Summary
Background
NVX-CoV2373, a Covid-19 vaccine was developed in the USA with ∼90% efficacy. The same vaccine is manufactured in India after technology transfer (called as SII-NVX-CoV2373), was evaluated in this phase 2/3 immuno-bridging study.
Methods
This was an observer-blind, randomised, phase 2/3 study in 1600 adults. In phase 2, 200 participants were randomized 3:1 to SII-NVX-CoV2373 or placebo. In phase 3, 1400 participants were randomized 3:1 to SII-NVX-CoV2373 or NVX-CoV2373 (940 safety cohort and 460 immunogenicity cohort). Two doses of study products (SII-NVX-CoV2373, NVX-CoV2373 or placebo) were given 3 weeks apart. Primary objectives were to demonstrate non-inferiority of SII-NVX-CoV2373 to NVX-CoV2373 in terms of geometric mean ELISA units (GMEU) ratio of anti-S IgG antibodies 14 days after the second dose (day 36) and to determine the incidence of causally related serious adverse events (SAEs) through 180 days after the first dose. Anti-S IgG response was assessed using an Enzyme-Linked Immunosorbent Assay (ELISA) and neutralizing antibodies (nAb) were assessed by a microneutralization assay using wild type SARS CoV-2 in participants from the immunogenicity cohort at baseline, day 22, day 36 and day 180. Cell mediated immune (CMI) response was assessed in a subset of 28 participants from immunogenicity cohort by ELISpot assay at baseline, day 36 and day 180. The total follow-up was for 6 months. Trial registration: CTRI/2021/02/031554.
Findings
Total 1596 participants (200 in Phase 2 and 1396 in Phase 3) received the first dose. SII-NVX-CoV2373 was found non-inferior to NVX-CoV2373 (anti-S IgG antibodies GMEU ratio 0.91; 95% CI: 0.79, 1.06). At day 36, there was more than 58-fold rise in anti-S IgG and nAb titers compared to baseline in both the groups. On day 180 visit, these antibody titers declined to levels slightly lower than those after the first dose (13–22 fold-rise above baseline). Incidence of unsolicited and solicited AEs was similar between the SII-NVX-CoV2373 and NVX-CoV2373 groups. No adverse event of special interest (AESI) was reported. No causally related SAE was reported.
Interpretation
SII-NVX-CoV2373 induced a non-inferior immune response compared to NVX-CoV2373 and has acceptable safety profile.
Funding
SIIPL, Indian Council of Medical Research, Novavax.
Keywords: SII-NVX-CoV2373, NVX-CoV2373, Safety, Immunogenicity, Non-inferiority
Research in context.
Evidence before this study
NVX-CoV2373 was originally manufactured in USA for which data on the safety, immunogenicity and efficacy is published. This vaccine was subsequently manufactured in India after technology transfer, (SII-NVX-CoV2373). We searched PubMed for research articles published from 01 January 2020 (before the start of the pandemic) until 19 November 2022, with no language restrictions, using the terms “SII-NVX-CoV2373”, “vaccine”, “Covovax” and “clinical trial” and no publications were found on SII-NVX-CoV2373.
Added value of this study
This is the first worldwide report on the safety and immunogenicity of SII-NVX-CoV2373 vaccine. The vaccine was found safe and well tolerated. Based on the immune response, SII-NVX-CoV2373 was successfully bridged to NVX-CoV2373.
Implications of all the available evidence
NVX-CoV2373 was found safe and efficacious in studies in USA, UK, South Africa. SII-NVX-CoV2373 was found non-inferior to NVX-CoV2373. Based on these data, SII-NVX-CoV2373 received regulatory approvals in many countries as well as WHO emergency use listing. As a result, the vaccine was supplied to many countries as well as to UNICEF and has been used in millions of doses. Moreover, the bulk of SII-NVX-CoV2373 was also used to make formulation of NVX-CoV2373. This formulation with bulk from India was approved in USA, UK, EU, Australia, Canada etc. and has been supplied to these countries in millions of doses.
Introduction
SARS-CoV-2 is a novel beta coronavirus originating in late 2019 leading to COVID-19 pandemic. SARS-CoV-2 is characterized by high transmission rates, at times leading to large outbreaks, mostly in indoor congregate settings. SARS-CoV-2 gets transmitted by droplet transmission and may also be by airborne transmission.1
Worldwide during the pandemic millions of COVID-19 cases and deaths have been reported.2 As of 19 November 2022, India has reported more than 44.5 million cases and more than 0.5 million deaths.3
In response, many vaccines emerged to limit COVID-19's ability to spread. In the United States of America (USA), a recombinant spike protein nanoparticle vaccine adjuvanted with Matrix M™ was developed (NUVAXOVID™, NVX-CoV2373, Novavax Inc.).4
In a Phase 1/2 study conducted in Australia and USA, NVX-CoV2373 was well tolerated and elicited immune responses that were 4-fold higher than those observed in COVID-19 convalescent sera.4 In the phase 2 study in USA and Australia, similar findings were seen.5 In the phase 3 study in UK, vaccine efficacy was 89.7% (95% CI, 80.2, 94.6).6 In another Phase 3 study in USA and Mexico, similar vaccine efficacy was reported: 90.4%; 95% CI, 82.9 to 94.6 for any strain and 92.6% (95% CI, 83.6 to 96.7) for any variant of concern or interest.7
After the technology transfer to Serum Institute of India Private Limited [SIIPL], the vaccine was also manufactured at SIIPL initially formulated from the bulk drug substance (DS) received from Novavax and eventually the vaccine was manufactured from bulk stage to fill finish (drug product; DP) (COVOVAX™, SII NVX-CoV2373) with the composition of the two vaccines being identical.
We performed an immunobridging study with the two vaccines to assess safety and immunogenicity in Indian adults.
Methods
Study design
This was a Phase 2/3, multi-center, observer-blind, randomized, controlled study in adults to evaluate the safety and immunogenicity of SII-NVX-CoV2373 in 1600 adults (1140 in safety cohort and 460 in immunogenicity/reactogenicity cohort). In all the cohorts, two doses of 0.5 ml of study vaccines/placebo were administered on day 1 and day 22 intramuscularly in the deltoid muscle.
The study was conducted in two parts as described later.
The study was approved by the Drugs Controller General of India (DCGI) and Institutional ethics committees of each of the participating study sites. The study was conducted at 20 hospitals across India in compliance with the ICH-GCP guidelines and the principles of the Declaration of Helsinki (2013). Written informed consent was provided by each participant before enrolment. Trial registration: CTRI/2021/02/031554.
Study procedure
In the Phase 2 of the study, 200 participants were enrolled in the safety cohort with 3:1 allocation to SII NVX-CoV2373 or placebo (Table 1). There was a telephone call at seven days after the first dose for safety assessment. This one-week safety data was reviewed by an independent Data Safety Monitoring Board (DSMB). After the DSMB's recommendation and the approval from the DCGI, the study progressed to the Phase 3 part. Simultaneously, the Phase 2 study participants were followed up as per protocol.
Table 1.
Treatment allocation.
| Phase | Cohorts | Investigational products | Number of Participants |
|---|---|---|---|
| Phase 2 (n = 200) | Safety Cohort (n = 200) | SII-NVX-CoV2373 | 150 |
| Placebo | 50 | ||
| Phase 3 (n = 1400) | Safety Cohort (n = 940) | SII-NVX-CoV2373 | 705 |
| Control vaccine (NVX-CoV2373) | 235 | ||
| Immunogenicity and Reactogenicity Cohort (n = 460) | SII-NVX-CoV2373 | 345 | |
| Control vaccine (NVX-CoV2373) | 115 |
In the Phase 3 of the study, remaining 1400 participants (940 in the safety cohort and 460 in the immunogenicity/reactogenicity cohort) were enrolled with 3:1 allocation to SII NVX-CoV2373 or NVX-CoV2373 (Table 1).
All the participants received 2 doses of study vaccine 3 weeks apart i.e. on day 1 and day 22 as per randomization. After day 1 visit, participants visited the sites on days 22, 36, 85 and 180 and were contacted telephonically on day 120 for safety follow up.
Study population
The study enrolled healthy adults ≥18 years of age. Acute illness with or without fever, history of laboratory confirmed COVID-19, prior receipt of a COVID-19 vaccine, history of severe allergic reactions after previous vaccinations, immunodeficiency or autoimmune conditions, pregnancy, lactation, bleeding disorder, chronic administration of immunosuppressive or immune modifying drugs, acute or chronic clinically unstable systemic disorders were the exclusion criteria. Individuals with clinically stable conditions were enrolled.
Study vaccines
In the Phase 2 part, SII-NVX-CoV2373 (DS manufactured by Novavax and DP fill and finished by SIIPL) or placebo were used. In the Phase 3 part, SII NVX-CoV2373 [DS and DP manufactured by SIIPL (COVOVAX™)] or NVX-CoV2373 (NUVAXOVID™) manufactured by Novavax were used.
Each dose of 0.5 ml of NVX-CoV2373/SII-NVX-CoV2373 contains 5 μg SARS-CoV-2 recombinant spike protein antigen and 50 μg Matrix M adjuvant. The other ingredients are 25 mM phosphate buffer (pH 7.2), 300 mM sodium chloride and 0.01% (v/v) polysorbate 80.
The placebo used in the Phase 2 part was 0.9% sodium chloride (manufactured by SIIPL).
Randomization and blinding
The randomization scheme was generated using SAS software version 9.4 (SAS Institute Inc, USA) for Interactive Response Technology (IRT).
The study participants, the study personnel responsible for the evaluation of any study endpoints and the laboratories involved in the immunological testing were blinded. Personnel involved in getting randomization code by accessing interactive web response system (IWRS), vaccine preparation and administration were unblinded and they did not conduct any study evaluations. The unblinded monitors and statisticians from the CRO could access the participants level unblinded data as per the need.
For the Phase 2 part, the blind was broken on or after day 85 visit and participants were offered SII-NVX-CoV2373, if they had received Placebo and they were continued in the study for safety follow-up.
Safety assessment
In the immunogenicity/reactogenicity cohort, pre-specified local and systemic adverse events (AEs) were actively solicited for 7 days after each vaccination by using structured diary cards.
In all the participants unsolicited AEs were collected until 2 weeks after the second dose, serious adverse events (SAEs) and adverse events of special interest (AESI) were collected throughout their participation.
For unsolicited AEs, SAEs and AESI, causality assessment was done by the investigators. All AEs were graded on a scale of 1–5 with pre-defined criteria based on the Division of AIDS (DAIDS) table for severity, corrected version 2.1, July 20, 2017 of the US National Institute of Health. The events that were not listed in the DAIDS table were graded as per pre-defined criteria.
At each study visit, the participants were assessed for any AEs and underwent general and systemic examinations.
Safety data was reviewed at periodic intervals by an independent DSMB comprising of two physicians and a biostatistician.
Immunogenicity assessment
In the immunogenicity cohort
Anti-S IgG assessments by a validated ELISA were performed at Novavax Inc., Gaithersburg, Maryland, USA on day 1, day 22, day 36, and day 180 in all participants of immunogenicity/reactogenicity cohort. The LLOQ for this assay was a titer of 200 ELISA units per mL (EU/mL), with titers below this level documented as 100 EU/mL. Neutralizing antibodies (nAb) were measured at 360biolabs, Melbourne, Australia on day 1 and day 36 in all participants of immunogenicity/reactogenicity cohort and in about one third of randomly selected participants (maintaining 3:1 allocation) on day 22 and day 180 using the validated virus neutralizing assay (VNA) with wild type virus (SARS-CoV-2 hCoV-19/Australia/VIC01/2020, GenBank MT007544.1). The LLOQ for this assay was a titer of 20, with titers below this level documented as 10. Seroconversion was defined as a four-fold increase in antibody titers from baseline.
CMI testing in a subset of 28 participants selected by stratified randomization was performed on day 1, day 36 and day 180 at ICMR-NARI, Pune, India using ELISpot assay and intracellular cytokines were detected by antibodies specific for T helper 1 (Th1) cytokines interferon gamma (IFN-γ) and T helper 2 (Th2) cytokine IL-5.
In all participants
Anti-nucleocapsid (anti-N) IgG testing was performed by Chemiluminescent Microparticle Immunoassay (CMIA) using ARCHITECT SARS-CoV-2 IgG kit (6R86) and ARCHITECT i2000 SR system from Abbott Laboratories at ICMR-NARI, Pune, India. The cutoff for seropositivity was S/C index ≥1.40. The test was performed on all 1600 participants on day 1, day 22, day 36, day 85 and day 180.
Participants with suspected COVID-19 or a contact with a COVID-19 case, were tested by RT-PCR at the site laboratory. All positive cases from 14 days after each vaccination were considered for analysis.
Statistical analysis
The study had 95% probability to detect at least one causally related SAE, if the frequency of such an event is 1/400. The study had at least 90% power to show non-inferiority of immune responses assuming a coefficient of variation of 1.35, a one-sided significance level of 0.025 and 0 difference in Anti-S IgG antibody titers between the two vaccine groups and that the proportion of non-evaluable participants in the immunogenicity/reactogenicity cohort was ≤20%. Non-inferiority was concluded if the lower limit of the 95% confidence interval (CI) for the geometric mean ELISA units (GMEU) ratio for Anti-S IgG antibodies between SII-NVX-CoV2373 and NVX-CoV2373 vaccine is more than 0.67, as per the WHO guidelines for clinical evaluation of vaccines.8 Sample size calculations were performed using a non-inferiority test for the ratio of two means in PASS 15.0.7 Version software.
For the co-primary immunogenicity analysis, ANCOVA fitted to the log transformed Anti-S IgG with terms for vaccine group, log baseline titer, co-morbidity, age group and sex to compare SII-NVX-CoV2373 against Control vaccine (NVX-CoV2373) for co-primary endpoint.
-
1)
Missing data generated as a result of hypothetical strategy was implemented for handling of intercurrent event's (death, immune modifiers, COVID-19/SARS-CoV-2 infection, missed second dose of vaccine) and for a participant who belong to immunogenicity analysis set and had a missing data at day 36 with assumption that data was missing at random. Only 3 participants had a missing data (2 in the SII-NVX-CoV2373 group and 1 in the NVX-CoV2373 group) which is less than 1% with respect to immunogenicity analysis population count in each treatment arm.
-
2)
Same ANCOVA model was used for analysing each of the imputed datasets to compute Least Square (LS) means and LS mean difference.
-
3)
The results from the analysis of 50 data sets were combined (using PROC MIANALYZE) per Rubin's rule to produce an overall estimate and a standard error (based on Rubin's formula) with associated 95% CI for the final statistical inference.
Categorical data are expressed as proportions while quantitative data are expressed as mean and SD.
All safety analyses were performed on the safety population which included all participants who received at least one dose of the study vaccines. All immunogenicity analyses were performed on the immunogenicity population which comprised of all participants who received the first dose of the study vaccine and provided an evaluable serum sample post vaccination for at least one assessment and had day 1 data available.
All statistical analyses were performed using SAS software Version 9.4.
Role of funding source
Funding by SIIPL, Indian Council of Medical Research the funder of the study was involved in study design, data interpretation, and writing of the report, but was not involved in data collection and data analysis.
Results
Baseline characteristics and demographics
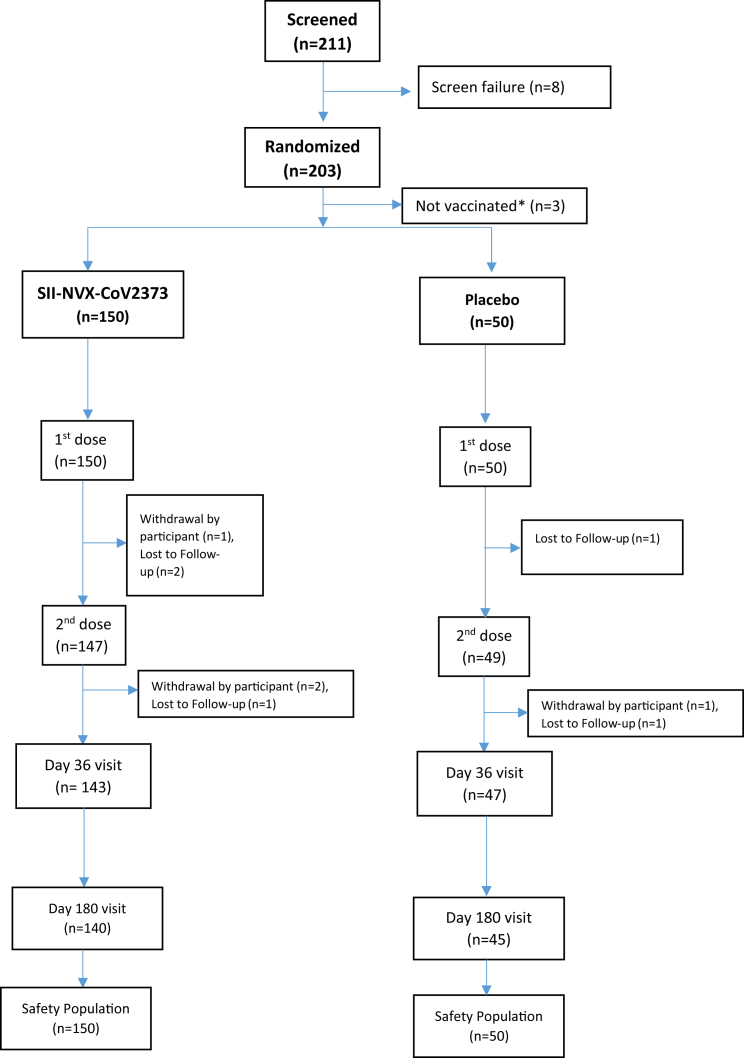
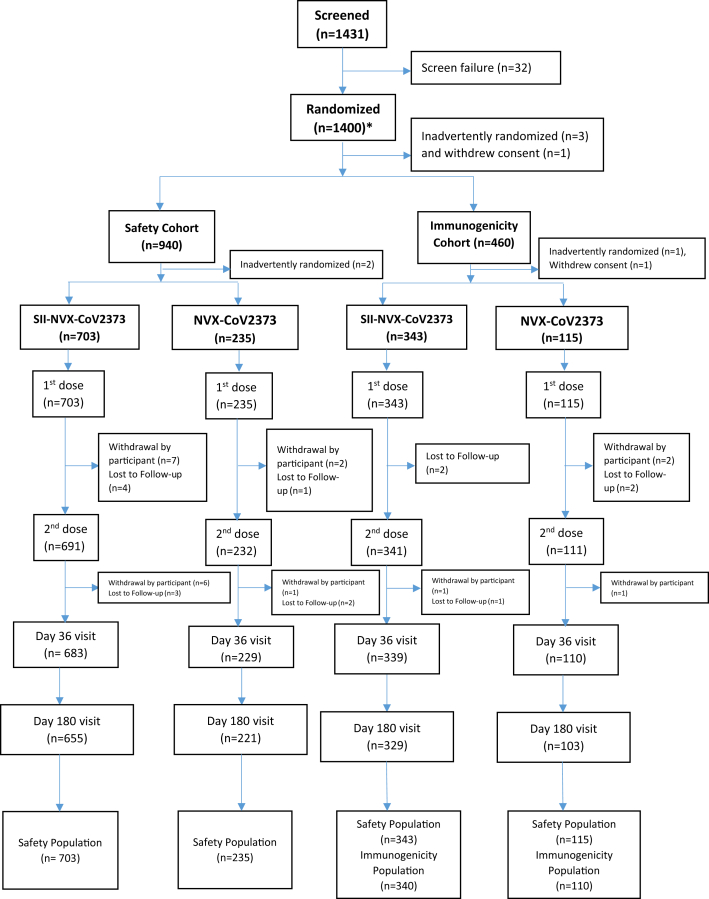
In the Phase 2 part in March 2021, 211 participants were enrolled of which 8 were screen failures, and 3 did not receive the vaccine. Thus, 200 participants received the first dose while 196 participants received second dose of the study vaccines (Fig. 1). In the Phase 3 part, 1431 participants were screened of which 32 were screen failures and 1400 were randomized (with 1 screen failure inadvertently randomized, but not vaccinated). While 1396 received the first dose, 1375 received the second dose of the study vaccines (Fig. 2). In the immunogenicity/reactogenicity cohort, 458 participants received the first dose while 452 participants received the second dose. In the safety cohort 938 participants received the first dose of which 923 received the second dose (Fig. 2).
Fig. 1.
CONSORT Flow Chart – Phase 2. ∗Due to temperature excursion reported at the site, 3 participants could not be vaccinated and by then target of 200 participants was completed at other study sites.
Fig. 2.
CONSORT Flow Chart – Phase 3. ∗Of total 1431 screened, 32 were screen failures with 1 screen failure inadvertently entered as randomized in the database. Thus, actually 1399 were randomized and 1 screen failure was inadvertently randomized.
Overall the demographic and baseline characteristics between the study groups were comparable in terms of age, sex, race and comorbidities. In the Phase 2 part, overall, 199 participants (99.5%) were between 18 and 59 years of age. The overall mean age was 33.7 (SD 9.36) years. 143 (71.5%) of the participants were male. In the Phase 3 part, overall 1364 participants (97.7%) were between 18 and 59 years of age. The mean age was 34.5 (SD 10.66) years. 811 (58.1%) of the participants were male (Table 2).
Table 2.
Demographics and baseline characteristics – (Safety population).
| Parameter | Phase 2 |
Phase 3 |
||
|---|---|---|---|---|
| SII-NVX-CoV2373 (N = 150) | Placebo (N = 50) | SII-NVX-CoV2373 (N = 1046) | NVX-CoV2373 (N = 350) | |
| Age (Years), Mean (SD) | 33.4 (9.47) | 34.5 (9.08) | 34.8 (10.80) | 33.4 (10.18) |
| Sex, n (%) | ||||
| Male | 111 (74.0) | 32 (64.0) | 602 (57.6) | 209 (59.7) |
| Female | 39 (26.0) | 18 (36.0) | 444 (42.4) | 141 (40.3) |
| Baseline SARS-CoV-2 serology and/or RT-PCR results, n (%)a | ||||
| Positive | 22 (14.7) | 6 (12.0) | 335 (32.0) | 118 (33.7) |
| Negative | 128 (85.3) | 44 (88.0) | 709 (67.8) | 231 (66.0) |
| Co-morbidities, n (%) | 20 (13.3) | 5 (10.0) | 134 (12.8) | 38 (10.9) |
Results missing in 2 and 1 participants in the two groups in Phase 3 study, respectively.
There were 25 (12.5%) and 172 (12.3%) participants with at least one comorbidity (Table 2). The most commonly reported (≥1%) conditions included surgical and medical procedures, hypothyroidism, gout, hyperlipidemia, type 2 diabetes mellitus and hypertension.
Immunogenicity results
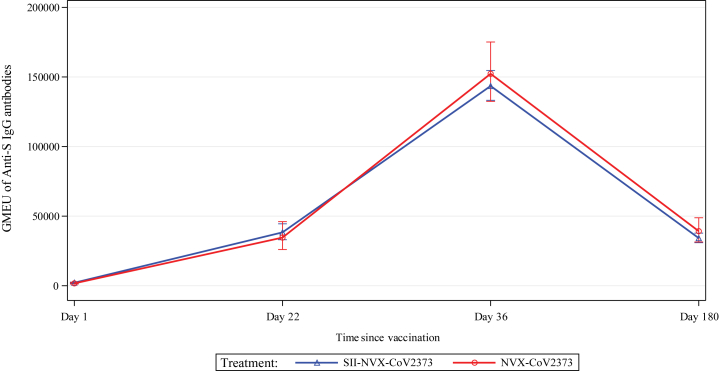
SII-NVX-CoV2373 met the co-primary endpoint of non-inferiority to NVX-CoV2373 vaccine with 0.79 as the lower bound of 95% CI for the anti-S IgG antibodies (GMEU ratio of 0.91, 95% CI 0.79, 1.06) (Table 3). Baseline GMEU of anti-S IgG antibodies were comparable between the groups and there was increase in GMEU in both the groups after each dose. There was more than 17-fold rise in GMEU at 21 days after the first dose and more than 65-fold rise at 14 days after the second dose in both the groups and more than 15-fold rise on day 180 (Fig. 3). In the baseline seronegative and those with negative baseline RT-PCR test, there was more than 129-fold rise in GMEU at 14 days after the second dose and more than 28-fold rise on day 180 in both the groups (Suppl. Table S1).
Table 3.
Non-inferiority of SII-NVX-CoV2373 to NVX-SARS-CoV-2 Vaccine in terms of Anti-S IgG Antibodies at 14 days after second dose– Immunogenicity analysis Population – Phase 3.
| Multiple Imputationa Results Using Rubin's Methodb |
SII-NVX-CoV2373 (N = 340) | NVX-CoV2373 (N = 110) |
|---|---|---|
| Estimated Mean | ||
| GMEUc | 139937.78 | 153200.54 |
| 95% Confidence Interval | (116839.75, 167602.05) | (124050.99, 189199.68) |
| Estimated Ratio | ||
| GMEU Ratioc | 0.91 | |
| 95% Confidence Interval | (0.79, 1.06) | |
Multiple Imputation model with classification variables vaccine, age group, sex, comorbidity status and continuous covariates log baseline titer used to impute 50 values for each missing value.
Rubin's method in PROC MIANALYZE used to pool estimates and SE across the 50 multiply imputed datasets.
Pooled ANCOVA results, LS Mean and it's 95% CI values by treatment were used to generate the GMEU and 95% CI and the differences in LS Means and corresponding 95% CI limits were used to obtain GMEU Ratio and 95% CI using back transforming to the original scale.
Fig. 3.
Line plots of GMEU (including 95% CIs) of Anti-S IgG - Immunogenicity Analysis Population.
At 21 days after the first dose, seroconversion for anti-S IgG antibodies was 82.6% in the SII-NVX-CoV2373 group and 83.6% in the NVX-CoV2373 group, which increased to 92.9% and 96.3%, respectively at 14 days after the second dose and then decreased to 78.0% and 78.2%, respectively on day 180 (Suppl. Table S2). In the participants with baseline seronegative status and negative RT-PCR, seroconversion after the first dose was more than 87.5%, more than 98.6% after the second dose and more than 90.4% on day 180 in both the groups (Suppl. Table S3).
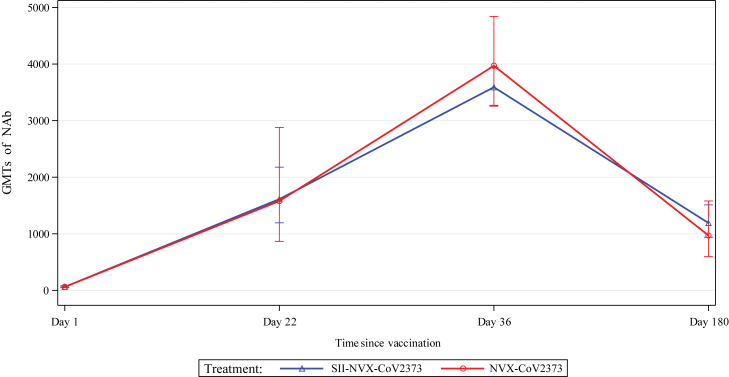
With respect to neutralizing antibodies, baseline GMTs were comparable between the groups. After the first dose, there was more than 22-fold rise while after the second dose, there was more than 57-fold rise in both the groups and more than 13-fold rise on day 180 (Fig. 4). In the baseline seronegative and negative RT-PCR participants, the GMTs increased by more than 79-fold after the second dose and more than 19-fold on day 180 in both the groups (Suppl. Table S1).
Fig. 4.
Line plots of GMT (including 95% CIs) of Neutralizing antibodies (nAb) against SARS-Cov-2 - Immunogenicity Analysis Population.
The seroconversion for neutralizing antibodies was more than 87% after the first dose, more than 92% after the second dose and more than 83% on day 180 in both the groups (Suppl. Table S2). In baseline seronegative and negative RT-PCR participants, the seroconversion was more than 85.7% after the first dose, more than 98.7% after the second dose and more than 90.0% on day 180 in both the groups (Suppl. Table S3).
In terms of CMI response, T cells secreting IFN-γ against whole length of spike protein antigen contained in the vaccine increased after the second dose and remained above the baseline values at day 180 in both the groups. T cells secreting IL-5 were less than those secreting IFN-γ, suggesting Th1 skewed CMI response (Suppl. Table S4).
At 14 days after the first dose, there was 1 each case of symptomatic COVID-19 (0.7%) and asymptomatic SARS CoV-2 infection (0.7%) in the SII-NVX-CoV2373 group and none in the placebo group in the Phase 2 part, and there were 2 cases of symptomatic COVID-19 (0.2%) in the SII-NVX-CoV2373 group in the Phase 3 part. From 14 days after the second dose until day 180 visit, there was only one case of COVID-19 reported in the SII-NVX-CoV2373 group (in the Phase 3 part). No case required ICU admission and all recovered completely.
Safety results
In the Phase 2 part, overall 70 participants (35%) reported 107 AEs after any dose. Of these, 61 participants (40.7%) reported 96 AEs in the SII-NVX-CoV2373 group and 9 participants (18%) reported 11 AEs in the placebo group. Most of the AEs were mild in the severity and resolved without any sequelae.
The incidence of vaccine related AEs was higher in the SII-NVX-CoV2373 group (51 participants, 34%) than in the placebo group (8 participants, 16%). The most commonly reported vaccine related unsolicited AEs with frequency ≥5% were injection site pain, pyrexia, headache, malaise and pain. One participant reported an SAE, which was COVID-19 pneumonia in the SII-NVX-CoV2373 group. It was not related to the study vaccine. No AESI was reported in the Phase 2 study.
In the Phase 3 part, overall 282 participants in the immunogenicity/reactogenicity cohort reported 1052 solicited AEs after any dose (Table 4). The incidence was 63% in the SII-NVX-CoV2373 group and 57.4% in the NVX-CoV2373 group. The incidence was higher after the second dose (49.8%) than after the first dose (37.6%). Majority of the solicited AEs were of mild severity.
Table 4.
Summary of solicited AEs (immunogenicity and reactogenicity cohort) – phase 3 (safety population).
| First dose |
Second dose |
|||
|---|---|---|---|---|
| SII-NVX-CoV2373 (N = 343) n (%) [E] |
NVX-CoV2373 (N = 115) n (%) [E] |
SII-NVX-CoV2373 (N = 343) n (%) [E] |
NVX-CoV2373 (N = 115) n (%) [E] |
|
| Participants with at Least One Solicited Adverse Event | 137 (39.9) [328] | 35 (30.4) [86] | 171 (50.1) [495] | 54 (48.6) [143] |
| Participants with at Least One Local Solicited AE | 94 (27.4) [153] | 28 (24.3) [44] | 116 (34.0) [184] | 28 (25.2) [34] |
| Injection Site Pain | 82 (23.9) [82] | 28 (24.3) [28] | 109 (32.0) [109] | 26 (23.4) [26] |
| Injection Site Tenderness | 34 (9.9) [34] | 8 (7.0) [8] | 39 (11.4) [39] | 7 (6.3) [7] |
| Injection Site Swelling | 15 (4.4) [15] | 4 (3.5) [4] | 13 (3.8) [13] | 0 |
| Injection Site Erythema | 12 (3.5) [12] | 3 (2.6) [3] | 12 (3.5) [12] | 0 |
| Injection Site Induration | 10 (2.9) [10] | 1 (0.9) [1] | 11 (3.2) [11] | 1 (0.9) [1] |
| Participants with at Least One Systemic Solicited AE | 91 (26.5) [175] | 21 (18.3) [42] | 145 (42.5) [311] | 49 (44.1) [109] |
| Headache | 54 (15.7) [54] | 8 (7.0) [8] | 68 (19.9) [68] | 28 (25.2) [28] |
| Fatigue | 30 (8.7) [30] | 14 (12.2) [14] | 61 (17.9) [61] | 21 (18.9) [21] |
| Myalgia | 29 (8.5) [29] | 8 (7.0) [8] | 54 (15.8) [54] | 13 (11.7) [13] |
| Malaise | 23 (6.7) [23] | 2 (1.7) [2] | 32 (9.4) [32] | 8 (7.2) [8] |
| Arthralgia | 15 (4.4) [15] | 5 (4.3) [5] | 34 (10.0) [34] | 13 (11.7) [13] |
| Fever | 12 (3.5) [12] | 3 (2.6) [3] | 49 (14.4) [49] | 20 (18.0) [20] |
| Nausea | 7 (2.0) [7] | 1 (0.9) [1] | 9 (2.6) [9] | 3 (2.7) [3] |
| Vomiting | 5 (1.5) [5] | 1 (0.9) [1] | 4 (1.2) [4] | 3 (2.7) [3] |
Overall, 425 participants (30.4%) reported 772 unsolicited AEs. With similar incidence in the SII-NVX-CoV2373 and NVX-CoV2373 groups (30.6% and 30.0%, respectively), most of the unsolicited AEs were mild in severity.
In the safety cohort, vaccine related unsolicited AEs (those AEs that were solicited in the immunogenicity/reactogenicity cohort) were reported by 39% and 39.1% participants in the SII-NVX-CoV2373 and NVX-CoV2373 groups, respectively. All were mild in severity except for 7 moderate severity events. In the immunogenicity/reactogenicity cohort, no vaccine related unsolicited AEs were reported.
Overall 13 SAEs were reported in 12 participants with only 1 SAE in the Phase 2 part and remainder in the Phase 3 part; 9 events in 9 participants in the SII-NVX-CoV2373 group and 4 events in 3 participants in the NVX-CoV2373 group. These included COVID-19 pneumonia (Phase 2 part) and dengue fever, gastroenteritis, femur fracture, head injury, limb crushing injury, pyrexia, hemolytic anemia, retinal vein occlusion, increased blood bilirubin, and joint effusion (Phase 3 part). None of the SAEs were related to the study vaccines and recovered without sequelae. No AESI was reported.
Discussion
The present study compared SII-NVX-CoV2373 with placebo/NVX-CoV2373 for safety and immunogenicity in adults. There were no causally related SAEs. The incidence of vaccine related AEs was higher in the SII-NVX-CoV2373 group than in the placebo group, but similar with the NVX-CoV2373 group. Most of the vaccine related AEs were mild in severity. SII-NVX-CoV2373 was non-inferior to NVX-CoV2373 in terms of GMEU ratio of anti-S IgG antibodies. Both anti-S IgG and nAb titers peaked at 14 days after the second dose and declined by day 180 to the levels slightly below to those seen after the first dose. The seroconversion for both anti-S IgG and nAb was ≥92% after the second dose which declined to more than 78% at day 180 in both the groups. SII-NVX-CoV2373 was found safe, well tolerated and immunogenic among adults in India. This waning of antibody titers is seen with all vaccines.9,10 It is known that neutralisation titers have a strong correlation with protection from SARS-CoV-2 parent strain as well as variants. Moreover, modelling of the effects of waning immunity predicts a loss of protection to the variants after vaccination.11 Solante R et al. reported that the real-world evidence from different COVID-19 vaccines (parent strain) supports the completion of primary series and booster vaccinations where appropriate, especially to restore waning vaccine effectiveness against the more infectious Omicron variant and protect populations from severe outcomes, hospital admissions, and longer lasting post-COVID-19 complications, as well as mortality.12 Considering this, booster doses are recommended at about 4–6 months or later after completion of primary immunization.
There are two COVID-19 vaccines majorly used in the adult immunization program of India, Covishield™ (ChAdOx1 nCoV-19, a simian adenovirus vectored COVID-19 vaccine, Serum Institute of India) and Covaxin™ (Whole virus inactivated vaccine with an adjuvant, Bharat Biotech). The seroconversion of Covaxin for neutralizing antibodies range from 91.9% to 96.6%.13,14 Covishield was tested in Phase 2/3 clinical trial in India. The seroconversion for neutralizing antibodies was 85.6%.15 The results in the present study for SII-NVX-CoV2373 as well as for NVX-CoV2373 are on the same lines. However, the efficacy of Covaxin and Covishield was 77.8% (95% CI 65.2–86.4)16 and 74.0% (95% CI, 65.3 to 80.5),17 respectively while the same of NVX-CoV2373 was 89.7% (95% CI, 80.2 to 94.6)6 and 90.4% (95% CI 82.9 to 94.6)7 in UK and USA/Mexico, respectively indicating that, in addition to humoral immunogenicity, that there may be other facets of the immune response contributing to the efficacy of the vaccine.
For NVX-CoV2373 vaccine, the seroconversion in anti-S IgG and for nAb was 100% in two Phase 1/2 studies.5,18 The present study shows similar results. However, in the Phase 2 study in USA and Australia, the GMT of anti-S IgG was 44,420.9 (95% CI, 37,929.1, 52,023.8) and that of neutralizing antibodies was 2200.8 (95% CI, 1342.6, 3607.5).5 In the Japan Phase 1/2 trial, the IgG GMT was 31,037 (95% CI, 26,837, 35,894) while neutralizing antibodies GMT was 884 (95% CI 749, 1044).18 The anti-S IgG GMTs in the present study were 143506.4 (133,203.2, 154,606.7) in the SII-NVX-CoV2373 group while 152,276.9 (95% CI, 132,441.4, 175,083.1) in the NVX-CoV2373 group. The respective neutralizing antibody GMTs were 3590.8 (95% CI, 3271.2, 3941.6) and 3970.1 (95% CI, 3255.8, 4841.0). These antibodies were measured at the same laboratories where all NVX-CoV2373 clinical trial samples were tested. The reasons for these apparent differences possibly are: about a third of participants (33.8%) enrolled in the present study were seropositive at baseline, while the same in the USA/Australia5 and Japan18 studies were 1.9% and all seronegative, respectively; the population in the NVX-CoV2373 trials was older [mean age 51.3 years (SD 17.47)] in the USA/Australia study5 while 52.6 years (range 20–77) in the Japan study,18 while the same in India trial was much lower (mean age 34.8 years [SD 10.80] in the SII-NVX-CoV2373 group and 33.4 [SD 10.18] in the NVX-CoV2373 group). Further, the Indian trial Phase 2 part was started in March 2021 and Phase 3 part started in end of June 2021. The Indian immunization program had started on 16 January 2021 targeting initially the health care workers and elderly people. By the time the Indian study started, many of the elderly had received the available vaccines and therefore, the number of elderly participants in the study was comparatively low. It is known that the immune responses are lower in the elderly population.19, 20, 21
SII-NVX-CoV2373 was found safe and well tolerated in the study. No SAE or AESI was caused by the vaccine. These findings are on the similar lines with the trials of NVX-CoV2373.4, 5, 6, 7
The reactogenicity of the mRNA vaccines is more with the second dose as compared to the first dose.22, 23, 24 With SII-NVX-CoV2373 as well, we find increased reactogenicity after the second dose. It is possible that matrix M adjuvant could be contributing to the increased reactogenicity. Increase in reactogenicity as the doses advance in vaccine series for adjuvanted vaccines like tetanus and diphtheria are known.25
We found a total of 13 COVID-19 cases from the first dose until two weeks after the second dose of vaccine in the study. There was only a single break-through case of symptomatic COVID-19 beyond two weeks after the second dose which is consistent with the ∼90% efficacy seen in UK and USA/Mexico studies,6,7 though the present study was not powered for efficacy.
The data from trials in US/Australia showed that the sera of NVX-CoV2373 recipients could neutralize Delta as well as Omicron variant, though there was a drop in neutralizing titers as compared to the original Wuhan strain.26 We do not currently have data on this from our study, though we expect results along similar lines of NVX-CoV2373 studies. This study was conducted during the second wave of COVID-19 in India with predominant delta variant cases emerging during phase 2 part and declining cases during phase 3 part.
For COVID-19 vaccines, neutralizing antibodies and binding antibodies correlate with clinical protection,27 though a protective cut off is not known. Hence, it is not possible to give seroprotection achieved by our study population. However, the high levels of seroconversion as well as GMTs for both types of antibodies that are in line with or higher than in the US/Australia trials of NVX-CoV2373, and efficacy of ≥90% in UK and USA/Mexico trials, SII-NVX-CoV2373 is expected to give the similarly high level protection.
Our study had a few potential limitations. We excluded previous known SARS-CoV-2 infection cases from the study. The study did not include participants with uncontrolled comorbid conditions, immunocompromised people, pregnant and lactating women and further studies are needed for these special populations. However, since our study population was representative of majority of population across the country, we believe these limitations do not affect the conclusions drawn on immunogenicity and safety of the vaccine from this study.
SII-NVX-CoV2373 was found non-inferior to NVX-CoV2373. At day 36, there was more than 58-fold rise in anti-S IgG and nAb titers compared to baseline in both the groups. Incidence of unsolicited and solicited AEs was similar between the SII-NVX-CoV2373 and NVX-CoV2373 groups. No AESI or causally related SAE was reported. The vaccine was found safe and highly immunogenic and should be an additional option for protection against COVID-19 parent strain as well as emerging variants such as Omicron. The vaccine has been evaluated in adults as a heterologous booster and will be evaluated as a booster in children.
Contributors
PSK and AK contributed equally as first authors. PSK, CSP, DK, BG, AK, US, SG, NG, SP and BB contributed to the study design and protocol development. PSK, DK, BG, CB, AD and AK accessed and verified the data. PSK, DK, BG, AD, AK and SG contributed to the manuscript preparation. Manuscript was finalized with considerable inputs from all the authors. VB, AR, SK, ST, PK, RL, MP, AB, NJG, SM, TK, SK, DHA, CS, GK, AG, DTS, SR, VH, and HLB contributed to data collection. US and MG contributed to immunogenicity experiments oversight and supervision. JSP, MZ, SCC, MP, SH, MT, and AS contributed to the immunogenicity experiments.
Data sharing statement
The anonymized study protocol is provided in appendix. Individual de-identified participant data will be made available on direct request to the corresponding author with an appropriate research proposal. This data will be shared upon approval of analysis proposals and signed data-access agreements are in place.
Declaration of interests
PSK, DK, BG, CB, AD, MG, and US are employees of SIIPL. JSP, MZ and SCC are employees of Novavax Inc. CSP is Chairman and Managing Director of SIIPL. All other authors declare no competing interests.
Acknowledgments
We gratefully acknowledge the contributions of our study participants. We acknowledge the DSMB members Dr Jayaprakash Muliyil, Dr Oommen John and Mr Charudatta Vaman Joglekar, and PPD for clinical monitoring and data management services.
Funding: SIIPL funded the contract research organisation and laboratory costs. The study site budgets, laboratory costs of RT-PCR testing by study sites, Anti-N IgG testing and CMI testing was funded by Indian Council of Medical Research. The study vaccines were supplied by SIIPL and Novavax.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2022.100139.
Contributor Information
Prasad S. Kulkarni, Email: drpsk@seruminstitute.com.
COVOVAX Study Group:
Shubhangi A. Kanitkar, Arjun L. Kakrani, Srikanth P. Tripathy, Abhijit V. Tilak, Akshay A. Dhamne, Shahzad Beg Mirza, Prachi V. Athavale, Mandakini Bhowmik, Parag J. Ratnakar, Subodh Gupta, Vijayshri Deotale, Jyoti Jain, Ashwini Kalantri, Vineet Jain, Nidhi Goyal, Alok Arya, Temsunaro Rongsen-Chandola, Shreyasi Dasgupta, Pratibha Periera, Vanmathi A, Anand Kawade, Arunkumar Gondhali, Palvi Kudyar, Abhishek Singh, Ravi Yadav, Alina Alexander, Venugopalan Gunasekaran, Sekar Dineshbabu, P.C. Samantaray, H.S. Ravish, Deepshikha Kamra, Shilpa Gaidhane, Quazi Syed Zahiruddin, Merlin Moni, Anil Kumar, Ameet Dravid, Anant Mohan, Tejas Suri, Tejas K. Patel, Surekha Kishore, Rahul Choche, Deepak Ghatage, and Sugam Salvi
Appendix A. Supplementary data
References
- 1.Priyanka Choudhary OP. Singh I, Patra G. Aerosol transmission of SARS-CoV-2: the unresolved paradox. Travel Med Infect Dis. 2020;37:101869. doi: 10.1016/j.tmaid.2020.101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) WHO Director-General’s opening remarks at the media briefing on COVID-19. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19-11-march-2020
- 3.2022. https://www.mygov.in/covid-19
- 4.Keech C., Albert G., Cho I., et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020 Dec 10;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Formica N., Mallory R., Albert G., et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med. 2021 Oct 1;18(10):e1003769. doi: 10.1371/journal.pmed.1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath P.T., Galiza E.P., Baxter D.N., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021 Sep 23;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkle L.M., Kotloff K.L., Gay C.L., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022 Feb 10;386(6):531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Guidelines on clinical evaluation of vaccines: regulatory expectations. WHO Technical Report Series 1004. Annex. October 21, 2020;9:2017. https://www.who.int/publications/m/item/WHO-TRS-1004-webannex-9 [Google Scholar]
- 9.Pérez-Alós L., Armenteros J.J.A., Madsen J.R., et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat Commun. 2022;13:1614. doi: 10.1038/s41467-022-29225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok S.L., Cheng S.M., Leung J.N., et al. Waning antibody levels after COVID-19 vaccination with mRNA Comirnaty and inactivated CoronaVac vaccines in blood donors, Hong Kong, April 2020 to October 2021. Euro Surveill. 2022;27(2):2101197. doi: 10.2807/1560-7917.ES.2022.27.2.2101197. Erratum in: Euro Surveill. 2022 Jan;27(3): PMID: 35027105; PMCID: PMC8759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cromer D., Steain M., Reynaldi A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022 Jan;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solante R., Alvarez-Moreno C., Burhan E., et al. Expert review of global real-world data on COVID-19 vaccine booster effectiveness and safety during the omicron-dominant phase of the pandemic. Expert Rev Vaccines. 2022:1–16. doi: 10.1080/14760584.2023.2143347. [DOI] [PubMed] [Google Scholar]
- 13.Ella R., Reddy S., Jogdand H., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021 Jul;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ella R., Vadrevu K.M., Jogdand H., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021 May;21(5):637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni P.S., Padmapriyadarsini C., Vekemans J., et al. A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India. eClinicalMedicine. 2021 Dec;42:101218. doi: 10.1016/j.eclinm.2021.101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ella R., Reddy S., Blackwelder W., et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021 Dec 11;398(10317):2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falsey A.R., Sobieszczyk M.E., Hirsch I., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda T., Murakami K., Sugiura K., Sakui S., Schuring R.P., Mori M. Safety and immunogenicity of NVX-CoV2373 (TAK-019) vaccine in healthy Japanese adults: interim report of a phase I/II randomized controlled trial. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.04.035. S0264–410X(22)00463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller L., Andrée M., Moskorz W., et al. Age-dependent immune response to the biontech/pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73(11):2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockman M.A., Mwimanzi F., Lapointe H.R., et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J Infect Dis. 2022;225(7):1129–1140. doi: 10.1093/infdis/jiab592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notarte K.I., Ver A.T., Velasco J.V., et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022:1–18. doi: 10.1080/10408363.2022.2038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vizcarra P., Haemmerle J., Velasco H., et al. BNT162b2 mRNA COVID-19 vaccine Reactogenicity: the key role of immunity. Vaccine. 2021;39(51):7367–7374. doi: 10.1016/j.vaccine.2021.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmani A., Dini G., Orsi A., Sticchi L., et al . Reactogenicity of BNT162b2 mRNA COVID-19 vaccine in a young working age population: a survey among medical school residents, within a mass vaccination Campaign, in a regional reference teaching hospital in Italy. Vaccines (Basel) 2021;9(11):1269. doi: 10.3390/vaccines9111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y.W., Lim S.Y., Lee J.H., et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in korea. J Korean Med Sci. 2021;36(21):e153. doi: 10.3346/jkms.2021.36.e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayas J.M., Vilella A., Bertran M.J., et al. Immunogenicity and reactogenicity of the adult tetanus-diphtheria vaccine. How many doses are necessary? Epidemiol Infect. 2001;127(3):451–460. doi: 10.1017/s095026880100629x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://ir.novavax.com/2021-12-22-Novavax-Announces-Initial-Omicron-Cross-Reactivity-Data-from-COVID-19-Vaccine-Booster-and-Adolescent-Studies. Accessed April 28, 2022.
- 27.Gilbert P.B., Montefiori D.C., McDermott A.B., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.