Abstract
In this study, we expand on the examination of genetically determined differences in host responses that correlate with clearance of Chlamydia trachomatis from the genital tract. We infected C57BL/6, BALB/c, and C3H/HeN mice with the mouse pneumonitis agent of C. trachomatis (MoPn). C57BL/6 mice had the shortest course of infection (22 days) and the lowest incidence of severe hydrosalpinx. BALB/c mice also had a short course of infection (25 days), but all developed hydrosalpinx. C3H/HeN mice had the longest course of infection (38 days), and all developed severe hydrosalpinx. Determination of local cytokine responses by enzyme-linked immunosorbent assay (ELISA) of genital tract secretions revealed that the levels of the proinflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) were significantly increased in the C57BL/6 and BALB/c strains compared to those in the C3H/HeN strain whereas the level of IL-6 was not different. The level of the neutrophil chemokine macrophage inflammatory protein 2 (MIP-2) was increased during the first week of infection in all three strains but was significantly higher in the BALB/c strain, the strain with the most rapid influx of neutrophils into the genital tract. Prolonged detection of MIP-2 in C3H/HeN mice was associated with a protracted presence of neutrophils in the genital tract. Early increases in the levels of the proinflammatory cytokines TNF-α and IL-1β are associated with earlier eradication of infection in the C57BL/6 and BALB/c strains than in the C3H/HeN strain. Increased levels of MIP-2 and neutrophils in BALB/c and C3H/HeN mice relative to C57BL/6 mice suggest that these responses may contribute to pathology.
Little is known about the pathogenesis of acute human chlamydial genital tract infection, and our knowledge of acute infection has been derived largely from animal models such as the mouse model with the agent of mouse pneumonitis (MoPn), a Chlamydia trachomatis biovar. The resolution of primary C. trachomatis genital tract infection in mice is highly dependent on T-cell-mediated immune responses (4, 14, 19–22), with Th1-type cytokines such as interleukin-12 (IL-12) and gamma interferon (IFN-γ) playing a critical role in controlling and resolving primary chlamydial infection (4, 5, 16). However, the immune response involves the activation of multiple cells and mediators, and an intricate balance is required for the inflammatory response to resolve infection and leave the host unharmed. Through detailed examination of this balancing act, we may begin to distinguish beneficial response profiles from detrimental ones.
It is a consistent observation in animal models that tubal dilatation may be an end result of primary chlamydial infection (9, 15, 26); therefore, at least one mechanism of tubal damage and infertility may be the inflammatory process resulting from an initial chlamydial insult. Genetically controlled differences in susceptibility to primary chlamydial infection have been observed among inbred mouse strains (9, 27, 29). Data reported by de le Maza et al. (9) revealed the development of higher degrees of infertility in C3H/HeN (C3H) and BALB/c mice than in C57BL/6 (C57) mice after intravaginal inoculation of MoPn. We determined that the C57 strain of mice sustained a shorter course of infection and less oviduct pathology than did the C3H strain after primary intravaginal infection with MoPn (7). Differences were seen in local tumor necrosis factor alpha (TNF-α) and neutrophil responses between the two strains, with both being significantly increased in the C57 mice compared to the C3H mice during the first week of infection (7). In a subsequent study, Stagg et al. (24) compared the course of chlamydial genital tract infection in BALB/c and C3H mice and found a prolonged infection in the C3H strain. The higher rate of clearance of chlamydial organisms in the BALB/c strain than in the C3H strain was associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue early in infection (day 7) in the BALB/c mice (24). This report and our prior study (7) suggest that mechanisms of innate immunity are important in determining the course of primary chlamydial genital tract infection.
The acute inflammatory response involves a network of mediators induced through a multistep process. This process is initiated by the release of the early-response cytokines, TNF-α and IL-1, which participate in the up-regulation of adhesion molecules needed for the first step in leukocyte extravasation into tissue. IL-6 is released in response to TNF-α or IL-1, and studies indicate that this cytokine may play a significant role in host defense against multiple infectious organisms (6, 28), although IL-6 knockout mice exhibit only a slight increase in MoPn genital tract infection on days 10 to 20 and no difference in the time to resolution of infection (17). In vitro studies reveal that primary human endocervical epithelial cells release IL-1α after C. trachomatis infection, which induces secretion of the neutrophil-stimulating cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as the chemokine IL-8 (the human equivalent of murine macrophage inflammatory protein 2 [MIP-2]) (23). MIP-2 is known to induce potent chemotaxis of neutrophils (1). MIP-2 and GM-CSF responses, and their relationship to neutrophil influx, have not been previously explored in an in vivo model of chlamydial genital tract disease. In this study, we continue our examination of genetically determined differences in early host responses that correlate with clearance of C. trachomatis from the genital tract and with a reduction in tissue pathology.
MATERIALS AND METHODS
Animals.
Female C57 (H-2b), BALB/c (H-2d), and C3H (H-2k) mice (6 weeks old) were purchased from Harlan-Sprague Dawley (Indianapolis, Ind.). The mice were given food and water ad libitum in an environmentally controlled room with a cycle of 12 h of light and 12 h of darkness. Female mice 7 to 10 weeks of age were used throughout the study.
Infection with chlamydiae and measurement of chlamydial shedding.
The agent of mouse pneumonitis (MoPn), a C. trachomatis biovar, was used for infection. This agent was originally obtained from American Type Culture Collection and is maintained in McCoy cells (18). Mice were infected by placing 30 μl of 250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (SPG) containing 107 inclusion-forming units (IFU) (1,700 50% infective doses for C57, 2,000 50% infective doses for BALB/c, and 1,500 50% infective doses for C3H) of MoPn into the vaginal vault. Infection was performed with the mice under sodium pentobarbital anesthesia. In selected experiments, control mice of each strain were inoculated with SPG. The mice received 2.5 mg of progesterone (Depo-Provera; Upjohn, Kalamazoo, Mich.) subcutaneously 7 days before vaginal infection; the progesterone was given to synchronize all mice in a state of anestrus.
Histopathology.
The mice were sacrificed on day 42 after infection or inoculation of buffer; and the entire genital tract was removed en bloc, fixed in 10% buffered formalin, and embedded in paraffin. Longitudinal 4-μm sections were cut, stained with hematoxylin and eosin, and evaluated by a pathologist blinded to the experimental design. Each anatomic site (exocervix, endocervix, uterine horn, oviduct, and mesosalpinx) was assessed independently for the presence of acute inflammation (neutrophils), chronic inflammation (lymphocytes), plasma cells, and fibrosis. Luminal distention of the uterine horns and dilatation of the oviducts were graded from 1 to 4, with grade 4 representing severe hydrosalpinx. The right and left uterine horns and the right and left oviducts were evaluated individually. A four-tiered semiquantitative scoring system were used to quantitate the inflammation and fibrosis: 0, normal; 1+, rare foci (minimal presence) of parameter; 2+, scattered (one to four) aggregates or mild diffuse increase in parameter; 3+, numerous aggregates (more than four) or moderate diffuse or confluent areas of parameter; 4+, severe diffuse infiltration or confluence of parameter.
Collection of genital tract secretions for cytokine analysis.
Genital tract secretions were collected from mice on multiple days throughout the course of infection and analyzed by enzyme-linked immunosorbent assay (ELISA) for various cytokines and chemokines of interest. At intervals before and after infection or inoculation of buffer, an aseptic surgical sponge (2 by 5 mm) (DeRoyal ear wicks; Powell, Ind.) was inserted into the vagina of an anesthetized animal and retrieved 30 min later. The sponges were held at −70°C until the day of the cytokine assay. Each sponge was placed in a Spin-X microcentrifuge tube (Fisher Scientific) containing a 0.2 μM cellulose acetate filter and incubated in 300 μl of sterile phosphate-buffered saline (PBS) plus 0.5% bovine serum albumin (BSA) and 0.05 Tween 20 for 1 h on ice and then centrifuged for 5 min. Spin-X filters were first preblocked with 0.5 ml of sterile PBS plus 2% BSA and 0.05% Tween 20 for 30 min at 25°C, centrifuged, and washed twice with 0.05 ml of sterile PBS. Samples were kept on ice and promptly loaded into an ELISA plate prepared for a specific cytokine assay.
Cytokine and chemokine protein assays.
Samples from sponges were assayed individually for cytokine (TNF-α, IL-1β, IL-6, GM-CSF, and MIP-2) activity by ELISA using commercial cytokine ELISA kits (R & D Systems, Minneapolis, Minn.).
Flow cytometry.
Single-cell suspensions (2 × 105 to 4 × 105 cells) were stained in Dulbecco's modified Eagle's medium containing 1% BSA (Sigma) and 0.1% sodium azide (staining buffer) by using the microplate method as previously described (12). Isolated cells were first incubated with rat monoclonal antibody to Ly-6G (clone RB6-8C5 [Pharmingen, San Diego, Calif.]) or with isotype control antibody (rat monoclonal immunoglobulin G2b antibody (A95-1 [Pharmingen]) for 25 min on ice and then washed twice with Dulbecco's modified Eagle's Medium containing 10% BSA. Ly-6G is a cell surface antigen found predominantly on neutrophils (10). The cells were then resuspended in goat anti-rat immunoglobulin G-conjugated fluorescein isothiocyanate (20 μg/ml; Biosource International, Camarillo, Calif.) with 10% autologous mouse serum for 25 min on ice. Following the washing step described above, the cells were fixed in PBS containing 1% paraformaldehyde and kept at 4°C until analyzed.
Flow cytometry was performed on a fluorescence-activated cell sorting analyzer equipped with a 488-nm argon laser and Lysys II software (FACScan; Becton Dickenson). The instrument was calibrated with beads (CaliBRITE; Becton Dickenson) using AutoCOMP software, and the same settings were used throughout the study. Dead cells were excluded on the basis of forward-angle and 90° light scatter, and 10,000 events were analyzed for each sample.
Statistics.
Statistical comparisons between the murine strains for level of infection and cytokine production over the course of infection were made by a two-factor (days and murine strain) analysis of variance with the post hoc Tukey test as a multiple-comparison procedure. The Wilcoxon rank sum test was used to compare the duration of infection in the respective strains over time. The z-test for determination of significant differences in sample proportions was used to compare the frequencies of pathological findings between specific groups. All experiments were repeated two or three times.
RESULTS
Course and outcome of chlamydial genital tract infection in C57, BALB/c, and C3H mice.
In three separate experiments, 10 mice of each of the strains were infected in parallel with 107 IFU of MoPn via intravaginal inoculation. Cervical swab isolations revealed that the rate of resolution of infection varied significantly between the C57 and C3H strains (P = 0.02 by the Wilcoxon rank sum test) and between the BALB/c and C3H strains (P = 0.04). There was no difference in the rate of resolution between the C57 and BALB/c strains (P = 0.72) (data not shown). In the C3H strain, 40% of the mice were still positive on day 25, when the C57 and BALB/c mice had both resolved their infections. The intensity of infection was also significantly increased in the C3H mice over the other two strains (P < 0.001 for C3H versus C57 and for C3H versus BALB/c by two-way analysis of variance). The course of infection was no different in C57 and BALB/c mice.
Histopathologic analysis was performed on genital tract tissues from mice sacrificed 42 days after infection with MoPn. Although there was some degree of oviduct dilation in all of the mice, 24 of 30 oviducts from the C3H group and 14 of 30 from the C57 group had severe oviduct dilatation (P = 0.03 for C57 versus C3H). In the BALB/c mice, 29 of 30 oviducts had severe dilatation or hydrosalpinx (P < 0.005 for C57 versus BALB/c). These data indicate that the C3H strain, with the longest course of infection, and the BALB/c strain, despite its shorter course of infection, are both more susceptible to oviduct pathology than is the C57 strain.
Kinetics of IL-1β, TNF-α, and IL-6 responses in the three strains.
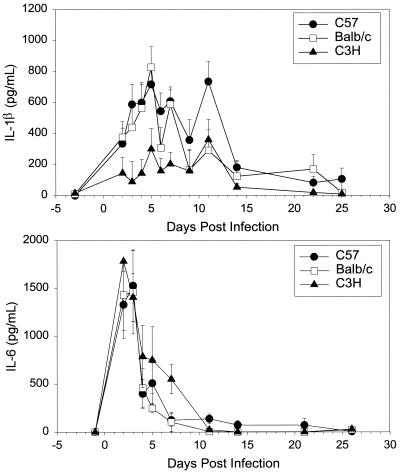
We compared the proinflammatory cytokine IL-1β in the three strains. By day 2 of infection, IL-1β levels were significantly increased over baseline in both the C57 and BALB/c mice (Fig. 1). A peak response was found on day 5 in these two strains of mice, with another peak occurring on day 12 in the C57 strain. Although increases in IL-1β levels occurred in the C3H mice in response to infection, they were significantly lower over the first week of infection than were the responses in the other two strains (P < 0.005 for C57 versus C3H, and P < 0.05 for BALB/c versus C3H). IL-1β was essentially at baseline levels in all three strains by day 14 of infection.
FIG. 1.
IL-1β and IL-6 levels (means and standard errors of the mean) in genital tract secretions of C57, BALB/c, and C3H mice over the course of primary infection with C. trachomatis MoPn. Levels of cytokines were determined with ELISA kits specific for murine IL-1β and IL-6. Data represent the combined results of three separate experiments (n = 5 or 6 per strain per experiment). Each sample was run in duplicate.
TNF-α response patterns paralleled those of IL-6 in the three strains, with C57 and BALB/c mice having significantly higher levels than C3H mice did. In all three strains, the TNF-α response had fallen to baseline by the end of the second week of infection (data not shown).
In contrast to IL-1β and TNF-α, the kinetics of the IL-6 response was very similar in the three strains, with levels in the C3H mice being equal in magnitude to those in the C57 and BALB/c mice (Fig. 1). IL-6 levels increased very rapidly, with extremely high levels being found on days 2 and 3 of infection, and then fell quickly back to baseline by day 7 of infection in C57 and BALB/c mice and by day 11 in C3H mice.
Neutrophil influx into the genital tract.
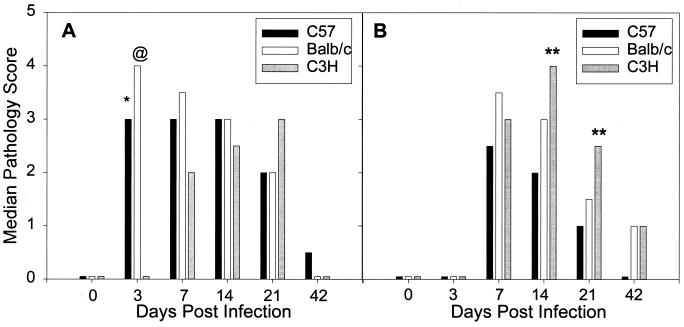
Median pathology scores were determined for the uterine horns, endocervix, and exocervix, as described in Materials and Methods. A rapid increase in the degree of neutrophil infiltration was seen in the uterine horns of C57 and BALB/c mice, in contrast to a notable lack of neutrophils in the C3H strain (Fig. 2A). Interestingly, the greatest influx of neutrophils was seen in the BALB/c strain, with a median pathology score of 4 on day 3 (P < 0.05 for BALB/c versus C57 and P < 0.005 for BALB/c and C57 versus C3H by the Student-Newman-Keuls multiple-comparison procedure). On day 7 of infection, the pathology scores for acute inflammation were still increased in C57 and BALB/c mice compared to C3H mice, although the difference was not statistically significant. Acute inflammation was similar in the three strains on day 14. On day 21, larger numbers of neutrophils were seen in the C3H strain than in the other two strains, although because of variability the difference was again not statistically significant. Scores for the endocervix and exocervix paralleled those for the uterine horns (data not shown).
FIG. 2.
Comparison of the relative degrees of neutrophil infiltrates in the uterine horns (A) and oviducts (B) of C57, BALB/c, and C3H mice over the course of primary infection with C. trachomatis MoPn. Bars represent median pathology scores calculated from 10 mice of each strain sacrificed at each time point. ∗, P < 0.05 for C57 versus C3H; @, P < 0.05 for BALB/c versus C57 and P < 0.005 for BALB/c versus C3H; ∗∗, P < 0.05 for C3H versus C57.
The lag in neutrophil influx into the lower genital tract of C3H mice was confirmed by flow cytometric techniques. A comparison of Ly-6G-positive cells in pooled cervicovaginal tissues from each of the strains on day 3 of infection revealed smaller numbers of neutrophils in the cervicovaginal tissues of the C3H strain than the other two strains, with a significant difference being seen between C3H and BALB/c mice (Ly-6G-positive cells per million genital tract cells = 4.5 × 103 ± 1.5 × 103 in C3H mice, 13.7 × 103 ± 2.7 × 103 in C57 mice, and 38.5 × 103 ± 6.5 × 103 in BALB/c mice, where the values are the means ± standard errors of the means of two independent pools of tissue). There were less than 5.0 × 103 Ly-6G-positive cells per million genital tract cells in tissues from uninfected mice of each strain. This trend for the largest numbers of neutrophils being detected in BALB/c mice and the smallest number being detected in C3H mice continued on day 7, although the differences were not statistically significant (Ly-6G-positive cells per million genital tract cells = 12.2 × 103 ± 12.0 × 103 in C3H mice, 22.2 × 103 ± 13.2 × 103 in C57 mice, and 65.8 × 103 ± 14.8 × 103 in BALB/c mice).
Histopathological grading of the oviducts for acute inflammation also revealed differences among the strains (Fig. 2B). Significant numbers of neutrophils were not observed until day 7, when there were slightly larger numbers in the BALB/c strain. There were increased numbers of neutrophils in the C3H strain on days 14 and 21, with significant differences being found between C3H and C57 mice on days 14 and 21 (P < 0.05 by the multiple-comparison procedure). The numbers of Ly-6G-positive cells determined by flow cytometric analysis of pooled oviduct tissues from each of the strains (data not shown) paralleled the degrees of neutrophil infiltration determined by histology on individual days (Fig. 2B).
By day 42, acute inflammation had resolved in the lower genital tract tissues of all three strains. The C57 strain had the lowest degree of acute inflammation in the oviducts on all days examined (Fig. 2B). The BALB/c strain developed a strong early neutrophil response that tended to resolve more slowly than that in the C57 strain, and acute inflammatory cells were most persistent in the oviducts of the C3H strain (Fig. 2B), the strain with delayed resolution of infection.
Kinetics of MIP-2 and GM-CSF in the three strains of mice.
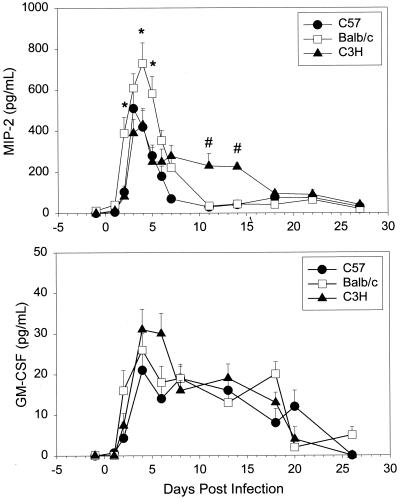
The murine C-X-C chemokine MIP-2 is the putative functional homologue of human IL-8 and is considered to be the principal neutrophil-attracting and -activating chemokine in mice (3, 13, 25). Since differences were determined in the influx and resolution of neutrophils in the genital tract tissues of the three strains, we sought to determine if the kinetics of MIP-2 secretion might be different as well. MIP-2 levels were examined in genital tract secretions prior to and intermittently for 27 days following vaginal inoculation of MoPn (Fig. 3A). The MIP-2 level increased rapidly in all three strains in response to infection, with significant increases over baseline being seen by day 2 in the BALB/c strain and by day 3 in the other two strains. MIP-2 levels were significantly higher in BALB/c mice than in C3H mice on days 2 to 5 and in BALB/c mice than in C57 mice on days 2, 4, and 5. These levels fell by day 7 in BALB/c and C57 mice but remained significantly elevated in C3H mice through day 13.
FIG. 3.
MIP-2 and GM-CSF levels (means and standard errors of the mean) in genital tract secretions of C57, BALB/c, and C3H mice over the course of primary infection with C. trachomatis MoPn. Levels of cytokines were determined with ELISA kits specific for murine MIP-2 and GM-CSF. Data represent the combined results of three separate experiments (n = 5 or 6 per strain per experiment). Each sample was run in duplicate.
The cytokine GM-CSF is known to enhance neutrophil influx and activity. Although increases in GM-CSF levels were found in all three strains in response to infection, there was no difference in the GM-CSF response over time among the strains of mice (Fig. 3B). GM-CSF levels increased rapidly and then remained relatively stable until day 18 in all three strains, when they fell to baseline. Thus, higher secretion of MIP-2 was seen in BALB/c mice during the first few days of infection and neutrophil numbers were increased in the lower genital tract of BALB/c mice relative to the other two strains on days 3 and 7. In addition, increased MIP-2 levels were noted for a longer period in the C3H strain, and this strain had larger numbers of neutrophils than the other two strains during the later times of infection. No differences were seen in the kinetics of the GM-CSF response in the three strains.
DISCUSSION
The study of cytokine networks is difficult due to the redundant nature of the cytokine response as well as the pleiotropic effects that an individual cytokine can exhibit. Through a comparison of the patterns of cytokine responses in three strains of mice that exhibit different courses and outcomes of infection, one can deduce instances of cytokine excess or deficiency which may tip the balance of the inflammatory response from one which results in benign resolution of infection to one which results in chronic tissue pathology. We have determined that the C57 mouse strain is the most resistant to genital tract infection, as evidenced by a short course of infection and less oviduct pathology than for BALB/c and C3H mice. Interestingly, although the BALB/c strain exhibits a course of infection in the genital tract similar to the C57 strain, it develops greater oviduct pathology, equal in severity and frequency to that in the C3H strain. The C3H strain has the longest duration of infection and severe oviduct pathology. Thus, one can deduce that the response profile observed in C57 mice is optimal for primary infection since it not only promotes early eradication of infection but also results in the least oviduct pathology.
In this study we chose to focus on the kinetics of early proinflammatory cytokines, the neutrophil chemokine MIP-2, and the neutrophil response itself. TNF-α and IL-1β levels were both significantly higher in the C57 and BALB/c strains than in the C3H strain during the first 10 days of infection, whereas IL-6 and GM-CSF levels were similar among the three strains. Increased neutrophil influx into the lower genital tracts of C57 and BALB/c mice paralleled the high TNF-α and IL-1β responses of these mice during the first week of infection. However, our previous data on depletion of TNF-α indicate that neutrophil chemotaxis is effectively induced in its absence (8). Moreover, the largest numbers of neutrophils were seen in the lower genital tract of the BALB/c strain during the first week of infection and were associated with significantly increased levels of MIP-2.
The C57 strain had the lowest degree of acute inflammation in the oviducts on all days examined. Similar to the response observed in the lower genital tract, the BALB/c strain developed a strong early neutrophil response in the oviducts that resolved more slowly than that in the C57 strain. Acute inflammatory cells were most persistent in the oviducts of the C3H strain and were associated with increased MIP-2 levels in C3H mice during week 2 of infection. A recent study by Huang et al. (11) found reduced severity of chlamydial pneumonia and no mortality in mice given IL-12 during C. psittaci lung infection. This was associated with reduced MIP-2 levels and neutrophil infiltration into lung tissues of the IL-12-treated mice. In another study with mice immunologically deficient for T cells, infection with MoPn caused severe hydrosalpinx associated with marked neutrophil infiltration (14). High concentrations of neutrophil proteases and reactive oxygen intermediates readily destroy structural proteins and can lead to direct tissue damage. Thus, the increased MIP-2 levels and neutrophil influx in the genital tracts of the BALB/c and C3H mice may promote the increased immunopathology observed in these two strains. However, it is also possible that the enhanced early MIP-2 and neutrophil response observed in BALB/c mice helps this strain to eradicate infection at a rate similar to that in the C57 strain. In a study by Barteneva et al. BALB/c mice given a granulocyte-depleting antibody developed a significantly more intense genital tract infection than did the controls (2).
Other mediators such as IL-12 and IFN-γ are known to be critical to resolution of chlamydial infection, and the kinetics of these responses may well be different in these three strains of mice. Yang et al. (29) described a prolonged duration of C. trachomatis MoPn pneumonia and increased mortality in BALB/c mice compared to C57 mice. Investigation of antigen-specific responses revealed marked differences in the IL-10 and IFN-γ responses between the resistant C57 strain and the more susceptible BALB/c strain, with the BALB/c strain exhibiting higher antigen-specific IL-10 responses and lower delayed-type hypersensitivity (DTH) responses as measured by footpad challenge with antigen. We did not see a prolonged genital infection in BALB/c mice compared to C57 mice. Since macrophages are numerous in the lungs, it is possible that macrophage and DTH responses play a more prominent role in a lung model of chlamydial infection than in the genital tract. Perhaps the robust early MIP-2 and neutrophil responses in the genital tract of BALB/c mice are able to compensate for their relative deficiency in DTH responses compared to those in C57 mice.
In this report, we have described three different patterns of select innate immune responses, each associated with a different consequence from chlamydial infection of the genital tract. Early innate responses not only regulate later adaptive effector responses but also serve to protect the host until an effective adaptive immune response develops. However, if these innate responses are too strong, they can be injurious. The robust neutrophil response seen in the BALB/c strain may help to clear the infection, but it may be too intense to be effective without promoting tissue damage. In contrast, in the C3H strain, delayed and decreased production of innate immune mediators is detrimental. Extrapolating this mouse model to human chlamydial genital tract disease, it is possible that persons who express the innate response patterns seen in either BALB/c or C3H mice will develop increased morbidity from primary chlamydial infection of the genital tract, regardless of later adaptive immunity. Local genital tract production of other cytokine and chemokine mediators must be compared in these three strains of mice to gain a more complete picture of the local response pattern that promotes their specific course and outcome of infection.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI43337.
REFERENCES
- 1.Appelberg R. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J Leukoc Biol. 1992;52:303–306. doi: 10.1002/jlb.52.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Barteneva N, Theodor I, Peterson E M, de la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacalano G, Lee J, Kikly K, Ryan A M, Pitts-Meek S, Hultgren B, Wood W I, Moore M W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 4.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis (MoPn) biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalrymple S E, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darville T, Andrews C W, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darville T, Andrews C W, Rank R G. Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infect Immun. 2000;68:5299–5305. doi: 10.1128/iai.68.9.5299-5305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Maza L, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming J T, Fleming M L, Malek T R. Selective expression of Ly6G on myeloid lineage cells in mouse bone marrow. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 11.Huang J, Wang M D, Lenz S, Gao D, Kaltenboeck B. IL-12 administered during Chlamydia psittaci lung infection in mice confers immediate and long-tem protection and reduces macrophage inflammatory protein-2 level and neutrophil infiltration in lung tissue. J Immuol. 1999;162:2217–2226. [PubMed] [Google Scholar]
- 12.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intraaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Cacalano G, Camerato T, Toy K, Moore M W, Wood W I. Chemokine binding activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 14.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patton D L, Kuo C-C. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85:647–656. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 16.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 17.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey K H, Newhall W J, Rank R G. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect Immun. 1989;57:2441–2446. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey K H, Rank R G. The role of T cell subpopulations in resolution of chlamydial genital infection of mice. In: Bowie W R, Caldwell H D, Jones R P, Mardh P-A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections. New York, N.Y: Cambridge University Press; 1990. pp. 241–244. [Google Scholar]
- 20.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rank R G, Soderberg L S F, Barron A L. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985;48:847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stagg A J, Tuffrey M, Woods C, Wunderink E, Knight S C. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535–3544. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Standiford T J, Strieter R M, Greenberger M J, Kunkel S L. Expression and regulation of chemokines in acute bacterial pneumonia. Biol Signals. 1996;5:203–208. doi: 10.1159/000109191. [DOI] [PubMed] [Google Scholar]
- 26.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol. 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 27.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–38. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 28.Van der Poll T, Keogh C V, Guirao X, Buurman W A, Kopf M, Lowry S F. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Hayglass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]