Abstract
Colistin is a last resort antimicrobial used for the treatment of gram-negative bacterial infections. Plasmid-mediated colistin resistance (mcr) genes are a cause of global concern, and, thus far, mcr-1–10 have been identified. In a previous study, we screened mcr-1–5 in Escherichia coli derived from diseased pigs in Japan and reported a high prevalence of mcr-1, -3 and -5. However, the previous report on the prevalence of mcr genes was inaccurate. In the present study, we aimed to clarify the prevalence of all reported variants of mcr in E. coli derived from the diseased pigs, which were previously screened for mcr-1–5. Additionally, we also characterized the mcr-9-positive E. coli , which was detected in this study. We screened mcr in 120 E. coli strains from diseased pigs and mcr-positive E. coli and an mcr-carrying plasmid were also characterized. One mcr-9-positive colistin-susceptible E. coli strain was detected (0.8 %). Plasmid-mediated mcr-9 was transferred to E. coli ML4909 as the recipient strain, and it was located on IncHI2/HI2A plasmid p387_L with other antimicrobial resistance genes (ARGs). The region harbouring ARGs including mcr-9, was similar to that on the Klebsiella pneumoniae chromosome harbouring mcr-9 isolated in Japan. mcr-3, -5 and -9 were detected (4.2 %) in colistin-susceptible strains. mcr-9 was found to be disseminated via the plasmid IncHI2/HI2A p387_L and transferred and inserted into chromosomes via a transposon. Our results suggest that mcr genes should be monitored regularly, regardless of their susceptibility to colistin.
Keywords: colistin, Escherichia coli, mcr, plasmid
Introduction
The plasmid-mediated colistin resistance (mcr-1) gene was first reported in 2015 and has since been identified in various bacterial species worldwide [1]. Colistin is used as a last resort antimicrobial for the treatment of multidrug-resistant gram-negative bacterial infections in clinical settings for humans [2]. In livestock, colistin has been used as a feed additive and also for the treatment of gram-negative bacterial gastrointestinal infections. mcr genes are frequently detected in swine-pathogenic Escherichia coli [3, 4]. Following reports of the prevalence of mcr genes in livestock, several governments, including Japan, have restricted or banned the usage of colistin in livestock [5].
To date, ten mcr genes (mcr-1–10) have been reported, with the last variant (mcr-10) reported in 2020 [6]. Globally, mcr-1 is the most frequently detected gene in colistin-resistant bacteria, followed by mcr-3 and mcr-5 [5, 7]. This trend has also been observed in Japan [3]. The effect of each mcr variant on colistin resistance is variable, with some mcr variants not conferring resistance to colistin in certain bacterial species and being silently disseminated [2, 8, 9]. However, overexpression of these genes can confer resistance to colistin [10]. Therefore, it is necessary to clarify the prevalence of mcr genes in both colistin-susceptible and colistin-resistant strains.
Following restrictions and bans on colistin usage in livestock, colistin resistance and mcr positivity have been reduced but not eliminated; a stable colistin resistance rate has been maintained [11]. Application of various types of antimicrobials can lead to selective pressure for maintaining mcr genes because of the co-localization of other antimicrobial resistance genes (ARGs) on plasmids harbouring mcr genes [12]. Specific types of mobile elements, including plasmids, are associated with the dissemination of specific ARGs [7]. Therefore, analysis of mcr-carrying plasmids is important to control the dissemination and maintenance of antimicrobial resistance.
We previously surveyed mcr-1–5 in diseased pigs in Japan and reported a high prevalence of mcr-1, -3 and -5 [3]. In the present study, we additionally screened for mcr-6–10 to clarify the prevalence and characteristics of these genes in the previously tested E. coli strains isolated from diseased pigs in Japan through retrospective analysis. We also characterized a newly identified mcr-9-positive E. coli strain.
Methods
Detection of mcr-6–10 in E. coli from diseased pigs
In our previous study, E. coli strains (n=120) were isolated from pigs, inhabiting 40 farms in Japan, with post-weaning diarrhoea. Of these strains, 73 (60.8 %) were resistant to colistin and 75 (62.5 %) harboured mcr-1, -3 and/or -5 (Table 1) [3]. In the present study, we screened for mcr-6–10 in these E. coli strains using PCR, as described previously [13, 14].
Table 1.
Susceptibility to colistin and the presence of mcr genes
|
Mcr gene* |
Colistin |
||
|---|---|---|---|
|
Resistance |
Susceptible |
Reference |
|
|
(n=73) |
(n=47) |
||
|
mcr-9 |
0 |
1 |
this study |
|
mcr-1 |
31 |
0 |
[3] |
|
mcr-3 |
8 |
2 |
|
|
mcr-5 |
27 |
2 |
|
|
mcr-1 and -5 |
5 |
0 |
|
|
nd |
2 |
42 |
*mcr-1–5 were screened in the previous study [3].
mcr-2, -4, -6, -7, -8 and -10 were not detected.
ND, not detected.
Antimicrobial susceptibility testing
MICs were determined for the newly detected mcr-positive E. coli strain Ec387 using the micro broth dilution method according to the Clinical Laboratory Standards Institute (CLSI) guidelines [15]. Antimicrobial susceptibility was tested for the following antimicrobial agents: colistin, ampicillin, cefazolin, cefotaxime, gentamicin, kanamycin, tetracycline, nalidixic acid, ciprofloxacin, chloramphenicol and trimethoprim (all obtained from Sigma-Aldrich, St. Louis, MO, USA). Resistance breakpoints were defined according to the CLSI guidelines for Enterobacterales. E. coli ATCC25922 and Pseudomonas aeruginosa ATCC27853 were used for quality control.
Conjugation experiments
Transferability of the newly detected mcr was tested using filter-mating methods with slight modification [16]. Briefly, the rifampicin-resistant K-12 ML4909 E. coli strain was used as the recipient strain, and mating was conducted at 37 °C. Transconjugants were selected on trypticase soy agar supplemented with 50 mg l−1 rifampicin (Sigma-Aldrich) and 1 mg l−1 colistin, 64 mg l−1 ampicillin, 32 mg l−1 kanamycin, 16 mg l−1 tetracycline, 32 mg l−1 chloramphenicol or 16 mg l−1 trimethoprim. Transconjugants were then tested for susceptibility to antimicrobials and the presence of mcr as described above.
Whole-genome sequencing and subsequent bioinformatics analysis
We performed whole-genome sequencing of the E. coli strain Ec387. Genomic DNA for short-read sequencing was extracted using QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany). The contig was mapped with 150 base pair (bp) paired-end reads obtained using Nextera XT and HiSeq sequencing platforms (Illumina, San Diego, CA, USA). Illumina reads were assembled de novo using spades 3.15.3 (https://github.com/ablab/spades) with default parameters. Genomic and plasmid DNA for long-read sequencing was extracted using Genomic-tip 20 G−1 and Genomic DNA Buffer Set (QIAGEN). The library was prepared using Rapid Barcoding Sequencing kit SQK-RBK004 (Oxford Nanopore Technologies, Oxford, UK) according to the manufacturer’s protocol. All bead washing steps were performed using AMPure XP beads (Beckman Coulter, Brea, CA, USA). Sequencing was performed on MinION with a FLO-MIN-106 R9.4 flow cell (Oxford Nanopore Technologies) using MinKNOW software with a 48 h run time and no alterations to any voltage scripts. Long-read sequencing reads were demultiplexed using Porechop v0.2.4 (https://github.com/rrwick/Porechop), and the reads were adaptor-trimmed and quality-filtered using NanoFilt (Q score, 9; minimum length, 1 000 bp). The reads were error-corrected using short-read sequencing reads with LoRDEC v0.9 software following default parameters [17]. De novo assembly was performed using Flye v2.9 with default parameters using error-corrected long-read sequencing reads [18]. Assembled contigs were error-corrected twice using short-read sequencing reads with Pilon v1.24 following default parameters [19]. The genome and plasmid sequences were annotated using DFAST (https://dfast.nig.ac.jp).
Multilocus sequence typing analysis was performed to determine the sequence type according to the PubMLST protocol and database (https://pubmlst.org/organisms/escherichia-spp). The plasmid replicon type and ARGs were detected using PlasmidFinder v2.1 and ResFinder v4.1, respectively, with default parameters, on the CGE server (http://www.genomicepidemiology.org). Mobile gene elements were detected using blast with the ISfinder database (https://github.com/ thanhleviet/ISfinder-sequences). We compared individual contigs using progressiveMauve [9]. Linear comparison of the sequence alignment was performed using blast and visualised using Easyfig v2.2.2 (https://mjsull.github.io/Easyfig/). A circular representation of the plasmid was visualized and compared using blast Ring Image Generator 0.95 (http://brig.sourceforge.net).
Results
One mcr-9-positive E. coli strain, Ec387, which had no other mcr genes, was detected among the 120 E. coli strains isolated in 2012 from diseased pigs with post-weaning diarrhoea (Table 1), representing a 0.8 % detection rate. However, mcr-6, -7, -8 and -10 were not detected in any of the tested strains. Ec387 was susceptible to colistin (MIC: 1 mg l−1), and resistant to ampicillin, cefazolin, kanamycin, tetracycline, nalidixic acid, ciprofloxacin, chloramphenicol and trimethoprim (Table 2). The mcr-9-harbouring transconjugants were obtained using selective agars supplemented with rifampicin, ampicillin, kanamycin, tetracycline, chloramphenicol or trimethoprim. mcr-9-harbouring transconjugants were not obtained using selective agar supplemented with rifampicin and colistin. All mcr-9-harbouring transconjugants were resistant to ampicillin, kanamycin, tetracycline, chloramphenicol and trimethoprim, but susceptible to colistin (Table 2).
Table 2.
Antimicrobial phenotypes of the mcr-9-harbouring Escherichia coli strain
|
MIC* (mg l−1) |
Donor |
Recipient |
Transconjugant |
|---|---|---|---|
|
Ec387 |
ML4909 |
TC_Ec387 |
|
|
Colistin |
1 |
0.5 |
0.25 |
|
Ampicilin |
>128 |
2 |
>128 |
|
Cefazolin |
8 |
<1 |
4 |
|
Cefotaxime |
<0.5 |
<0.5 |
<0.5 |
|
Gentamicin |
1 |
<0.5 |
1 |
|
Kanamycin |
>64 |
4 |
>64 |
|
Tetracycline |
>16 |
2 |
>16 |
|
Nalidix acid |
>32 |
4 |
4 |
|
Ciprofloxacin |
>4 |
<0.03 |
<0.03 |
|
Chloramphenicol |
>32 |
8 |
>32 |
|
Trimethoprim |
>8 |
0.5 |
>8 |
*Bold type indicates resistant.
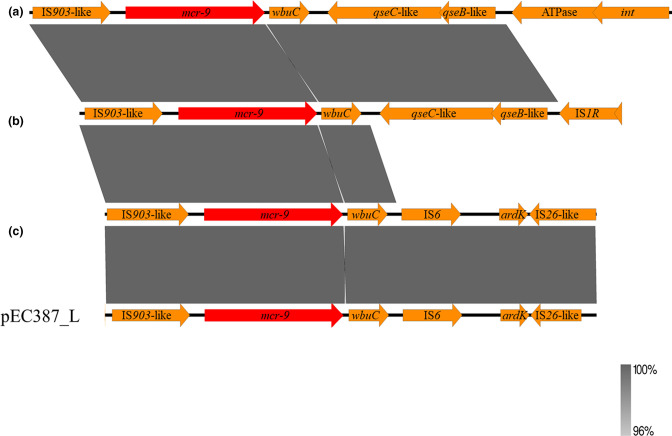
Whole-genome sequencing analysis revealed that Ec387 belonged to ST1196 and carried two plasmids: p387_L and p387_2 [accession no. AP024582 (chromosome), AP024583 (p387_L), and AP024584 (p387_2)]. P387_L was a 29 1362 bp plasmid classified as IncHI2/HI2A, which carried mcr-9, bla TEM-1B, aph(3')-Ia, aph(3'')-Ib, aph(6)-Id, aadA2b, tetD, catA2, floR, sul1, sul2, and dfrA19. p387_2 was an 88 903 bp plasmid classified as IncFIA/K/X1, which carried the bla TEM-1B, aadA1, aadA2, aph(3'')-Ib, aph(6)-Ib, tetM, cmlA, floR, sul3, and dfrA12. The region surrounding mcr-9 in Ec387 was flanked by IS903-like and IS26-like mobile elements, but the qseBC-like element was not observed (Fig. 1).
Fig. 1.
Linear comparison of regions surrounding mcr-9 located in plasmid p387_L with (a) CP031102.1, (b) CP026661.1 and (c) CP020529.1. Red colour indicates antimicrobial resistance genes.
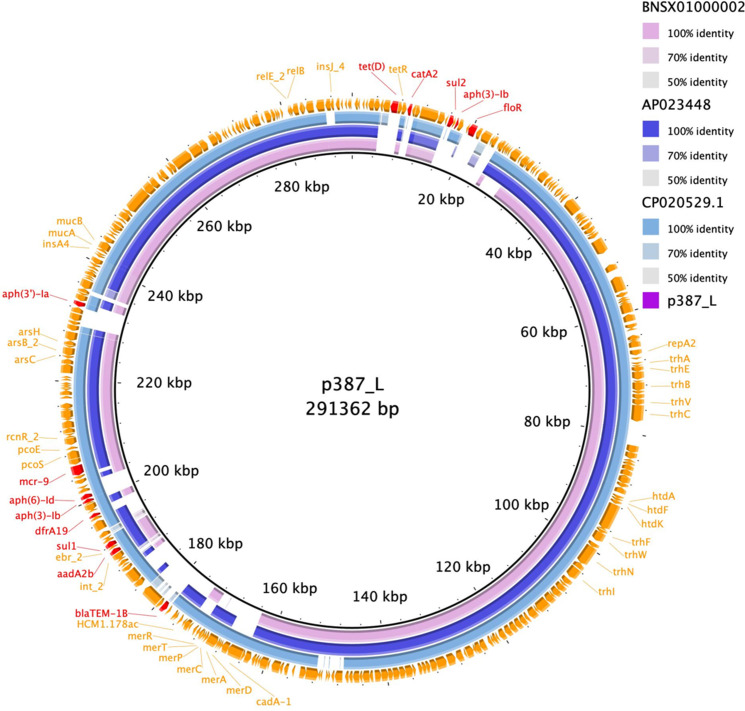
Overall comparison of the plasmids showed that p387_L was highly similar to the InHI2/HI2A plasmid CP020529.1, which was isolated in the USA, and also similar to plasmids isolated in Japan (accession no. BNSX01000002 and AP023448) (Fig. 2). In addition, the p387_plasmid region harbouring ARGs, including mcr-9, was highly similar to that of the Klebsiella pneumoniae chromosome harbouring mcr-9 isolated in Japan (accession no. BNSV01000001) (Fig. S1, available with the online version of this article).
Fig. 2.
Comparison of mcr-9-harbouring IncHI2/HI2A plasmid p387_L with BNSX01000002, AP023448, and CP020529.1. Red colour indicates antimicrobial resistance genes.
Discussion
In the present study, one mcr-9-positive colistin-susceptible E. coli strain (0.8 %) was detected among E. coli strains (n=120) derived from diseased pigs in Japan. Our previous study on these strains demonstrated the presence of the majority of mcr genes (mcr-1, -3 and -5) in colistin-resistant strains, with a small number also detected in colistin-susceptible strains [3]. The expression of mcr-9 can be induced in the presence of colistin when it is located upstream of qseBC, which was not detected in p387_L of strain Ec387 [20]. mcr-9 has been detected worldwide, including in Japan, and a high prevalence of the gene was reported in colistin-susceptible Salmonella isolated from turkeys in the USA [5, 8, 21]. Generally, studies investigating the prevalence of ARGs in isolates first analyse their susceptibility to antimicrobials, and then investigate ARGs in the resistant isolates. Additional resistance mechanisms, including chromosomal mutation related to antimicrobial resistance, were investigated in resistant isolates in which the ARGs were not detected. However, in some cases, important ARGs, such as mcr, can be overlooked if only resistant isolates are analysed, which poses a risk of silent dissemination.
mcr-9-harbouring IncH12/HI2A plasmids also carried other ARGs, which could be transferred under the selection of antimicrobials without colistin. Globally, and in Japan, mcr-9-harbouring IncHI2/HI2A plasmids have been detected in Enterobacterales derived from humans and livestock. In some cases, these plasmids carry extended-spectrum β-lactamase- and carbapenemase-producing genes [9, 21, 22]. These results suggest that mcr-9 can persist even when colistin is not used, through specific plasmids along with various other types of ARGs. Dissemination of these plasmids can propagate multidrug resistance and substantially restrict the choice of antimicrobials for treating bacterial infectious diseases.
Similarly, regions harbouring mcr-9 and several other types of ARGs were previously confirmed in the plasmid p387_L and in the chromosome of K. pneumoniae isolated in Japan (accession no. BNSV01000001) [21]. Moreover, mcr-9 located upstream of qseBC was detected in the chromosome of Enterobacter spp. [21]. These results suggest that mcr-9 is transferred to various bacterial species and inserted into their chromosomes via a transposon.
Conclusions
One mcr-9-harbouring colistin-susceptible E. coli strain was detected among 120 E. coli strains isolated from diseased pigs in Japan in 2012. The mcr-9-harbouring plasmid identified in the present study was similar to the previously reported global dissemination types. Therefore, surveillance of mcr genes, including among colistin-susceptible strains, is essential to accurately determine the prevalence of mcr genes.
Supplementary Data
Funding information
The author(s) received no specific grant from any funding agency.
Author contributions
A.F. and M.U. designed and performed the experiments. H.N. performed the experiments. All authors analysed the data. A.F. and M.U. wrote the manuscript.
Conflicts of interest
The author(s) declare that there are no conflicts of interest.
Footnotes
Abbreviations: ARG, antimicrobial resistance genes; CLSI, Clinical Laboratory Standards Institute; MIC, minimum inhibitory concentration; PCR, polymerase chain reaction.
A supplementary figure is available with the online version of this article.
References
- 1.Nang SC, Li J, Velkov T. The rise and spread of mcr plasmid-mediated polymyxin resistance. Crit Rev Microbiol. 2019;45:131–161. doi: 10.1080/1040841X.2018.1492902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghapour Z, Gholizadeh P, Ganbarov K, Bialvaei AZ, Mahmood SS, et al. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect Drug Resist. 2019;12:965–975. doi: 10.2147/IDR.S199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda A, Sato T, Shinagawa M, Takahashi S, Asai T, et al. High prevalence of mcr-1, mcr-3 and mcr-5 in Escherichia coli derived from diseased pigs in Japan. Int J Antimicrob Agents. 2018;51:163–164. doi: 10.1016/j.ijantimicag.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Rhouma M, Beaudry F, Letellier A. Resistance to colistin: what is the fate for this antibiotic in pig production? Int J Antimicrob Agents. 2016;48:119–126. doi: 10.1016/j.ijantimicag.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Valiakos G, Kapna I. Colistin resistant mcr genes prevalence in livestock animals (swine, bovine, poultry) from a multinational perspective. A systematic review. Vet Sci. 2021;8:265. doi: 10.3390/vetsci8110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Feng Y, Liu L, Wei L, Kang M, et al. Identification of novel mobile colistin resistance gene mcr-10 . Emerg Microbes Infect. 2020;9:508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbediwi M, Li Y, Paudyal N, Pan H, Li X, et al. Global burden of colistin-resistant bacteria: mobilized colistin resistance genes study (1980-2018) Microorganisms. 2019;7:461. doi: 10.3390/microorganisms7100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyson GH, Li C, Hsu C-H, Ayers S, Borenstein S, et al. The mcr-9 gene of Salmonella and E. coli is not associated with colistin resistance in the United States. Antimicrob Agents Chemother. 2020;64:e00573–20. doi: 10.1128/AAC.00573-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macesic N, Blakeway LV, Stewart JD, Hawkey J, Wyres KL, et al. Silent spread of mobile colistin resistance gene mcr-9.1 on IncHI2 “superplasmids” in clinical carbapenem-resistant Enterobacterales. Clin Microbiol Infect. 2021;27:1856. doi: 10.1016/j.cmi.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, et al. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype typhimurium isolate using a combination of high-throughput, in silico screening and functional analysis. MBio. 2019;10:e00853–19. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usui M, Nozawa Y, Fukuda A, Sato T, Yamada M, et al. Decreased colistin resistance and mcr-1 prevalence in pig-derived Escherichia coli in Japan after banning colistin as a feed additive. J Glob Antimicrob Resist. 2021;24:383–386. doi: 10.1016/j.jgar.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Yang R-S, Zhang Q, Feng Y, Fang L-X, et al. Co-transfer of bla NDM-5 and mcr-1 by an IncX3-X4 hybrid plasmid in Escherichia coli . Nat Microbiol. 2016;1:16176. doi: 10.1038/nmicrobiol.2016.176. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Usui M, Harada K, Fukushima Y, Nakajima C, et al. Complete genome sequence of an mcr-10-possessing Enterobacter roggenkampii Strain Isolated from a Dog in Japan. Microbiol Resour Announc. 2021;10:e0042621. doi: 10.1128/MRA.00426-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Zhang Z, Feng Y, Hu H, Yu Y, et al. Molecular detection of the mcr genes by multiplex PCR. Infect Drug Resist. 2020;13:3463. doi: 10.2147/IDR.S256320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standard Institute Performance standards for antimicrobial susceptibility testing. 30th. Wayne, PA: CLSI; edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo H, Usui M, Nagafuji W, Oka K, Takahashi M, et al. Inhibition effect of flavophospholipol on conjugative transfer of the extended-spectrum β-lactamase and vanA genes. J Antibiot (Tokyo) 2019;72:79–85. doi: 10.1038/s41429-018-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmela L, Rivals E. LoRDEC: accurate and efficient long read error correction. Bioinformatics. 2014;30:3506–3514. doi: 10.1093/bioinformatics/btu538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 19.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieffer N, Royer G, Decousser J-W, Bourrel A-S, Palmieri M, et al. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob Agents Chemother. 2019;63:e00965-19. doi: 10.1128/AAC.00965-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura A, Nakamura T, Niki M, Kuchibiro T, Nishi I, et al. Genomic characterization of ESBL- and carbapenemase-positive Enterobacteriaceae Co-harboring mcr-9 in Japan. Front Microbiol. 2021;12:665432. doi: 10.3389/fmicb.2021.665432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umeda K, Nakamura H, Fukuda A, Matsumoto Y, Motooka D, et al. Genomic characterization of clinical Enterobacter roggenkampii co-harbouring blaIMP-1- and blaGES-5-encoding IncP6 and mcr-9-encoding IncHI2 plasmids isolated in Japan. J Glob Antimicrob Resist. 2021;24:220–227. doi: 10.1016/j.jgar.2020.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.