IMPORTANCE:
Troponin I is frequently elevated in sepsis, but optimal clinical approaches to diagnosis and management of troponin I during sepsis are unclear.
OBJECTIVES:
We aimed to describe the variation in troponin I measurement and the cardiovascular diagnostic and therapeutic approach to elevated troponin I among critically ill adults with sepsis.
DESIGN, SETTING, AND PARTICIPANTS:
Observational cohort study of the hospital-level variation in serial troponin I measurement, trending troponin I to peak, echocardiography, cardiac stress test, cardiac catheterization, antiplatelet agents, therapeutic anticoagulation, beta-blockers, and statins quantified using hospital median odds ratios—the median odds of receiving an intervention at randomly selected higher versus lower rate hospitals—derived from multivariable-adjusted random-effects logistic regression models with hospital site as the random effect. The Premier Healthcare Database was used. Patients were adults aged greater than 18 years admitted to the ICU with sepsis from 2016 to 2020.
MAIN OUTCOMES AND MEASURES:
The hospital-level median odds ratios of troponin I measurement as well as cardiovascular diagnostics and therapeutics.
RESULTS:
Among 85,830 adults with sepsis, 53,058 (61.8%) had a troponin I measured, with a median odds ratio of troponin measurement across hospitals of 5.30 (95% CI, 4.98–5.67). Among 27,665 adults (32.2%) with sepsis and an elevated troponin I level, 84.8% had serial troponin I measurements, 66.0% had troponin trended to peak level, 66.7% had an echocardiogram, 4.1% had a cardiac stress test, 6.6% underwent cardiac catheterization, 48.3% received antiplatelet agents, 8.3% received therapeutic anticoagulation, 50.5% received beta-blockers, and 38.1% received statins. The median odds ratios between hospitals for cardiovascular diagnostics and therapeutics ranged from 1.28 (95% CI, 1.24–1.32) for use of beta-blockers to 7.58 (95% CI, 6.43–8.77) for use of therapeutic anticoagulation.
CONCLUSIONS AND RELEVANCE:
Both troponin I measurement and the approach to an elevated troponin I among critically ill adults with sepsis varied widely across hospitals consistent with disparate practice and care efficiency. Prospective studies are needed to guide an informed approach to troponin I measurement and cardiovascular evaluation in sepsis.
Keywords: healthcare utilization, infection, sepsis, troponin I
KEY POINTS
Question: We hypothesize that troponin I measurement among critically ill patients with sepsis and the subsequent cardiovascular management response to an elevated troponin I varies widely and idiosyncratically across hospitals resulting in disparate practice and care efficiency.
Findings: In an observational cohort study of 85,830 critically ill adults with sepsis, 53,058 patients (61.8%) had troponin I measured, with a median odds ratio of troponin I measurement across hospitals of 5.30 (95% CI, 4.98–5.67). Among 27,665 patients (32.2%) with sepsis and an elevated troponin I level, the median odds ratios between hospitals for cardiovascular diagnostics and therapeutics ranged from 1.28 (95% CI, 1.24–1.32) for use of beta-blockers to 7.58 (95% CI, 6.43–8.77) for use of therapeutic anticoagulation.
Meaning: Establishing contemporary cardiovascular practice and identifying the determinants of practice variation can help guide future comparative effectiveness studies in myocardial injury during sepsis and subsequently guide implementation and/or deimplementation efforts toward an evidenced-based cardiovascular management approach to critically ill patients with sepsis.
Sepsis is the life-threatening organ dysfunction due to dysregulated host response to infection and is responsible for 1.7 million hospitalizations in the United States annually (1, 2). Cardiac troponin I, a cardiac regulatory protein used as a sensitive indicator of myocardial injury, is elevated in 50% of patients hospitalized with sepsis (3–5). Modest troponin I elevation during sepsis, colloquially termed “troponin leak,” is prognostic for hospital mortality and risks of cardiovascular complications following sepsis hospitalization (6, 7). Despite the frequency of troponin I elevation and the associated long-term cardiovascular morbidity, there are few recommendations on troponin I measurement or the management approach to elevated troponin I during sepsis, likely resulting in disparate practice, clinical outcomes, and care efficiency.
In order to inform knowledge gaps in the cardiovascular management approach to critically ill patients with sepsis, we sought to describe contemporary troponin I measurement practices as well as the cardiovascular diagnostic and therapeutic practice patterns among patients with sepsis and an elevated troponin I. We hypothesized that troponin I measurement among critically ill patients with sepsis as well as the use of cardiovascular diagnostic testing and cardiovascular therapeutics among critically ill patients with sepsis and an elevated troponin I would vary widely and idiosyncratically across hospitals after adjusting for patient- and hospital-level characteristics.
MATERIALS AND METHODS
This study was approved by the Boston Medical Center Institutional Review Board (H-41201, approved January 27, 2022, Characterizing contemporary practices in the approach to elevated troponin I levels during sepsis) with a waiver of informed consent due to the de-identified nature of the study. All research procedures were followed in accordance with the ethical standards of the Boston Medical Center Institutional Review Board and with the Helsinki Declaration of 1975.
Data Source
We used the Premier Healthcare Database (Premier Inc., Charlotte, NC) to perform a retrospective cohort study of critically ill adults with sepsis and an elevated troponin I. The Premier Healthcare Database is a United States hospital-based, service-level, all-payer database that contains de-identified administrative, healthcare utilization, and billing data from participating hospitals (8). The data contained within the Premier Healthcare Database represents approximately 20% of all hospitalized patients in nonfederal hospitals within the United States.
Sepsis Cohort
We identified a cohort of adults greater than 18 years old who were admitted to the medical ICU with sepsis between January 1, 2016, and December 31, 2020. Sepsis was defined using the sepsis clinical surveillance definition consisting of suspected serious infection (blood culture obtained and greater than 4 antibiotic days with at least 1 IV antibiotic day) and presence of acute organ dysfunction defined as a Sequential Organ Failure Assessment (SOFA) score of greater than 2 (1, 2, 9). Patient with sepsis near ICU admission were included if they had blood cultures and antibiotics within 48 hours of ICU admission and a SOFA score greater than 2 within 48 hours of antibiotic administration. We excluded patients who did not have laboratory or vital sign data available within the database needed to identify sepsis. Patients were excluded if they had a cardiac catheterization on the day of admission to exclude patients admitted for a primary coronary process rather than sepsis. In order to stabilize estimates of cardiovascular management practices, hospitals with less than 25 patients meeting sepsis criteria were excluded from the analysis. This “total sepsis cohort” was used in the first primary analysis to assess the practice variation in troponin I measurement within the first 14 days of ICU admission among all critically ill patients with sepsis.
Elevated Troponin I in Sepsis Cohort
Among those critically ill patients with sepsis and troponin I measured, a second cohort was created to include only patients with an elevated troponin I measured during sepsis admission. Patients with an elevated troponin I were identified by the presence of at least one troponin I level that exceeded the upper limit of normal of their individual hospital assay measured within 14 days of sepsis admission. Similar to the total sepsis cohort, hospitals with fewer than 25 patients meeting both sepsis and elevated troponin I criteria were excluded in order to stabilize estimates of hospital cardiovascular practice. This “sepsis with elevated troponin I” cohort was used to assess the practice patterns and hospital-level variation in cardiovascular diagnostic testing and cardiovascular therapeutics among patients with an elevated troponin I level during sepsis. Cardiovascular diagnostic testing of interest included: 1) serial troponin I measurement; 2) trending troponin I to peak; 3) echocardiography; 4) cardiac stress testing; and 5) cardiac catheterization. Cardiovascular therapeutics of interest included use of: 1) antiplatelet agents; 2) therapeutic anticoagulants; 3) beta-blockers; and 4) statins (cardiovascular diagnostics and therapeutics further defined in e-Table 1, http://links.lww.com/CCX/B127). Cardiovascular diagnostic testing and therapeutics were identified via charge codes within 7 days of the first elevated troponin I measurement in order to allow for associations between outcomes and the elevated troponin I level. Therapeutic anticoagulation was measured within 1 day of the first elevated troponin I to evaluate clinical interpretation of troponin I elevation for an acute coronary syndrome.
Covariates
Models were adjusted for variables, which could influence the cardiovascular management approach including admission year, patient characteristics (i.e., patient demographics, past medical comorbid conditions [10], organ support therapies received, and maximum admission SOFA score) and hospital characteristics (i.e., hospital community type, teaching status, hospital size, and geographic location). Organ support therapies were included if occurring at admission within the “total sepsis cohort” or prior to the first elevated troponin I within the “elevated troponin I in sepsis cohort.” Given the influence the magnitude of the first elevated troponin I level may have on subsequent troponin I measurements, the first elevated troponin I level was used as a covariate in models of serial troponin I measurement and models of trending troponin I levels to peak, whereas the maximum troponin I level was used in models of echocardiography, cardiac stress testing, cardiac catheterization, and cardiovascular therapeutics. Adjustment for both the first elevated troponin I level and maximum elevated troponin I level were not used for each model due to collinearity.
Statistical Analysis
Dichotomous and categorical variables were reported as counts with percentages, while continuous variables were reported as means with sds or median with interquartile range (IQR) based on the distribution. Multivariable-adjusted, random-effects logistic regression models, with hospital site as the random effect, were used to identify associations between patient- and hospital-level factors with troponin I measurement, cardiovascular diagnostics, and therapeutics. The hospital-level variation in troponin I measurement, cardiovascular diagnostics and cardiovascular therapeutics was quantified using the median odds ratio (11), which represents the median increase in odds of a patient receiving a cardiovascular test or treatment when moving from a randomly selected hospital with lower rates of cardiovascular testing or treatment to a hospital with higher rates of cardiovascular testing or treatment. All tests were two-sided and conducted using a significance level of 0.05. Statistical analyses were conducted using R (Version 4.1.0; RCore Team; Vienna, Austria).
Subgroup Analyses
We performed multiple subgroup analyses to assess the robustness of results. We assessed crude rates of cardiovascular diagnostics and therapeutics among all critically ill patients with sepsis stratified by the presence of a normal versus an elevated troponin I level with differences compared using Pearson chi-square test. Since those with prior structural or ischemic heart disease may be more likely to have an elevated troponin I when measured during sepsis, we evaluated cardiovascular diagnostic and therapeutic practice pattern variation stratified by history of prior cardiovascular disease with and without inclusion of cardiovascular disease equivalents (cardiovascular disease and equivalents defined in e-Table 2, http://links.lww.com/CCX/B127).
RESULTS
Patient Characteristics
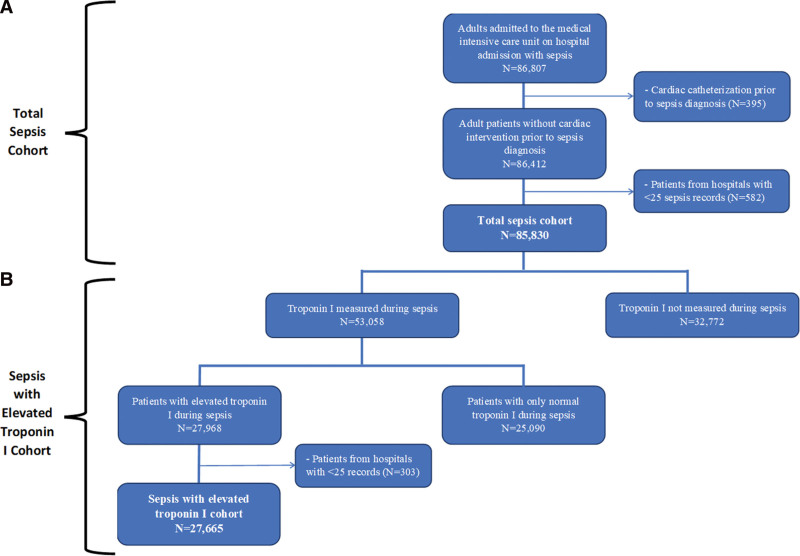
We identified 85,830 adults admitted to the ICU with sepsis between 2016 and 2020, across 149 U.S. hospitals who met study criteria (Fig. 1A). Patients were a median age of 66 years (IQR, 56–76 yr); 46.7% were female and 79.9% were White (Table 1). Among the 85,830 adults admitted to the ICU with sepsis, 53,058 (61.8%) had a troponin I measured and 27,665 (32.2%) had an elevated troponin I level within the first 14 days of sepsis hospitalization (Fig. 1B). The median time from sepsis diagnosis to troponin I measurement was 0 days (IQR, 0–0 d) with both sepsis diagnosis and troponin I measurement occurring predominantly on day 1 of ICU admission (IQR, 1–1). Baseline characteristics of sepsis with elevated troponin I cohort stratified by prior cardiovascular disease history and stratified by receipt of cardiovascular diagnostic or therapeutics are seen in e-Table 3 (http://links.lww.com/CCX/B127) and e-Tables 4 and 5 (http://links.lww.com/CCX/B127).
Figure 1.
Cohort assembly of critically ill adults with sepsis and troponin I measurement. A flow diagram identifying the two separate cohorts used within our study. A, The “Total Sepsis Cohort” identifies adults age greater than 18 yr admitted to the medical ICU with sepsis at admission. B, The “Sepsis with Elevated Troponin I Cohort” identifies adults age greater than 18 yr admitted to the medical ICU with sepsis at admission and an elevated troponin I within the first 14 d of sepsis hospitalization.
TABLE 1.
Baseline Characteristics of Critically Ill Adults With Sepsis
| Characteristics | Total Sepsis Cohort, n = 85,830 (100%) | Sepsis With Elevated Troponin I Cohort, n = 27,665 (32.2%) |
|---|---|---|
| Age (yr), median (IQR) | 66 (56–76) | 69 (59–78) |
| Sex (female), n (%) | 40,110 (46.7) | 12,530 (45.3) |
| Race, n (%) | ||
| White | 68,607 (79.9) | 21,852 (79.0) |
| Black | 10,561 (12.3) | 4,017 (14.5) |
| Other | 6,662 (7.8) | 1,796 (6.5) |
| Ethnicity (Hispanic), n (%) | 5,183 (6.0) | 1,571 (5.7) |
| Past medical comorbidities, n (%) | ||
| Myocardial infarction | 8,909 (10.4) | 7,104 (25.7) |
| Congestive heart failure | 33,485 (39.0) | 14,594 (52.8) |
| Cardiac arrhythmia | 30,712 (35.8) | 12,080 (43.7) |
| Peripheral vascular disease | 7,341 (8.6) | 2,971 (10.7) |
| Cerebrovascular disease | 4,217 (4.9) | 1,840 (6.7) |
| Pulmonary circulation disease | 10,679 (12.4) | 4,318 (15.6) |
| Diabetes mellitus | 37,369 (43.5) | 12,815 (46.3) |
| Chronic kidney disease | 30,078 (35.0) | 11,445 (41.4) |
| Hypertension | 62,799 (73.2) | 21,743 (78.6) |
| Chronic pulmonary disease | 34,200 (39.8) | 11,449 (41.4) |
| Liver disease | 13,766 (16.0) | 4,505 (16.3) |
| Vasopressors, n (%) | 31,033 (36.2) | 12,010 (43.4) |
| Invasive mechanical ventilation, n (%) | 32,146 (37.5) | 12,802 (46.3) |
| Hospital dialysis, n (%) | 2,914 (3.4) | 1,572 (5.7) |
| Maximum admission Sequential Organ Failure Assessment score, median (IQR) | 3 (2–5) | 4 (2–6) |
| Hospital community type: urban, n (%) | 72,995 (85.0) | 24,344 (88.0) |
| Teaching status: academic, n (%) | 42,903 (50.0) | 13,607 (49.2) |
| Hospital beds, n (%) | ||
| > 500 | 33,907 (39.5) | 9,557 (34.5) |
| 200–499 | 34,350 (40.0) | 12,383 (44.8) |
| < 200 | 17,573 (20.5) | 5,725 (20.7) |
| Geographic region, n (%) | ||
| South | 52,727 (61.4) | 17,783 (64.3) |
| Northeast | 10,877 (12.7) | 2,168 (7.8) |
| Midwest | 20,334 (23.7) | 7,633 (27.6) |
| West | 1,892 (2.2) | 81 (0.3) |
IQR = interquartile range.
Variation in Troponin I Measurement
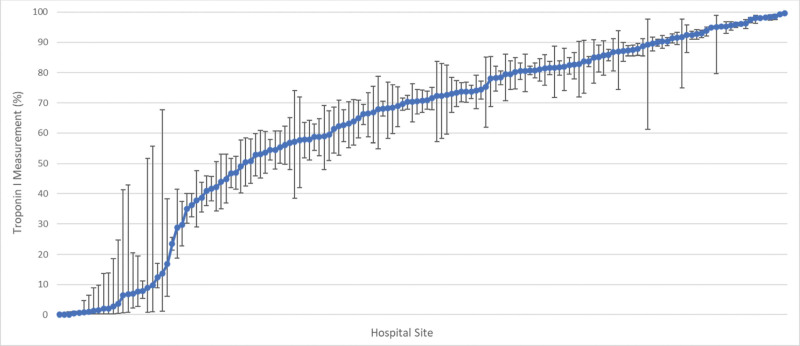
The multivariable-adjusted hospital-level percentage of troponin I measurement among critically ill patients with sepsis was 70.7% (IQR, 49.0–85.7%) (Fig. 2). The median odds ratio for troponin I measurement was 5.30 (95% CI, 4.98–5.67), representing the median odds of having a troponin I measured when being treated at randomly selected higher versus lower troponin I measurement rate hospitals. The patient- and hospital-level fixed effects of troponin I measurement among critically ill patients with sepsis are seen in e-Table 6 (http://links.lww.com/CCX/B127).
Figure 2.
Variation in troponin I measurement among critically ill adults with sepsis. Caterpillar plot of hospital-level troponin I measurement use adjusted by patient demographics, comorbidities, admission year, hospital site, organ support therapies received, maximum admission Sequential Organ Failure Assessment score, hospital mortality, hospital community type, hospital size, hospital teaching status, and hospital geographic region among critically ill adults with sepsis. The y-axis shows the adjusted hospital-level percent rate of troponin I measurement for each hospital. The x-axis shows individual hospitals treating critically ill adults with sepsis at admission, sorted in order of increasing percentage rate of troponin I measurement.
Cardiovascular Diagnostics and Therapeutics Among Patients With an Elevated Troponin I in Sepsis
Among the 27,665 (32.2%) of critically ill adults with sepsis and elevated troponin I, 84.8% had serial troponin I measurements, 66.0% had troponin trended to a peak level, 66.7% had an echocardiogram, 4.1% underwent cardiac stress testing, 6.6% underwent cardiac catheterization, 48.3% received antiplatelet agents, 8.3% received therapeutic anticoagulation, 50.5% received beta-blockers, and 38.1% received statins (Table 2). Of the 23,447 patients who had serial troponin I measured, 18,255 (77.9%) trended troponin I until a peak level was reached. Compared with patients with normal troponin I levels, those with elevated troponin I had significantly higher rates of serial troponin I measurement (84.6% vs 34.8%), troponin trended to peak (65.9% vs 21.8%), echocardiography (71.6% vs 45.9%), cardiac stress test (4.7% vs 1.9%), and cardiac catheterization (7.8% vs 0.8%). Similarly, crude rates of all cardiovascular therapeutics were greater among adults who had an elevated troponin I measured during sepsis compared with those with only normal troponin I levels (e-Table 7, http://links.lww.com/CCX/B127). Crude rates of all cardiovascular diagnostics and therapeutics were also greater among adults with a prior cardiovascular disease history when compared with those without a prior cardiovascular disease history (e-Table 8, http://links.lww.com/CCX/B127).
TABLE 2.
Cardiovascular Diagnostics and Therapeutics Among Critically Ill Adults With Sepsis and Elevated Troponin I
| Cardiovascular Management Approach | Sepsis With Elevated Troponin I |
|---|---|
| Total, n (%) | 27,665 (100) |
| Cardiovascular diagnostic testing | |
| Hospital day of first elevated troponin I, median (IQR) | 1 (1–1) |
| First elevated troponin I level (ng/mL), median (IQR) | 0.14 (0.07–0.47) |
| Hospital day of maximum troponin I level, median (IQR) | 1 (1–2) |
| Maximum troponin I level (ng/mL), median (IQR) | 0.28 (0.09–1.30) |
| Serial troponin I, n (%) | 23,447 (84.8) |
| Number of serial troponin I measurements after the first elevated troponin I, median (IQR) | 2 (1–3) |
| Trend troponin I to peak, n (%) | 18,255 (66.0) |
| Echocardiography, n (%) | 18,455 (66.7) |
| Cardiac stress test, n (%) | 1,128 (4.1) |
| Cardiac catheterization, n (%) | 1,829 (6.6) |
| Cardiovascular therapeutics, n (%) | |
| Antiplatelet agents, n (%) | 13,349 (48.3) |
| Therapeutic anticoagulation, n (%) | 2,293 (8.3) |
| Beta-blockers, n (%) | 13,967 (50.5) |
| Statins, n (%) | 10,541 (38.1) |
IQR = interquartile range.
Variation in Cardiovascular Diagnostics and Therapeutics in Patients With an Elevated Troponin I in Sepsis
There was wide variation among hospitals for all measured cardiovascular diagnostics and therapeutics. The median odds ratios between hospitals ranged from 1.28 (95% CI, 1.24–1.32) for use of beta-blockers to 7.58 (95% CI, 6.43–8.77) for use of therapeutic anticoagulation (Table 3). Subgroup analyses of model cohorts showed that the large variation seen in cardiovascular diagnostics and therapeutics among patients with an elevated troponin I did not differ when stratified by prior cardiovascular disease history (Table 3). The fixed effects for primary models are seen in e-Tables 9 and 10 (http://links.lww.com/CCX/B127).
TABLE 3.
Variation in Cardiovascular Management Strategies Among Critically Ill Adults With Sepsis and Elevated Troponin I
| Cardiovascular Management Approach | |||||
|---|---|---|---|---|---|
| Primary Analysisa | Subgroup Analysesa,b | ||||
| Sepsis With Elevated Troponin I | Cardiovascular Disease | No Prior Cardiovascular Disease | Cardiovascular Disease or Cardiovascular Disease Equivalents | No Prior Cardiovascular Disease or Cardiovascular Disease Equivalents | |
| Median OR (95% CI) | Median OR (95% CI) | Median OR (95% CI) | Median OR (95% CI) | Median OR (95% CI) | |
| Total, n (%) | 27,665 (100) | 21,974 (79.4) | 5,691 (20.6) | 24,816 (89.7) | 2,849 (10.3) |
| Cardiovascular diagnostics | |||||
| Serial troponin I | 1.95 (1.84–2.06) | 1.95 (1.83–2.08) | 1.78 (1.65–1.90) | 1.92 (1.82–2.05) | 1.62 (1.52–1.72) |
| Trend troponin I to peak | 1.71 (1.63–1.80) | 1.84 (1.74–1.94) | 1.63 (1.54–1.73) | 1.81 (1.73–1.91) | 1.64 (1.54–1.76) |
| Echocardiography | 1.50 (1.45–1.55) | 1.56 (1.50–1.62) | 1.56 (1.48–1.65) | 1.55 (1.49–1.61) | 1.55 (1.46–1.65) |
| Cardiac stress test | 3.30 (2.94–3.66) | 3.63 (3.18–4.13) | 3.92 (3.31–4.67) | 3.41 (3.01–3.91) | 4.38 (3.64–5.30) |
| Cardiac catheterization | 2.16 (1.99–2.34) | 2.18 (2.02–2.37) | 2.50 (2.23–2.82) | 2.20 (2.02–2.41) | 1.90 (1.73–2.09) |
| Cardiovascular therapeutics | |||||
| Antiplatelet agents | 1.28 (1.24–1.31) | 1.28 (1.24–1.31) | 1.26 (1.22–1.30) | 1.27 (1.23–1.30) | 1.40 (1.34–1.46) |
| Therapeutic anticoagulation | 7.58 (6.43–8.77) | 8.98 (7.65–10.69) | 5.59 (4.61–6.70) | 9.02 (7.65–10.89) | 7.10 (5.67–8.87) |
| Beta-blockers | 1.28 (1.24–1.32) | 1.29 (1.25–1.33) | 1.19 (1.16–1.22) | 1.28 (1.25–1.32) | 1.19 (1.16–1.23) |
| Statins | 1.32 (1.28–1.36) | 1.35 (1.30–1.39) | 1.18 (1.15–1.20) | 1.34 (1.30–1.38) | 1.04 (1.03–1.05)c |
OR = odds ratio.
Covariates: Patient-level factors such as age, sex, race, ethnicity, history of myocardial infarction, congestive heart failure, cardiac arrhythmia, peripheral vascular disease, cerebrovascular disease, pulmonary circulation disease, diabetes mellitus, chronic kidney disease, hypertension, chronic pulmonary disease, liver disease, admission year, organ support therapies received, maximum admission Sequential Organ Failure Assessment score, and either first elevated troponin I level or maximum troponin I level. Facility-level factors such as hospital community type, hospital teaching status, hospital beds, and hospital geographic region.
Hospital geographic region excluded from all subgroup analyses to improve model fit.
Facility-level variables excluded from statin models of the no cardiovascular disease or cardiovascular disease equivalents cohort to avoid model singularity.
DISCUSSION
In a large, multicenter cohort study of more than 85,000 critically ill adults with sepsis, there was wide variation in troponin I measurement and in the cardiovascular management approach to patients with an elevated troponin I. The variation seen in the cardiovascular practice remained large across subgroup analyses after stratifying patients by the presence of preexisting cardiovascular disease. While the characteristics of the patient should determine variation in clinical practice, our findings quantify highly variable cardiovascular practices driven largely by hospital site of admission and highlights the need for an evidence-based cardiovascular management approach for patients with sepsis.
Consistent with prior studies of critically ill adults with sepsis, troponin I was measured in the majority of patients, with greater than 50% having an elevated troponin I (5). Our study is novel in quantifying the wide variation in troponin I measurement rates across hospitals after adjusting for patient- and hospital-level characteristics. The variation exemplifies the ongoing debate over the utility of troponin I measurement in sepsis (12–15). Outside of its utility for prognostication of mortality and post-sepsis cardiovascular events (6, 7), proponents of troponin I measurement in sepsis support its use as a marker of sepsis-induced organ damage and a possible perfusion target for resuscitation when elevated, based on limited observational studies (12, 13). Conversely, indiscriminate troponin I ordering is associated with significant hospital cost and healthcare resource utilization without evidence that it changes clinical management or results in improved patient outcomes (14–16). The unclear etiology of troponin I release in sepsis muddles the interpretation of the test’s clinical significance in sepsis. Potential mechanisms include myocardial injury from infection or inflammation, oxygen supply/demand mismatch, microvascular dysfunction, or renal dysfunction (17–19). Although acute coronary syndrome is rarely thought to be the source of an elevated troponin I level in sepsis (20), elevated troponin during sepsis is associated with incident cardiovascular events in the year after sepsis (7). However, patients with elevated troponin are more likely to receive acute coronary syndrome therapies such as antiplatelet and anticoagulant therapies, which have unclear benefit in this population and likely have patient-level risks. Given the unclear mechanisms of troponin I release in sepsis, the optimal cardiovascular management approach during sepsis hospitalization and following hospital discharge is unclear.
The lack of clarity in mechanisms and management of troponin elevation during sepsis was shown in the wide variation in approaches to measurement of troponin and cardiovascular interventions among patients with an elevated troponin seen within our study. The observed high rates of troponin ordering with low rates of subsequent evaluation of elevated troponin levels or change in treatment suggests potential for overuse of troponin testing in sepsis. However, evidence for troponin as a prognostic marker of short- and long-term cardiovascular complications warrants further evaluation of the optimal diagnostic and treatment approaches to troponin elevation in sepsis. The practice variation following elevated troponin levels in both patients with and without prior cardiovascular disease suggests that clinicians may also consider additional clinical information (e.g., electrocardiogram findings) or have differing troponin I level thresholds to trigger further cardiovascular intervention, regardless of a patient’s prior cardiovascular disease history. Further studies could leverage the observed cardiovascular practice variation as natural experiments to identify likely effective approaches that optimize targeted cardiovascular interventions to improve both inpatient and post-hospitalization cardiovascular morbidity. Additionally, qualitative interviews with practicing clinicians are needed to enhance our understanding of the driving factors behind the cardiovascular practice variation, which can then inform future studies.
Strengths
The Premier Healthcare Database is an extensive administrative database that allowed for development of a robust and detailed sepsis cohort. Models were adjusted for by a wide range of patient and facility characteristics, which could influence cardiovascular practice in sepsis with the use of median odds ratios to quantify the contribution of hospital-driven practice variation. We performed robust subgroup analyses, which showed wide variation in outcomes even when stratified by past cardiovascular disease history. Additionally, we identified elevated troponin I using each hospitals’ upper limit of normal to evaluate cardiovascular practices within each hospital.
Limitations
There are several limitations to this study. First, our findings likely underestimate the practice variation seen with use of high-sensitivity troponin I testing, where the improved analytic sensitivity of the assay can identify lower levels of troponin I that may not necessarily represent cardiac injury and likely result in more variable practice (21). Second, unmeasured clinical factors may have impacted practice and effect estimates of risk-adjusted practice variation. Third, the hospitals within the Premier Healthcare Database may not be representative of all U.S. hospitals. Last, without access to outpatient pharmacy records, we could not differentiate chronic cardiovascular medications from new prescriptions during sepsis. However, subgroup analyses stratified by prior cardiovascular disease history showed similar wide variation in cardiovascular therapeutics, and therapies among patients with sepsis and elevated troponin differed from patients with sepsis but without elevated troponin.
CONCLUSIONS
Among a cohort of over 85,000 critically ill adults admitted to the medical ICU with sepsis, there was wide variation in troponin I measurement attributable largely to the hospital site of admission. Furthermore, there was large variation in the cardiovascular diagnostic testing and therapeutic management approach to elevated troponin I in sepsis. Overall, the idiosyncratic practice patterns suggest potential overuse of troponin testing in sepsis without a change in subsequent cardiovascular diagnostics and therapeutics. Benchmarking cardiovascular practice and identifying the determinants of variation is essential to guide future comparative effectiveness studies in myocardial injury during sepsis and subsequently direct implementation and/or deimplementation efforts toward an evidenced-based cardiovascular management approach to critically ill patients with sepsis. Prospective investigation leveraging the observed practice variation is needed to identify the optimal cardioprotective management approach to minimize sepsis-associated cardiovascular morbidity without undue harm and cost.
Supplementary Material
Footnotes
Dr. Garcia receives funding from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant F32HL160062. Dr. Bosch receives funding from NIH/NHLBI grants OT2HL156812 and R01HL151607, Department of Defense grant W81XWH2110593, and Gilead Sciences. Dr. Peltan receives funding from the NIH/National Institute of General Medical Studies grant K23GM129661, Centers for Disease Control and Prevention, and Janssen and support to his institution from Asahi Kasei Pharma and Regeneron, all outside the present work. Dr. Walkey receives funding from the NIH/NHLBI grants R01HL151607, R01HL139751, R01HL136660, Agency of Healthcare Research and Quality, R01HS026485, Boston Biomedical Innovation Center/NIH/NHLBI 5U54HL119145-07, and royalties from UptoDate.
Drs. Garcia and Walkey were involved in conceptual design, data acquisition, analysis, interpretation, and article preparation. Dr. Bosch was involved in data acquisition and analysis. All authors listed have been involved in revising the article for important intellectual content, approved the final version submitted for publication and are in agreement with the accuracy and integrity of the submitted work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Singer M, Deutschman CS, Seymour C, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program: Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017; 318:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S, Jackson PG, Makan J: Cardiac troponins. J Clin Pathol 2004; 57:1025–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim W, Qushmaq I, Devereaux PJ, et al. : Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med 2006; 166:2446–2454 [DOI] [PubMed] [Google Scholar]
- 5.Ammann P, Fehr T, Minder E, et al. : Elevation of troponin I in sepsis and septic shock. Intensive Care Med 2001; 27:965–969 [DOI] [PubMed] [Google Scholar]
- 6.Ou SM, Chu H, Chao PW, et al. : Long-term mortality and major adverse cardiovascular events in sepsis survivors. A nationwide population-based study. Am J Respir Crit Care Med 2016; 194:209–217 [DOI] [PubMed] [Google Scholar]
- 7.Garcia MA, Rucci JM, Thai KK, et al. : Association between troponin I levels during sepsis and postsepsis cardiovascular complications. Am J Respir Crit Care Med 2021; 204:557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premier Healthcare Database: Data That Informs and Performs. Premier Applied Sciences®, Premier. 2020. Available at: https://learn.premierinc.com/white-papers/premier-healthcaredatabase-whitepaper. Accessed May 8, 2022
- 9.Vincent J-L, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 11.Merlo J, Chaix B, Ohlsson H, et al. : A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frencken JF, van Baal L, Kappen TH, et al. ; Members of the MARS Consortium: Myocardial injury in critically ill patients with community-acquired pneumonia. A cohort study. Ann Am Thorac Soc 2019; 16:606–612 [DOI] [PubMed] [Google Scholar]
- 13.Bonk MP, Meyer NJ: Troponin I: A new marker of sepsis-induced hypoperfusion? Ann Am Thorac Soc 2019; 16:552–553 [DOI] [PubMed] [Google Scholar]
- 14.Aberegg SK, Kaufman DA: Troponin in sepsis. Ann Am Thorac Soc 2019; 16:1335–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siuba MT, Farkas JD: Against another nonspecific marker of perfusion. Ann Am Thorac Soc 2019; 16:1334–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson N, Ives ST, Carlson MD, et al. : Should we monitor troponin up to peak value when evaluating for acute coronary syndrome? Cleve Clin J Med 2020; 87:480–482 [DOI] [PubMed] [Google Scholar]
- 17.Favory R, Neviere R: Significance and interpretation of elevated troponin in septic patients. Crit Care 2006; 10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammarsten O, Mair J, Möckel M, et al. : Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018; 23:725–734 [DOI] [PubMed] [Google Scholar]
- 19.Frencken JF, van Smeden M, van de Groep K, et al. ; MARS Consortium: Etiology of myocardial injury in critically ill patients with sepsis: A cohort study. Ann Am Thorac Soc 2022; 19:773–780 [DOI] [PubMed] [Google Scholar]
- 20.Altmann DR, Korte W, Maeder MT, et al. : Elevated cardiac troponin I in sepsis and septic shock: No evidence for thrombus associated myocardial necrosis. PLoS One 2010; 5:e90171–e90175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brush JE, Kaul S, Krumholz HM: Troponin testing for clinicians. J Am Coll Cardiol 2016; 68:2365–2375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.