In our study, day 30 was determined as the end of the experiment. In the microscopic evaluations, it was observed that the chitosan gel persisted in the tissue on day 30. Furthermore, evisceration was not observed in the group that was repaired only with chitosan gel. Although no significant difference could be detected in terms of capsule formation, the capsule was observed to be thinner and less fibrotic in the 5-FU applied groups and the control groups to which chitosan gel was added. This finding can be attributed both to the antifibrotic and antiadhesive effects of chitosan gel and to the fact that 5-FU strengthened the antifibrotic and antiadhesive effects in 5-FU-loaded groups.
Conclusions
Intraperitoneal adhesions due to mesh are still a current and important problem in surgical practice. 5-FU released from mesh materials coated with chitosan gel had a positive effect by reducing post-operative intra-abdominal adhesions and capsule formation. Applications for meshes to be placed on peritoneal surfaces, especially in the intra-abdominal region, may be beneficial toward reducing complications that may occur after hernia surgery by preventing intraperitoneal adhesions. Additional studies on dose and release patterns are needed for 5-FU-loaded chitosan gel.
Footnotes
Cite this article as: Balas S, Demir Dora D, Erol T, Akyollu B, Korkusuz P, Hamaloglu E. Effects of 5-fluorouracil released from different prosthetic meshes on post-operative adhesion formation in rats. North Clin Istanb 2022;9(6):565–575.
Ethics Committee Approval
The Hacettepe University Experimental Animals Ethics Committee granted approval for this study (date: 01.04.2004, number: 2004/21).
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure
This work was partially supported by Hacettepe University Research Fund (project no: 05D07101005).
Authorship Contributions
Concept – EH, SB; Design – EH, SB; Supervision – BA, TE; Fundings – SB, PK; Materials – DDD, SB; Data collection and/or processing – BA, TE, PK; Analysis and/or interpretation – SB, EH; Literature review – DDD, SB; Writing – DDD, SB, PK; Critical review – SB, BA, TE.
References
- 1.Brüggmann D, Tchartchian G, Wallwiener M, Münstedt K, Tinneberg HR, Hackethal A. Intra-abdominal adhesions: definition, origin, significance in surgical practice, and treatment options. Dtsch Arztebl Int. 2010;107:769–75. doi: 10.3238/arztebl.2010.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menzies D, Ellis H. Intestinal obstruction from adhesions--how big is the problem? Ann R Coll Surg Engl. 1990;72:60–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Yılmaz KB, Akıncı M, Doğan L, Karaman N, Özaslan C, Atalay C. A prospective evaluation of the risk factors for development of wound dehiscence and incisional hernia. Ulus Cerrahi Derg. 2013;29:25–30. doi: 10.5152/UCD.2013.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin M, Saydam M, Yilmaz KB, Ozturk D, Demir P, Arıkok AT, et al. Comparison of incisional hernia models in rats: an experimental study. Hernia. 2020;24:1275–81. doi: 10.1007/s10029-020-02234-2. [DOI] [PubMed] [Google Scholar]
- 5.Akinci M, Yilmaz KB, Kulah B, Seker GE, Ugurlu C, Kulacoglu H. Association of ventral incisional hernias with comorbid diseases. Chirurgia (Bucur) 2013;108:807–11. [PubMed] [Google Scholar]
- 6.Mathes T, Walgenbach M, Siegel R. Suture versus mesh repair in primary and incisional ventral hernias: a systematic review and meta-analysis. World J Surg. 2016;40:826–35. doi: 10.1007/s00268-015-3311-2. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart K, Dunn D, Teo S, Ng JY, Dhillon M, Teo E, et al. Mesh versus non-mesh for inguinal and femoral hernia repair. Cochrane Database Syst Rev. 2018;9:CD011517. doi: 10.1002/14651858.CD011517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBord JR. The historical development of prosthetics in hernia surgery. Surg Clin North Am. 1998;78:973–1006. doi: 10.1016/S0039-6109(05)70365-0. [DOI] [PubMed] [Google Scholar]
- 9.Luijendijk RW, de Lange DC, Wauters CC, Hop WC, Duron JJ, Pailler JL, et al. Foreign material in postoperative adhesions. Ann Surg. 1996;223:242–8. doi: 10.1097/00000658-199603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baptista ML, Bonsack ME, Felemovicius I, Delaney JP. Abdominal adhesions to prosthetic mesh evaluated by laparoscopy and electron microscopy. J Am Coll Surg. 2000;190:271–80. doi: 10.1016/s1072-7515(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 11.Gavlin A, Kierans AS, Chen J, Song C, Guniganti P, Mazzariol FS. Imaging and treatment of complications of abdominal and pelvic mesh repair. Radiographics. 2020;40:432–53. doi: 10.1148/rg.2020190106. [DOI] [PubMed] [Google Scholar]
- 12.Hu M, Lin X, Huang R, Yang K, Liang Y, Zhang X, et al. Lightweight, highly permeable, biocompatible, and antiadhesive composite meshes for intraperitoneal repairs. Macromol Biosci. 2018;18:e1800067. doi: 10.1002/mabi.201800067. [DOI] [PubMed] [Google Scholar]
- 13.Sabino FD, Campos CF, Caetano CE, Trotte MN, Oliveira AV, Marques RG. Effects of TachoSil and 5-fluorouracil on colonic anastomotic healing. J Surg Res. 2014;192:375–82. doi: 10.1016/j.jss.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 14.Ang BCH, Seen S, Kumaran A, De Leon JMS, Seah SKL, Foster PJ, et al. Visual field progression 8 years after trabeculectomy in Asian eyes: results from The Singapore 5-Fluorouracil Study. Br J Ophthalmol. 2020;104:1690–6. doi: 10.1136/bjophthalmol-2019-314121. [DOI] [PubMed] [Google Scholar]
- 15.Nazifi O, Stuart AL, Nikkhah D. The use of 5-fluorouracil in the prevention of tendon adhesions: A systematic review. Animal Model Exp Med. 2020;3:87–92. doi: 10.1002/ame2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaş HS. Chitosan: properties, preparations and application to microparticulate systems. J Microencapsul. 1997;14:689–711. doi: 10.3109/02652049709006820. [DOI] [PubMed] [Google Scholar]
- 17.de la Portilla F, Ynfante I, Bejarano D, Conde J, Fernández A, Ortega JM, et al. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: an experimental study in rats. Dis Colon Rectum. 2004;47:2157–61. doi: 10.1007/s10350-004-0741-6. [DOI] [PubMed] [Google Scholar]
- 18.Bölgen N, Vargel I, Korkusuz P, Menceloğlu YZ, Pişkin E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J Biomed Mater Res B Appl Biomater. 2007;81:530–43. doi: 10.1002/jbm.b.30694. [DOI] [PubMed] [Google Scholar]
- 19.Dietz UA, Menzel S, Lock J, Wiegering A. The treatment of incisional hernia. Dtsch Arztebl Int 2018;115:31-7. Erratum. Dtsch Arztebl Int. 2018;115:98. doi: 10.3238/arztebl.2018.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Cano M, Martin-Dominguez LA, Pereira JA, Armengol-Carrasco M, García-Alamino JM. Balancing mesh-related complications and benefits in primary ventral and incisional hernia surgery. A meta-analysis and trial sequential analysis. PLoS One. 2018;13:e0197813. doi: 10.1371/journal.pone.0197813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kämmerling K, Fisher LE, Antmen E, Simsek GM, Rostam HM, Vrana NE, et al. Mitigating the foreign body response through ‘immune-instructive’ biomaterials. Journal of Immunology and Regenerative Medicine. 2021;12:100040. [Google Scholar]
- 22.Duci SB, Arifi HM, Ahmeti HR, Manxhuka-Kerliu S, Neziri B, Mekaj AY, et al. Biomechanical and macroscopic evaluations of the effects of 5-fluorouracil on partially divided flexor tendon injuries in rabbits. Chin Med J (Engl) 2015;128:1655–61. doi: 10.4103/0366-6999.158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuseir AF, Alsalem M, Alzahr M, Ababneh M, Alomari AI, Alzoubi F. The effect of topical 5-flurouracil application post endoscopic inferior turbinoplasty. Am J Otolaryngol. 2017;38:135–8. doi: 10.1016/j.amjoto.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick RE. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg. 1999;25:224–32. doi: 10.1046/j.1524-4725.1999.08165.x. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Kim YC. Topical delivery of 5-fluorouracil-loaded carboxymethyl chitosan nanoparticles using microneedles for keloid treatment. Drug Deliv Transl Res. 2021;11:205–13. doi: 10.1007/s13346-020-00781-w. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Li S, Wang N, Liu W, Liu W. A comparative study of the safety and efficacy effect of 5-fluorouracil or mitomycin C mounted biological delivery membranes in a rabbit model of glaucoma filtration surgery. Clin Ophthalmol. 2013;7:655–62. doi: 10.2147/OPTH.S34200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim Canter H, Konas E, Bozdogan O, Vargel I, Ozbatir B, Oner F, et al. Effect of slow-release 5-Fluorouracil on capsule formation around silicone breast implants: an experimental study with mice. Aesthetic Plast Surg. 2007;31:674–9. doi: 10.1007/s00266-006-0172-y. [DOI] [PubMed] [Google Scholar]
- 28.Ren C, Zhao D, Zhu L. Use of N,O-carboxymethyl chitosan to prevent postsurgical adhesions in a rabbit double uterine horn model: a randomized controlled design. Sci China Life Sci. 2016;59:504–9. doi: 10.1007/s11427-016-5019-4. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Ren G, Zhang W. Reduction of abdominal adhesions with elecrospun fiber membranes in rat models. J Invest Surg. 2018;31:210–7. doi: 10.1080/08941939.2017.1310961. [DOI] [PubMed] [Google Scholar]
- 30.Lauder CI, Garcea G, Strickland A, Maddern GJ. Use of a modified chitosan-dextran gel to prevent peritoneal adhesions in a rat model. J Surg Res. 2011;171:877–82. doi: 10.1016/j.jss.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Jayanth ST, Pulimood A, Abraham D, Rajaram A, Paul MJ, Nair A. A randomized controlled experimental study comparing chitosan coated polypropylene mesh and Proceed™ mesh for abdominal wall defect closure. Ann Med Surg (Lond) 2015;4:388–94. doi: 10.1016/j.amsu.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]