Abstract
Patients with metabolic syndrome (MetS) have a higher risk for NASH and significant fibrosis. Presence of NASH and advanced fibrosis are associated with adverse outcomes in patients with NAFLD. Using a noninvasive method, we determined the prevalence of at-risk NASH and its association with MetS components in a large population-based analysis. We used the 2017–2018 National Health and Nutrition Examination Survey and included adults ≥18 years with NAFLD (controlled attenuation parameter ≥274 dB/m). Pregnancy, subjects with other causes of liver disease or missing data were excluded. FibroScan-AST (FAST) score was calculated using aspartate aminotransferase, liver stiffness measurement, and controlled attenuation parameter. Patients with a FAST score >0.35 were considered to have at-risk NASH, defined as NASH with NAFLD activity score ≥4 and fibrosis stage ≥2 on liver biopsy. The sample included 687 patients. The overall prevalence of at-risk NASH was 11.6% (95% CI: 8.8–15.1) and was higher in males than females (15.8% vs. 6.5%; p < 0.001). Subjects with comorbidities (diabetes mellitus, obesity, MetS, and insulin resistance) had between 1.3 and 1.7 times higher prevalence than the general population. Among MetS components, elevated glucose/diabetes, large waist circumference, and low HDL were independent risk factors for at risk-NASH. The number of MetS components was also important—one additional component increased the odds of at-risk NASH by 2 times. The FAST score had the highest correlation with alanine aminotransferase (r= 0.70; p < 0.001). We estimated ~9 million people in the US have at-risk NASH and may benefit from active surveillance and therapy.
INTRODUCTION
NAFLD encompasses a spectrum from simple fat accumulation to liver fibrosis.1 It can also progress to more advanced diseases such as advanced fibrosis, cirrhosis, or liver cancer.2 The prevalence of NAFLD is estimated to be 25% globally and 32% in the US.3,4 Underlying comorbidities, specifically components of metabolic syndrome (MetS), are risk factors for NASH/progression of NAFLD. Some patient subgroups, for example, those with type 2 diabetes mellitus (T2DM) or obesity, had a higher prevalence of NASH than the general population with NAFLD.3,5 In particular, the presence of MetS was associated with more than 3 times the odds of having NASH or significant fibrosis (stage 3 or higher).6 Within patients with NAFLD, ∼25%–35% have the progressive form of NASH.7 The rising prevalence of NASH has resulted in an increase of 170% in liver transplant registrants with NASH between 2004 and 2013.8 It has surpassed viral hepatitis in becoming one of the leading causes of liver transplantation.9
Both NASH and advanced fibrosis are associated with worse liver-related outcomes. The estimated annual incidence rate of HCC in patients with NASH was nearly 12 times higher than in the general NAFLD patient population.3 Patients with NASH also have increased liver-specific and all-cause mortality compared with those with simple steatosis.3 The risk of all-cause mortality increased in NAFLD patients with each additional MetS condition compared with those with no condition.10 With the increasing prevalence of MetS and NAFLD, it is important to identify patients at high-risk of NASH and advanced fibrosis to provide the best opportunity for early intervention.11,12
Although liver biopsy remains the gold standard for diagnosis of NASH and fibrosis, it is not suitable for population-based risk stratification. Noninvasive methods, therefore, have been increasingly used to identify at-risk patients.13 The fibrosis-4 (FIB-4) index and NAFLD fibrosis score (NFS) are among the most commonly used tools for the noninvasive diagnosis of advanced fibrosis.14 A meta-analysis reported a FIB-4 cutoff between 1.51 and 2.24 for advanced fibrosis diagnosis, with a sensitivity of 77.0% (range: 70.6%–89.5%) and specificity of 79.2% (range: 67.1%–93.6%).15 For the NFS score with a threshold of −1.455, the meta-analysis reported a sensitivity of 72.9% (range: 22.7%–96.0%) and a specificity of 73.8% (range: 42.9%–100%).15 Although these noninvasive methods are helpful in detecting simple steatosis and advanced fibrosis, they lack the ability to detect the presence or severity of NASH, or progression in NAFLD such as changes within fibrosis stages.16,17 In recent years, there is increasing interest in liver vibration-controlled transient elastography (VCTE), an ultrasound-based modality for assessing liver fibrosis, with an AUROC of 0.87 (95% CI: 0.83–0.90) for detecting advanced fibrosis and AUROC of 0.93 (95% CI: 0.90–0.94) for detecting liver cirrhosis.15,18 FibroScan can measure liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) in a single scan.19 The advantages of VCTE include being noninvasive, ability to measure a larger region of the liver, its reproducibility, and a short duration of the test.18 VCTE is a potential noninvasive tool for population-based epidemiological studies for liver fibrosis.20
To improve the ability to noninvasively detect patients with at-risk NASH, defined as progressive NASH [NAFLD activity score (NAS) ≥4 and fibrosis stage 2 or higher], a FibroScan-AST (FAST) score based on LSM, CAP, and aspartate aminotransferase (AST) has been used.21 Multiple studies have shown the FAST score to have an excellent test performance with an AUROC ranging from 0.74 to 0.95.21–23 With no current approved treatments for NAFLD/NASH, it is important to identify patients who are at risk of progression to cirrhosis or HCC and to conduct longitudinal follow-up studies to allow for prioritization of resource allocation in managing these patients and evaluating therapeutic response. While a biopsy is generally believed to be required for the diagnosis of NASH, noninvasive indices that include an elevated alanine aminotransferase (ALT) are increasingly recognized as a surrogate for underlying NASH.24
The National Health and Nutrition Examination Survey (NHANES), for the first time in 2017–2018, includes VCTE data, providing an opportunity to use composite noninvasive tests for determining the epidemiology of NAFLD at the population level.25 A recent publication using 2017–2018 NHANES showed that among US adults, the prevalence of fatty liver was 47.8% (95% CI: 45.3%–50.3%) and the prevalence of fibrosis (F≥F2) for those with fatty liver was 13.8% (95% CI: 10.4%–15.9%).26 Other studies using the same data estimated the prevalence of NAFLD and fibrosis within different subgroups.27,28 However, no studies to date have examined at-risk NASH or the contribution of different MetS components to disease severity. Our primary aim was to estimate the prevalence of at-risk NASH and examine the hierarchical effect of different MetS components on at-risk NASH in US adults using the FAST score and 2017–2018 NHANES. We also assessed the correlation among FAST score, ALT, FIB-4, and NFS.
METHODS
Study
Design and population
A cross-sectional analysis of 2017–2018 NHANES was performed. NHANES combines interviews and physical examinations to assess the health and nutritional status of a representative sample of noninstitutionalized adults and children in the US.25 All research was conducted in accordance with both the Declarations of Helsinki and Istanbul. All survey participants provided informed consents before their data were collected for NHANES. Because NHANES data is publically available and de-identified, our study was exempt from Institutional Review Board approval. Our study population included participants aged ≥18 years old with NAFLD. We defined NAFLD as having a CAP score of ≥274 dB/m.29 We excluded pregnant women, patients with incomplete VCTE measurements, missing or excessive alcohol consumption (defined as >2 drinks/day for males and >1 drink/day for females, hepatitis B or C, ALT or AST >500 IU/L), or missing values for AST and MetS components.
Regarding comorbidities, we defined T2DM as either having ever been diagnosed with T2DM, HbA1C ≥6.5%, fasting glucose >125 mg/dL, or taking medication. MetS was defined as consisting of at least 3 of the 5 criteria: waist circumference over 40 inches in men or 35 inches in women, blood pressure >140/90 mm Hg or taking medication, fasting triglyceride ≥150 mg/dL or taking medication, fasting HDL cholesterol <40 mg/dL in men or 50 mg/dL in women, and fasting glucose >100 mg/dL or with T2DM.30 We defined obesity as a body mass index (BMI) of ≥30 kg/m2 (≥25 kg/m2 for Asians) and insulin resistance as a HOMA-IR score of ≥3. The noninvasive tests were calculated as31,32:
Outcome measures
NHANES used FibroScan model 502 V2 Touch equipped with medium and extra-large probes to noninvasively detect liver disease. We utilized the FAST score to identify patients with at-risk NASH, defined as having NASH with a NAS of ≥4 and fibrosis stage ≥F2 following the original paper of the FAST score. The FAST score was calculated using the equation21:
Patients with a FAST score of >0.35 were considered to have at-risk NASH and ≤0.35 were considered low-risk NASH.21
Statistical analysis
Descriptive statistics were used to compare patient characteristics between those with at-risk versus low-risk NASH. We estimated the prevalence of at-risk NASH for the overall study population and stratified by sex, age groups, race/ethnicity, comorbidities, and medications. For T2DM patients, we calculated the prevalence by patients using metformin only, insulin only, and other diabetes medications only. In patients with hypertension, we assessed those using angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). In patients with hypertriglyceridemia, prevalence was estimated for those using statins versus nonstatin medications. To assess the association between at-risk NASH and MetS, we used univariate logistic models to examine MetS criteria individually. We then examined the association of MetS components in 2 multivariable logistics regression models: model 1 included each individual MetS criteria as predictors, and model 2 included each individual MetS criteria and patient demographics (age, sex, and race/ethnicity). To examine whether the impact of MetS differed by the number of criteria met, we used the number of MetS criteria as a predictor in addition to demographic factors in model 3. Finally, we assessed the association between advanced fibrosis (F≥F3, defined as LSM≥9.7 kPA) and MetS using a similar modeling approach.29 ORs and 95% CIs were calculated. Finally, we assessed the correlation between FAST score, FIB-4, NFS, and ALT. Appropriate survey weights were applied for all analyses which were performed using Stata version 17.33
RESULTS
We identified 687 subjects with NAFLD, representing 31.4 million US adults (Figure 1). A total of 4719 NHANES participants in the 2017–2018 cycle had missing values for excessive alcohol consumption. Because patients with a larger body weight might be more likely to have partial or incomplete transient elastography results and hence excluded from analysis, we compared participants’ mean body weight across the 4 categories of elastography exam status (complete, partial, ineligible, and not done). We found significant differences in weight amongst the groups (p < 0.05). On average, the partial group weighted 7.3 kg (95% CI: 2.7–11.8) more than the completed group. However, after imputing excessive alcohol consumption, we were able to include an addition of 1480 people (16% of NHANES participants) in the analytical sample. The imputed sample included patients with incomplete elastography exams. Because the estimates using imputed data were not significantly different from those using original data, we reported results based on the latter.
FIGURE 1.
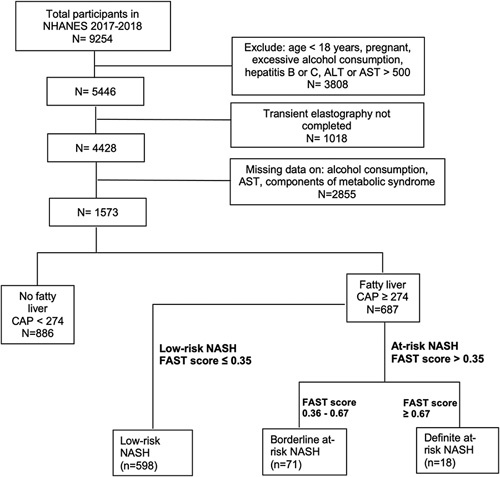
Flow chart of participants in the study. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; FAST, FibroScan-AST; NHANES, National Health and Nutrition Examination Survey.
Overall, the mean age was 51.4 years (95% CI: 49.4–53.4), the mean BMI was 33.4 kg/m2 (95% CI: 32.6–34.2), and 54.5% were male (Table 1). Patients with at-risk NASH were more likely to be male and had a higher BMI than low-risk patients. Among US adults with NAFLD, 26.4% (95% CI: 21.9–31.4) had T2DM, 65.7% (95% CI: 61.0–70.11) were obese, 64.1% (95% CI: 56.3–71.3) had insulin resistance, and 64.9% (95% CI: 58.0–71.2) had MetS (Table 2). Compared with patients with low-risk, those with at-risk NASH had significantly higher prevalence of comorbidities including MetS (93.3% vs. 60.3%) and its individual components except for hypertension and hypertriglyceridemia. In addition, patients with at-risk NASH had higher AST, ALT, GGT, and ferritin levels than the low-risk group (p < 0.001) (Table 3).
TABLE 1.
Baseline characteristics in US adults (≥18 y) with NAFLD
| All patients, N=687 | Low risk NASH (FAST score ≤0.35), N=598 | At-risk NASH (FAST score >0.35), N=89 | p | |
|---|---|---|---|---|
| Age (y)—mean (95% CI) | 51.4 (49.4–53.4) | 51.8 (49.4–54.2) | 48.5 (45.2–51.8) | 0.14 |
| Male—% (95% CI) | 54.5 (47.3–61.6) | 25.7 (18.4–34.6) | 74.3 (65.4–81.6) | <0.001 |
| BMI (kg/m2)—mean (95% CI) | 33.4 (32.6–34.2) | 32.7 (31.9–33.5) | 38.7 (35.9–41.6) | <0.001 |
| Race/ethnicity—% (95% CI) | ||||
| Non-Hispanic White | 64.5 (59.4–69.2) | 65.4 (60.3–70.2) | 57.1 (45.2–68.2) | 0.15 |
| Non-Hispanic Black | 8 (5.6–11.3) | 8 (5.8–10.8) | 8.1 (3.5–18) | |
| Hispanic | 18.9 (13.2–26.2) | 17.8 (12.1–25.3) | 27.1 (16.4–41.2) | |
| Other | 8.7 (5.9–12.6) | 8.8 (5.7–13.3) | 7.7 (4.5–12.8) | |
| Education—% (95% CI) | ||||
| <High school | 9.6 (7.4–12.3) | 9.3 (7–12.3) | 11.7 (5.5–23.2) | 0.19 |
| High school or GED | 30.4 (26.3–34.8) | 30.9 (26.2–35.9) | 26.7 (15.2–42.6) | |
| Associate degree or some college | 31.4 (24.8–38.9) | 29.8 (22.6–38.2) | 43.5 (26.9–61.8) | |
| ≥College | 28.6 (20.5–38.4) | 30 (21–40.9) | 18 (12.3–25.6) | |
| Income-to-poverty ratio—% (95% CI) | ||||
| <1 | 12.8 (9.3–17.3) | 13 (9.1–18.3) | 11.1 (5.4–21.4) | 0.29 |
| 1–2 | 20.2 (16.1–25) | 20.6 (16.2–26) | 16.6 (9.9–26.6) | |
| 2–4 | 28.1 (21.8–35.4) | 26.2 (20.1–33.4) | 42.8 (22.5–66) | |
| ≥4 | 38.9 (30.3–48.3) | 40.1 (30.9–50) | 29.5 (11.4–57.5) | |
| Insurance type—% (95% CI) | ||||
| None | 10.4 (7.3–14.5) | 9.5 (6.4–13.8) | 17 (7.4–34.5) | 0.11 |
| Private | 41.3 (34.8–48.1) | 40.2 (32.3–48.7) | 49.4 (36.1–62.7) | |
| Government | 48.4 (41.7–55) | 50.3 (42.7–57.9) | 33.6 (25.3–43) | |
| Smoking status—% (95% CI) | ||||
| Never | 56.4 (51.6–61) | 56.7 (51.6–61.6) | 54.1 (38.5–69) | 0.46 |
| Former | 29.7 (24.1–35.9) | 28.8 (22.8–35.7) | 36.1 (21.4–54) | |
| Current | 13.9 (10.6–18.1) | 14.5 (10.7–19.3) | 9.8 (4.9–18.4) | |
Abbreviations: BMI, body mass index; FAST, FibroScan-AST.
TABLE 2.
Comorbidities in US adults (≥18 y) with NAFLD
| All patients | Low risk NASH (FAST score ≤0.35) | At-risk NASH (FAST score >0.35) | p | |
|---|---|---|---|---|
| Comorbidities—% (95% CI) | N=687 | N=598 | N=89 | |
| T2DM | 26.4 (21.9–31.4) | 23.9 (19–29.6) | 45.2 (31.9–59.2) | 0.01 |
| Obesity | 65.7 (61–70.1) | 63 (57.9–67.8) | 86.1 (74.9–92.8) | 0.002 |
| Insulin resistant | 64.1 (56.3–71.3) | 60.3 (52.1–68) | 93.3 (83.2–97.5) | <0.001 |
| MetS criteria | ||||
| Elevated glucose/diabetes | 79.5 (75.1–83.3) | 77.7 (72.7–82) | 93.3 (83.9–97.4) | 0.01 |
| Large waist circumference | 80.8 (75.7–85) | 79 (73.8–83.3) | 94.3 (84.5–98.1) | 0.01 |
| Hypertension | 53.7 (45.4–61.8) | 52.5 (43.3–61.5) | 63.2 (49.8–74.7) | 0.19 |
| Hypertriglyceridemia | 50.3 (42.8–57.8) | 49.2 (40.8–57.5) | 58.8 (45.4–71) | 0.23 |
| Low HDL cholesterol | 35.8 (30.8–41.2) | 32.5 (27.8–37.6) | 60.9 (39.3–79) | 0.01 |
| MetSa | 64.9 (58.0–71.2) | 62 (54.8–68.8) | 87 (66.4–95.7) | 0.02 |
| Number of MetS criteria | ||||
| 0 | 2 (0.9–4.6) | 2.3 (1–5.2) | NA | 0.03 |
| 1 | 11.4 (7.5–16.8) | 12.4 (8.5–17.9) | 3.1 (0.5–16.2) | |
| 2 | 21.7 (17.1–27.1) | 23.2 (18.5–28.7) | 9.9 (2.6–31.5) | |
| 3 | 26.4 (20.5–33.2) | 27 (21.7–33) | 21.7 (9.9–41) | |
| 4 | 26.5 (20–34.2) | 24.2 (18.7–30.7) | 43.8 (25.4–64.1) | |
| 5 | 12.1 (8.7–16.5) | 10.8 (7.3–15.8) | 21.4 (10.5–39) | |
Note: Defined as having ≥3 criteria.
Abbreviations: MetS, metabolic syndrome; NA, not applicable; T2DM, type 2 diabetes mellitus.
TABLE 3.
Lab values and noninvasive tests in US adults (≥18 y) with NAFLD
| All patients, N=687 | Low risk NASH (FAST score ≤0.35), N=598 | At-risk NASH (FAST score >0.35), N=89 | p | |
|---|---|---|---|---|
| Lab values—mean (95% CI) | ||||
| Total bilirubin (mg/dL) | 0.49 (0.46–0.52) | 0.48 (0.45–0.51) | 0.52 (0.45–0.6) | 0.30 |
| AST (IU/L) | 22.15 (21.16–23.14) | 20.03 (19.09–20.97) | 38.31 (34.11–42.51) | <0.001 |
| ALT (IU/L) | 26.25 (25.03–27.46) | 22.89 (21.51–24.26) | 51.89 (44.19–59.59) | <0.001 |
| GGT (IU/L) | 32.8 (28.73–36.86) | 28.3 (24.57–32.02) | 67.15 (47.33–86.96) | 0.001 |
| Albumin (g/dL) | 4 (3.94–4.06) | 4.01 (3.94–4.07) | 3.96 (3.9–4.03) | 0.25 |
| Alkaline phosphatase (IU/L) | 78.68 (75.42–81.95) | 78.72 (75.44–82) | 78.42 (71.2–85.64) | 0.93 |
| Platelet count (103 cells/µL) | 243.92 (235.08–252.76) | 246.43 (236.51–256.36) | 224.77 (212.82–236.72) | 0.01 |
| Total cholesterol (mg/dL) | 187.44 (176.16–198.72) | 187.49 (176.1–198.88) | 187.08 (172.15–202) | 0.94 |
| Triglyceride (mg/dL) | 142.19 (124.75–159.63) | 135.71 (118.84–152.59) | 191.61 (132.71–250.52) | 0.06 |
| HDL cholesterol (mg/dL) | 49.28 (47.11–51.45) | 50.34 (48.01–52.67) | 41.22 (38.4–44.04) | 0.001 |
| LDL cholesterol (mg/dL) | 110.98 (102.4–119.56) | 110.68 (101.85–119.5) | 113.49 (99.65–127.33) | 0.65 |
| Fasting plasma glucose (mg/dL) | 119.8 (114.84–124.75) | 117.86 (112.49–123.23) | 134.54 (126.1–142.99) | 0.002 |
| Insulin (uU/mL) | 18.54 (16.65–20.43) | 16.85 (15.12–18.58) | 31.43 (23.41–39.45) | 0.002 |
| HOMA score | 5.83 (5.18–6.48) | 5.24 (4.58–5.9) | 10.31 (7.77–12.86) | 0.001 |
| HbA1C (%) | 5.94 (5.82–6.06) | 5.89 (5.75–6.02) | 6.36 (6.1–6.62) | 0.01 |
| Serum iron (µg/dL) | 89.36 (83.52–95.2) | 89.55 (83.79–95.32) | 87.86 (76.22–99.5) | 0.75 |
| Ferritin (µg/L) | 163.56 (143.2–183.92) | 149.97 (131.57–168.36) | 267.21 (201.54–332.88) | <0.001 |
| Transferritin receptor (mg/L) | 3.32 (3.1–3.54) | 3.32 (3.09–3.55) | 3.36 (2.91–3.81) | 0.83 |
| Noninvasive test—mean (95% CI) | ||||
| FAST score | 0.16 (0.14–0.18) | 0.11 (0.1–0.12) | 0.55 (0.52–0.59) | <0.001 |
| FIB-4 | 1.04 (0.96–1.12) | 1.01 (0.92–1.09) | 1.29 (1.12–1.46) | 0.01 |
| NAFLD fibrosis score | −0.35 (−0.51, −0.19) | −0.42 (−0.57, −0.27) | 0.15(−0.09, 0.39) | <0.001 |
| VCTE—mean (95% CI) | ||||
| Controlled attenuation parameter score (dB/m) | 321.48 (317.12–325.84) | 316.28 (312.59–319.98) | 361.17 (350.07–372.27) | <0.001 |
| Interquartile range, mean | 34.44 (32.90–35.99) | 35.32 (34.08–36.56) | 27.78 (18.55–37.01) | 0.10 |
| Liver stiffness measurement (kPa) | 6.27 (5.65–6.89) | 5.42 (5.17–5.67) | 12.78 (9.22–16.33) | <0.001 |
| Interquartile range, mean | 0.96 (0.79–1.12) | 0.78 (0.72–0.85) | 2.28 (1.27–3.29) | 0.01 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; FAST, FibroScan-AST; FIB-4, fibrosis-4; GGT, gamma-glutamyl transferase; VCTE, vibration controlled transient elastography.
The overall prevalence of at-risk NASH in patients with NAFLD was 11.6% (95% CI: 8.8–15.1). The prevalence did not differ by age group (p = 0.22) or race/ethnicity (p = 0.28) but was significantly higher in males than females, 15.8% versus 6.5% (p = 0.002). Patients with comorbidities had a higher prevalence of at-risk NASH than the general NAFLD population. Specifically, patients with coexisting NAFLD and T2DM had the highest prevalence of at-risk NASH, followed by NAFLD patients with insulin resistance and MetS (20.8% vs. 16.9% vs. 15.5%, respectively) (Table 4). Among MetS components, patients having low HDL had the highest prevalence, 19.7% (95% CI: 13.4–28.0) (Figure 2). In addition, the higher the number of MetS criteria the patients had, the higher the prevalence of at-risk NASH. Patients who had all 5 components of MetS had the highest prevalence, which was 2 times higher than those with 3 components. Among those taking medications, NAFLD participants with T2DM who took metformin had a prevalence of 10.5% (95% CI: 4.9–21.2). (Table 4). Participants with hypertriglyceridemia and taking statins had a prevalence of 10.5% (95% CI: 6.9–15.6). Finally, patients with hypertension taking ACE inhibitors or ARBs had a prevalence of 16.8% (95% CI: 10.2–26.5) and 10.3% (3.8–25.2), respectively.
TABLE 4.
Prevalence of probable at-risk NASH (%) by patient characteristics
| N | At-risk NASH (FAST score >0.35) | p | |
|---|---|---|---|
| All patients | 687 | 11.6 (8.8–15.1) | |
| Age group—% (95% CI) | |||
| 18–39 | 155 | 14.2 (8.9–21.9) | 0.22 |
| 40–64 | 333 | 12.2 (8.4–17.5) | |
| 65+ | 199 | 7.3 (4–13.1) | |
| Sex—% (95% CI) | |||
| Female | 312 | 6.5 (4.7–9.1) | 0.002 |
| Male | 375 | 15.8 (11.7–21) | |
| Race/ethnicity—% (95% CI) | |||
| Non-Hispanic White | 255 | 10.3 (6.9–14.9) | 0.28 |
| Non-Hispanic Black | 121 | 11.8 (6.2–21.3) | |
| Hispanic | 199 | 16.6 (11.2–24) | |
| Other | 112 | 10.3 (5.3–19) | |
| Comorbidities—% (95% CI) | |||
| T2DM | 251 | 19.9 (15–26) | 0.01 |
| Without T2DM | 435 | 8.6 (5.3–13.7) | |
| Obesity | 428 | 15.2 (11.3–20.2) | 0.003 |
| Without obesity | 258 | 4.7 (2.5–8.7) | |
| Insulin resistant | 468 | 16.9 (12.6–22.2) | <0.001 |
| Without insulin resistant | 218 | 2.2 (0.9–5) | |
| Metabolic syndrome | 475 | 15.5 (12.3–19.3) | 0.03 |
| Without metabolic syndrome | 212 | 4.3 (1.3–13.3) | |
| Medications—% (95% CI) | |||
| Patients with T2DM | |||
| Metformin | 83 | 10.5 (4.9–21.2) | 0.84 |
| Insulin | 14 | 6.5 (1.4–25.3) | |
| Not metformin or insulin | 15 | 13.3 (2–53.9) | |
| Patients with hypertension | |||
| Angiotensin receptor blockers | 86 | 10.3 (3.8–25.2) | 0.19 |
| ACE inhibitors | 137 | 16.8 (10.2–26.5) | |
| Patients with hypertriglyceridemia | |||
| Statin | 193 | 10.5 (6.9–15.6) | 0.22 |
| Nonstatin | 18 | 2.9 (0.3–21.8) | |
Abbreviations: ACE, angiotensin-converting enzyme; FAST, FibroScan-AST; T2DM, type 2 diabetes mellitus.
FIGURE 2.
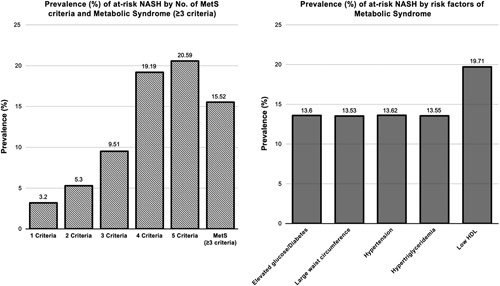
Prevalence (%) of at-risk NASH in NAFLD patients by a number of metabolic syndrome (MetS) criteria, by having MetS (≥3 criteria), and by MetS risk factors.
The association between at-risk NASH and MetS components are shown in Table 5. In univariate analysis, being male, having elevated glucose or diabetes, large waist circumference, low HDL, or a higher number of MetS criteria were associated with higher odds of having at-risk NASH (p < 0.05). When all MetS components were controlled for (model 1), elevated glucose/diabetes, large waist circumference, or low HDL remained significant, with elevated glucose/diabetes having the highest adjusted odds (OR: 3.46, 95% CI: 1.15–10.37). When age, sex, and race/ethnicity were added to the model (model 2), the effect sizes of these 3 MetS components were enhanced, with large waist circumference having the highest effect (OR: 4.66; 95% CI: 1.53–14.17). In model 3, having one additional MetS component was associated with 2.27 (95% CI: 1.64–3.15) times the odds of having at-risk NASH.
TABLE 5.
Association between at-risk NASH and metabolic syndrome in US adults with NAFLD
| Variables | Univariate | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Age | 0.99 (0.97–1) | 0.97 (0.95–0.99) | 0.96 (0.94–0.98) | |
| Sex—reference: female | ||||
| Male | 2.68 (1.74–4.13) | 3.35 (1.67–6.72) | 2.85 (1.74–4.68) | |
| Race/ethnicity—reference: non-Hispanic White | ||||
| Non-Hispanic Black | 1.17 (0.62–2.21) | 1.46 (0.7–3.06) | 1.38 (0.76–2.52) | |
| Hispanic | 1.74 (0.95–3.19) | 1.64 (0.73–3.69) | 1.63 (0.77–3.47) | |
| Other | 1 (0.47–2.16) | 0.87 (0.32–2.37) | 0.83 (0.32–2.14) | |
| Comorbidities | ||||
| MetS criteria | ||||
| Elevated glucose/diabetes | 4.02 (1.36–11.91) | 3.46 (1.16–10.37) | 3.9 (1.37–11.11) | |
| Large waist circumference | 4.42 (1.55–12.63) | 3.23 (1.09–9.63) | 4.66 (1.53–14.17) | |
| Hypertension | 1.55 (0.78–3.08) | 1.27 (0.64–2.53) | 1.85 (0.74–4.62) | |
| Hypertriglyceridemia | 0.39 (−0.28, 1.06) | 1.02 (0.54–1.92) | 1.21 (0.62–2.35) | |
| Low HDL | 3.24 (1.3–8.06) | 2.76 (1.15–6.63) | 2.97 (1.11–8) | |
| Number of MetS criteria (per 1 criterion increase) | 1.76 (1.3–2.38) | 2.27 (1.64–3.15) | ||
Note: Data are presented as OR (95% CI).
Model 1 included 5 MetS components as independent variables.
Model 2 included age, sex, race/ethnicity, and 5 MetS components as independent variables.
Model 3 included age, sex, race/ethnicity, and the number of MetS components as independent variables.
Abbreviation: MetS, metabolic syndrome.
Regarding advanced fibrosis (F≥F3), the associations are shown in Table 6. In univariate analysis, having a higher number of MetS criteria was associated with higher odds of having F≥F3 (p < 0.05). When all MetS components were controlled for (model 1), none of the variables were significant. Similarly, when age, sex, and race/ethnicity were added to the model (model 2), none of the variables were significant. In model 3, having one additional MetS component was associated with 1.45 (95% CI: 1.07–1.98) times the odds of having F≥F3 fibrosis.
TABLE 6.
Association between F≥F3 fibrosis (liver stiffness measurement ≥9.7 kPA) and metabolic syndrome in US adults with NAFLD
| Variables | Univariate | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Age | 1.01 (0.99–1.02) | 0.99 (0.98–1.02) | 1.00 (0.98–1.02) | |
| Sex—reference: female | ||||
| Male | 1.75 (0.69–4.47) | 1.86 (0.76–4.53) | 1.71 (0.66–4.38) | |
| Race/ethnicity—reference: non-Hispanic White | ||||
| Non-Hispanic Black | 0.65 (0.24–1.76) | 0.71 (0.25–2.00) | 0.72 (0.26–2.03) | |
| Hispanic | 1.31 (0.65–2.61) | 1.49 (0.66–3.35) | 1.42 (0.65–3.09) | |
| Other | 0.63 (0.18–2.25) | 0.66 (0.17–2.61) | 0.60 (0.16–2.28) | |
| Comorbidities | ||||
| MetS criteria | ||||
| Elevated glucose/diabetes | 2.03 (0.46–8.90) | 1.71 (0.34–8.62) | 1.49 (0.26–8.47) | |
| Large waist circumference | 2.59 (0.50–13.52) | 2.17 (0.42–11.24) | 2.57 (0.53–12.60) | |
| Hypertension | 1.74 (0.74–4.08) | 1.53 (0.67–3.49) | 1.52 (0.59–3.94) | |
| Hypertriglyceridemia | 1.27 (0.45–3.57) | 0.98 (0.29–3.37) | 0.97 (0.29–3.22) | |
| Low HDL | 1.68 (0.72–3.95) | 1.51 (0.59–3.90) | 1.56 (0.59–4.11) | |
| Number of MetS criteria (per 1 criterion increase) | 1.41 (1.07–1.89) | 1.45 (1.07–1.98) | ||
Note: Data are presented as OR (95% CI).
Model 1 included 5 MetS components as independent variables.
Model 2 included age, sex, race/ethnicity, and 5 MetS components as independent variables.
Model 3 included age, sex, race/ethnicity, and the number of MetS components as independent variables.
Abbreviation: MetS, metabolic syndrome.
Finally, the FAST score was highly correlated with ALT (r=0.70, p < 0.001) and LSM (r=0.61, p < 0.001) but not as much with FIB-4 (r=0.23, p < 0.001) or NFS (r=0.18, p < 0.001) (Figure 3). The other 2 noninvasive scores, FIB-4 and NFS, were highly correlated with each other (r =0.72, p < 0.001) but not with ALT.
FIGURE 3.
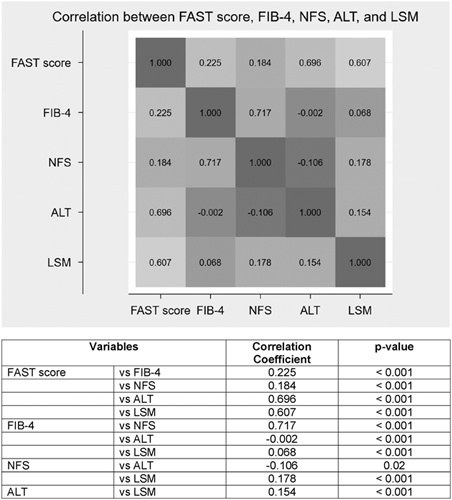
Heatmap showing correlation between FAST score, LSM FIB-5, NFS, and ALT level in US adults with NAFLD. Abbreviations: ALT, alanine aminotransferase; FAST, FibroScan-AST; FIB-4, fibrosis-4; LSM, liver stiffness measurement; NFS, NAFLD fibrosis score.
DISCUSSION
The average annual rate of progression in NASH globally has been estimated to be 0.09/1000 person-years (95% CI: 0.06–0.12) and the liver-specific mortality among NASH patients to be 11.77/1,000 person-years (95% CI: 7.10–19.53).3 The highest increase in liver transplant waitlist registration since 2004–2018 was related to a diagnosis of NASH, the leading cause for liver transplant for women in the US.8,9 Using 2017–2018 NHANES and the noninvasive FAST score, we examined the population-based prevalence of at-risk NASH in patients with NAFLD and in those with comorbidities. We found the overall prevalence was 12% and about 1 in 6 patients with at-risk NASH was identified as Hispanic. However, given the increasing recognition of heterogeneity within the group of Hispanics, more details are needed to identify which specific Hispanic subpopulations are at higher risk.34 The proportion of Hispanic population in our final sample was 18.9%, which may appear to be overrepresented demographically. However, the prevalence was not significantly different by race/ethnicity in our study although a previous meta-analysis showed a higher risk of NASH in Hispanics than Whites.35 In addition, we found that subjects with comorbidities, such as T2DM, obesity, insulin resistance, or MetS had a prevalence 1.3–1.7 times higher than the overall population. Our univariate logistic regression showed being male, Hispanic, elevated glucose/diabetes, large waist circumference, and low HDL increased the odds of having at-risk NASH. The prevalence of at-risk NASH also increases in parallel with increasing number of MetS components. Comparing subjects with only 1 MetS component to all 5 MetS components, the prevalence of at-risk NASH jumped from 3.2% to 20.6%. The multivariable analyses confirmed the importance of elevated glucose/diabetes, large waist circumference, low HDL in developing at-risk NASH. Furthermore, each additional MetS component doubled the odds of at-risk NASH. Our logistic regression also showed having a higher number of MetS criteria increased the odds of having F≥F3 fibrosis. However, the multivariable analysis did not show a significant association between F≥F3 fibrosis and individual MetS components.
Numerous studies have validated the efficiency of FibroScan to diagnose fibrosis and steatosis using LSM and CAP values.29,36,37 In patients with NAFLD, the FAST score offers good discrimination with an AUROC of 0.85 (95% CI: 0.83–0.87) for pooled cohort and 0.86 (95% CI: 0.80–0.93) for US cohort.21 Due to limited sample size, we stratified our cohort into FAST >0.35 which included patients with rule-in or gray-zone NASH score (at-risk NASH) versus ≤0.35 (low-risk NASH). Future studies with larger sample size may provide more granular details for the highest risk population (FAST ≥0.67). The use of noninvasive measures, such as the FAST score, to diagnose at-risk NASH presents a great opportunity to identify patients in the general population eligible for interventions to prevent progression of the disease to cirrhosis and complications related to advance liver disease. The 2017–2018 NHANES is the first national survey containing transient elastography data for the multiethnic population in the US. Using this unique dataset, our study was among the first to examine the epidemiology of at-risk NASH (NASH with NAS ≥4 and fibrosis stage ≥2) and its association with different components of MetS.
The prevalence of NASH among patients with NAFLD has been estimated at 60.64% (95% CI: 49.56–70.72) in North America and 69.75% (95% CI: 37.29–96.83) among those with coexisting NAFLD and T2DM.3,5 Meanwhile, the proportion of patients with combined NASH and advanced fibrosis in a large US patient registry was 40% compared with 12% in our study.38 Besides the different diagnostic methods, liver biopsy versus FAST score, the registry included patients from 8 university medical research centers who presumably had more severe disease than the general adult population that was included in our study.
MetS has been shown to be associated with NAFLD, NASH, and advanced fibrosis.6,27,39–41 Patients with MetS had about 3 times the odds of NASH or significant fibrosis.6 They had a higher prevalence of fibrosis and mean fibrosis score than those without MetS.41 In addition, the increase in number of MetS components led to an increased risk of NASH and/or fibrosis. Using the Spanish HEPAmet Registry, Ampuero et al.39 showed that the odds of having NASH increased significantly for patients with each additional component of MetS compared with those without MetS (OR: 1.66–4.88). These findings are comparable to our study, which confirmed the independent impact of elevated glucose/diabetes, large waist circumference, and low HDL, as well as the number of MetS components on the odds of at-risk NASH. The strength of our study was that we extended our understanding of at-risk NASH and MetS in the general US population rather than limiting to patients from large specialty care centers. In addition, we found a high correlation between FAST score and ALT level (r=0.70, p < 0.001), which was similar to a study in a Japanese cohort.42 Other studies have investigated the prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD).43,44 Kim and colleagues found MAFLD had a higher risk of all-cause mortality but NAFLD did not after adjusting for metabolic risk factors.43 Wong and Cheung44 estimated the prevalence of MAFLD using NHANES 2017–2018 as 38% and our estimate for NAFLD was 41%. Future studies performing a comparative analysis using both definitions should be considered.
Studies using NHANES data before the 2017–2018 cycle mostly estimated the prevalence of NAFLD or advanced fibrosis based on other noninvasive methods, for example the Fatty Liver Index, FIB-4, NFS, or gallbladder ultrasound images.45–47 These studies could not examine at-risk NASH. Similarly, other studies using 2017–2018 NHANES only reported the prevalence of NAFLD and/or advance fibrosis.26,27,48 A recent study by Zhang et al.27 estimated the prevalence of active fibrotic NASH for participants ≥ 20 years old using both 0.35 and 0.67 cutoffs for the FAST score. They reported a prevalence of 6.4% (95% CI: 5.4–7.5) with >0.35 as the cutoff, which is about half of our estimate. It’s important to note that the prevalence was estimated for their whole study cohort, comprising of all eligible adults (N=4424) instead of among patients with NAFLD as in our study. Also, alcohol consumption was calculated using dietary total nutrients data instead of the alcohol questionnaire data, which may not be as accurate. Furthermore, we used a CAP ≥274 dB/m to identify fatty liver, which has sensitivity of 0.90 (95% CI: 0.87–0.93) and specificity of 0.60 (0.44–0.74).29 and excluded participants with any missing MetS components to ensure robust evaluation of the effects of MetS components. No previous study including Zhang and colleagues’ evaluated the hierarchical effect of MetS components on at-risk NASH.
Limitations to this survey data include recall bias from participants, especially regarding alcohol usage. More robust methods of alcohol use may add strength to the NHANES data, but a diagnosis of NAFLD in clinical practice and in most clinical studies to date depends on patient-reported alcohol consumption. NHANES does not provide liver biopsy data to reliably diagnose steatosis, NASH, or fibrosis staging. However, even though VCTE is not the current gold standard for NAFLD diagnosis, its reliability has been shown in a number of publications.49 In addition, we used a validated CAP cutoff ≥274 dB/m with a sensitivity of 90% and cutoffs for FAST score which were shown by others to correlate well with liver biopsy findings of NASH.19,21 Noureddin et al.50 proposed adopting sequential testing after FAST score with enhanced liver fibrosis or magnetic resonance elastography. Combining different options reduces dependency on a single score and should reduce liver biopsy.50 Finally, we excluded a large number of patients due to missing data on MetS components. However, we compared the mean CAP, LSM, and FAST scores between the excluded population and our final sample, and found no statistically significant difference between the groups.
Our study, using a large population-based dataset and a composite noninvasive score, allows for the generalizability of the results to the US adult population. We examined the hierarchical effect of MetS components on at-risk NASH at the population level rather than on selected patients who visited large specialty medical centers. Our findings highlight the high prevalence of at-risk NASH in those with NAFLD, especially among participants with different comorbidities. In addition, we showed that patients with NAFLD who also had MetS or its individual components including elevated glucose/diabetes, large waist circumference, and low HDL, and those with a high number of MetS components had higher odds of at-risk NASH. Our analyses would allow for risk stratification of patients with NAFLD in clinical practice. Furthermore, our study suggested clinical focus on addressing these components of the MetS in primary care and specialty management of patients with NAFLD. Because each additional component of the MetS doubles the odds of at-risk NASH, having any of the components under control would benefit the patients, suggesting patients and physicians could choose to work on the components that are easier to manage first. This is especially critical given that there is increasing recognition of NAFLD, more patients are being managed by nonhepatologists and nonendocrinologists, and there are currently no approved pharmacologic treatments. Based on our analyses, we estimated that ~9 million people would benefit from active screening or possible treatments for NASH. Interventions to address the progression of NASH within these populations would be beneficial and lessen the burden on the health care system.
Acknowledgments
AUTHOR CONTRIBUTIONS
J.P. conducted data analysis and drafted the manuscript. N.A. and P.L. contributed to analytical methodology, interpretation the data, reviewed and edited the manuscript. M.R. interpreted the data, reviewed and edited the manuscript, and obtained funding. P.P. and C.S. interpreted the data, reviewed and edited the manuscript. S.D. conceptualized the study, interpreted the data, reviewed and edited the manuscript. All authors approved the final draft submitted.
CONFLICT OF INTEREST
Dr. S.D. is funded by grants NIH RO1 GM119174; RO1 DK113196; P50 AA024333; RO1 AA021890; 3U01AA026976; UO1 AA 026976; R56HL141744; UO1 DK061732; 5U01 DK062470-17S2; R21 AR 071046 which are independent of the submitted work. Dr. N.A. is a speaker for Echosens (makers of FibroScan) and received research funding from Gilead, Intercept, Allergan, Cirius, Madrigal, and Genfit which was not related to this study. All other authors reported no conflict of interest.
Footnotes
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CAP, controlled attenuation parameter; FAST, FibroScan-AST; FIB-4, fibrosis-4; LSM, liver stiffness measurement; MAFLD, metabolic dysfunction-associated fatty liver disease; MetS, metabolic syndrome; NAS, NAFLD activity score; NFS, NAFLD fibrosis score; NHANES, National Health and Nutrition Examination Survey; T2DM, type 2 diabetes mellitus; VCTE, vibration controlled transient elastography.
Funding information The study is funded by AHRQ R01HS026937.
Contributor Information
Julia Y. Payne, Email: paynej5@ccf.org.
Naim Alkhouri, Email: nalkhouri@azliver.com.
Phuc Le, Email: lep@ccf.org.
Michael B. Rothberg, Email: rothbem@ccf.org.
Prido Polanco, Email: ppolanco@azliver.com.
Celine Sakkal, Email: celinesakkal@gmail.com.
Srinivasan Dasarathy, Email: dasaras@ccf.org.
REFERENCES
- 1.Gadiparthi C, Spatz M, Greenberg S, Iqbal U, Kanna S, Satapathy S, Broder A, Ahmed A. NAFLD Epidemiology, Emerging Pharmacotherapy, Liver Transplantation Implications and the Trends in the United States. J clin transl hepatol. 2020;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesini G, Day CP, Dufour JF, Canbay A, Nobili V, Ratziu V, Tilg H, Roden M, et al. EASL -EASD -EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 4.Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J Intern Med. 2020;288:139–51. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. [DOI] [PubMed] [Google Scholar]
- 7.Harrison SA, Gawrieh S, Roberts K, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75:284–291. [DOI] [PubMed] [Google Scholar]
- 8.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the United States. Gastroenterology. 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 9.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am. J. Gastroenterol. 2018;113:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD. Medicine. 2018;97:e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2020;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323:2526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui MS, Yamada G, Vuppalanchi R, Natta MV, Loomba R, Guy C, Brandman D, Tonascia J, et al. Diagnostic Accuracy of Non-Invasive Fibrosis Models to Detect Change in Fibrosis Stage. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019;17:1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–501. [DOI] [PubMed] [Google Scholar]
- 16.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease—Where do we stand. World J Gastroenterol. 2016;22:7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqui MS, Vuppalanchi R, van Natta ML, Jallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri B, Loomba R, et al. Vibration-controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019;17:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Wong GLH, Wong VWS. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri P, Jain S, Fuchs M. Use of FibroScan–AST score to stratify high-risk nonalcoholic steatohepatitis in US veterans. Clin Gastroenterol Hepatol. 2020;18:3060–1. [DOI] [PubMed] [Google Scholar]
- 23.Oeda S, Takahashi H, Imajo K, Seko Y, Kobayashi T, Ogawa Y, Moriguchi M, Yoneda M, et al. Diagnostic accuracy of FibroScan-AST score to identify non-alcoholic steatohepatitis with significant activity and fibrosis in Japanese patients with non-alcoholic fatty liver disease: Comparison between M and XL probes. Hepatology Research. 2020;50:831–839. [DOI] [PubMed] [Google Scholar]
- 24.Anstee QM, Lawitz EJ, Alkhouri N, Wong VWS, Romero-Gomex M, Okanoue T, Trauner M, Kersey K, et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology. 2019;70:1521–1530. [DOI] [PubMed] [Google Scholar]
- 25.NHANES Questionnaires, Datasets, and Related Documentation. Accessed November 18, 2021. https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
- 26.Kim D, Cholankeril G, Loomba R, Ahmed A. Prevalence of fatty liver disease and fibrosis detected by transient elastography in adults in the United States, 2017-2018. Clin Gastroenterol Hepatol. 2021;19:1499–1501.e2. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Heredia NI, Balakrishnan M, Thrift AP. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: results from NHANES 2017–2018. PLOS One. 2021;16:e0252164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciardullo S, Monti T, Perseghin G. Prevalence of liver steatosis and fibrosis detected by transient elastography in adolescents in the 2017–2018 National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2021;19:384–390.e1. [DOI] [PubMed] [Google Scholar]
- 29.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717–1730. [DOI] [PubMed] [Google Scholar]
- 30.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski MS, Torriani FJ, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 32.Angulo P, Hui JM, Marchesini G, Bugianesi E, Geroge J, Farrell GC, Enders F, Saksena S, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 33.StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021. [Google Scholar]
- 34.Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the multi-ethnic study of atherosclerosis. World J Gastroenterol. 2014;20:4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG. Racial and Ethnic Disparities in Non-alcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of Transient Elastography for the Staging of Liver Fibrosis: A Meta-Analysis. Gastroenterology. 2008;134:960–974 e8. [DOI] [PubMed] [Google Scholar]
- 37.Wong VWS, Vergniol J, Wong GLH, Foucher J, Chan HLY, Bail BL, Choi PCL, Kowo M, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. [DOI] [PubMed] [Google Scholar]
- 38.Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Network Open. 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ampuero J, Aller R, Gallego-Durán R, Banales JM, Crespo J, García-Monzón C, Pareja M, Vilar-Gómez E, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment. Pharmacol. Ther. 2018;48:1260–1270. [DOI] [PubMed] [Google Scholar]
- 40.Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent Predictors of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2009;7:1224–1229 e2. [DOI] [PubMed] [Google Scholar]
- 41.Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond Pv. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1222–4. [DOI] [PubMed] [Google Scholar]
- 42.Fujii H, Fukumoto S, Enomoto M, Uchida-Kobayashi S, Kimura T, Tamori A, Nadatani Y, Takashima S, et al. The FibroScan-aspartate aminotransferase score can stratify the disease severity in a Japanese cohort with fatty liver diseases. Scientific Reports. 2021;11:13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–91. [DOI] [PubMed] [Google Scholar]
- 44.Wong RJ, Cheung R. Trends in the Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in the United States, 2011-2018. Clin Gastroenterol Hepatol. 2022;20:e610–e613. [DOI] [PubMed] [Google Scholar]
- 45.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65–76. [DOI] [PubMed] [Google Scholar]
- 46.Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12:e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Kim WR, Kim HJ, Therneau TM. Association between non-invasive fibrosis markers and mortality among adults with non-alcoholic fatty liver disease in the United States. Hepatology (Baltimore, MD). 2013;57:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D, Cholankeril G, Loomba R, Ahmed A. Prevalence of Nonalcoholic Fatty Liver Disease and Hepatic Fibrosis Among US Adults with Prediabetes and Diabetes, NHANES 2017-2018. J Gen Intern Med. 2022;37:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, Levick CK, Young LAJ, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2021;75:770–785. [DOI] [PubMed] [Google Scholar]
- 50.Noureddin N, Alkhouri N, Brown KA, Noureddin M. Driving nonalcoholic steatohepatitis forward using the FibroScan aspartate aminotransferase score, but obey the traffic lights. Hepatology. 2020;72:2228–30. [DOI] [PubMed] [Google Scholar]


