Abstract
Although there are many assessment tools for upper extremity (UE) function, it is still difficult to select an appropriate outcome measurement for the rehabilitation process of individuals with stroke. This review aims to classify each tool within the International Classification of Functioning, Disability and Health (ICF) framework and provide an overview of UE assessments. Through a comprehensive understanding of assessments based on ICF, health care professionals will be able to choose suitable measurement tools for individuals, facilitating their rehabilitation.
Keywords: Stroke, Outcome Assessment, Upper Extremities, Rehabilitation, Stroke Rehabilitation
Highlights
• This review contains overview of upper extremity assessment according to International Classification on Functioning, Disability and Health framework.
• We provide information about the availability in Korean version of the test.
INTRODUCTION
The comprehensive functional assessment of the upper extremity (UE) is important in both clinical and research settings, since it allows an in-depth understanding of individuals’ function, rather than of the disease per se, and facilitates setting appropriate goals, planning interventions based on function, and tracking progress [1]. Numerous assessments of the UE have been developed and evaluated in terms of reliability and validity; nonetheless, it remains difficult to choose the most suitable outcome measure for individuals [2].
The International Classification of Functioning, Disability and Health (ICF), which is established by the World Health Organization (WHO), classifies human functioning and disability rather than disease, which is the role of the International Classification of Diseases. Outcome measures can be categorized into three ICF domains: body functions/structures, activities, and participation [3]. Referring to a previous systematic review that identified the most frequently cited UE measures in stroke [2], thirteen outcome measures were chosen for this study. The present review aims to provide an overview of UE functional assessments and categorize each measure within the ICF framework.
IMPAIRMENT (BODY FUNCTIONS AND BODY STRUCTURES)
According to the WHO [3], body functions are defined as “the physiological functions of body systems (including psychological functions)” (p.49). Impairment means having troubles in body function or structure due to an abnormal condition or critical injuries. Most standardized assessments quantify the extent to which the body function and structure are impaired [3].
Fugl-Meyer Assessment (FMA)
The FMA is the most commonly used tool in the field of stroke rehabilitation to measure overall motor impairment [4]. It follows the concept of recovery stages among people with stroke, which originated from Twitchell and Brunnstrom [5,6]. It measures the isolated joint control ability, which is hampered by synergies, as well as muscle strength, with a hypothesized ranked ordering of difficulty. This assessment is composed of five components: motor function, balance, sensory function, joint range of motion (ROM), and joint pain [7]. At present, only the motor function domain of the FMA is commonly measured, but not other components. The upper and lower extremity section of the FMA are frequently administered separately, and the entire assessment takes around 30–35 minutes to conduct [8]. The FMA-UE is divided into total, proximal, and distal parts, and these subtests are examined separately to detect the movement of body components because the authors suggested that each part of the UE recovers independently. The FMA has also shown excellent inter- and intra-rater reliability, as well as construct validity. Furthermore, the FMA is applicable to every recovery stage from acute to chronic [9]. The minimal clinically important difference (MCID) of the FMA-UE depends on the level of impairment; in those with minimal to moderate chronic stroke, it is 5.25 points, while in those with moderate to severe subacute stroke, it has a range from 4.9 to 12.4 depending on the body part of the UE: 12.4 for the upper limb, 5.6 for the upper arm, and 4.9 for the wrist/hand [10,11]. Furthermore, for those with severe subacute stroke, the MCID is 9 to 10 [12].
To cover both the domains of body function/structures and activity/participation in the ICF model, the FMA is utilized with other measurements of activity/participation such as the Action Research Arm Test (ARAT), Wolf Motor Function Test (WMFT), and Motor Activity Log (MAL). The most commonly used combination in research is the ARAT and FMA [13]. Although special training or certificates to administer the FMA are not necessary, See and Dodakian [14] provided a standardized training on the FMA in order to improve the accuracy of clinical assessments and maximize its use. Kim and Hwang [15] recently developed the Korean version of the FMA with a standardized training manual and demonstrated its reliability and validity. It is posted on the website of the University of Gothenburg (https://www.gu.se/en/neuroscience-physiology/fugl-meyer-assessment) along with versions in other languages [14].
Kinematics/Kinetics
Several studies have indicated that kinematic/kinetic parameters are classified into the body function and structure domain in the ICF [16,17,18]. Kinematic/kinetic assessments are needed for objective and sensitive measurements of UE movement and to distinguish true recovery (or restitution) from behavioral compensation [18]. Kwakkel et al. [18] established a standardized assessment of the quality of UE movements in stroke patients. They recommended that 2-dimensional kinematic assessments such as planar reaching tasks and finger individuation should be carried out on both the affected and the less affected arms at 1, 12, and 26 weeks after stroke onset, and at least 15 times per target in order to obtain accurate, reliable, and valid statistics. Furthermore, they explained a detailed assessment protocol, including appropriate postures and the measurement method for the standardized assessment. Along with the FMA, kinematic assessments have been increasingly used in research [13], and the rapid development of sensing technologies may accelerate kinematic and kinetic assessments to capture changes in body function/structure more sensitively.
ACTIVITY
Activity is defined as “the execution of a task or action by an individual” p.127. Activity limitations can be termed as a condition in which individuals have trouble carrying out activities [3].
ARAT
The ARAT was designed to assess arm motor function in terms of movement quality. It observes 19 items, containing four categories of functional movement (grip, grasp, pinch, and gross movements) on a 4-point scale, from 0 (no movement) to 3 (the movement is performed normally). A strength of this assessment is that the items in each subscale are organized in descending order of difficulty; in other words, it allows individuals to perform the most difficult task first, consequently enabling the prediction of success with the next items and thereby improving the efficiency of the assessment. As a result, the time for administration is about 5–15 minutes in total, depending on the number of items performed [19]. The intra-and inter-rater reliability of the ARAT was demonstrated to be high (intraclass correlation coefficient [ICC] > 0.098), and ARAT scores were proven to be closely correlated with other UE assessments such as the FMA. Furthermore, the clinically meaningful difference using this tool was determined to be 5.7 points [20,21]. The ARAT also includes a subscale that evaluates several musculoskeletal conditions, for which reason it also overlaps with the body function and structure category in the ICF classification [2]. No training for administration is required, but a quick instruction video is available on the official website (http://www.aratest.eu) if needed. Researchers at Soonchunhyang University recently developed standardized instructions in Korean (SCH-ARAT) to ensure accurate assessments. To administer the ARAT, it is necessary to purchase the corresponding equipment.
WMFT
The WMFT consists of 15 functional tasks, including proximal joint movements, hand function, and 2 strength measurements. An advantage of the WMFT is that clinicians are able to assess diverse aspects of UE function, including ROM, strength, manipulation, and coordination [22]. The WMFT can be scored with the time to finish each task and a 6-point functional ability scale from 0 (cannot be performed) to 5 (normal performance). Items are arranged from simple to complex joint movements, in order of difficulty, and each task should be completed within 120 seconds; if a subject fails to complete a task in time, a score of 1 is given [22,23]. Thus, it complements the limitations of the Jebsen-Taylor Hand Function Test (JHFT), which measures only performance time, so that the assessment can be used to understand patients’ functional ability and changes therein. Moreover, it has been reported that the WMFT and the MAL are commonly used together in many studies to cover two domains of the ICF [13]. According to Morris and Uswatte [22], the WMFT demonstrates high inter-rater reliability, internal consistency, and test-retest reliability. Additionally, the estimated MCID of the WMFT in the stroke population is 1 point for the dominant hand and 1.2 points for the non-dominant hand [24]. The overall score of the WMFT considering both the mean score of each item and the median score of performance time could predict individuals’ functional ability. Even though the WMFT requires particular materials for administration, such as checkers, a face towel, and a basket, these are available without the need to purchase test-specific equipment. Furthermore, the standardized set-up template is needed in order to place test objects in an appropriate spot when administering the test. The Korean version of the WMFT has been developed, and it has been confirmed to have only high inter- and intra-rater reliability, but also an excellent correlation with the FMA [25,26].
JHFT
The JHFT consists of seven items that require subjects to execute several functional hand tasks in relation to activities of daily living (ADL) while the required time is recorded for each item. The test can be administered quickly and easily; it takes approximately 15 minutes to complete, and the materials needed for testing are generally accessible [27,28]. The JHFT has normative measurement data for standardized performance; thus, the performance of one subject can be determined using the test. A systematic review reported that the use of the JHFT has been decreasing over time; nevertheless, it is frequently used as an indicator of outcomes in both research and clinical settings, especially for patients with stroke or cerebral palsy [29,30]. Each subscale of the JHFT has demonstrated adequate to excellent test-retest reliability, and the total score of the JHFT showed good to excellent test-restest reliability for both dominant and non-dominant hands (ICCs = 0.84–0.97) [27,31]. Since research has proven that performance ability increases if the JHFT is administered more than four times, it is recommended to consider the practice-effect when interpreting outcome results obtained with the JHFT [32]. In Korea, a standardized scoring system was established for both non-disabled adults and children considering the physical characteristics of Koreans [33,34].
Purdue Pegboard Test (PPT)
The PPT was originally developed as a screening test for manual dexterity in industrial workers performing simple manual labor [35], but it has been broadly used to evaluate hand dexterity in many clinical settings with diverse populations, especially among patients who have cerebral lesions and deficits [36]. It comprises 4 subtests, including placing pins at the holes and assembling each component, and it takes less than 10 minutes to perform, including the instructions. Interestingly, a study discovered that the PPT required more cognitive speed and attention management with hand function in comparison to other UE assessment tools and was useful for anticipating the complex dexterity function needed for daily activities [37]. In Korea, Kang [38] developed a standardized version of the PPT for occupational evaluations of individuals with disability. Since the norms of the PPT in the original article are outdated, however, a study updated the norms for only the assembly task of the PPT with 150 healthy individuals [39].
Nine Hole Peg Test (NHPT)
The NHPT is reported to be the second most frequently used evaluation tool among occupational therapists following the FMA [30]. The NHPT was developed to examine hand dexterity by asking patients to place nine pegs into the holes of a small container as quickly as possible using one hand [40]. It is a cost-effective, simple, and efficient measurement that is applicable to all age groups [41]. The NHPT revealed excellent test-retest reliability for both acute and chronic stroke patients (ICC = 0.85) [42] and adequate correlations with the PPT (r = −0.75 to −0.74) [41]. However, it might not be sensitive enough to find subtle changes because it tracks relatively simple repetitive movements [43].
Box and Block Test (BBT)
The BBT is commonly used in both clinical and research settings because it is a simple, low-cost, and effective outcome measure to assess manual dexterity. Participants are required to transfer as many small cubes as possible from one box to the other within 60 seconds [44,45]. The score can be calculated by counting the number of blocks successfully transferred by the participants and comparing them with normative data; a higher score indicates better dexterity of the hand. Chen et al. [42] reported that the number of boxes considered as a minimal detectable change (MDC) was 5.5 in the stroke population. The time to administer the BBT is less than 5 minutes, and training to administer the BBT is not mandatory. The test-retest reliability and construct validity of the BBT have been verified in diverse populations, including older adults, children, and patients with stroke [44,45,46,47]. Because a previous study found that the score of the BBT was not sensitive to the change of UE function until 5 weeks after onset for stroke patients in the acute phase [48], the BBT is an effective tool to identify patterns of change in the hand dexterity of stroke patients in at least the subacute stage.
MAL
The MAL is a semi-structured interview measure of an individual’s functional UE performance in real life to examine the amount of use (AOU) and quality of movement (QOM). The original version of the MAL consists of 30 items, and there are several different versions of the MAL with different numbers of items [49,50]. Each subscale of AOU and QOM is scored using a 6-point system ranging from 0 (never use) to 5 (same use as pre-stroke). A score closer to 5 suggests greater movement quality and increased usage of the affected arm and hand. The MAL requires minimum 10 minutes to administer, and any health professional who has thoroughly reviewed the instructions can administer it [49]. The MDC for the AOU and QOM is estimated as 16.8% and 15.4% of the original scores, respectively. Its correlation with the ARAT is 0.63, and the test-retest reliability of the QOM scale is 0.91 [51,52]. The MAL measurements can be easily conducted because the MAL does not require any equipment other than evaluation sheets and can be administered in both clinical and home settings. Since no specific training is required to administer the MAL, any therapist who reviews the instructions and relevant literature can use this tool.
Functional Independence Measure (FIM)
The FIM is extensively used as a functional-level assessment tool to monitor changes in patients’ functional status during the rehabilitation process [53]. A systematic review reported that the FIM is useful in clinical practice because it can predict the post-stroke prognosis quite accurately [54]. The FIM contains 18 items, including self-care, sphincter control, transfer, locomotion, communication, social interaction, and cognition; 13 items are related to motor function impairment and 5 items assess cognitive function. Each item can be rated on a 7-point scale ranging from 1 to 7. A score of 1 means “total assistance” (performs less than 25% of the task), while a score of 7 is defined as “complete independence” [55]. According to Black, Soltis [56], a FIM score of 80 or above at discharge showed high specificity and sensitivity when it came to patients being discharged to their homes. In a quantitative review of studies analyzing the reliability of the FIM, the median inter-rater reliability and test-retest reliability were both 0.95 [57]. Another study indicated that the correlation between motor FIM and the Barthel Index (BI) in post-stroke patients was 0.92 at admission and 0.94 at discharge [58]. The MCID of the FIM in patients with stroke was identified as 22, 17, and 3 for the total FIM, motor subscale, and cognitive subscale, respectively [59]. The FIM is designed to be completed in 30 to 45 minutes, which is very time-consuming and laborious. Moreover, this instrument should be administered by trained professionals [58]. At present, there are several restrictions to using the FIM in Korea since it is necessary to pay a fee to the original author and be trained [60].
Modified Barthel Index (MBI)
The MBI is an assessment tool that has been modified from the BI to evaluate the extent to which individuals independently perform ADL. It covers 10 items in the ADL area, including feeding, personal hygiene, bathing, dressing, toilet transfer, bladder and bowel control, chair/bed transfers, stair climbing, and ambulation. Each item has a 5-point scale and the range of the total score is from 0 to 100. It measures what patients actually do, not what they could do; they are allowed to use assistive aids during the test. Higher scores indicate more independence, while lower scores imply less independence in performing ADL [61]. Hsieh et al. [62] suggested that the estimated MCID was 1.85 points in patients with stroke. The Korean version of the MBI has been developed, with excellent inter-rater reliability (with Kendall coefficients ranging from 0.93 to 0.98, p < 0.01) and good intra-rater reliability (0.87 to 1.00, p < 0.01) [60]. This assessment could be administered via self-report and observation by professionals, and the administration time varies depending on the client’s ability [63].
PARTICIPATION
Participation refers to being involved in a life circumstance from the standpoint of society. The term “participation restriction” denotes problems that an individual may experience when participating in life situations [3].
Stroke Impact Scale (SIS)
The SIS is a self-report questionnaire that assesses disability and health-related quality of life after stroke, covering both the activity and participation domains in the ICF framework. It assesses eight domains (strength, hand function, ADL/instrumental ADL [IADL], mobility, communication, emotion, memory and thinking, and participation) on a 5-point scale ranging from 1 to 5, as well as a visual analog scale of 0 to 100 on which patients rate their overall level of recovery from stroke [64]. The latest version, SIS 3.0, is typically administered within 15 to 20 minutes and does not require any professional training. However, a short-form SIS consisting of 16 questions is also frequently used to reduce the administration time and relieve respondents’ fatigue [65,66,67]. The MCIDs for four domains in relation to physical function (strength, hand function, ADL/IADL, and mobility) are 9.2, 5.9, 4.5 and 17.8 points, respectively [68]. Duncan et al. [65] demonstrated that the SIS was more valid, reliable, and sensitive to changes in patients with stroke than other measurements such as the BI and Short Form-36. Since the SIS is a self-reported assessment, it is not appropriate for those in the acute phase or who have cognitive/communication problems. Consequently, the SIS is expected to result in an insightful outcome when administered to patients with minimal to no persistent deficits [69]. The Korean version of the SIS 3.0 has been developed and confirmed to have high reliability and validity [70].
The Assessment of Motor and Process Skills (AMPS)
The AMPS is an observation-based assessment that occupational therapists use to evaluate the quality of ADL task performance in terms of effort, efficiency, safety, and need for assistance in a familiar environment. The assessment includes 2 areas of performance skills: motor and process skills. Motor skills are observable goal-directed actions performed by individuals to move or handle their bodies and objects during task performance (e.g., walking, reaching, manipulating, and lifting), while process skills refer to the ability to manage actions in sequential order to complete tasks, such as choosing appropriate tools, gathering ingredients, and organizing spaces. Although the AMPS has not been translated into Korean, it can be utilized in the domestic environment because it was designed to be applicable internationally and cross-culturally (with flexible available options for cultural differences). Only therapists who are trained by the CIOTS (the AMPS provider) can administer the AMPS according to the standardized manual. Special equipment is not needed, and the AMPS can be administered within 30–40 minutes in any setting in relation to the task. As a disadvantage of the AMPS, documentation must be conducted using computer-scoring software in order to obtain valid determinations of the quality of performance and severity compared to normative data [71]. Another limitation is that the AMPS manual and software are not available in Korean.
Canadian Occupation Performance Measure (COPM)
The COPM is a semi-structured, client-centered, and individualized assessment that focuses on clients’ own roles and role expectations within a personal context, measuring outcomes in three occupational performance areas: self-care, productivity, and leisure. Clients are asked to prioritize important and challenging aspects of occupational performance and to score their level of satisfaction with current performance, using a 10-point scale ranging from 1 to 10. Problems identified from the COPM can be a basis for the individual’s goals and incorporated into the intervention plan [72]. The test-retest reliability of the COPM showed excellent results for both performance (r = 0.89) and satisfaction areas (r = 0.88) in stroke patients, while its validity relative to other assessment tools, such as the Klein-Bell ADL (KB-ADL), FIM, and Satisfaction with Performance Scale Questionnaire (SPSQ) was not significant [73,74,75]. A change of 2 or more points in both performance and satisfaction areas is clinically significant [76]. Aside from the fact that the COPM is time-consuming (about 20–40 minutes to perform) and difficult to administer, it has the advantage of being applicable to all clients regardless of diagnosis. Moreover, formal COPM training is not required [77]. Currently, an official Korean version of the COPM measure and manual are available for purchase (https://www.thecopm.ca/buy/translations/).
CONCLUSION
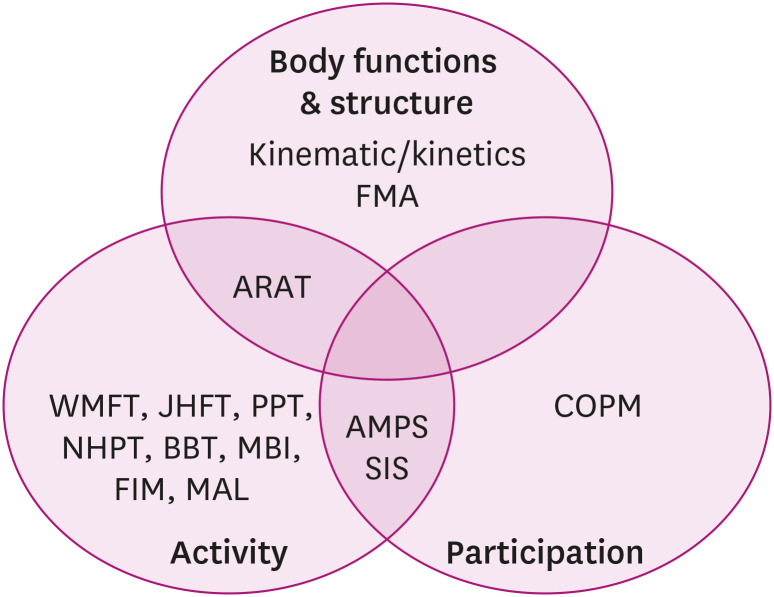
In summary, numerous UE assessments with solid validity and reliability are available in the field of rehabilitation. This paper identifies the characteristics of each UE assessment, categorizes each tool within the ICF framework (Figs. 1 and 2) and provides information about the availability of a Korean version of the test (Table 1). A comprehensive approach to assessments based on the ICF domains is necessary for health care professionals to thoroughly determine individuals’ optimal status in an objective manner. Therefore, an understanding of those assessments allows health care professionals to select appropriate assessment tools for people with specific conditions in accordance with a specific purpose.
Fig. 1. Upper extremity outcome measures according to the ICF framework.
ICF, International Classification of Functioning, Disability and Health; FMA, Fugl-Meyer Assessment; ARAT, Action Research Arm Test; WMFT, Wolf Motor Function Test; JHFT, Jebsen-Taylor Hand Function Test; PPT, Purdue Pegboard Test; NHPT, Nine Hole Peg Test; SIS, Stroke Impact Scale; BBT, Box and Block Test; AMPS, Assessment of Motor and Process Skills; MBI, Modified Barthel Index; FIM, Functional Independence Measure; MAL, Motor Activity Log; COPM, Canadian Occupational Performance Measure.
Fig. 2. A diagram of overlapping upper extremity outcome measures within the ICF framework.
ICF, International Classification of Functioning, Disability and Health; FMA, Fugl-Meyer Assessment; ARAT, Action Research Arm Test; WMFT, Wolf Motor Function Test; JHFT, Jebsen-Taylor Hand Function Test; PPT, Purdue Pegboard Test; NHPT, Nine Hole Peg Test; BBT, Box and Block Test; MBI, Modified Barthel Index; FIM, Functional Independence Measure; MAL, Motor Activity Log; AMPS, Assessment of Motor and Process Skills; SIS, Stroke Impact Scale; COPM, Canadian Occupational Performance Measure.
Table 1. An overview of upper extremity functional assessments.
| UE assessment | ICF domain | Year of development | Time to administer (min) | Required training | Score range | Total score | Normative data | MCID | Equipment purchase | Korean version |
|---|---|---|---|---|---|---|---|---|---|---|
| FMA (UE) | Body function & Structure | 1975 | < 30 | No | 0–2 | 66 | No | 4.9–12.4 | No | Yes |
| ARAT | Body function & Structure activity | 1981 | 5–15 | No | 0–3 | 57 | No | 5.7 | Yes | Yes |
| WMFT | Activity | 1995 | 30 | No | 0–5 | 75 | No | 1–1.2 | No | Yes |
| JHFT | Activity | 1969 | 15 | No | Yes | Yes | Yes | |||
| PPT | Activity | 1948 | < 10 | No | Yes | Yes | ||||
| NHPT | Activity | 1971 | < 10 | No | Yes | Yes | ||||
| BBT | Activity | 1985 | < 5 | No | Yes | 5.5 | Yes | |||
| MAL | Activity | 1993 | > 10 | No | 0–5 | 150 | No | 25.2 (AOU) | No | No |
| 23.1 (QOM) | ||||||||||
| FIM | Activity | 1996 | 30–45 | Yes | 1–7 | 126 | No | 22 (total) 17 (motor) 3 (cognition) | No | Yes |
| MBI | Activity | 1989 | 20 | No | 0–5,10,15 | 0–100 | No | 1.85 | No | Yes |
| SIS 3.0 | Activity & Participation | 2003 | 15–20 | No | 1–5 | 0–100 | No | 4.5–17.8 | No | Yes |
| AMPS | Activity & Participation | 1999 | 30–40 | Yes | 1–4 | Yes | No | No | ||
| COPM | Participation | 1991 | 20–40 | No | 1–10 | 20 | No | 2 | No | Yes |
UE, upper extremity; ICF, International Classification of Functioning, Disability and Health; MCID, minimal clinically important difference; FMA, Fugl-Meyer Assessment; ARAT, Action Research Arm Test; WMFT, Wolf Motor Function Test; JHFT, Jebsen-Taylor Hand Function Test; PPT, Purdue Pegboard Test; NHPT, Nine Hole Peg Test; BBT, Box and Block Test; MAL, Motor Activity Log; AOU, amount of use; QOM, quality of movement; FIM, Functional Independence Measure; MBI, Modified Barthel Index; SIS 3.0, Stroke Impact Scale 3.0; AMPS, Assessment of Motor and Process Skills; COPM, Canadian Occupational Performance Measure.
Footnotes
Funding: None.
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Long AF, Scott DL. Measuring health status and outcomes in rheumatoid arthritis within routine clinical practice. Br J Rheumatol. 1994;33:682–685. doi: 10.1093/rheumatology/33.7.682. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf C, Adams J, Burridge J, Yule V, Chappell P. A review of clinical upper limb assessments within the framework of the WHO ICF. Musculoskelet Care. 2007;5:160–173. doi: 10.1002/msc.108. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) International Classification of Functioning, Disability and Health (ICF) Geneva: World Health Organization; 2001. [Google Scholar]
- 4.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer Assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 5.Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- 6.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 7.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 8.Poole JL, Whitney SL. Assessments of motor function post stroke. Phys Occup Ther Geriatr. 2001;19:1–22. [Google Scholar]
- 9.Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, Saposnik G, Winstein C, van Wegen EE, Wolf SL, Krakauer JW, Bernhardt J. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12:451–461. doi: 10.1177/1747493017711813. [DOI] [PubMed] [Google Scholar]
- 10.Hiragami S, Inoue Y, Harada K. Minimal clinically important difference for the Fugl-Meyer Assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J Phys Ther Sci. 2019;31:917–921. doi: 10.1589/jpts.31.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 12.Arya KN, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil. 2011;18(Suppl 1):599–610. doi: 10.1310/tsr18s01-599. [DOI] [PubMed] [Google Scholar]
- 13.Santisteban L, Térémetz M, Bleton JP, Baron JC, Maier MA, Lindberg PG. Upper limb outcome measures used in stroke rehabilitation studies: a systematic literature review. PLoS One. 2016;11:e0154792. doi: 10.1371/journal.pone.0154792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer DJ, Cramer SC. A standardized approach to the Fugl-Meyer Assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27:732–741. doi: 10.1177/1545968313491000. [DOI] [PubMed] [Google Scholar]
- 15.Kim TL, Hwang SH, Lee WJ, Hwang JW, Cho I, Kim EH, Lee JA, Choi Y, Park JH, Shin JH. The Korean version of the Fugl-Meyer Assessment: reliability and validity evaluation. Ann Rehabil Med. 2021;45:83–98. doi: 10.5535/arm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran VD, Dario P, Mazzoleni S. Kinematic measures for upper limb robot-assisted therapy following stroke and correlations with clinical outcome measures: a review. Med Eng Phys. 2018;53:13–31. doi: 10.1016/j.medengphy.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Sivan M, O’Connor RJ, Makower S, Levesley M, Bhakta B. Systematic review of outcome measures used in the evaluation of robot-assisted upper limb exercise in stroke. J Rehabil Med. 2011;43:181–189. doi: 10.2340/16501977-0674. [DOI] [PubMed] [Google Scholar]
- 18.Kwakkel G, Van Wegen E, Burridge JH, Winstein CJ, van Dokkum L, Alt Murphy M, Levin MF, Krakauer JW. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2019;14:783–791. doi: 10.1177/1747493019873519. [DOI] [PubMed] [Google Scholar]
- 19.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the Action Research Arm Test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 20.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the Action Research Arm Test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the Action Research Arm Test in stroke patients. Age Ageing. 1998;27:107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- 22.Morris DM, Uswatte G, Crago JE, Cook EW, 3rd, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 24.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park CS, Park SW, Kim KM, Son MO, Yoo JH, Jang SJ, Park BK. The interrater and intrarater reliability of Korean Wolf Motor Function Test. J Korean Acad Rehabil Med. 2005;29:317–322. [Google Scholar]
- 26.Park CS, Park SW, Kim KM, Son MO, Yoo JH, Jang SJ, Park BK. Validity and reliability of Korean Wolf Motor Function Test. J Korean Soc Occup Ther. 2004;12:49–60. [Google Scholar]
- 27.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 28.Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys Ther. 1992;72:373–377. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- 29.Culicchia G, Nobilia M, Asturi M, Santilli V, Paoloni M, De Santis R, Galeoto G. Cross-cultural adaptation and validation of the Jebsen-Taylor Hand Function Test in an Italian population. Rehabil Res Pract. 2016;2016:8970917. doi: 10.1155/2016/8970917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SH, Bosch PR, Rowe VT, Fasoli SE, Langan J. Use of standardized assessments and online resources in stroke rehabilitation. Open J Occup Ther. 2019;7:1–22. [Google Scholar]
- 31.Sığırtmaç İC, Öksüz Ç. Investigation of reliability, validity, and cutoff value of the Jebsen-Taylor Hand Function Test. J Hand Ther. 2021;34:396–403. doi: 10.1016/j.jht.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer SY, Saba A, Baird JF, Kolar MB, Duff K, Stewart JC. Within-session practice effects in the Jebsen Hand Function Test (JHFT) Am J Occup Ther. 2018;72:7206345010p1–7206345010p5. doi: 10.5014/ajot.2018.024745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim B, Jang C, Kim Y. Assessment of hand function in normal Korean children by Jebsen Hand Function Test. J Korean Acad Rehabil Med. 1987;11:102–106. [Google Scholar]
- 34.Kim Y, Choi M, Kim B. Assessment of hand function in normal Korean adults by Jebsen Hand Function Test. J Korean Acad Rehabil Med. 1984;8:109–114. [Google Scholar]
- 35.Tiffin J, Asher EJ. The Purdue Pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 36.Reddon JR, Gill DM, Gauk SE, Maerz MD. Purdue Pegboard: test-retest estimates. Percept Mot Skills. 1988;66:503–506. doi: 10.2466/pms.1988.66.2.503. [DOI] [PubMed] [Google Scholar]
- 37.Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. New York, NY: Oxford University Press; 2006. p. xvii.p. 1216-xvii. [Google Scholar]
- 38.Kang Y. Purdue Pegboard standardization for occupational evaluation of persons with disabilities. Disabil Employ. 2004;14:85–96. [Google Scholar]
- 39.Lindstrom D, Veenstra N. Examining the Purdue Pegboard Test for occupational therapy practice. Open J Occup Ther. 2015;3 [Google Scholar]
- 40.Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134–139. doi: 10.1161/01.STR.0000105386.05173.5E. [DOI] [PubMed] [Google Scholar]
- 41.Wang YC, Magasi SR, Bohannon RW, Reuben DB, McCreath HE, Bubela DJ, Gershon RC, Rymer WZ. Assessing dexterity function: a comparison of two alternatives for the NIH Toolbox. J Hand Ther. 2011;24:313–320. doi: 10.1016/j.jht.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23:435–440. doi: 10.1177/1545968308331146. [DOI] [PubMed] [Google Scholar]
- 43.da Silva LC. Nine-hole peg test for evaluation of hand function: the advantages and shortcomings. Neurol India. 2017;65:1033–1034. doi: 10.4103/neuroindia.NI_739_17. [DOI] [PubMed] [Google Scholar]
- 44.Desrosiers J, Bravo G, Hébert R, Dutil E, Mercier L. Validation of the Box and Block Test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75:751–755. [PubMed] [Google Scholar]
- 45.Mathiowetz V, Federman SM, Wiemer DM. Box and Block Test of manual dexterity: norms for 6-19 year olds. Can J Occup Ther. 1985;52:241–245. [Google Scholar]
- 46.Liang KJ, Chen HL, Shieh JY, Wang TN. Measurement properties of the Box and Block Test in children with unilateral cerebral palsy. Sci Rep. 2021;11:20955. doi: 10.1038/s41598-021-00379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira CS, Almeida CS, Freitas LC, Santana R, Fernandes G, Junior PRF, Moura RCF. Use of the Box and Block Test for the evaluation of manual dexterity in individuals with central nervous system disorders: a systematic review. Man Ther Posturolog Rehabil J. 2016;14:436. [Google Scholar]
- 48.Higgins J, Mayo NE, Desrosiers J, Salbach NM, Ahmed S. Upper-limb function and recovery in the acute phase poststroke. J Rehabil Res Dev. 2005;42:65–76. doi: 10.1682/jrrd.2003.10.0156. [DOI] [PubMed] [Google Scholar]
- 49.Ashford S, Slade M, Malaprade F, Turner-Stokes L. Evaluation of functional outcome measures for the hemiparetic upper limb: a systematic review. J Rehabil Med. 2008;40:787–795. doi: 10.2340/16501977-0276. [DOI] [PubMed] [Google Scholar]
- 50.Taub E, Uswatte G. Constraint-induced movement therapy and massed practice. Stroke. 2000;31:986–988. doi: 10.1161/01.str.31.4.983-c. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Wolf SL, Zhang Q, Thompson PA, Winstein CJ. Minimal detectable change of the actual amount of use test and the Motor Activity Log: the EXCITE Trial. Neurorehabil Neural Repair. 2012;26:507–514. doi: 10.1177/1545968311425048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke. 2005;36:2493–2496. doi: 10.1161/01.STR.0000185928.90848.2e. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–ee181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 54.Chumney D, Nollinger K, Shesko K, Skop K, Spencer M, Newton RA. Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: systematic review. J Rehabil Res Dev. 2010;47:17–29. doi: 10.1682/jrrd.2009.08.0140. [DOI] [PubMed] [Google Scholar]
- 55.Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 56.Black TM, Soltis T, Bartlett C. Using the Functional Independence Measure instrument to predict stroke rehabilitation outcomes. Rehabil Nurs. 1999;24:109–114. doi: 10.1002/j.2048-7940.1999.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 57.Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the Functional Independence Measure: a quantitative review. Arch Phys Med Rehabil. 1996;77:1226–1232. doi: 10.1016/s0003-9993(96)90184-7. [DOI] [PubMed] [Google Scholar]
- 58.Hsueh IP, Lin JH, Jeng JS, Hsieh CL. Comparison of the psychometric characteristics of the Functional Independence Measure, 5 item Barthel Index, and 10 item Barthel Index in patients with stroke. J Neurol Neurosurg Psychiatry. 2002;73:188–190. doi: 10.1136/jnnp.73.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87:32–39. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 60.Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, Kim SH, Kim TH, Han TR. Development of the Korean version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007;31:283–297. [Google Scholar]
- 61.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 62.Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair. 2007;21:233–238. doi: 10.1177/1545968306294729. [DOI] [PubMed] [Google Scholar]
- 63.Audebert HJ, Schenkel J, Heuschmann PU, Bogdahn U, Haberl RL Telemedic Pilot Project for Integrative Stroke Care Group. Effects of the implementation of a telemedical stroke network: the Telemedic Pilot Project for Integrative Stroke Care (TEMPiS) in Bavaria, Germany. Lancet Neurol. 2006;5:742–748. doi: 10.1016/S1474-4422(06)70527-0. [DOI] [PubMed] [Google Scholar]
- 64.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 65.Duncan PW, Bode RK, Min Lai S, Perera S Glycine Antagonist in Neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/s0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 66.Jenkinson C, Fitzpatrick R, Crocker H, Peters M. The Stroke Impact Scale: validation in a UK setting and development of a SIS short form and SIS index. Stroke. 2013;44:2532–2535. doi: 10.1161/STROKEAHA.113.001847. [DOI] [PubMed] [Google Scholar]
- 67.Mulder M, Nijland R. Stroke Impact Scale. J Physiother. 2016;62:117. doi: 10.1016/j.jphys.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Lin KC, Fu T, Wu CY, Wang YH, Liu JS, Hsieh CJ, Lin SF. Minimal detectable change and clinically important difference of the Stroke Impact Scale in stroke patients. Neurorehabil Neural Repair. 2010;24:486–492. doi: 10.1177/1545968309356295. [DOI] [PubMed] [Google Scholar]
- 69.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44:1111–1116. doi: 10.1161/STROKEAHA.111.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi SU, Lee HS, Shin JH, Ho SH, Koo MJ, Park KH, Yoon JA, Kim DM, Oh JE, Yu SH, Kim DA. Stroke Impact Scale 3.0: reliability and validity evaluation of the Korean version. Ann Rehabil Med. 2017;41:387–393. doi: 10.5535/arm.2017.41.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher AG, James KB. Assessment of Motor and Process Skills: Volume 1: Development, standardization, and administration manual. Chicago, IL: Three Star Press; 2010. [Google Scholar]
- 72.Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57:82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- 73.Yang SY, Lin CY, Lee YC, Chang JH. The Canadian Occupational Performance Measure for patients with stroke: a systematic review. J Phys Ther Sci. 2017;29:548–555. doi: 10.1589/jpts.29.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan CC, Lee TM. Validity of the Canadian Occupational Performance Measure. Occup Ther Int. 1997;4:231–249. [Google Scholar]
- 75.Cup EH, Scholte op Reimer WJ, Thijssen MC, van Kuyk-Minis MA. Reliability and validity of the Canadian Occupational Performance Measure in stroke patients. Clin Rehabil. 2003;17:402–409. doi: 10.1191/0269215503cr635oa. [DOI] [PubMed] [Google Scholar]
- 76.Jansa J, Sicher Z, Angleitne K, Law M. The use of Canadian Occupational Performance Measure (COPM) in clients with an acute stroke. World Fed Occup Ther Bull. 2004;50:18–23. [Google Scholar]
- 77.Toomey M, Nicholson D, Carswell A. The clinical utility of the Canadian Occupational Performance Measure. Can J Occup Ther. 1995;62:242–249. doi: 10.1177/000841749506200503. [DOI] [PubMed] [Google Scholar]