Abstract
Background
Data are needed on the use of oral anticoagulation in patients with atrial fibrillation (AF) in rural versus urban areas, including the initiation of direct oral anticoagulants (DOACs).
Objective
We used Medicare data to examine rural/urban differences in anticoagulation use in patients with AF.
Methods
We identified incident AF in a 20% sample of fee-for-service Medicare beneficiaries (aged ≥ 65 years) from 2011 to 2016 and collected ZIP code and covariates at the time of AF. We identified the first anticoagulant prescription filled, if any, following AF diagnosis. We categorized beneficiaries into four rural/urban areas using rural–urban commuting area codes and used Poisson regression models to compare anticoagulant use.
Results
We included 447,252 patients with AF (mean age 79 ± 8 years), of which 82% were urban, 9% large rural, 5% small rural, and 4% isolated. The percentage who initiated an anticoagulant rose from 34% in 2011 to 53% in 2016, paralleling the uptake of DOACs. In a multivariable-adjusted analysis, those in rural areas (vs. urban) were more likely to initiate an anticoagulant. However, rural beneficiaries (vs. urban) were less likely to initiate a DOAC; those in isolated areas were 17% less likely (95% confidence interval [CI] 13–20), those in small rural areas were 12% less likely (95% CI 9–15), and those in large rural areas were 10% less likely (95% CI 8–12).
Conclusion
Among Medicare beneficiaries with AF, anticoagulation use was low but increased over time with the introduction of DOACs. Rural beneficiaries were less likely to receive a DOAC.
1. Introduction
Individuals with atrial fibrillation (AF), a common cardiac arrhythmia, have a fivefold increased risk of stroke compared with those without AF, so the mainstay of stroke prevention in AF is the initiation and maintenance of anticoagulant therapies [1]. The 2014 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS) guidelines for the management of patients with AF recommended that either warfarin or one of the direct oral anticoagulants (DOACs) be prescribed for those with nonvalvular AF and a CHA2DS2-VASc sore ≥ 2 [2]. Since 2010, the US FDA has approved four DOACs for stroke prevention in AF, including the direct thrombin inhibitor dabigatran and the direct factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The DOACs have many advantages over warfarin, including fewer drug interactions, more predictable pharmacological profiles, an absence of major dietary effects, and a reduced risk of intracranial bleeding and ischemic stroke [2]. Therefore, the 2019 ACC/AHA/HRS guidelines stated that DOACs are preferred over warfarin in most cases [3]. Currently, DOACs account for > 50% of anticoagulants prescribed for patients with AF and have directly contributed to the rising percentage of patients with AF treated with anticoagulants [4, 5].
In the USA, nearly 60 million people (19% of the population) are living in rural areas according to the US Census Bureau. Those in rural areas have higher rates of adverse cardiovascular disease and risk factors such as cigarette smoking, hypertension, diabetes, and obesity, and poorer outcomes such as coronary heart disease and stroke [6–9]. Despite AF being an established risk factor for stroke, and stroke risk being higher in rural areas, little is known regarding anticoagulation rates in patients with AF in rural versus urban areas. Furthermore, no data have been published addressing the adoption of DOAC prescriptions in rural areas of the USA. Rural patients face additional challenges with the distance to a care facility, and a medication such as warfarin, with its need for frequent monitoring, may place additional burden on the patient. Differences in the initiation of anticoagulation and DOAC use by rural/urban status may identify an area of practice improvement for providers to reduce the burdens of stroke and healthcare utilization in a population of older adults.
Using a sample of Medicare beneficiaries, which included detailed patient geographic location information, we described trends in oral anticoagulant prescription fills, including the initiation of DOACs, in patients with AF from 2011 to 2016. We also compared type of anticoagulation treatment in Medicare beneficiaries with AF living in rural versus urban areas.
2. Methods
2.1. Study Population
We conducted a retrospective study using healthcare utilization claims data from a 20% sample of Medicare beneficiaries from 2011 to 2016. We limited the cohort to beneficiaries receiving fee-for-service Medicare who were aged ≥ 65 years living in the USA, and—to capture all medication fills—beneficiaries must have been enrolled in a stand-alone Part D prescription drug plan. To be included, beneficiaries must have had at least 90 days of continuous enrollment in Medicare Parts A/B/D without supplemental coverage during the years 2011–2016. We required at least the first 90 days of a beneficiaries’ observation time to be free of AF diagnosis codes and anticoagulation codes to (1) capture incident AF events, (2) capture the first anticoagulation prescription following an AF event, and (3) to serve as a run-in period to capture patient health information and comorbidities prior to an AF event. If a beneficiary enrolled in supplemental coverage after the first 90 days, we censored them at the time of supplemental enrollment. For this analysis, we also required at least a 30-day follow-up period after AF diagnosis to allow an appropriate time window for the beneficiary to fill an anticoagulant prescription.
The initial sample included 910,649 patients with AF aged 65–112 years. The exclusion flow chart is depicted in Fig. 1. We excluded those with an AF diagnosis or prescription fill for an anticoagulant during the first 90 days of enrollment (n = 412,076), those initiating edoxaban (because of small numbers; n = 296), those with less than 30 days of follow-up (n = 50,026), and those with a missing ZIP code or a ZIP code in a US territory (n = 819). Our final analytic sample for the trends analysis overall was 447,252; for the analyses comparing rural versus urban, 202,074 of those were successfully matched. This study was approved by the University of Minnesota Institutional Review Board as exempt because deidentified data were used.
Fig. 1.

Analysis flowchart of the 20% sample of traditional fee-for-service Medicare Beneficiaries, 2011–2016. AF atrial fibrillation, DC District of Columbia, ICD International Classification of Diseases
2.2. Ascertainment of Atrial Fibrillation
This analysis included patients aged ≥ 65 years with at least one inpatient claim for AF or two outpatient claims for AF 7–365 days apart. AF claims were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes 427.3, 427.31, and 427.32 and ICD-10-CM codes starting 1 October 2015 of I48.x in any position, which is a standard definition used in claims analysis [10, 11]. The validity of ICD-9-CM codes for the identification of AF has been well-established, with a systematic review of studies showing a positive predictive value (PPV) of approximately 90% and a sensitivity of approximately 80% [12]. We defined the AF diagnosis date as the earlier of (1) the earliest discharge date for an inpatient claims or (2) the earliest service date of the outpatient or physician claim. Consistent with prior research, two outpatient claims were required to diagnose outpatient AF to minimize the impact of rule-out diagnosis and to improve specificity [11].
2.3. Defining Rural and Urban Beneficiaries
We captured the beneficiary ZIP code at the time of AF diagnosis. We cross-walked ZIP codes to Rural-Urban Communing Area (RUCA) codes, which are approximation codes developed by the University of Washington Rural Health Research Center [13] commonly used to define rural and urban areas [14]. RUCA codes combine standard Census definitions with area commuting behaviors to capture functional and work relationships between regions.
We used a common four-category classification to access the rurality of beneficiaries: urban (RUCA codes 1–3, 4.1, 5.1, 7.1, 8.1, 10.1), large rural (RUCA codes 4.0, 4.2, 5.0, 5.2, 6.0, 6.1), small rural (RUCA codes 7.0, 7.2, 7.3, 7.4, 8.0, 8.2, 8.3, 8.4, 9.0, 9.1, 9.2), and isolated (RUCA codes 10, 10.2, 10.3, 10.4, 10.5, 10.6) [14]. In a secondary analysis, we reported rural–urban trends in oral anticoagulation use by stratifying the USA into four US Census Bureau-designated regions: northeast (CT, ME, MA, NH, RI, VT, NJ, NY, PA), midwest (IN, IL, MI, OH, WI, IA, KA, MN, MO, NE, ND, SD), south (DE, D.C., FL, GA, MD, NC, SC, VA, WV, AL, KY, MS, TN, AR, LA, OK, TX), and west (AZ, CO, ID, NM, MT, UT, NV, WY, AK, CA, HI, OR, WA).
2.4. Anticoagulation Treatment Definitions
We identified filled prescriptions for oral anticoagulation using Part D pharmaceutical claims data, which included the prescription fill date, the strength, and the number of days supplied. Beneficiaries were assigned to the first anticoagulant filled in either the 30 days prior to or anytime following their first AF claim. We included prescriptions initiated for warfarin, dabigatran, rivaroxaban, and apixaban in this analysis. We excluded edoxaban users because numbers were small. The validity of warfarin claims in administrative databases is excellent, with a sensitivity of 94% and a PPV of 99% [15]. Validation studies of DOAC claims have not yet been conducted.
2.5. Covariates
Using the Medicare files, we identified covariates prevalent at the time of AF diagnosis. Race was self-reported, and we categorized it into categories of white, Black, and other/unknown (because numbers were small). We defined predetermined covariates based on inpatient, outpatient, carrier, and pharmacy claims using validated published algorithms [16–18]. These included demographic characteristics, comorbidities, and prior or current pharmacy prescription fills. Comorbidities of interest were ascertained with published algorithms from inpatient and outpatient claims and included prior stroke/transient ischemic attack (TIA), hemorrhagic stroke, heart failure, myocardial infarction, hypertension, diabetes, peripheral arterial disease, liver disease, kidney disease, chronic pulmonary disease, malignancies (except malignant skin neoplasm), metastatic cancer, history of bleeding, hematological disorders (anemia, coagulation defects), dementia, depression, and alcohol abuse [16, 17]. ICD codes for the comorbidity variables are listed in Table 1 in the electronic supplementary material (ESM). We captured prescription fills for the following medication groups: clopidogrel, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, β-blockers, calcium channel blockers, antiarrhythmics, and statins. We calculated the CHA2DS2-VASc score [19] at AF date; this consisted of congestive heart failure, hypertension, age (1 point for age 65–74 years; 2 points for ≥ 75 years), diabetes, prior stroke or TIA (2 points), vascular disease, and female sex. The HAS-BLED score [20] was calculated using the variables hypertension, abnormal renal/liver function, stroke, bleeding history or disposition, elderly (age > 65 years), and drugs/alcohol concomitantly. The variable international normalized ratio (INR) is normally included in the HAS-BLED score but was not available for this cohort in Medicare. We used Medicare carrier claims to identify provider specialty at outpatient visits. Beneficiaries who saw a cardiology provider within a predetermined period (30 days prior to or 90 days after AF diagnosis) were classified in the cardiology group, whereas patients seen exclusively by internal medicine, family practice, or medical doctor or an unspecified multispecialty group were classified as primary care. Patients seen by a cardiologist were included in the cardiology provider group, regardless of whether they also saw noncardiology providers. Lastly, to take into account the biased propensity of an individual to seek care, we incorporated a variable of seeking preventive service, which we captured as receipt of the influenza vaccine within the year prior to AF diagnosis.
2.6. Statistical Analysis
We examined the anticoagulant prescription fill patterns in AF Medicare patients in rural versus urban areas. We compared baseline characteristics at the time of AF diagnosis between the four rurality groups. We evaluated the proportion of patients with AF who filled oral anticoagulant prescriptions using graphs, first overall by year and quarter, and then in each rural/urban category by year. We also determined oral anticoagulant prescriptions within the CHA2DS2-VASc score by rural category.
To compare proportions of anticoagulants, we first created a propensity score for levels of rurality. We used multivariable logistic regression to predict the probability of living in each classification of rural area (vs. the urban area) based on the aforementioned covariates. We matched beneficiaries based on AF date (± 30 days), age (± 1 years), sex, CHA2DS2-VASc score (± 0), and propensity score (± 0.01). We used a greedy matching algorithm to match one beneficiary from each of the three rural categories with up to two beneficiaries in the urban category [21]. We used Poisson regression models with robust variance estimates to compute risk ratios (RRs) and 95% confidence intervals (CIs) [22]. The model adjusted for age (continuous), race (white, Black, other), sex, CHA2DS2-VASc score (categorical, 0–9), HAS-BLED score (continuous), specialist care (cardiology: yes/no), and the additional covariates listed above and in Table 1.
Table 1.
Characteristics at the time of atrial fibrillation diagnosis by urban/rural classification for the entire cohort, Medicare, 2011–2016
| Characteristic | Urban/rural classification |
|||
|---|---|---|---|---|
| Urban (n = 369,357) | Large rural (n = 38,167) | Small rural (n = 21,934) | Isolated rural (n = 17,794) | |
| Age, years | 79.0 ± 8.4 | 78.6 ± 8.2 | 78.7 ± 8.1 | 78.7 ± 8.2 |
| Female | 55 | 56 | 56 | 54 |
| White race | 84 | 92 | 92 | 94 |
| Black race | 7 | 4 | 4 | 2 |
| Other race | 9 | 4 | 3 | 3 |
| CHA2DS2-VASC score | 5.0 ± 1.9 | 4.8 ± 1.8 | 4.9 ± 1.8 | 4.8 ± 1.8 |
| HAS-BLED score | 3.1 ± 1.2 | 3.0 ± 1.1 | 3.0 ± 1.1 | 2.9 ± 1.1 |
| Cardiology involvement | 84 | 77 | 74 | 74 |
| Received influenza vaccine | 53 | 50 | 43 | 40 |
| Comorbidities | ||||
| Hypertension | 88 | 88 | 88 | 87 |
| Diabetes | 42 | 39 | 40 | 39 |
| Myocardial infarction | 12 | 14 | 15 | 14 |
| Heart failure | 36 | 36 | 38 | 37 |
| Ischemic stroke/TIA | 35 | 31 | 31 | 30 |
| Peripheral artery disease | 36 | 31 | 32 | 30 |
| Hemorrhagic stroke | 2 | 2 | 2 | 2 |
| Dementia | 9 | 7 | 7 | 6 |
| Renal disease | 26 | 24 | 24 | 24 |
| Chronic pulmonary disease | 31 | 35 | 36 | 35 |
| Liver disease | 10 | 8 | 7 | 7 |
| Hematological disorders | 26 | 23 | 22 | 20 |
| Gastrointestinal bleed | 35 | 34 | 33 | 32 |
| Other bleed | 48 | 42 | 43 | 41 |
| Malignancy | 21 | 19 | 18 | 19 |
| Metastatic cancer | 4 | 4 | 3 | 4 |
| Depression | 21 | 21 | 21 | 20 |
| Alcohol abuse | 1 | 1 | 1 | 1 |
| Medications | ||||
| Digoxin | 0.4 | 0.4 | 0.4 | 0.4 |
| Clopidogrel | 14 | 15 | 15 | 14 |
| Antiplatelet agents | 0.3 | 0.4 | 0.3 | 0.5 |
| ACE inhibitors | 26 | 29 | 29 | 29 |
| Angiotensin-receptor blockers | 15 | 14 | 13 | 12 |
| β-blockers | 34 | 37 | 38 | 38 |
| Calcium channel blockers | 28 | 28 | 28 | 26 |
| Anti-arrhythmic agents | 2 | 2 | 2 | 3 |
| Statins | 41 | 41 | 41 | 41 |
| Diabetes medications | 6 | 7 | 7 | 7 |
| Oral anticoagulants | ||||
| Warfarin | 23 | 26 | 28 | 29 |
| Dabigatran | 4 | 4 | 4 | 4 |
| Rivaroxaban | 10 | 9 | 9 | 8 |
| Apixaban | 10 | 9 | 9 | 8 |
Data are presented as percentages or mean ± standard deviation
ACE angiotensin-converting enzyme, CHA2DS2-VASC congestive heart failure, hypertension, age (1 point for age 65–74; 2 points for ≥75 years), diabetes, prior stroke or TIA (2 points), vascular disease, and female sex, HAS-BLED hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly (age >65 years), drugs/alcohol concomitantly, TIA transient ischemic attack
We examined effect modification by sex, race, and age (< 75 years, ≥ 75 years) by adding a multiplicative interaction term in the model. A sensitivity analysis was limited to patients with AF who qualified for oral anticoagulants (CHA2DS2-VASc scores ≥ 2); because of the advanced age and multimorbidity of Medicare patients, we had to exclude only a small percentage (< 2%) of beneficiaries because of low CHA2DS2-VASc scores. We conducted an additional sensitivity analysis requiring a 180-day run-in time instead of 90 days.
3. Results
After exclusion criteria were applied, our study included 447,252 beneficiaries with AF (mean age 79 ± 8 years), of which 369,357 (83%) lived in an urban area, 38,167 (9%) lived in a large rural area, 21,934 (5%) lived in a small rural area, and 17,794 (4%) lived in an isolated rural area. Characteristics of the total cohort of patients with AF are listed in Table 1. Those in urban areas were slightly older, were more likely to be a non-white race, were more likely to have seen a cardiologist, and had a higher CHA2DS2-VASc score than those in rural areas.
We present several figures to graphically depict anticoagulation initiation over the study period for the entire cohort. Overall temporal trends of the anticoagulants by year and quarter are depicted in Fig. 1 in the ESM. In 2011, only 34% of beneficiaries used anticoagulants; by 2016, the percentage was 53%. The proportion of warfarin users decreased every year after 2012, whereas the uptake of DOACs increased every year. By 2016, apixaban was the most commonly used anticoagulant. Figure 2 shows the temporal trends of anticoagulation initiation by urban/rural category. Total anticoagulation increased in a similar manner in each of the four rural/urban categories over time. Warfarin use is depicted with a clear gradient across rurality, and for every year, the highest percentage of patients with AF prescribed warfarin were in the rural areas, with those in isolated areas appearing to be the most likely to receive warfarin and the least likely to receive a DOAC. This pattern persisted when we pooled years and stratified anticoagulation initiation by CHA2DS2-VASc score, as presented in Fig. 3. Overall, anticoagulation frequency was higher in those in more rural areas, but those in rural areas were more likely to be receiving warfarin than were those in urban areas. In Fig. 2 in the ESM, we present patterns of anticoagulant initiation by rural/urban category in four areas of the USA. Total anticoagulation was highest in the northeast region and lowest in the southern region. DOAC use was highest in the southern region.
Fig. 2.
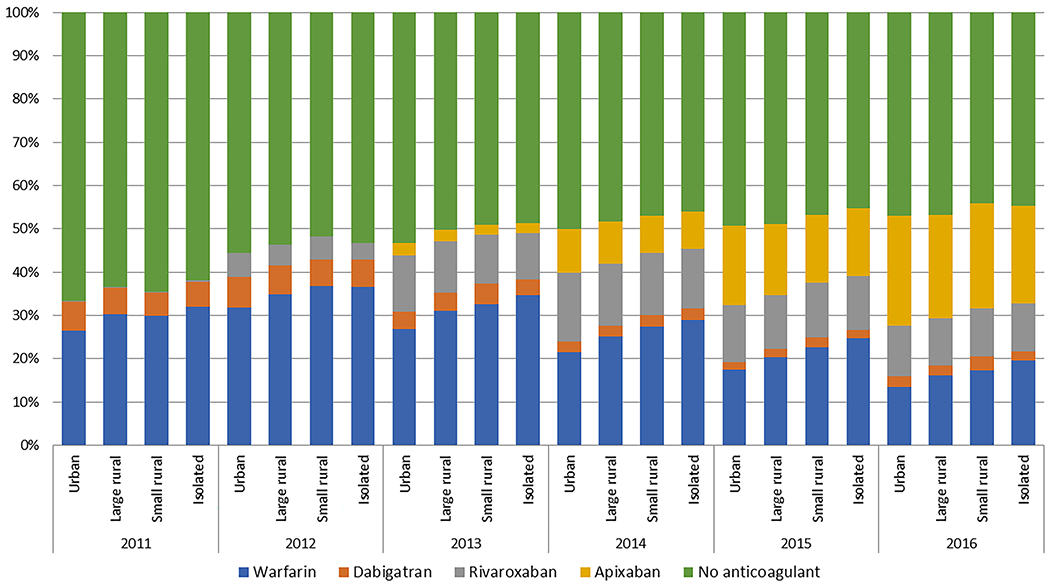
Temporal trends of anticoagulant initiation for the treatment of atrial fibrillation in Medicare beneficiaries by urban/rural category, 2011–2016
Fig. 3.
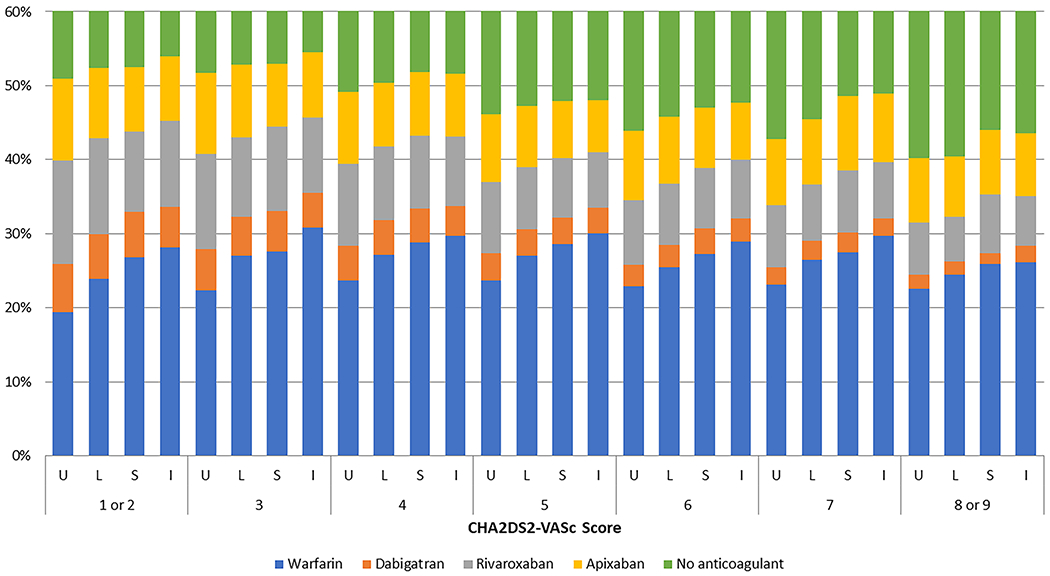
Overall temporal trends of oral anticoagulant initiation for the treatment of atrial fibrillation, by CHA2DS2-VASc score, Medicare beneficiaries, pooled 2011–2016. The Y-axis is depicted to 60%, and all percentages above that are beneficiaries on no anticoagulants. I isolated rural, L large rural, S small rural, U urban
The characteristics of patients after the 2 (urban) to 1 (rural) matching are listed in Table 2 in the ESM. Patients were well-matched with regards to age and comorbidities. Those in rural areas were more likely to be of white race and, overall, had slightly less cardiology involvement than did those in urban areas. Statistical comparisons between rural/urban areas using the matched sample are listed in Table 2. Compared with urban areas, those in isolated areas were 8% more likely to use an anticoagulant (RR 1.08; 95% CI 1.05–1.11). However, they were 17% less likely to use a DOAC than those in urban areas (RR 0.83; 95% CI 0.80–0.87). In those taking DOACs, there were no statistically significant differences in prescription fills between isolated rural and urban areas. A similar pattern was seen in the two other rural categories: those in small rural areas were 5% more likely (95% CI 3–8) to be receiving anticoagulants but 12% less likely (95% CI 9–15) to use a DOAC. Those in large rural areas were 2% more likely (95% CI 0–4) to be receiving anticoagulants but 10% less likely (95% CI 8–12) to use a DOAC. In those taking DOACs, patients in the large rural areas were more likely to receive dabigatran than those in urban areas.
Table 2.
Anticoagulation fill patterns of matched atrial fibrillation patients by rural/urban classification, Medicare, 2011–2016
| Isolated vs. urban | Total population | Isolated rural | Matched urban (Ref) | RR (95% CI) |
|---|---|---|---|---|
| N | 50,662 | 17,107 | 35,555 | |
| Any anticoagulant | 24,254 (48) | 8627 (50) | 15,627 (47) | 1.08 (1.05–1.11) |
| Warfarin | 12,797 (53) | 5042 (58) | 7755 (50) | 1.16 (1.11–1.20) |
| DOAC | 11,457 (47) | 3585 (42) | 7872 (50) | 0.83 (0.80–0.87) |
| Dabigatran | 2112 (18) | 687 (19) | 1425 (18) | 1.04 (0.95–1.15) |
| Rivaroxaban | 4954 (43) | 1535 (43) | 3419 (43) | 0.98 (0.93–1.05) |
| Apixaban | 4391 (38) | 1363 (38) | 3028 (38) | 0.99 (0.93–1.05) |
| Small rural vs. urban | Small rural | Matched urban (Ref) | RR (95% CI) | |
| N | 62,575 | 21,125 | 41,450 | |
| Any anticoagulant | 30,270 (48) | 10,530 (50) | 19,740 (48) | 1.05 (1.03–1.08) |
| Warfarin | 15,569 (51) | 5888 (56) | 9681 (49) | 1.12 (1.09–1.16) |
| DOAC | 14,701 (49) | 4642 (44) | 10,059 (51) | 0.88 (0.85–0.91) |
| Dabigatran | 2793 (19) | 923 (20) | 1870 (19) | 1.06 (0.98–1.15) |
| Rivaroxaban | 6338 (43) | 1971 (42) | 4367 (43) | 0.98 (0.93–1.04) |
| Apixaban | 5570 (38) | 1748 (38) | 3822 (38) | 0.98 (0.93–1.04) |
| Large rural vs. urban | Large rural | Matched urban (Ref) | RR (95% CI) | |
| N | 109,488 | 36,978 | 72,510 | |
| Any anticoagulant | 52,553 (48) | 17,957 (48) | 34,596 (48) | 1.02 (1.00–1.04) |
| Warfarin | 26,525 (50) | 9,718 (54) | 16,807 (49) | 1.10 (1.07–1.13) |
| DOAC | 26,028 (50) | 8,239 (46) | 17,789 (51) | 0.90 (0.88–0.92) |
| Dabigatran | 4790 (18) | 1598 (19) | 3192 (18) | 1.08 (1.02–1.15) |
| Rivaroxaban | 11,038 (42) | 3451 (42) | 7587 (43) | 0.98 (0.94–1.02) |
| Apixaban | 10,200 (39) | 3190 (39) | 7010 (39) | 0.98 (0.94–1.02) |
Relative risk of prescription fills for rural status vs. urban (reference). Warfarin and DOAC are considered only within the population receiving anticoagulants. Each DOAC is considered only within the population receiving DOACs. Adjusted for age, race, sex, CHA2DS2-VASc score, HAS-BLED score, cardiology involvement, receipt of influenza vaccine, hypertension, diabetes, myocardial infarction, heart failure, ischemic stroke/transient ischemic attack, hemorrhagic stroke, peripheral artery disease, dementia, renal disease, chronic pulmonary disease, liver disease, hematological disorders, gastrointestinal bleeding, other bleeding, malignancy, metastatic cancer, depression, alcohol abuse, and use of digoxin, clopidogrel, antiplatelets, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, statins, and diabetes medications
CI confidence interval, DOAC direct oral anticoagulant, HAS-BLED hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly (age > 65 years), drugs/alcohol concomitantly, RR relative risk
Results were nearly identical when limited to those with a CHA2DS2-VASc score ≥ 2, and results were similar when we required a 180-day run-in period instead of the 90-day run-in period (Table 3 in the ESM). We observed lower overall anticoagulation rates in women (8% lower) than in men, but these rates did not differ by rural/urban status. Similar to other reports, we observed lower anticoagulation rates in beneficiaries of Black race (16% lower) and other/unknown race (13% lower) compared with whites, but these rates did not differ by rural/urban status. We also did not observe a significant interaction for age by rural/urban status.
4. Discussion
In this retrospective administrative claims analysis of Medicare patients with AF, the proportion of patients with AF prescribed anticoagulants remained low but increased over time to 53% in 2016. This increase corresponded with the introduction of the DOACs. Overall, any anticoagulation use was higher among those in rural areas than in those in urban areas; however, DOAC initiation was lower in rural areas than in urban areas, with those in isolated areas least likely to be using a DOAC. This pattern persisted across all CHA2DS2-VASC scores. Regional variations in the proportion of beneficiaries using anticoagulants were modest, as were differences in proportions initiating DOACs.
Studies from the early 2000s showed that warfarin was underused among Medicare beneficiaries [23]. Our study used updated Medicare data, and our results indicated that anticoagulation was still underutilized in the Medicare population, but the introduction of DOACs to the market was associated with an increased percentage of patients with AF being prescribed anticoagulants. Hernandez et al. [24] evaluated the regional variation in anticoagulant use in Medicare patients in 2013–2014 and found the adjusted probability of receiving any anticoagulant use was lowest in the south, and DOAC use was lowest in the northern USA. Our study indicated that a similar regional treatment effect held true through 2016. Similar to previous studies, we found that any oral anticoagulation use, including DOAC initiation, was lower in beneficiaries of Black race and other/unknown race than in whites and was also lower in females [25, 26]. In our study, these race and sex patterns held true across all rural/urban categories. We also observed that, in those receiving a DOAC, those in rural areas were slightly more likely to receive dabigatran than were those in urban areas, but this was only significantly different in large rural areas in our main analysis. This may reflect the years included in the study (2011–2016) and that we categorized patients according to the first prescription fill following their AF event, so our analysis would not capture those who switched to a different DOAC later in the study. More recent data on DOAC prescriptions are needed.
Our study adds to the literature by showing that DOAC initiation in Medicare patients remains lower in isolated and rural areas than in urban areas. European and North American guidelines for the management of AF incorporate recommendations on using DOACs as an alternative to warfarin [2, 27], and the updated 2019 ACC/AHA/HRS guidelines clarified that DOACs are preferred over warfarin except in moderate to severe mitral stenosis and mechanical heart valves [3]. Currently, recommendations specifically for rural patients are not mentioned in the guidelines. However, because of the individualized approaches to INR monitoring that require frequent visits (approximately monthly) for patients receiving warfarin, along with barriers faced by rural patients with AF, such as distance to coagulation clinics, it has been suggested that rural patients should be considered for DOACs instead of warfarin [28]. One barrier to DOAC initiation might be the higher cost and copayment for DOACs versus warfarin. However, the evidence suggests that long-term therapy with DOACs may be more cost effective than warfarin treatment [29, 30], primarily because of lower monitoring costs and reduced numbers of patients with strokes and systemic embolism. Given that our study population consisted of only Medicare beneficiaries with a stand-alone Part D plan, further research should be conducted in privately insured populations and in those with supplemental insurance to determine whether similar DOAC prescription patterns exist in those rural patients. If DOAC prescription patterns in rural areas differ by insurance type, the patterns observed in our study could be the result of costs and copayment options unique to this Medicare population and would identify an area for improvement.
Reports suggest cardiology providers are more likely to prescribe oral anticoagulants than are primary care providers [31–34], which possibly results in a lower risk of stroke among patients who are managed by cardiology specialists [32]. Our study took into account whether patients had seen a cardiology provider in the time period around AF diagnosis, and those in the most isolated areas were less likely to have seen a cardiologist. We observed that cardiology providers did prescribe DOACs at a higher rate than did primary care providers. However, most patients in our Medicare cohort had seen a cardiology provider around the time of AF (80%), so adjusting for provider specialty did not influence our estimates. Still, because of differences in the initiation of DOACs and because those in isolated areas were less likely to see a cardiology provider, educating providers in rural areas to prescribe DOACs over warfarin may reduce the burdens of stroke and healthcare utilization for older adults in rural areas.
4.1. Limitations
This study had several limitations that should be considered. First, this analysis was limited to fee-for-service Medicare beneficiaries with a stand-alone Part D plan, and this is a subset of all Medicare beneficiaries that is known to have a lower socioeconomic status and more comorbidities than those with supplemental coverage. Therefore, our results may not be generalizable to the entire Medicare (aged ≥ 65 years) population. Second, we used ICD-9 and -10 codes to identify AF cases and comorbid conditions, and some misclassification is unavoidable despite using validated algorithms. Third, unmeasured confounding is a known limitation in observational studies using administrative claims data. Although we attempted to account for many measured patient characteristics in our multivariable model that may have accounted for differences in rural/urban patients, unmeasured factors (e.g., socioeconomic status, distance from a clinic) possibly influenced our findings. Fourth, more up-to-date data are needed to examine recent trends. Lastly, we had information only on prescriptions filled by patients, not on the medication prescribed by the provider or compliance with therapy. Despite these limitations, our study had numerous key strengths, including a large sample size of Medicare beneficiaries that allowed us to detect differences between groups. Also, Medicare data contain individual ZIP codes, which allowed us to compare rural status on a patient level, which has not been done in other claims-based datasets. Using this large sample of Medicare data allowed us to identify important differences between rural and urban populations.
5. Conclusion
In this Medicare population with AF, anticoagulation use remained low but increased over time, paralleling the introduction of DOACs. However, those in rural areas were less likely to receive a DOAC than those in urban areas, with the lowest DOAC use occurring in the most isolated areas. Increasing the use of anticoagulants in general and of DOACs in particular in patients with AF living in rural areas may reduce the burdens of stroke and healthcare utilization for older adults in rural areas.
Supplementary Material
Key Points.
Among Medicare beneficiaries with atrial fibrillation from 2011 to 2016, anticoagulation use was low but increased over time with the introduction of direct oral anticoagulants (DOACs).
Rural beneficiaries were more likely to receive an anticoagulant but less likely to receive a DOAC.
The use of anticoagulants should be improved in all geographical areas of the USA, with a concentrated effort to improve DOAC use in rural areas.
Acknowledgement
This manuscript is part of Dr. Faye Norby’s PhD dissertation (manuscript 3). A non-peer-reviewed, full-text version of the dissertation is available online at Proquest, LLC.
Funding
Alvaro Alonso has received funding from American Heart Association grant 16EIA26410001 and NHLBI grant K24HL148521.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s40256-021-00502-9.
Conflict of interest Faye L. Norby, Pamela L. Lutsey, Nathan D. Shippee, Lin Y. Chen, Carrie Henning-Smith, Alvaro Alonso, Rob F. Walker, and Aaron R. Folsom have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval This study was approved by the University of Minnesota Institutional Review Board as exempt because deidentified data were used.
Consent to participate Not applicable.
Consent for publication Not applicable.
Availability of data and material The authors are unable to share the data since they were obtained under a data use agreement that does not allow data sharing.
Code availability Available upon request.
References
- 1.Katsnelson M, Koch S, Rundek T. Stroke prevention in atrial fibrillation. J Atrial Fibrillation. 2010;3(3):279. 10.4022/jafib.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–32. 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300–5. 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med. 2014;127(11):1075–82 e1. 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Moy GM, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas—United States, 1999-2014. MMWR Surveill Summ. 2017;66(SS-1):1–8. 10.15585/mmwr.ss6601a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813–20. 10.1016/j.puhe.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Howard G, Kleindorfer DO, Cushman M, Long DL, Jasne A, Judd SE, et al. Contributors to the excess stroke mortality in rural areas in the United States. Stroke J Cerebr Circ. 2017;48(7):1773–8. 10.1161/STROKEAHA.117.017089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mainous AG 3rd, King DE, Garr DR, Pearson WS. Race, rural residence, and control of diabetes and hypertension. Ann Fam Med. 2004;2(6):563–8. 10.1370/afm.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso A, MacLehose RF, Chen LY, Bengtson LG, Chamberlain AM, Norby FL, et al. Prospective study of oral anticoagulants and risk of liver injury in patients with atrial fibrillation. Heart. 2017. 10.1136/heartjn1-2016-310586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141–7. 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rural-Urban Commuting Area Codes (RUCAs): rural health research center. http://depts.washington.edu/uwruca/index.php Accessed 20 Apr 2019.
- 14.USDA: Rural-urban communting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx Accessed 27 Feb 2020.
- 15.Garg RK, Glazer NL, Wiggins KL, Newton KM, Thacker EL, Smith NL, et al. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiol Drug Saf. 2011;20(3):313–6. 10.1002/pds.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–6. 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 18.Norby FL, Bengtson LGS, Lutsey PL, Chen LY, MacLehose RF, Chamberlain AM, et al. Comparative effectiveness of rivaroxaban versus warfarin or dabigatran for the treatment of patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. 2017;17(1):238. 10.1186/s12872-017-0672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 20.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100. 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 21.Bergstralh EKJ. GMATCH macro. 2003. http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros. Accessed.
- 22.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 23.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke J Cerebr Circ. 2000;31(4):822–7. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez I, Saba S, Zhang Y. Geographic variation in the use of oral anticoagulation therapy in stroke prevention in atrial fibrillation. Stroke J Cerebr Circ. 2017;48(8):2289–91. 10.1161/STROKEAHA.117.017683. [DOI] [PubMed] [Google Scholar]
- 25.Waddy SP, Solomon AJ, Becerra AZ, Ward JB, Chan KE, Fwu CW, et al. Racial/ethnic disparities in atrial fibrillation treatment and outcomes among dialysis patients in the United States. J Am Soc Nephrol. 2020;31(3):637–49. 10.1681/ASN.2019050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Essien UR, Magnani JW, Chen N, Gellad WF, Fine MJ, Hernandez I. Race/ethnicity and sex-related differences in direct oral anticoagulant initiation in newly diagnosed atrial fibrillation: a retrospective study of medicare data. J Natl Med Assoc. 2020;112(1):103–8. 10.1016/j.jnma.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47. 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 28.Hayden K, Schweinle W. Demographic considerations in anticoagulation therapy for atrial fibrillation. S D Med J S D State Med Assoc. 2015;68(3):116–9. [PubMed] [Google Scholar]
- 29.Shields GE, Bates AE, Chapman AM. Implementing guidelines: the cost and clinical impact of anticoagulants in the UK atrial fibrillation population. Appl Health Econ Health Policy. 2015;13(5):543–51. 10.1007/s40258-015-0180-7. [DOI] [PubMed] [Google Scholar]
- 30.Vestergaard AS, Ehlers LH. A health economic evaluation of stroke prevention in atrial fibrillation: guideline adherence versus the observed treatment strategy prior to 2012 in Denmark. Pharmacoeconomics. 2015;33(9):967–79. 10.1007/s40273-015-0281-z. [DOI] [PubMed] [Google Scholar]
- 31.Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165(1):93–101 e1. 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Perino AC, Fan J, Schmitt SK, Askari M, Kaiser DW, Deshmukh A, et al. Treating specialty and outcomes in newly diagnosed atrial fibrillation: from the TREAT-AF study. J Am Coll Cardiol. 2017;70(1):78–86. 10.1016/j.jacc.2017.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fosbol EL, Holmes DN, Piccini JP, Thomas L, Reiffel JA, Mills RM, et al. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. J Am Heart Assoc. 2013;2(4):e000110. 10.1161/JAHA.113.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neal WT, Sandesara PB, Claxton JS, MacLehose RF, Chen LY, Bengtson LGS, et al. Provider specialty, anticoagulation prescription patterns, and stroke risk in atrial fibrillation. J Am Heart Assoc. 2018. 10.1161/JAHA.117.007943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


