Abstract
A new species of Cytospora was isolated from cankered wood of Prunus spp. during a survey of orchards exhibiting symptoms of fruit tree decline syndrome in southern Ontario, Canada. We found isolates that are morphologically similar to species in the Cytosporaceae family, which is characterized by single or labyrinthine locules, filamentous conidiophores or clavate to elongate obovoid asci and allantoid, hyaline conidia. Multi-gene phylogenetic analysis of ITS, LSU, act and tef1- α showed that the isolates form a distinct clade, sister to Cytospora plurivora. Morphologically, our isolates showed differences in the length of conidia and culture characteristics compared to C. plurivora, suggesting the establishment of a new species. The species is described as Cytospora paraplurivora sp. nov. and placed in the family Cytosporaceae of Diaporthales. Additionally, we sequenced, assembled and characterized the genome of the representative isolate for this new species. The phylogenomic analysis confirms the species order and family level classification. C. paraplurivora sp. nov. has the potential to severely affect stone fruits production, causing cankers and dieback in stressed trees, and eventually leads to tree decline. Pathogenicity tests show that the species is pathogenic to Prunus persica var. persica.
Introduction
Stone fruit trees are economically important crops cultivated in Ontario, Canada. The Niagara Peninsula produces more than 90% of its peaches, nectarines and apricots [1]. Peach occupy the largest production areas, followed by nectarines and apricots. Fruit tree decline syndrome (FTDS) has been recently discovered in several orchards in this area of the province. The common symptoms include stem canker and dieback, wilting, increased suckering, leaf discolouration and eventual total collapse of the tree. Disease incidence ranged from 49 to 72% for apricots and nectarines (Fig 1). Similar disease symptoms were observed in stone fruits and apple trees in other regions of North America [2, 3]. A number of studies reported that Cytospora spp. was among the causative agents of canker and dieback diseases in fruit trees including Prunus spp. [4, 5]. Generally, members of the Cytospora spp. infect trees that are stressed by extreme weather conditions (drought or freezing), and by invading wounds in the bark caused by insect damage and improper pruning. These fungi commonly overwinter in a form of pycnidia embedded in the bark of cankered branches [6].
Fig 1. Nectarine trees showing fruit tree decline syndrome (FTDS) in southern Ontario, Canada.
The genus Cytospora (Cytosporacea, Diaporthales) was established by Ehrenberg in 1818. There are currently 676 records of Cytospora spp. registered in the IndexFungorum (www.indexfungorum.org; accessed Nov 2021). However, 163 species of the genus with available nucleotide sequences are listed in the NCBI taxonomy database (www.ncbi.nlm.nih.gov/taxonomy; accessed May 2022). Approximately, 150 species in the genus have been reported to cause diseases in more than 120 woody plants, which can result in significant commercial losses for growers [7–9]. The taxonomic species identification of Cytospora spp. is mainly based on morphological characteristics and molecular phylogenetic analysis. Recent studies described a number of new species of Cytospora on different hosts using multi-gene (ITS, LSU, act, tef1- α, tub2, and rpb2) phylogenetic analysis [5, 10–12]. This approach allows for the identification of cryptic and novel species within the genus. Obtaining high-quality, cost-effective genomic data is now possible through rapidly developing sequencing technologies. Whole-genome sequence data used in phylogenomics can contribute to resolving taxonomic uncertainties or support species reclassifications [13]. One such study found that a polyphagous plant pathogen, Corynespora olivacea was previously misclassified on family level [14].
The objective of this paper was to identify and characterize a novel Cytopora species associated with the decline of Prunus armeniaca, P. persica var. persica, P. persica var. nucipersica in Ontario, Canada. The characterization of the new species was performed following recently published guidelines [15, 16].
Materials and methods
Sample collection and isolation
Wood samples were collected in 2018–2021 from 30 apricot, 6 peach and 6 nectarine trees exhibiting extensive tree fruit decline symptoms from nine commercial orchards located in southern Ontario. Roots were symptom-free. The cankered and diseased wood sections from trunk and rootstock were cut into 1 cm pieces, and surface disinfested with 70% ethanol for 30 sec, followed by 1% NaClO for 20 min and three rinses in sterile distilled water. The samples were air-dried and placed on a 2% potato dextrose agar (PDA, Difco, USA) supplemented with kanamycin (50 mg L−1). The PDA plates were incubated at 22°C for 5 days in the dark. All fungal colony-forming units were hyphal-tip transferred to individual PDA plates and incubated at 22°C for 7 days in the dark. Purified mycelial isolates were classified into morphotypes prior to molecular identification. C. paraplurivora was characterized by fast-growing, white to cream with uneven lobate growth margin colonies.
Tree branches with fruiting structures (conidiomata) were checked separately. Single conidia isolations were performed using the protocol described by Chomnunti et al. [17]. For long-term preservation, fungal cultures were stored at −80°C in 30% glycerol. The holotype specimen was deposited in the Canadian National Mycological Herbarium (DAOM) and the living culture collection maintained by the Canadian Collection of Fungal Cultures (DAOMC).
Morphological examination
Conidiomata formed on the tree branches were described prior to sectioning with a sterile surgical scalpel. The macro-morphological structures were measured using a dissecting microscope (OMAX 2000X Infinity Compound Siedentopf Microscope with a Built-in Camera). In order to calculate the mean size of the structures, 10 conidiomata, 20 conidiogenous cells and 50 conidia were measured. The obtained measurements were recorded with minimum and maximum values and means were calculated. Radial growth of fungal colonies was estimated with two perpendicular measurements after 5 days of incubation. Morphology description [18] and color characterisation [19] were performed after 7 days.
DNA extraction, PCR amplification and Sanger sequencing
Genomic DNA was extracted from mycelium using the Plant/Fungi DNA Isolation Kit (Norgen Biotech Corp., Thorold, Canada) with the following modifications: fungal tissue was vortexed for 15 minutes with 1 mm glass beads and 500 μL lysis buffer and 1 μL RNase A prior to incubation at 65°C. After incubation on ice, the fungal mixture was centrifuged at 10,000 rpm to separate the lysate from the beads and biomass. During the column wash, the resin was dried by spinning for 10 minutes at 14,000 rpm. DNA was eluted at 10,000 rpm for 2 minutes. PCR amplifications were executed using C1000 Touch PCR thermal cycler (Bio-Rad, Hercules, USA) under conditions described in the references for each region. The internal transcribed spacer (ITS) region was amplified with the primer pair ITS1/ITS4 [20]. The primer pair LR0R/LR5 [21] was used to amplify the large subunit rRNA gene (LSU). The partial actin (ACT) region was amplified with the primer pair ACT512F/ACT783R [22], and the primer pair EF1-728F/ EF1-986R [22] was used to amplify partial translation elongation factor 1-alpha (tef1-α) gene sequences. The quality of the PCR products was examined using electrophoresis in 1% agarose gel. Sanger sequencing was carried out at Genome Quebec’s Sequencing Facility (Montreal, Canada).
Sequence alignment and phylogenetic analysis
The consensus sequences were built from Sanger chromatograms using BioEdit [23]. The ITS, LSU, act, tef1-α sequences from our study were uploaded on GenBank (http://www.ncbi.nlm.nih.gov/) on the Basic Local Alignment Search Tool (BLAST) [24] to find other similar sequences. The sequences of Cytospora spp. from the top BLAST hits were then extracted and added to recently published sequence datasets [5, 9]. Diaporthe vaccinii (CBS 160.32) of Diaporthaceae was used as the outgroup. All the sequences were initially aligned using CLUSTAL-X2 [25] and edited manually in MEGA-X [26]. Some characters were trimmed from both ends of the alignments. Randomized Accelerated Maximum Likelihood (RAxML) v. 8.0 [27] was used for Maximum Likelihood (ML) analysis [28] and MrBayes v. 3.2.7 for Bayesian Inference (BI) analysis [29].
ML analysis was performed using a general time reversible substitution (GTR) model with gamma-distributed rate of heterogeneity and proportion of invariant sites [30]. The model was selected with ModelTest-NG v. 0.1.7 [31] based on the Akaike Information Criterion [32]. Branch support was estimated with bootstrapping of 1000 replicates [33]. Bayesian probabilities (BP) values were defined by Markov Chain Monte Carlo (MCMC) sampling with the GTR model. Six simultaneous Markov Chains were run for 100,000 generations. The first 500 trees were discarded, and the remaining trees were used to calculate BP in the majority rule consensus tree. Phylograms were visualized using FigTree v. 1.4.4 [34]. The newly generated sequences were deposited in GenBank (Table 1). The alignments used in the analyses were submitted into TreeBase (www.treebase.org; ID: 29116).
Table 1. Strains used in the phylogenetic analysis with their culture accession and GenBank numbers.
Strains from this study are in bold and ex-types are marked with*. NA: not available.
| Species | Strain | Host | Origin | GenBank accession numbers | |||
|---|---|---|---|---|---|---|---|
| ITS | LSU | act | tef1-α | ||||
| Cytospora ailanthicola | CFCC 89970* | Ailanthus altissima | China | MH933618 | MH933653 | MH933526 | MH933494 |
| C. ampulliformis | MFLUCC 16–0583* | Sorbus intermedia | Russia | KY417726 | KY417760 | KY417692 | NA |
| MFLUCC 16–0629 | Acer platanoides | Russia | KY417727 | KY417761 | KY417693 | NA | |
| C. amygdali | LH357* | Prunus dulcis | USA | MG971853 | NA | MG972002 | MG971659 |
| C. atrocirrhata | CFCC 89615 | Juglans regia | China | KR045618 | KR045700 | KF498673 | KP310858 |
| CFCC 89616 | Juglans regia | China | KR045619 | KR045701 | KF498674 | KP310859 | |
| C. beilinensis | CFCC 50493* | Pinus armandii | China | MH933619 | MH933654 | MH933527 | MH933495 |
| CFCC 50494 | Pinus armandii | China | MH933620 | MH933655 | MH933528 | MH933496 | |
| C. berberidis | CFCC 89927* | Berberis dasystachya | China | KR045620 | KR045702 | KU710990 | KU710913 |
| CFCC 89933 | Berberis dasystachya | China | KR045621 | KR045703 | KU710991 | KU710914 | |
| C. bungeanae | CFCC 50495* | Pinus bungeana | China | MH933621 | MH933656 | MH933529 | MH933497 |
| CFCC 50496 | Pinus bungeana | China | MH933622 | MH933657 | MH933530 | MH933498 | |
| C. californica | 9C-24* | Juglans regia | USA | MG971935 | NA | MG972083 | MG971645 |
| C. carbonacea | CFCC 89947 | Ulmus pumila | China | KR045622 | KP310812 | KP310842 | KP310855 |
| C. carpobroti | CMW 48981* | Carpobrotus edulis | S. Africa | MH382812 | MH411216 | NA | MH411212 |
| C. celtidicola | CFCC 50497* | Celtis sinensis | China | MH933623 | MH933658 | MH933531 | MH933499 |
| CFCC 50498 | Celtis sinensis | China | MH933624 | MH933659 | MH933532 | MH933500 | |
| C. ceratosperma | CFCC 89624 | Juglans regia | China | KR045645 | KR045724 | NA | KP310860 |
| CFCC 89625 | Juglans regia | China | KR045646 | KR045725 | NA | KP31086 | |
| C. ceratospermopsis | CFCC 89626* | Juglans regia | China | KR045647 | KR045726 | KU711011 | KU710934 |
| CFCC 89627 | Juglans regia | China | KR045648 | KR045727 | KU711012 | KU710935 | |
| C. chrysosperma | CFCC 89629 | Salix psammophila | China | KF765673 | KF765689 | NA | NA |
| CFCC 89981 | Populus alba | China | MH933625 | MH933660 | MH933533 | MH933501 | |
| C. coryli | CFCC 53162* | Corylus mandshurica | China | MN854450 | MN854661 | NA | MN850758 |
| C. davidiana | YW2014* | Populus davidiana | China | KM034870 | NA | NA | NA |
| CXY 1374 | Populus davidiana | China | KM034869 | NA | NA | NA | |
| C. elaeagni | CFCC 89632 | Elaeagnus angustifolia | China | KR045626 | KR045706 | KU710995 | KU710918 |
| CFCC 89633 | Elaeagnus angustifolia | China | KF765677 | KF765693 | KU710996 | KU710919 | |
| C. elaeagnicola | CFCC 52883 | Elaeagnus angustifolia | China | MK732342 | NA | MK732345 | NA |
| CFCC 52884 | Elaeagnus angustifolia | China | MK732343 | NA | MK732346 | NA | |
| C. erumpens | CFCC 50022 | Prunus padus | China | MH933627 | MH933661 | MH933534 | MH933502 |
| MFLUCC 16–0580* | Salix x fragilis | Russia | KY417733 | KY417767 | KY417699 | NA | |
| C. eucalypti | KARE1585 | Eucalyptus globulus | USA | MG971907 | NA | MG972056 | MG971617 |
| C. euonymicola | CFCC 50499* | Euonymus kiautschovicus | China | MH933628 | MH933662 | MH933535 | MH933503 |
| CFCC 50500 | E. kiautschovicus | China | MH933629 | MH933663 | MH933536 | MH933504 | |
| C. euonymina | CFCC 89993* | E.kiautschovicus | China | MH933630 | MH933664 | MH933537 | MH933505 |
| C. fraxinigena | MFLU 17–0880 | Fraxinus ornus | Italy | MF190134 | MF190079 | NA | NA |
| C. fugax | CXY 1381 | Populus ussuriensis | China | KM034853 | NA | NA | NA |
| C. gigalocus | CFCC 89620* | Juglans regia | China | KR045628 | KR045708 | KU710997 | KU710920 |
| CFCC 89621 | Juglans regia | China | KR045629 | KR045709 | KU710998 | KU710921 | |
| C. gigaspora | CFCC 50014 | Juniperus procumbens | China | KR045630 | KR045710 | KU710999. | KU710922 |
| CFCC 89634* | Salix psammophila | China | KF765671 | KF765687 | KU711000 | KU710923 | |
| C. granati | 6F-45* | Punica granatum | USA | MG971799 | NA | MG971949 | MG971514 |
| C. hippophaes | CFCC 89639 | Hippophae rhamnoides | China | KR045632 | KR045712 | KU711001 | KU710924 |
| CFCC 89640 | Hippophae rhamnoides | China | KF765682 | KF765698 | KF765730 | KP310865 | |
| C. japonica | CFCC 89956 | Prunus cerasifera | China | KR045624 | KR045704 | KU710993 | KU710916 |
| C. joaquinensis | KARE975* | Populus deltoides | USA | MG971895 | NA | MG972044 | MG971605 |
| C. junipericola | BBH 42444 | Juniperus communis | Italy | MF190126 | MF190071 | NA | MF377579 |
| MFLU 17–0882* | Juniperus communis | Italy | MF190125 | MF190072 | NA | MF377580 | |
| C. juniperina | CFCC 50502 | Juniperus przewalskii | China | MH933633 | MH933667 | MH933540 | MH933508 |
| CFCC 50503 | Juniperus przewalskii | China | MH933634 | MH933668 | MH933541 | MH933509 | |
| C. leucosperma | CFCC 89622 | Pyrus bretschneideri | China | KR045616 | KR045698 | KU710988 | KU710911 |
| CFCC 89894 | Pyrus bretschneideri | China | KR045617 | KR045699 | KU710989 | KU710912 | |
| C. leucostoma | HigginsLake4 | Alnus incana | USA | JX475137 | NA | NA | JX438600 |
| C. longiostiolata | MFLUCC 16–0628* | Salix fragilis | Russia | KY417734 | KY417768 | KY417700 | NA |
| C. longispora | 10F-57* | Prunus domestica | USA | MG971905 | NA | MG972054 | MG971615 |
| C. mali | CFCC 50028 | Malus pumila | China | MH933641 | MH933675 | MH933548 | MH933513 |
| CFCC 50030 | Malus pumila | China | MH933643 | MH933677 | MH933550 | MH933524 | |
| C. melnikii | CFCC 89984 | Rhus typhina | China | MH933644 | MH933678 | MH933551 | NA |
| MFLUCC 15–0851* | Malus domestica | Russia | KY417735 | KY417769 | KY417701 | NA | |
| C. nivea | MFLUCC 15–0860 | Salix acutifolia | Russia | KY417737 | KY417771 | KY417703 | NA |
| C. oleicola | KARE1021* | Olea europaea | USA | MG971944 | NA | MG972098 | MG971660 |
| C. palm | CXY 1276 | Cotinus coggygria | China | JN402990 | NA | NA | KJ781296 |
| C. parakantschavelii | MFLUCC 15–0857* | Populus sibirica | Russia | KY417738 | KY417772 | KY417704 | NA |
| MFLUCC 16–0575 | Pyrus pyraster | Russia | KY417739 | KY417773 | KY417705 | NA | |
| C. parapistaciae | KARE270* | Pistacia vera | USA | MG971804 | NA | MG971954 | MG971519 |
| C. paraplurivora | FDS-439 | Prunus armeniaca | Canada | OL640182 | OL640184 | OL631586 | OL631589 |
| FDS-564* | Prunus persica var. nucipersica | Canada | OL640183 | OL640185 | OL631587 | OL631590 | |
| FDS-623 | Prunus persica var. persica | Canada | OL640181 | OL640123 | OL631588 | OL631591 | |
| C. parasitica | MFLUCC 15–0507* | Malus domestica | Russia | KY417740 | KY417774 | KY417706 | NA |
| XJAU 2542–1 | Malus sp. | China | MH798884 | MH798897 | NA | MH813452 | |
| C. paratranslucens | MFLUCC 15–0506* | Populus alba var. bolleana | Russia | KY417741 | KY417775 | KY417707 | NA |
| MFLUCC 16–0627 | Populus alba | Russia | KY417742 | KY417776 | KY417708 | NA | |
| C. pistaciae | KARE443* | Pistacia vera | USA | MG971802 | NA | MG971952 | MG971517 |
| C. platanicola | MFLU 17–0327* | Platanus hybrida | Italy | MH253451 | MH253452 | MH253449 | NA |
| C. platyclade | CFCC 50504* | Platycladus orientalis | China | MH933645 | MH933679 | MH933552 | MH933516 |
| CFCC 50505 | Platycladus orientalis | China | MH933646 | MH933680 | MH933553 | MH933517 | |
| C. platycladicola | CFCC 50039 | Platycladus orientalis | China | KR045642 | KR045721 | KU711008 | KU710931 |
| C. plurivora | KARE1452* | Olea europaea | USA | MG971861 | NA | MG972010 | MG971572 |
| 5L-29 | Prunus persica | USA | MG971856 | NA | MG972005 | MG971567 | |
| C. populicola | KARE973* | Populus deltoides | USA | MG971891 | NA | MG972040 | MG971601 |
| C. populina | CFCC 89644 | Salix psammophila | China | KF765686 | KF765702 | KU711007 | KU710930 |
| C. populinopsis | CFCC 50032* | Sorbus aucuparia | China | MH933648 | MH933683 | MH933556 | MH933520 |
| C. pruinopsis | CFCC 50034* | Ulmus pumila | China | KP281259 | KP310806 | KP310836 | KP310849 |
| CFCC 50035 | Ulmus pumila | China | KP281260 | KP310807 | KP310837 | KP310850 | |
| C. pruinose | CFCC 50036 | Syringa oblata | China | KP310800 | KP310802 | KP310832 | KP310845 |
| C. prunicola | MFLU 17–0995* | Prunus sp. | Italy | MG742350 | MG742351 | MG742353 | NA |
| C. quercicola | MFLU 17–0881 | Quercus sp. | Italy | MF190128 | MF190074 | NA | NA |
| MFLUCC 14–0868* | Quercus sp. | Italy | MF190129 | MF190073 | NA | NA | |
| C. ribis | CFCC 50026 | Ulmus pumila | China | KP281267 | KP310813 | KP310843 | KP310856 |
| CFCC 50027 | Ulmus pumila | China | KP281268 | KP310814 | KP310844 | KP310857 | |
| C. rosae | MFLUCC 14–0845* | Rosa canina | Italy | MF190131 | MF190075 | NA | NA |
| C. rostrata | CFCC 89909* | Salix cupularis | China | KR045643 | KR045722 | KU711009 | KU710932 |
| C. rusanovii | MFLUCC 15–0854* | Salix babylonica | Russia | KY417744 | KY417778 | KY417710 | NA |
| C. salicacearum | MFLUCC 15–0861 | Salix x fragilis | Russia | KY417745 | KY417779 | KY417711 | NA |
| MFLUCC 15–0509* | Salix alba | Russia | KY417746 | KY417780 | KY417712 | NA | |
| C. salicicola | MFLUCC 15–0866 | Salix alba | Russia | KY417749 | KY417783 | KY417715 | NA |
| MFLUCC 14–1052* | Salix alba | Russia | KU982636 | KU982635 | KU982637 | NA | |
| C. salicina | MFLUCC 15–0862* | Salix alba | Russia | KY417750 | KY417784 | KY417716 | NA |
| MFLUCC 16–0637 | Salix x fragilis | Russia | KY417751 | KY417785 | KY417717 | NA | |
| C. schulzeri | CFCC 50040 | Malus domestica | China | KR045649 | KR045728 | KU711013 | KU710936 |
| CFCC 50042 | Malus asiatica | China | KR045650 | KR045729 | KU711014 | KU710937 | |
| C. sibiraeae | CFCC 50045* | Sibiraea angustata | China | KR045651 | KR045730 | KU711015 | KU710938 |
| CFCC 50046 | Sibiraea angustata | China | KR045652 | KR045731 | KU711015 | KU710939 | |
| C. sophorae | CFCC 50048 | Magnolia grandiflora | China | MH820401 | MH820394 | MH820409 | MH820405 |
| C. sophoricola | CFCC 89596 | Styphnolobium japonicum var. pendula | China | KR045656 | KR045735 | KU711020 | KU710943 |
| CFCC 89595* | Styphnolobium japonicum var. pendula | China | KR045655 | KR045734 | KU711019 | KU710942 | |
| C. sophoriopsis | CFCC 89600* | Styphnolobium japonicum | China | KR045623 | KP310804 | KU710992 | KU710915 |
| C. sorbicola | MFLUCC 16–0584* | Acer pseudoplatanus | Russia | KY417755 | KY417789 | KY417721 | NA |
| MFLUCC 16–0633 | Cotoneaster melanocarpus | Russia | KY417758 | KY417792 | KY417724 | NA | |
| C. spiraeae | CFCC 50049* | Spiraea salicifolia | China | MG707859 | MG707643 | MG708196 | NA |
| CFCC 50050 | Spiraea salicifolia | China | MG707860 | MG707644 | MG708197 | NA | |
| C. spiraeicola | CFCC 53138* | Spiraea salicifolia | China | MN854448 | MN854659 | NA | MN850756 |
| CFCC 53139 | Tilia nobilis | China | MN854449 | MN854660 | NA | MN850757 | |
| C. tamaricicola | CFCC 50507 | Rosa multifolora | China | MH933651 | MH933686 | MH933559 | MH933525 |
| CFCC 50508* | Tamarix chinensis | China | MH933652 | MH933687 | MH933560 | MH933523 | |
| C. tanaitica | MFLUCC 14–1057* | Betula pubescens | Russia | KT459411 | KT459412 | KT459413 | NA |
| C. translucens | CXY 1351 | Populus davidiana | China | KM034874 | NA | NA | NA |
| C. ulmi | MFLUCC 15–0863* | Ulmus minor | Russia | KY417759 | NA | NA | NA |
| Diaporthe vaccinii | CBS 160.32 | Vaccinium macrocarpon | USA | KC343228 | NA | JQ807297 | KC343954 |
DNA isolation, genome sequencing and characterization
Highly purified genomic DNA from the isolate FDS-564 was extracted from fungal mycelium grown in a liquid Potato Dextrose Broth for 5 days at room temperature, filtered and freeze-dried before following the modified DNA extraction protocol as previously described (Norgen Biotech Comp., Thorold, Canada). The library was prepared using a SMRT bell Express Template Prep Kit (PacBio, Menlo Park, USA). The library pool was sequenced on one SMRT cell using the PacBio Sequel II platform in the SickKids sequencing facility (Toronto, ON, Canada). The reads were assembled into contigs with the Canu assembler v. 2.1.1 [35]. The quality of genome assemblies was accessed using QUAST v. 5.0.2 [36]. The completeness of assembly was estimated with BUSCO v.4.0.5 employing the dataset Ascomycota_odb10 [37]. Repeat sequences were identified with RepeatMasker v. 4.0.9 [38] using the library of repeats for fungi obtained from Repbase [39]. Gene prediction was executed using Augustus v. 3.4.0 [40]. Augustus was trained with the gene structures from the representative genome of C. mali 03–8 (GCA_000818155) obtained using the MAKER2 pipeline v. 2.31.11 [41].
Phylogenomic analysis and genome alignment
The proteomes of 17 species of ascomycetes, including five species in the Cytosporaceae, were retrieved from the Mycocosm portal [42] (accessed May 2022) and the NCBI Genome database (accessed Dec 2021). Orthofinder v. 2.5.4 [43] was used to identify the single-copy orthogroups (SCO) in the species included in the analysis. Multiple sequence alignments were performed using MAFFT v. 7.489 [44]. The ML tree was produced with FastTree v. 2.1.10 [45]. All runs were performed using the default parameters. Mollisia scopiformis CBS 120377 (Leotiomycetes) was used as an outgroup (Table 2). The tree was visualized in FigTree v. 1.4.4 [34]. Genome alignment was performed with minimap2 v. 2.23 [46] and displayed as a dot-plot graph using D-Genies [47].
Table 2. Genomes used in phylogenomic analyses.
Strains from this study are in bold. NA: not available.
| Species name | Strain | GenBank assembly accession | Host | Country | Reference |
|---|---|---|---|---|---|
| Botryosphaeria dothidea | sdau11-99 | GCA_011503125 | Malus sp. | China | [48] |
| Colletotrichum higginsianum | IMI 349063 | GCA_001672515 | Brassica rapa subsp. chinensis | Trinidad and Tobago | [49] |
| Cytospora chrysosperma | CFL2056 | NA | NA | Canada | PI permission |
| Cytospora leucostoma | SXYLt | GCA_003795295 | Prunus persica | China | [50] |
| Cytospora (Valsa) mali | 03–8 | GCA_000818155 | Malus sp. | China | [51] |
| Cytospora (Valsa) mali var. pyri | SXYL134 | GCA_000813385 | Malus sp. | China | [51] |
| Cytospora paraplurivora | FDS-564 | GCA_021272945 | Prunus persica var. nucipersica | Canada | This study |
| Cytospora piceae | CFCC52841 | GCA_016508685 | Picea crassifolia | China | PI permission |
| Diaporthe ampelina | DA912 | GCA_001006365 | Vitis vinifera | USA | [52] |
| Diaporthe helianthi | 7/96 | GCA_001702395 | Helianthus sp. | France | [53] |
| Macrophomina phaseolina | MS6 | GCA_000302655 | Corchorus olitorius | Bangladesh | [54] |
| Mollisia scopiformis | CBS 120377 | GCA_001500285 | Picea glauca | Canada | [55] |
| Neofusicoccum parvum | UCRNP2 | GCA_000385595 | Vitis vinifera | USA | [56] |
| Neurospora crassa | FGSC 73 | GCA_000786625 | NA | USA | [57] |
| Peltaster fructicola | LNHT1506 | GCA_001592805 | Malus sp. | China | [58] |
| Pyrenophora tritici-repentis | Ptr | GCA_000149985 | Triticum sp. | USA | [59] |
| Pyricularia oryzae | 70–15 | GCA_000002495 | Oryza sativa | USA | [60] |
| Zymoseptoria tritici | IPO323 | GCA_000219625 | Triticum sp. | Netherlands | [61] |
Excised branch pathogenicity trials
Excised branches collected from non-symptomatic apricot, nectarine and peach trees were used to test the pathogenicity of C. paraplurivora isolates following the protocol by Arzanlou and Narmani [62]. Thirteen green lateral branches, with a mean diameter of 1 cm and 20 cm in length, were excised from each tree species. The leaves were removed, and the branches were surface disinfested with 70% ethanol for 10 min, rinsed 3 times with sterile water and air-dried. Ten branches were wounded and inoculated with 4 mm mycelium agar plug from a 5-day old cultures of each isolate and wrapped with Parafilm (Parafilm® "M", MilliporeSigma, Canada). Three controls were inoculated with sterile PDA plugs. Excised branches were placed inside a clear plastic container with moist paper towels and incubated at room temperature in the dark. The branches were checked 12 days post-inoculation to measure the necrotic lesions. Longitudinal sections were made from the inoculation point for further lesion examination.
Living plants pathogenicity trials
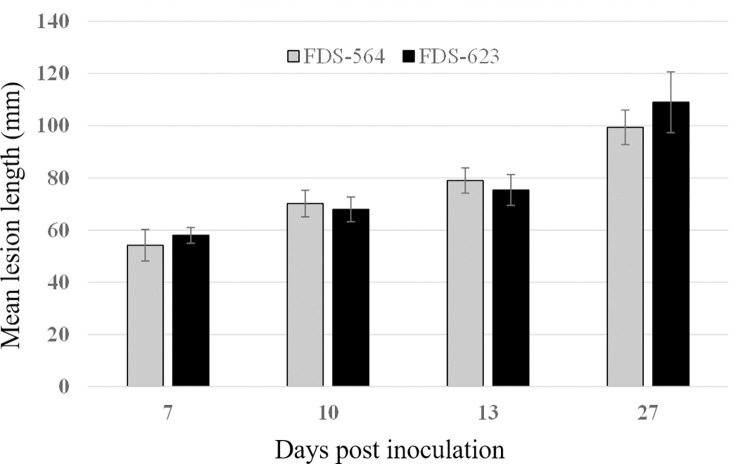
The peach seedlings (cv. Loring) were grown from seeds and placed in a biosafety plant growth chamber under a temperature of 23°C and a photoperiod of 16 h. A completely randomized design was used for the experiment. Inoculations were performed on four-month-old seedlings. A total of five seedlings were wounded and inoculated with 4 mm mycelium agar plug from a 5-day old cultures of each isolate and wrapped with Parafilm (Fig 7). Five seedlings were inoculated with sterile PDA plugs as negative control. The developing lesions were measured 7, 10, 13 and 27 days post-inoculation (dpi).
Fig 7.
Symptoms of Cytospora paraplurivora infection on Prunus persica var. persica seedlings including A) 5-day old canker (ck) and gummosis (g); B) 7-day old canker; and C) 27-day old canker, conidiomata (cd) and gummosis.
Re-Isolation of the pathogen
C. paraplurivora was re-isolated from the excised branches and seedlings stem fragments and from fruiting structures (conidiomata). Three tissue fragments (0.5 cm long) were taken from the advanced necrosis and from the lesion edge of each branch and seedling. The tissue fragments were surface disinfested with 70% ethanol for 30 sec, followed by 1% NaClO for 20 min and three rinses in sterile distilled water. The samples were air-dried and placed on a 2% PDA supplemented with kanamycin (50 mg L−1). The PDA plates were incubated at 22°C for 5 days in the dark. All fungal colony-forming units were hyphal-tip transferred to individual PDA plates and incubated at 22°C for 7 days in the dark. One conidiomata was taken from each infected seedling and the pathogen was re-isolated using the protocol described by Chomnunti et al. [17]. The identification of C. paraplurivora re-isolated from lesions and fruiting structures was made on the basis of morphological features using reference cultures and PCR with the primer pair EF1-728F/ EF1-986R [22], as described above, to fulfil Koch’s postulates.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi and plants, and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
In addition, new names contained in this work have been submitted to Index Fungorum and MycoBank from where they will be made available to the Global Names Index. The unique Index Fungorum number can be resolved and the associated information viewed through any standard web browser by appending the Index Fungorum number contained in this publication to the prefix www.indexfungorum.org/. The online version of this work is archived and available from the following digital repositories: PubMed Central, LOCKSS.
Results
The decline disease has affected up to 72% of the trees in the surveyed stone fruits orchards in southern Ontario. C. paraplurivora was the only Cytospora spp. isolated from symptomatic trees in all surveyed orchards. The incidence of C. paraplurivora on symptomatic trees was 50% in peaches, 57% in apricots and 100% in nectarines.
Phylogenetic analysis
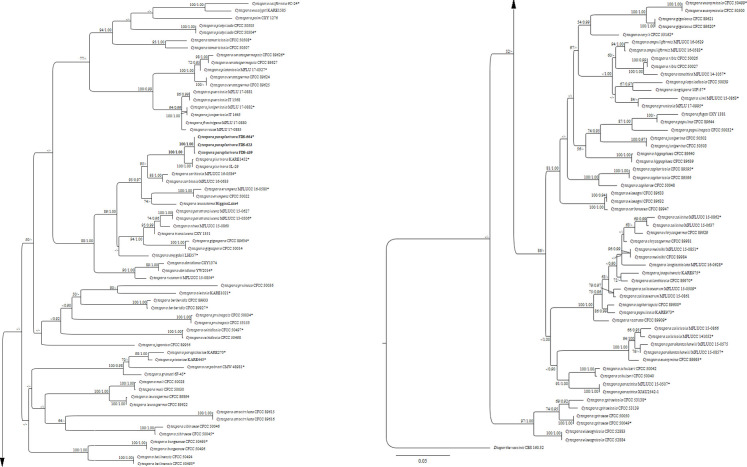
The ML and BI analyses based on a concatenated alignment of ITS, LSU, act and tef1- α from 119 strains of Cytosporaceae produced phylogenetic trees with similar topology to those in recent taxonomic studies of Cytospora [5, 9, 12]. The best scoring RAxML tree is depicted in Fig 2. The strains of C. paraplurivora obtained in this study are clustered together and formed a distinct clade with strong bootstrap support values (100% ML, 1.00 BP). The closest species, C. plurivora is grouped with the new species in the well supported clade (100% ML, 1.00% BP). Broadly, the taxa with leucocytosporoid condiomata (C. amydgali, C. davidiana, C. erumpens, C. gigaspora, C. leucostoma, C. nivea, C. paratranslucens, C. rusanovii, C. sorbicola, C. translucens) also constitute a separate clade (88% ML, 1.00 BP).
Fig 2. Maximum likelihood (ML) phylogeny based on a concatenated ITS, LSU, act, tef1-α sequence alignment.
Bootstrap support values for ML ≥ 50% and BP ≥ 0.90 are defined as ML/BP above and below the nodes. Strains of the new species are in bold, and ex-type strains are marked with*. The tree is rooted to Diaporthe vaccinii (CBS 160.32).
Taxonomy
Cytospora paraplurivora Ilyukhin & Ellouze, sp. nov. (Fig 3).
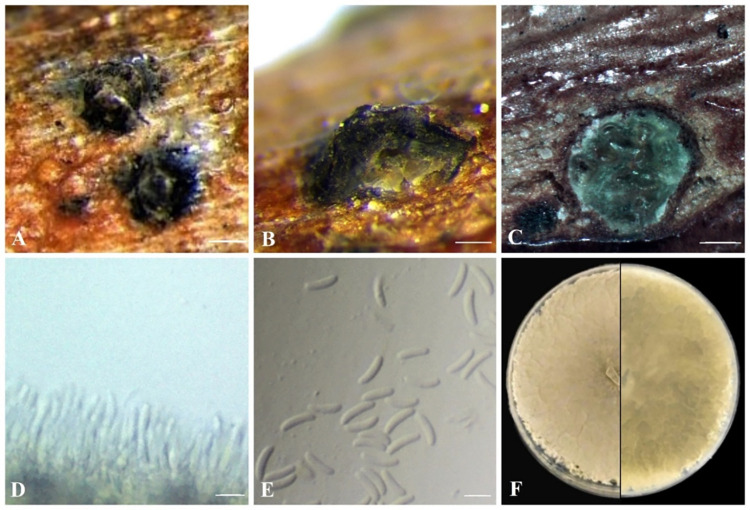
Fig 3. C. paraplurivora on P. persica var. nucipersica (FDS-564).
A. Habit of conidiomata on branches. B. Longitudinal section through conidiomata. C. Transverse section of conidiomata. D. Conidiogenous cells. E. Conidia. F. Seven-day-old culture on PDA. Scale bars: A = 200 μm B-C = 100 μm, D-E = 5 μm.
Index Fungorum No: IF559353
Mycobank No: MB842135
Facesoffungi No: FoF19613
Etymology—the species epithet refers to Greek prefix “para-” meaning “close” and latin “plurivora” is the species name of Cytospora plurivora.
Holotype—DAOM 984922
Pathogenic on seedlings and branches of Prunus persica var. persica.
Sexual morph: not observed. Asexual morph: Conidiomata 440–720 × 220–310 μm diameter (n = 10), semi-immersed in host tissue, erumpent, discoid, solitary, circular to ovoid, scattered, multiloculate, with long ostiolar neck. Ostioles 125–160 μm diameter, at the same level as the disc or higher. Conidiophores unbranched, reduced to conidiogenous cells. Conidiogenous cells 7.5–10 μm (n = 20), blastic, phialidic, originate from inner layer of pycnidial wall, hyaline, smooth-walled. Conidia (4.8–)5.5–7.2(–7.4) × 1.2–1.5(–1.7) μm ( = 6.4 × 1.3 μm, n = 50), hyaline, allantoid, somewhat elongate, aseptate.
Culture characteristics: colonies on PDA are fast-growing, reaching 6.2 cm in diameter after 5 days at 25°C in the dark; initially white turning olivaceous after 5–7 days with thick texture at center, uneven lobate growth margin, lacking aerial mycelium; irregular, abundant pycnidia develop after 14 days. Hyphae hyaline, smooth, branched and septate.
Notes: based on morphological characteristics and phylogenetic analysis, C. paraplurivora is closest to C. plurivora isolated from different hosts in California (USA). This species is found to be associated with a canker disease of fruit (including Prunus spp.) and nut trees. Both species have common morphological characteristics but conidia of C. paraplurivora are notably longer than those of C. plurivora (6.4 × 1.3 μm versus 4.1 × 1.0 μm). Lack of olivaceous color of the C. plurivora cultures grown on PDA and incubated at the same conditions also differentiates these species [5].
Material examined: CANADA, southwestern Ontario, isolated from main stem of Prunus armeniaca, 2019, W. Ellouze, K.E. Schneider, FDS-439; southern Ontario, isolated from single conidium, pycnidia collected from branches of Prunus persica var. nucipersica, Nov. 2019, E. Ilyukhin, W. Ellouze FDS-564 (DAOM 984922 = holotype; DAOMC 252466 = ex-type living culture); southern Ontario, isolated from main stem of Prunus persica var. persica, Apr. 2021, W. Ellouze, K.E. Schneider, FDS-623 (DAOMC 252525 = ex-type living culture).
Genome assembly
A total of 6 854 783 single-end long reads (NCBI SRA accession no. SRR17267775) were generated from PacBio sequencing of FDS-564 and assembled into 211 contigs (> 1000 bp in size), with an N50 of 658,741 bp, and a genome size of 39.7 Mb, which is within the size range of other species of Cytosporaceae (35.2 Mb for C. mali var. pyri SXYL134 to 41. 9 Mb for the representative genome of C. mali 03–8). Assessment of the completeness of the genome using BUSCO groups for fungi resulted in values of C:98.9% [D: 1.0%], F: 0.1%, n: 1 706 (C:complete [D:duplicated], F:fragmented, n: number of genes) which makes the genome sequence suitable for further analysis. The repeat content was 2.9% in the assembly of C. paraplurivora FDS-564. A total of 10,249 protein-coding genes were predicted, which is 9.2% fewer genes than predicted for the genome of C. mali 03–8 (11,284).
Phylogenomic analysis
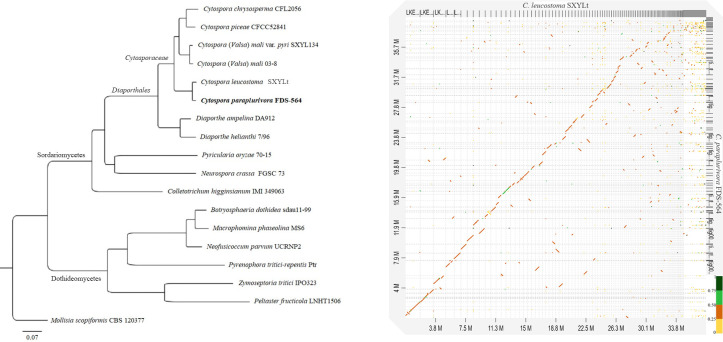
A total of 3,482 orthogroups present in all species included in the analysis with 2,279 SCOs. The phylogenomic tree (Fig 4A) constructed from a concatenated amino acid alignment of SCOs showed that C. paraplurivora was closest to C. leucostoma SXYLt. This analysis placed C. paraplurivora within the Cytosporaceae family of the order Diaporthales with strong bootstrap support (100% ML). However, whole genome sequence alignment between C. paraplurivora and C. leucostoma indicated rather low similarity, where 84.31% of the syntenic blocks were less than 50% similar with a number of small gaps and some repeats (Fig 4B).
Fig 4.
A. ML tree indicating the placement of C. paraplurivora FDS-564 among 17 species of Ascomycetes. Bootstrap support for all nodes is 100. B. Dot plot graph showing syntenic blocks between genome sequences of C. leucostoma SXYLt and C. paraplurivora FDS-564.
Pathogenicity tests
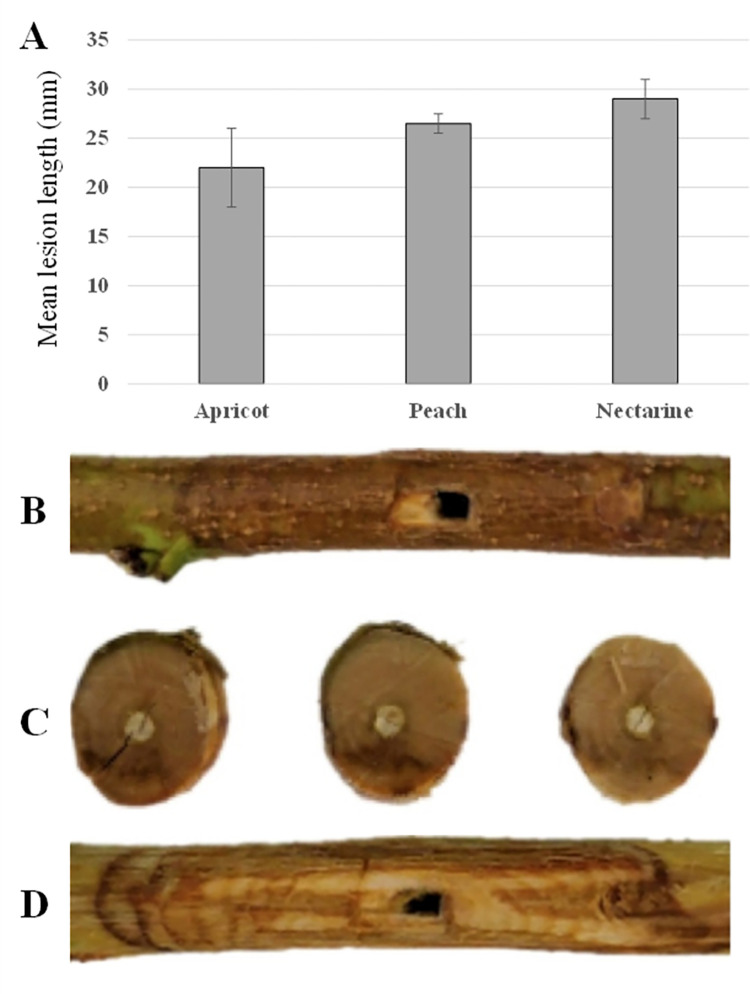
Twelve days post-inoculation, the length of the necrotic lesions caused by C. paraplurivora on apricot, peach and nectarine excised branches were 22, 26 and 29 mm respectively. Lesions were significantly larger in nectarine cuttings compared to those in apricot (Fig 5A). No significant differences were observed between the mean lesion length caused by C. paraplurivora isolates FDS-564 and FDS-623 on peach seedlings 7, 10, 13 and 27 dpi (Fig 6). The canker lesions enlarged on both sides of inoculated incision. The infected peach seedlings start wilting 15 dpi and collapsed 30 dpi. The symptoms observed in the inoculated cuttings and seedlings, included flattening and discolouration of the bark, light brown wedge-shaped cankers, gummosis and abundant fruiting structures (conidiomata) development were similar to those observed on trees in the field (Figs 5B–5D and 7). C. paraplurivora was re-isolated from 90–100% of the inoculated cuttings and seedlings, fulfilling Koch’s postulates. Control cuttings and seedlings had no symptoms, and the fungus was not isolated from the wood.
Fig 5.
A. Mean lesion length caused by C. paraplurivora in Prunus spp. 12 days post-inoculation. B-D. Symptoms caused by C. paraplurivora (FDS-564) in excised branches of P. persica var. nucipercica 12 days post-inoculation.
Fig 6. Mean lesion length caused by C. paraplurivora isolates FDS-564 and FDS-623 in Prunus persica var. persica seedlings 7, 10, 13 and 27 days post-inoculation.
Discussion
Cytospora spp. are important plant pathogens with worldwide distribution causing canker and dieback diseases in woody plants. The genus has a broad host range that includes Castanea, Corylus. Eucaliptus, Juglans, Malus, Olea, Pistacia, Platanus, Prunus, and Salix. [5, 10, 12, 63, 64]. Pathogenic species of Cytospora were mainly isolated from wood cankers. The present study identifies C. paraplurivora sp. nov. isolated from both single conidia and cankered wood sampled from symptomatic apricot, nectarine, and peach trees in southern Ontario. Abundant conidiomata of C. paraplurivora sp. nov. were observed on trunk and branches of declining trees. C. paraplurivora sp. nov. spores dissemination mechanism might be similar to that of the sister species C. plurivora [65], other Cytospora spp. and fungal species causing Botryosphaeriaceae canker [66, 67]. The presence of conidiomata suggest that this species might spread across fruit tree orchards through spores dissemination by rain splash, wind and insect [65].
The symptoms caused by the new species resemble the leucostoma canker disease (perennial canker) of stone fruits, a widespread plant disease affecting crop yield in Canada and some regions of USA. C. leucostoma and C. cincta were found to be associated with this disease [68] and the species were identified solely by morphological characteristics. The studies on Cytospora spp. with the ITS sequence data refined the species identification of the leucocytosporoid species [69]. However, the later studies revealed that the protein-coding genes included in phylogenetic analysis allow for discrimination of closely related species of Cytospora [70]. In this study, using the sequence data of act and tef1-α genes suggested by Urbez-Torres et al. [39] improved the phylogenetic resolution of this species complex. This approach has also been extensively used to resolve phylogenies of other plant-associated fungal pathogens such as Diaporthe [71], Valsaria [72] and Diplodia [73].
Phylogenomic analysis using the genome sequence data of C. paraplurivora FDS-564 confirmed order and family level classification of the species. It is interesting to note, that the genome size of the new species is slightly larger than the average for Ascomycota (36.9Mb) [41] but significantly smaller compared to plant pathogens of Diaporthales such as Daiporthe citri 63.6Mb [74]. The high-quality genome assembly of C. paraplurivora will provide a valuable resource for the further study of host-pathogen interactions, pathogenicity-related genes, fungal biology, as well as comparative and population genomics analyses of Cytospora spp. and other fungal taxa.
The pathogenicity tests of C. paraplurivora on Prunus spp. performed in this study can be comparable to the trials of other pathogenic species of Cytospora on Malus spp. conducted in vitro under the same conditions with different timelines [75]. The phylogenetically close species C. paratranslucens caused necrotic lesions up to 10 cm on detached apple branches after 21 days [75]. The lesions up to 3.9 cm developed on twig segments of Malus sieversii inoculated with mycelium of C. parasitica after 8 days. This study showed that, highly virulent model species C. mali was able to cause the lesions up to 8.1 cm for the same time period [76]. Considering the time of post-inoculation with C. paraplurivora, it is assumed that the species can quickly colonize the plant tissue and cause the decline of infected trees. The pathogenicity tests on living peach seedlings confirmed the rapid spread of the canker lesions on the trunk of the inoculated plants, leading to the collapse of peach seedlings 30 dpi.
This paper is the first formal report for a new Cytospora species causing the canker of Prunus spp. associated with FTDS in Ontario. The findings suggest that C. paraplurivora has the potential to severely affect stone fruits production in Ontario. Accurate identification of pathogen(s) associated with stone fruit trees decline will support management of the disease. Further research needs to be conducted on emerging fungal pathogens and their associated infection mechanisms, to develop effective disease management strategies applicable to fruit tree orchards.
Acknowledgments
The authors would like to thank Karin Schneider who provided help and support with logistic and field work. Thanks are also due to Dr. Antonet Svircev, Agriculture and Agri-Food Canada, Vineland Station, ON for her helpful comments on the manuscript. The authors acknowledge the following principal investigators for permission to use genomic data: Dr. R. Hamelin (C. chrysosperma CFL2056), Dr. L. Huang (C. leucostoma SXYLt), Dr. X. Fan (C. piceae CFCC52841).
Data Availability
Alignment of DNA data are available in Treebase (submission ID: 29116) (https://www.treebase.org/treebase-web/home.html).Nucleotide sequences of Cytospora paraplurivora strains FDS-439, FDS-564 and FDS-623 were submitted to NCBI repository (https://www.ncbi.nlm.nih.gov) under accession numbers: OL640182, OL640183, OL640181 (ITS); OL640184, OL640185, OL640123 (LSU); OL631586, OL631587, OL631588 (act) and OL631589, OL631590, OL631591 (tef1-α). Cytospora paraplurivora strains FDS-564 Whole Genome Sequencing data was submitted to Genomes NCBI repository under accession numbers: JAJSPL000000000 (WGS), GCA_021272945 (Assembly) and SAMN24182651 (BioSample).
Funding Statement
This research was funded by Agriculture and Agri-Food Canada, grant number J-002199 awarded to Dr. Walid Ellouze. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gardner J, Slingerland K, Fisher P. What you should know about fruit production In Ontario Ontario, Canada: Ontario Ministry of Agriculture, Food and Rural Affairs; 2016. [2022-10-23]. Available from: http://www.omafra.gov.on.ca/english/crops/facts/04-045.htm. [Google Scholar]
- 2.Brown-Rytlewski DE, McManus PS. Outbreak of Leucostoma canker caused by Leucostoma cincta on McIntosh Apple trees in Wisconsin. Plant Dis. 2000;84(8):923–. doi: 10.1094/pdis.2000.84.8.923b [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Iezzoni A, Adams G. Genetic heterogeneity of Leucostoma species in Michigan peach orchards. Phytopathology. 1998;88(5):376–81. doi: 10.1094/phyto.1998.88.5.376 [DOI] [PubMed] [Google Scholar]
- 4.Bagherabadi S, Zafari D, Soleimani MJ. Morphological and molecular identification of Cytospora chrysosperma causing canker disease on Prunus persica. Australasian Plant Dis Notes. 2017;12(1):26. doi: 10.1007/s13314-017-0250-9 [DOI] [Google Scholar]
- 5.Lawrence DP, Holland LA, Nouri MT, Travadon R, Abramians A, Michailides TJ, et al. Molecular phylogeny of Cytospora species associated with canker diseases of fruit and nut crops in California, with the descriptions of ten new species and one new combination. IMA Fungus. 2018;9:333–70. doi: 10.5598/imafungus.2018.09.02.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams G, Wingfield M, Common R, Roux J. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus2005 01/01. 144 p. [Google Scholar]
- 7.Senanayake IC, Crous PW, Groenewald JZ, Maharachchikumbura SSN, Jeewon R, Phillips AJL, et al. Families of Diaporthales based on morphological and phylogenetic evidence. Stud Mycol 2017;86:217–96. doi: 10.1016/j.simyco.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Alvarez LV, Bonthond G, Tian C, Fan X. Cytospora elaeagnicola sp. nov. associated with narrow-leaved oleaster canker disease in China. Mycobiology. 2019;47(3):319–28. doi: 10.1080/12298093.2019.1633902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan XL, Bezerra JDP, Tian CM, Crous PW. Cytospora (Diaporthales) in China. Persoonia. 2020;45:1–45. doi: 10.3767/persoonia.2020.45.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norphanphoun C, Raspé O, Jeewon R, Wen T-C, Hyde KD. Morphological and phylogenetic characterisation of novel Cytospora species associated with mangroves. MycoKeys. 2018;38:93–120. doi: 10.3897/mycokeys.38.28011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang N, Yang Q, Fan X-L, Tian C-M. Identification of six Cytospora species on Chinese chestnut in China. MycoKeys. 2020;62:1–25. doi: 10.3897/mycokeys.62.47425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan M, Zhu H, Tian C, Huang M, Fan X. Assessment of Cytospora isolates from conifer cankers in China, with the descriptions of four new Cytospora species. Front Plant Sci. 2021;12:636460. doi: 10.3389/fpls.2021.636460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haridas S, Albert R, Binder M, Bloem J, LaButti K, Salamov A, et al. 101 Dothideomycetes genomes: A test case for predicting lifestyles and emergence of pathogens. Stud Mycol. 2020;96:141–53. doi: 10.1016/j.simyco.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dal’Sasso TCdS Rody HVS, Grijalba PE Oliveira LOd. Genome sequences and in silico effector mining of Corynespora cassiicola CC_29 and Corynespora olivacea CBS 114450. Arch Microbiol. 2021;203(8):5257–65. doi: 10.1007/s00203-021-02456-7 [DOI] [PubMed] [Google Scholar]
- 15.Jeewon R. Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere. 2016;7:1669–77. doi: 10.5943/mycosphere/7/11/4 [DOI] [Google Scholar]
- 16.Aime MC, Miller AN, Aoki T, Bensch K, Cai L, Crous PW, et al. How to publish a new fungal species, or name, version 3.0. IMA Fungus. 2021;12(1):11. doi: 10.1186/s43008-021-00063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, et al. The sooty moulds. Fungal Divers. 2014;66(1):1–36. doi: 10.1007/s13225-014-0278-5 [DOI] [Google Scholar]
- 18.Senanayake IC, Rathnayaka AR, Marasinghe DS, Calabon MS, Gentekaki E, Lee HB, et al. Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere. 2020;11(1):2678–754. doi: 10.5943/mycosphere/11/1/20 [DOI] [Google Scholar]
- 19.Rayner RW. A mycological colour chart. Kew, Surrey, England: Commonwealth Mycological Institute and British Mycological Society; 1970. [Google Scholar]
- 20.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego, CA: Academic Press; 1990. p. 315–22. [Google Scholar]
- 21.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 1990;172(8):4238–46. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91(3):553–6. doi: 10.1080/00275514.1999.12061051 [DOI] [Google Scholar]
- 23.Hall T, editor BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser; 1999. [Google Scholar]
- 24.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 25.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–82. doi: 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swofford DL. PAUP*. Phylogenetic analysis using parsimony (∗and Other Methods). Sunderland, MA: Sinauer Associates. 2003. [Google Scholar]
- 29.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinform. 2001;17(8):754–5. doi: 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- 30.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–21. doi: 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 31.Darriba D, Posada D, Kozlov AM, Stamatakis A, Morel B, Flouri T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol Biol Evol. 2019;37(1):291–4. doi: 10.1093/molbev/msz189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akaike H, editor Information theory as an extension of the maximum likelihood principle. Second International Symposium on information theory; 1973; Budapest: Akademiai Kiado. [Google Scholar]
- 33.Hillis DM, Bull JJ. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol. 1993;42(2):182–92. doi: 10.1093/sysbio/42.2.182 [DOI] [Google Scholar]
- 34.Rambaut A. FigTree v1.4.4. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh; 2018. [Google Scholar]
- 35.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27(5):722–36. doi: 10.1101/gr.215087.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikheenko A, Prjibelski A, Saveliev V, Antipov D, Gurevich A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics. 2018;34(13):i142–i50. doi: 10.1093/bioinformatics/bty266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2. doi: 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- 38.Smit AFA, Hubley R, Green P. RepeatMasker Open-4.0. 2013–2015. < http://www.repeatmasker.org > 2015.
- 39.Bao W, Kojima KK, Kohany O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mobile DNA. 2015;6(1):11. doi: 10.1186/s13100-015-0041-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33(suppl_2):W465–W7. doi: 10.1093/nar/gki458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinform. 2011;12(1):491. doi: 10.1186/1471-2105-12-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, et al. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 2013;42(1):699–704. doi: 10.1093/nar/gkt1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol 2015;16(1):157. doi: 10.1186/s13059-015-0721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price MN, Dehal PS, Arkin AP. FastTree 2 –Approximately Maximum-Likelihood Trees for Large Alignments. PLOS ONE. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–100. doi: 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabanettes F, Klopp C. D-GENIES: dot plot large genomes in an interactive, efficient and simple way. PeerJ 2018;6:e4958. doi: 10.7717/peerj.4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu C, Diao Y, Lu Q, Zhao J, Cui S, Peng C, et al. Genome assembly and annotation of Botryosphaeria dothidea sdau11-99, a latent pathogen of apple fruit ring rot in China. Plant Dis. 2021;105(5):1555–7. doi: 10.1094/pdis-06-20-1182-a [DOI] [PubMed] [Google Scholar]
- 49.Zampounis A, Pigné S, Dallery J-F, Wittenberg AHJ, Zhou S, Schwartz DC, et al. Genome sequence and annotation of Colletotrichum higginsianum, a causal agent of crucifer anthracnose disease. Genome Announc. 2016;4(4):e00821–16. doi: 10.1128/genomeA.00821-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Q, Bakhshi M, Balci Y, Broders KD, Cheewangkoon R, Chen SF, et al. Genera of phytopathogenic fungi: GOPHY 4. Stud Mycol. 2022;101:417–564. doi: 10.3114/sim.2022.101.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin Z, Liu H, Li Z, Ke X, Dou D, Gao X, et al. Genome sequence of Valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytol. 2015;208(4):1202–16. doi: 10.1111/nph.13544 [DOI] [PubMed] [Google Scholar]
- 52.Morales-Cruz A, Amrine KCH, Blanco-Ulate B, Lawrence DP, Travadon R, Rolshausen PE, et al. Distinctive expansion of gene families associated with plant cell wall degradation, secondary metabolism, and nutrient uptake in the genomes of grapevine trunk pathogens. BMC Genom. 2015;16(1):469. doi: 10.1186/s12864-015-1624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baroncelli R, Scala F, Vergara M, Thon MR, Ruocco M. Draft whole-genome sequence of the Diaporthe helianthi 7/96 strain, causal agent of sunflower stem canker. Genom Data. 2016;10:151–2. doi: 10.1016/j.gdata.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islam MS, Haque MS, Islam MM, Emdad EM, Halim A, Hossen QMM, et al. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012;13(1):493. doi: 10.1186/1471-2164-13-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker AK, Frasz SL, Seifert KA, Miller JD, Mondo SJ, LaButti K, et al. Full genome of Phialocephala scopiformis DAOMC 229536, a fungal endophyte of spruce producing the potent anti-insectan compound rugulosin. Genome Announc. 2016;4(1):e01768–15. doi: 10.1128/genomeA.01768-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanco-Ulate B, Rolshausen P, Cantu D. Draft genome sequence of Neofusicoccum parvum Isolate UCR-NP2, a fungal vascular pathogen associated with grapevine cankers. Genome Announc 2013;1(3):e00339–13. doi: 10.1128/genomeA.00339-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker SE, Schackwitz W, Lipzen A, Martin J, Haridas S, LaButti K, et al. Draft genome sequence of Neurospora crassa Strain FGSC 73. Genome Announc. 2015;3(2):e00074–15. doi: 10.1128/genomeA.00074-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu C, Chen H, Gleason ML, Xu J-R, Liu H, Zhang R, et al. Peltaster fructicola genome reveals evolution from an invasive phytopathogen to an ectophytic parasite. Sci Rep. 2016;6(1):22926. doi: 10.1038/srep22926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manning VA, Pandelova I, Dhillon B, Wilhelm LJ, Goodwin SB, Berlin AM, et al. Comparative genomics of a plant-pathogenic fungus, Pyrenophora tritici-repentis, reveals transduplication and the impact of repeat elements on pathogenicity and population divergence. G3-Genes Genom Genet. 2013;3(1):41–63. doi: 10.1534/g3.112.004044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434(7036):980–6. doi: 10.1038/nature03449 [DOI] [PubMed] [Google Scholar]
- 61.Goodwin SB, Ben M’Barek S, Dhillon B, Wittenberg AHJ, Crane CF, Hane JK, et al. Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet. 2011;7(6):e1002070. doi: 10.1371/journal.pgen.1002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arzanlou M, Narmani A. ITS sequence data and morphology differentiate Cytospora chrysosperma associated with trunk disease of grapevine in northern Iran. J Plant Prot Res. 2015;55(2):117–25. doi: 10.1515/jppr-2015-0015 [DOI] [Google Scholar]
- 63.Adams GC, Roux J, Wingfield MJ. Cytospora species (Ascomycota, Diaporthales, Valsaceae): introduced and native pathogens of trees in South Africa. Australasian Plant Pathology. 2006;35(5):521–48. doi: 10.1071/AP06058 [DOI] [Google Scholar]
- 64.Úrbez-Torres JR, Lawrence DP, Hand FP, Trouillas FP. Olive twig and branch dieback in California caused by Cytospora oleicola and the newly described species Cytospora olivarum sp. nov. Plant Dis. 2020;104(7):1908–17. doi: 10.1094/pdis-09-19-1979-re [DOI] [PubMed] [Google Scholar]
- 65.Miller ST. Management and epidemiology of Cytospora perennial canker, Cytospora plurivora, in western Colorado [Ph.D. Dissertation]. Fort Collins, Colorado: Colorado State University; 2021. [Google Scholar]
- 66.Ilyukhin E, Schneider K, Ellouze W. First report of Botryosphaeria dothidea causing stem canker and dieback of apple trees in Ontario, Canada. Plant Dis. 2022. doi: 10.1094/pdis-12-21-2838-pdn 35350900 [DOI] [Google Scholar]
- 67.Luo Y, Niederholzer FJA, Felts DG, Puckett RD, Michailides TJ. Inoculum quantification of canker-causing pathogens in prune and walnut orchards using real-time PCR. Journal of applied microbiology. 2020;129(5):1337–48. doi: 10.1111/jam.14702 [DOI] [PubMed] [Google Scholar]
- 68.Biggs AR, Grove GG. Leucostoma canker of stone fruits. The Plant Health Instructor. 2005. doi: 10.1094/PHI-I-2005-1220-01 [DOI] [Google Scholar]
- 69.Adams GC, Surve-Iyer RS, Iezzoni AF. Ribosomal DNA sequence divergence and group I introns within the Leucostoma species L. cinctum, L. persoonii, and L. parapersoonii sp. nov., ascomycetes that cause Cytospora canker of fruit trees. Mycologia. 2002;94(6):947–67. doi: 10.1080/15572536.2003.11833153 [DOI] [PubMed] [Google Scholar]
- 70.Lawrence DP, Travadon R, Pouzoulet J, Rolshausen PE, Wilcox WF, Baumgartner K. Characterization of Cytospora isolates from wood cankers of declining grapevine in North America, with the descriptions of two new Cytospora species. Plant Pathol. 2017;66(5):713–25. doi: 10.1111/ppa.12621 [DOI] [Google Scholar]
- 71.Manawasinghe IS, Dissanayake AJ, Li X, Liu M, Wanasinghe DN, Xu J, et al. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front Microbiol. 2019;10. doi: 10.3389/fmicb.2019.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pem D, Hyde KD, Doilom M, Camporesi E, Hongsanan S, Rampadarath S, et al. Multigene phylogenetic analyses to establish new Valsaria species and taxonomic significance of spore ornamentation. PLOS ONE. 2019;14(6):e0217982. doi: 10.1371/journal.pone.0217982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferreira SL, Stauder CM, Martin DKH, Kasson MT. Morphological and phylogenetic resolution of Diplodia corticola and D. quercivora, emerging canker pathogens of oak (Quercus spp.), in the United States. Plant Dis. 2021;105(5):1298–307. doi: 10.1094/pdis-05-20-0977-re [DOI] [PubMed] [Google Scholar]
- 74.Gai Y, Xiong T, Xiao X, Li P, Zeng Y, Li L, et al. The genome sequence of the Citrus Melanose Pathogen Diaporthe citri and Two Citrus-Related Diaporthe Species. Phytopathology. 2021;111(5):779–83. doi: 10.1094/phyto-08-20-0376-sc [DOI] [PubMed] [Google Scholar]
- 75.Azizi R, Ghosta Y, Ahmadpour A. Morphological and molecular characterization of Cytospora species involved in apple decline in Iran. Mycol Iran. 2020;7(2):205–18. doi: 10.22043/mi.2021.123907 [DOI] [Google Scholar]
- 76.Liu X, Li X, Bozorov TA, Ma R, Ma J, Zhang Y, et al. Characterization and pathogenicity of six Cytospora strains causing stem canker of wild apple in the Tianshan Forest, China. For Path. 2020;50(3):e12587. doi: 10.1111/efp.12587 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Alignment of DNA data are available in Treebase (submission ID: 29116) (https://www.treebase.org/treebase-web/home.html).Nucleotide sequences of Cytospora paraplurivora strains FDS-439, FDS-564 and FDS-623 were submitted to NCBI repository (https://www.ncbi.nlm.nih.gov) under accession numbers: OL640182, OL640183, OL640181 (ITS); OL640184, OL640185, OL640123 (LSU); OL631586, OL631587, OL631588 (act) and OL631589, OL631590, OL631591 (tef1-α). Cytospora paraplurivora strains FDS-564 Whole Genome Sequencing data was submitted to Genomes NCBI repository under accession numbers: JAJSPL000000000 (WGS), GCA_021272945 (Assembly) and SAMN24182651 (BioSample).