Background:
HVPG measurement is the gold standard for assessing portal hypertension. Many patients decline HVPG measurements due to associated pain. According to previous studies, propofol sedation during HVPG measurements potentially alters HVPG readings. However, opioid analgesics’ effects on HVPG await full elucidation. This study aimed to evaluate fentanyl analgesia’s effects on HVPG measurement accuracy in patients with cirrhosis.
Methods:
This prospective, multicenter study included patients with cirrhosis undergoing HVPG measurements, which were performed preanalgesia and under analgesia with fentanyl injection (1.0 or 1.5 μg/kg).
Results:
Of the 48 enrolled patients with cirrhosis, 23 were administered 1.0 μg/kg fentanyl analgesia during HVPG measurement. The HVPG was 13.4±4.9 mm Hg in preanalgesia and 13.5±5.2 mm Hg under analgesia. HVPG measurement accuracy was not altered after fentanyl analgesia (p = 0.801). The following measures also did not change: heart rate (p = 0.132), mean arterial pressure (p = 0.348), and blood oxygen saturation (p = 0.748); however, respiratory rate (p = 0.001) changes occurred. The Verbal Numerical Rating Score for comfort under analgesia was higher than that in preanalgesia (p = 0.001). Twenty-five patients were administered 1.5 μg/kg fentanyl analgesia during HVPG measurement. The HVPG was 19.5±5.7 mm Hg in preanalgesia and 19.6±5.6 mm Hg under analgesia. HVPG measurement accuracy did not alter after fentanyl analgesia (p = 0.469). Similarly, the following measures did not change: mean arterial pressure (p = 0.871) and oxygen saturation (p = 0.327); nevertheless, respiratory rate (p = 0.015) and heart rate (p = 0.019) changes occurred. The Verbal Numerical Rating Score for comfort under analgesia was higher than that in preanalgesia (p < 0.001).
Conclusion:
Fentanyl analgesia did not alter HVPG measurement accuracy, and fentanyl improved comfort in patients with cirrhosis during HVPG measurements.
INTRODUCTION
Chronic liver disease results from long-term liver injury and liver-tissue wound-healing mechanisms potentially lead to fibrosis, which can gradually progress to cirrhosis.1 Portal hypertension develops as a result of increased intrahepatic vascular resistance and hepatic microcirculatory dysfunction caused by chronic liver disease.2,3 Portal hypertension is a considerably frequent and severe complication of liver cirrhosis and is predominantly responsible for its negative outcomes, including ascites, gastroesophageal variceal bleeding, and encephalopathy. The evaluation of portal hypertension and its complications has always been significant.4,5 The measurement of the HVPG is a recommended method for evaluating the presence and severity of portal hypertension in patients with liver fibrosis.6 An HVPG >5 mm Hg indicates portal hypertension, and that ≥10 mm Hg indicates clinically significant portal hypertension.4 Although it is a technique for minimally adverse clinical complications, HVPG measurement is invasive. Therefore, many patients find it painful, causing them to be reluctant to undergo HVPG measurements.6–8 Sedation or general anesthesia is necessary for patients who are anxious and experience pain during HVPG measurements. Regrettably, previous studies have noted that propofol or propofol in combination with remifentanil sedation during HVPG measurements potentially leads to alterations in HVPG readings.6,7 Moreover, researchers recommend that patients undergo the procedure without sedative agents to obtain accurate HVPG measurements.7 An earlier study was conducted to assess the effects of midazolam on HVPG measurements, and the results indicated that a small dose of midazolam sedation (0.02 mg/kg) does not affect hepatic venous pressure; nevertheless, higher dose midazolam (0.03 mg/kg) was associated with significant changes in hepatic venous pressure.9 According to common knowledge, propofol and benzodiazepine midazolam are both sedatives and possess almost no analgesic properties. In clinical practice, opioids are the predominant anesthetic drugs for almost all types of surgery, and they carry several advantages in the treatment of pain during an invasive procedure: they potentially provide excellent analgesia with minimal hemodynamic fluctuations and can be antagonized in situations where they cause side effects.10 However, the effects of opioid analgesics on HVPG measurements have not yet been elucidated. Therefore, this study aimed to evaluate the effects of fentanyl, a classical yet robust opioid, on the accuracy of HVPG measurements in patients with cirrhosis.
MATERIALS AND METHODS
Patients
This prospective, multicenter study enrolled 48 patients (18 years≤age≤75 years) with liver cirrhosis and suspected sinusoidal portal hypertension undergoing HVPG measurement (American Society of Anesthesiologists class: I–III) between June 2020 and June 2022 (ClinicalTrials.gov ID: NCT04724148). The exclusion criteria were as follows: (1) inability to obtain a reliable HVPG measurement due to vein-to-vein collaterals; (2) PVT; (3) known allergy to fentanyl; (4) unclear etiology of clinical and/or pathological diagnosis; and (5) the presence of autoimmune hepatitis, drug-induced hepatitis, or congenital liver cirrhosis. The study was performed in compliance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the First Hospital of Lanzhou University (approval number: LDYYLL2020-251). The patients who participated in this study provided written informed consent.
Procedure
HVPG measurements were performed by experienced hepatologists according to a standard operating procedure. Each patient’s respiratory rate (RR), blood oxygen saturation (SpO2), heart rate (HR), and mean arterial pressure (MAP) were monitored during the procedure. After disinfection, 1% lidocaine was administered for local anesthesia. A 5.5-Fr catheter introducer (Fogarty 12TLW805F35; Edwards Lifesciences) was inserted into the right jugular vein under ultrasound guidance. After successful insertion, a catheter sheath was placed; it was introduced into the inferior vena cava through the right atrium using a guidewire. The catheter was introduced into the hepatic vein (the middle or right hepatic vein), and hepatic venography was performed to confirm the patency of the vein and absence of an obvious vein-vein collateral shunt. Subsequently, the hepatic vein was selected as the pressure-measuring vessel. Thereafter, free hepatic venous pressure (FHVP) and hepatic venous wedge pressure (WHVP) values were acquired separately according to the guidelines.4 All pressures were recorded in triplicates after a stable value was obtained. HVPG was calculated using the following equation: HVPG = WHVP−FHVP. First, the HVPG measurement was performed in the preanalgesia state. Upon measurement completion, the Verbal Numerical Rating Score (VNRS) for comfort was determined and rated as follows: 0 = no comfort, and 10 = most comfort.11 Then fentanyl was diluted to 10 μg/mL with saline, and 1.0 or 1.5 μg/kg fentanyl was administered intravenously as a bolus injection. Five minutes later, the same patients underwent a second HVPG measurement, and the VNRS for comfort was determined again. Each patient’s RR, SpO2, HR, and MAP were monitored using a GE Dash 4000 multiparameter monitor (GE Medical Systems Information Technologies Inc.) during the procedure in both preanalgesia and under-analgesia states.
Study outcomes
This study’s primary aim was to evaluate the diagnostic accuracy of HVPG measurements with and without fentanyl analgesia. The secondary aim was to assess changes in RR, SpO2, HR, MAP, and VNRS for comfort in both preanalgesia and under-analgesia states during the procedure.
Statistical analysis
Categorical data are expressed as numbers and percentages and quantitative data as the mean±SD, unless otherwise stated. Correlation analyses were performed using the Pearson correlation coefficients. The paired-samples t test was used to compare normally distributed quantitative data. A Bland–Altman plot was constructed for agreement analysis. Statistical analyses were conducted using SPSS 23.0 statistical software, and graphs were drawn using GraphPad Prism 9.0. Statistical significance was set at p value < 0.05.
RESULTS
Patient characteristics
The demographic and baseline clinical characteristics of the 48 patients enrolled in our study are presented in Table 1. Twenty-three and 25 patients were administered 1.0 and 1.5 μg/kg fentanyl analgesia for HVPG measurement, respectively. The main causes of cirrhosis included HBV (79.1%), HCV (8.4%), and alcoholic cirrhosis (12.5%). The mean HVPG value was 16.7±6.2 mm Hg, and the duration of HVPG measurement was 37.8±7.8 minutes. The main hepatic veins targeted for HVPG measurement included the right (60.4%) and middle (39.6%) hepatic veins. One patient with obstructive sleep apnea-hypopnea syndrome experienced transient respiratory depression during fentanyl analgesia (1.5 µg/kg) administration for HVPG measurement.
TABLE 1.
Baseline patient characteristics
| Variables | N=48, n (%) |
|---|---|
| Male sex | 32 (66.7) |
| Age (years) | 52.5 (22–75) |
| BMI (kg/m2) | 23.0 (14.7–29.4) |
| Etiology of liver cirrhosis | |
| HBV | 38 (79.1) |
| HCV | 4 (8.4) |
| Alcoholic cirrhosis | 6 (12.5) |
| Child–Pugh grade | |
| A | 28 (58.3) |
| B | 18 (37.5) |
| C | 2 (4.2) |
| ASA class | |
| I | 8 (16.6) |
| II | 14 (29.2) |
| III | 26 (54.2) |
| OSAHS | 3 (6.3) |
| Respiratory depression | 1 (2.1) |
| The duration of HVPG measurement (mean±SD) (minutes) | 37.8±7.8 |
| The HVPG value (mean±SD) (mm Hg) | 16.7±6.2 |
| Hepatic veins for HVPG measurement | |
| Middle hepatic vein | 19 (39.6) |
| Right hepatic vein | 29 (60.4) |
| Length of hospitalization (mean±SD) (days) | 7.2±2.1 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; OSAHS, obstructive sleep apnea-hypopnea syndrome.
Hemodynamic, respiratory, and VNRS-for-comfort changes with 1.0-μg/kg fentanyl analgesia administration for HVPG measurement
Twenty-three patients were administered 1.0 μg/kg fentanyl analgesia during HVPG measurement. The HVPG values were 13.4±4.9 and 13.5±5.2 mm Hg in the preanalgesia and under-analgesia states, respectively. The preanalgesia and under-analgesia RRs were 17.5±3.5 and 14.4±3.5/minute, respectively. The preanalgesia and under-analgesia SpO2 values were 99.2±2.2% and 99.4±2.1%, respectively. The preanalgesia and under-analgesia HRs were 75.9±13.9 and 72.4±14.0/minute, respectively. The preanalgesia and under-analgesia MAP values were 88.7±12.7 and 88.8±11.6 mm Hg, respectively. The preanalgesia and under-analgesia VNRS-for-comfort values were 5.13±0.9 and 6.13±0.8, respectively. The accuracy of HVPG measurements was not altered after fentanyl analgesia (p = 0.801) (Figure 1A). The representative hepatic vein pressure is shown in Figure 2. The following measures did not change: SpO2 (p = 0.748) (Figure 1C), HR (p = 0.132) (Figure 1D), and MAP (p = 0.348) (Figure 1E). However, changes in RR (p = 0.001) were observed (Figure 1B). The VNRS for comfort under analgesia was higher than that in preanalgesia (p = 0.001) (Figure 1F). A strong correlation was observed between preanalgesia and under-analgesia HVPG results (r=0.921, p < 0.001) (Figure 3A), and similar results were yielded by the Bland–Altman plot (Figure 3B).
FIGURE 1.
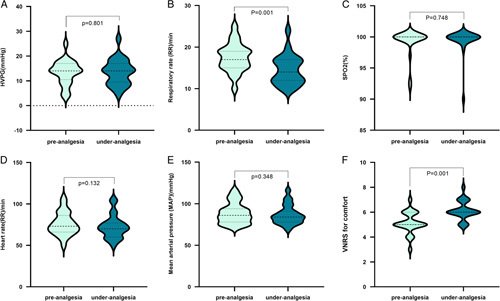
Hemodynamic, respiratory, and Verbal Numerical Rating Score-for-comfort changes with 1.0-μg/kg fentanyl analgesia administration for HVPG measurement.
FIGURE 2.

Representative hepatic vein pressure was registered during the preanalgesia (A) and under-analgesia (B) states with 1.0 µg/kg fentanyl administration, and no marked oscillation of HVPG readings was observed during HVPG measurement.
FIGURE 3.
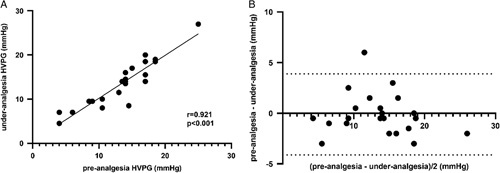
Correlation between the preanalgesia and under-analgesia HVPG with 1.0 µg/mg fentanyl administration. (A) The scatterplot shows the agreement between the preanalgesia and under-analgesia HVPG. (B) The Bland–Altman plot shows the difference between the preanalgesia and under-analgesia HVPG.
Hemodynamic, respiratory, and VNRS-for-comfort changes with 1.5-μg/kg fentanyl analgesia administration for HVPG measurement
Twenty-five patients were administered 1.5 μg/kg fentanyl analgesia during HVPG measurement. The preanalgesia and under-analgesia HVPG values were 19.5±5.7 and 19.6±5.6 mm Hg, respectively. The preanalgesia and under-analgesia RRs were 20.1±4.4 and 18.7±3.6/minute, respectively. The preanalgesia and under-analgesia SpO2 values were 98.8±1.6% and 99.4±1.1%, respectively. The preanalgesia and under-analgesia HRs were 79.8±15.2 and 74.8±15.5/minute, respectively. The preanalgesia and under-analgesia MAP values were 83.6±13.3 and 83.8±15.3 mm Hg, respectively. The pre-analgesia and under-analgesia VNRS-for-comfort values were 5.12±0.8 and 6.40±0.9, respectively. The accuracy of HVPG measurements was not altered after fentanyl analgesia administration (p = 0.469) (Figure 4A). The representative hepatic vein pressure is shown in Figure 5. The following measures did not change: SpO2 (p = 0.327) (Figure 4C) and MAP (p = 0.871) (Figure 4E). However, changes in RR (p = 0.015) (Figure 4B) and HR (p = 0.019) (Figure 4D) were observed under analgesia. The VNRS for comfort under analgesia was higher than that in preanalgesia (p = 0.001) (Figure 2F). A strong correlation between pre-analgesia and under-analgesia HVPG results was observed (r=0.982, p = 0.001) (Figure 3A), and similar results were yielded by the Bland–Altman plot (Figure 6B).
FIGURE 4.

Hemodynamic, respiratory, and Verbal Numerical Rating Score-for-comfort changes with 1.5-μg/kg fentanyl analgesia administration for HVPG measurement.
FIGURE 5.
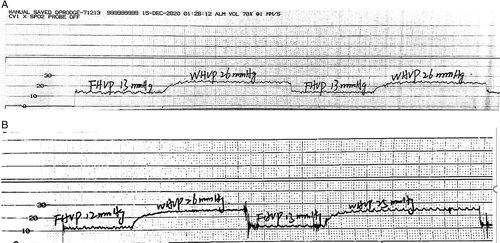
Representative hepatic vein pressure was registered during the preanalgesia (A) and under-analgesia (B) states with 1.5 µg/kg fentanyl administration, and no marked oscillation of HVPG readings was observed.
FIGURE 6.
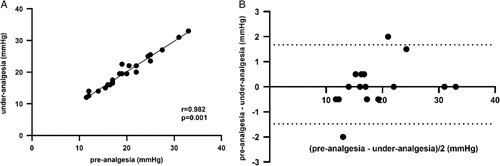
Correlation between the pre-analgesia and under-analgesia HVPG with 1.5 µg/mg fentanyl administration. (A) The scatterplot shows the agreement between the pre-analgesia and under-analgesia HVPG. (B) The Bland–Altman plot shows the difference between the pre-analgesia and under-analgesia HVPG.
DISCUSSION
An increase in portal pressure in a patient with cirrhosis potentially causes life-threatening complications, and the degree of portal hypertension is associated with the patient’s prognosis.12 At present, the HVPG value is the optimal indirect measure of portal pressure in patients with liver fibrosis.13 HVPG measurement is an invasive procedure; hence, some patients find it painful and unpleasant. Previous studies have noted that propofol sedation potentially affects the accuracy of HVPG readings.6,7 However, the effects of opioid analgesics on HVPG have not yet been fully elucidated. Therefore, this study evaluated the effects of fentanyl analgesia on the accuracy of HVPG measurements in patients with cirrhosis. In clinical practice, 1.0–2.0 µg/kg fentanyl is the frequently recommended dose for minor surgery according to drug instructions, and our study demonstrated that analgesia with 1.0 or 1.5 µg/kg fentanyl does not affect HVPG readings. This conclusion may have important implications for clinical decision-making, since fentanyl analgesia is a potential option for pain-sensitive patients who are undergoing HVPG measurements. The anesthetic regime or drugs may affect hepatic blood flow,14–16 and the changes in hepatic blood flow are dose-dependent.17 Previous studies have revealed that propofol or propofol in combination with remifentanil sedation during HVPG measurements potentially leads to relevant alterations of HVPG readings.6,7 The foregoing conclusion is conceivable because sedation or general anesthesia may inhibit myocardial contraction, thus causing hemodynamic changes.18 Furthermore, liver blood flow may be affected by changes in systemic blood pressure. Perhaps, for this reason, HVPG measurements have always been performed without anesthetics.7 However, the selection of appropriate anesthetic drugs or regimes is significantly essential for invasive procedures.19 Our study indicated that recommended doses of the opioid fentanyl analgesia do not influence the accuracy of HVPG measurements.
Fentanyl is a robust agonist of the µ-opioid receptor.20 It has emerged as the most popular opioid for intraoperative analgesia worldwide. As fentanyl is a considerably old and classical opioid, its synthesis is quite reasonably priced. Moreover, anesthesiologists or clinicians working in pain situations are generally familiar with it. More importantly, fentanyl has a minimal cardiovascular effect and relatively brief working and drug-effect durations.21 Based on the properties of fentanyl described above, the present study proposes that low-dose fentanyl may have minimal effects on HVPG values as well. This study is potentially significant because, according to Chinese consensus, fentanyl analgesia should be avoided during HVPG measurements to prevent inaccurate HVPG readings,22 which may be inconsistent with the properties of fentanyl and our clinical anesthetic experience. Fentanyl can maintain its effect for 30 minutes according to its clinical pharmacokinetics,23 and the duration of HVPG measurement lasted 37 minutes in this study. Therefore, we considered a single intravenous injection of fentanyl to be appropriate for HVPG measurements.
In this study, we observed the effects of 1.0 and 1.5 µg/kg fentanyl on hepatic venous pressure, RR, SpO2, HR, MAP, and the VNRS for comfort. The results revealed that both doses did not affect the accuracy of HVPG readings, MAP, and SpO2; nevertheless, they improved patient comfort. We also found that both 1.0 and 1.5 µg/kg fentanyl slowed down RR, while 1.5 µg/kg fentanyl slowed down HR. RR reductions must be considered when using fentanyl. Despite the safety of current doses for most patients, opioids can attenuate the CO2 responsiveness of the respiratory center in a dose-dependent manner, thus potentially leading to a decrease in RR. Respiratory depression is generally well tolerated; however, in patients with respiratory disease, it potentially causes hypoxemia and generates other risks.10 In this study, only 1 patient with obstructive sleep apnea-hypopnea syndrome suffered minor respiratory depression. After adjusting the patient’s head position, breathing returned to normal. Therefore, respiratory depression-related side effects associated with fentanyl must be strongly considered when deciding to use fentanyl during HVPG measurements. Reverter et al.6 found that a marked fluctuation in breathing may affect HVPG measurements during deep sedation with a combination of propofol and remifentanil. In our opinion, breathing issues not only affect the accuracy of hepatic venous pressure readings but also the safety of patients with cirrhosis. Therefore, we suggest that an experienced anesthesiologist should be available to monitor patients’ vital signs when using fentanyl analgesia for HVPG measurements.
In addition, we found that 1.5 µg/kg fentanyl slowed down HR in patients with cirrhosis during HVPG measurements; nonetheless, it did not affect HVPG and MAP values. A plausible reason for this is that 1.5 µg/kg fentanyl efficiently suppresses a patient’s pain or tension via analgesic and sedative effects. HR is a sensitive indicator of pain, and a meta-analysis demonstrated that HR variability is a potentially valuable marker for evaluating the nociceptive response in experimentally induced pain.24 Our study revealed that both 1.0 and 1.5 µg/kg fentanyl increased patients’ VNRS-for-comfort values during HVPG measurement. We applied the comfort score rather than the pain score because a previous study suggested that the use of the word “comfort” as opposed to “pain” may be beneficial to patients’ recovery.11 Regrettably, we could not evaluate patients’ Visual analog Scale scores for comfort as their heads were covered by sterile surgical drapes during HVPG measurements. Therefore, determining the VNRS for comfort is more patient-friendly than the VAS score. Moreover, certain flaws regarding the comfort score might have been present in this trial, since VNRS-for-comfort values were not equivalent between the preanalgesia and under-analgesia states. This trial was a “before-after” study on the same patients. The discomfort-associated HVPG measurements entail local anesthesia, paracentesis, catheterization, and measurement.25 To observe the changes in HVPG readings, the first HVPG measurement was performed in the preanalgesia state. Upon measurement completion, the VNRS for comfort was determined. Five minutes later, the same patients underwent a second HVPG measurement under analgesia with fentanyl, and the VNRS-for-comfort value was determined again. Therefore, the VNRS for comfort in preanalgesia might have involved local anesthesia, paracentesis, catheterization, and measurement, whereas that under analgesia only involved measurement. Steinlauf examined the degree of comfort and relaxation in relation to midazolam sedation for HVPG measurements and noted an increase in comfort and relaxation after midazolam administration compared with that in the placebo group.9 However, we considered it potentially unreasonable to compare comfort levels since the preanalgesia and under-sedation procedures were different.
The effect of general anesthesia or sedation on the accuracy of HVPG measurements remains controversial. Reverter et al.6 and Ebrahimi et al.7 noted that propofol sedation affected HVPG readings; however, an earlier study by Mandell et al.17 demonstrated that general anesthesia with propofol did not change the HVPG variables. Furthermore, we noticed that Mandell performed tracheal intubation and mechanical ventilation on patients with HVPG measurements, thus potentially eliminating the effects of respiratory fluctuations on HVPG readings due to sedation. If an artificial airway is not established after sedation, respiratory fluctuations may be obvious, and this potentially affects the accuracy of HVPG measurements. This phenomenon was also demonstrated in Reverter et al.’s6 study. From an anesthesiologist’s perspective, deep sedation without an artificial airway during HVPG measurement may increase safety risks in patients with cirrhosis. Since a large radiology suite typically constitutes a “remote environment,” the conditions of airway management are always a challenge for anesthesiologists.26 Therefore, in our opinion, if deep sedation is required during HVPG measurements, tracheal intubation with general anesthesia would be safe and reasonable. However, if analgesia alone is required, low-dose fentanyl is suitable and effective for patients undergoing HVPG measurements, although it may not provide sufficient depth of anesthesia.
This study has certain limitations. MAP measurements are noninvasive, and they may not be synchronized with HVPG. Owing to concerns regarding respiratory depression, only 1.0 and 1.5 µg/kg fentanyl doses were used in this trial, which may be insufficient for some pain-sensitive patients, and no higher doses of fentanyl were attempted for HVPG measurements.
In summary, fentanyl analgesia can be administered to pain-sensitive patients who are reluctant to undergo HVPG measurements. Doses of 1.0 and 1.5 µg/kg fentanyl were found to have no effect on HVPG readings; moreover, they were associated with respiratory and circulatory safety. More importantly, fentanyl improved comfort in patients with cirrhosis during the HVPG measurements.
ACKNOWLEDGMENTS
The authors thank Chuan Liu and Yifei Huang for his support on study registration and design. They also thank Editage (www.editage.cn) for English language editing.
CONFLICT OF INTEREST
Nothing to report.
Footnotes
Funding information This work was supported by the National Natural Science Foundation (82060119), Gansu Province Health Industry Project (GSWSKY2016-27), The First Hospital of Lanzhou University Funds (ldyyyn2020-29).
Haijun Zhang, Lili Yang, and Ziniu Yu contributed equally to this work.
Contributor Information
Haijun Zhang, Email: ldyy_haijunzhang@lzu.edu.cn.
Lili Yang, Email: 763887273@qq.com.
Ziniu Yu, Email: 1515094@zju.edu.cn.
REFERENCES
- 1.Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, Robert C, Ortega-Ribera M, Fernández-Iglesias A, et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74:1188–1199. [DOI] [PubMed] [Google Scholar]
- 2.McConnell M, Iwakiri Y. Biology of portal hypertension. Hepatol Int. 2018;12(suppl 1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gracia-Sancho J, Marrone G, Fernández-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 2019;16:221–234. [DOI] [PubMed] [Google Scholar]
- 4.Franchis R de, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII Faculty. Baveno VII—Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reverter E, Cirera I, Albillos A, Debernardi-Venon W, Abraldes J G, Llop E, et al. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J Hepatol. 2019;71:942–950. [DOI] [PubMed] [Google Scholar]
- 6.Reverter E, Blasi A, Abraldes JG, Martínez-Palli G, Seijo S, Turon F, et al. Impact of deep sedation on the accuracy of hepatic and portal venous pressure measurements in patients with cirrhosis. Liver Int. 2014;34:16–25. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahimi F, Semela D, Heim M. Impact of propofol sedation on the diagnostic accuracy of hepatic venous pressure gradient measurements in patients with cirrhosis. Hepatol Int. 2021;16:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X, Berzigotti A, Cardenas A, Sarin SK. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3:708–719. [DOI] [PubMed] [Google Scholar]
- 9.Steinlauf AF, Garcia-Tsao G, Zakko MF, Dickey K, Gupta T, Groszmann. RJ. Low-dose midazolam sedation: an option for patients undergoing serial hepatic venous pressure measurements. Hepatology. 1999;29:1070–1073. [DOI] [PubMed] [Google Scholar]
- 10.Brody H. Opioids. Nature. 2019;573:S1. [DOI] [PubMed] [Google Scholar]
- 11.Chooi CSL, White AM, Tan SGM, Dowling K, Cyna AM. Pain vs comfort scores after caesarean section: a randomized trial. Br J Anaesth. 2013;110:780–787. [DOI] [PubMed] [Google Scholar]
- 12.Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359–1376. [DOI] [PubMed] [Google Scholar]
- 13.Sauerbruch T. Assessment of portal pressure in cirrhosis: how and when can we sedate? Liver Int. 2014;34:4–5. [DOI] [PubMed] [Google Scholar]
- 14.Meierhenrich R, Wagner F, Schütz W, Rockemann M, Steffen P, Senftleben U, et al. The effects of thoracic epidural anesthesia on hepatic blood flow in patients under general anesthesia. Anesth Analg. 2009;108:1331–1337. [DOI] [PubMed] [Google Scholar]
- 15.Alva N, Palomeque J, Carbonell T. Nitric oxide induced by ketamine/xylazine anesthesia maintains hepatic blood flow during hypothermia. Nitric Oxide. 2006;15:64–69. [DOI] [PubMed] [Google Scholar]
- 16.Iber T, Hecker K, Vagts DA, Roesner JP, Otto B, Steinicke A, et al. Xenon anesthesia impairs hepatic oxygenation and perfusion in healthy pigs. Minerva Anestesiol. 2008;74:511–519. [PubMed] [Google Scholar]
- 17.Mandell MS, Durham J, Kumpe D, Trotter JF, Everson GT, Niemann CU. The effects of desflurane and propofol on portosystemic pressure in patients with portal hypertension. Anesth Analg. 2003;97:1573–1577. [DOI] [PubMed] [Google Scholar]
- 18.Fargen KM, Spiotta AM, Hyer M, Lena J, Turner RD, Turk AS, et al. Comparison of venous sinus manometry gradients obtained while awake and under general anesthesia before venous sinus stenting. J Neurointerv Surg. 2017;9:990–993. [DOI] [PubMed] [Google Scholar]
- 19.Fortea JI, Puerto M, Fernández-Mena C, Asensio I, Arriba M, Almagro J, et al. Sevoflurane versus ketamine+diazepam anesthesia for assessing systemic and hepatic hemodynamics in rats with non-cirrhotic portal hypertension. PLoS ONE. 2020;15:e0233778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vo QN, Mahinthichaichan P, Shen J, Ellis CR. How μ-opioid receptor recognizes fentanyl. Nat Commun. 2021;12:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley TH. The fentanyl story. J Pain. 2014;15:1215–1226. [DOI] [PubMed] [Google Scholar]
- 22.Consensus on clinical application of hepatic venous pressure gradient in China (2018). Zhonghua Gan Zang Bing Za Zhi. 2018;26:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholz J, Steinfath M, Schulz M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin Pharmacokinet. 1996;31:275–292. [DOI] [PubMed] [Google Scholar]
- 24.Forte G, Troisi G, Pazzaglia M, De Pascalis V, Casagrande M. Heart rate variability and pain: a systematic review. Brain Sci. 2022;12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J-H, Zhao H, Zhang H, Li L, Örmeci N, Yu Z-N, et al. Tolerance and acceptance of hepatic venous pressure gradient measurement in cirrhosis (CHESS1904): an international multicenter study. Portal Hypertension & Cirrhosis. 2022;1:7–14. [Google Scholar]
- 26.DeGasperi A, Corti A, Corso R, Rampoldi A, Roselli E, Mazza E, et al. Transjugular intrahepatic portosystemic shunt (TIPS): the anesthesiological point of view after 150 procedures managed under total intravenous anesthesia. J Clin Monit Comput. 2009;23:341–346. [DOI] [PubMed] [Google Scholar]


