Abstract
Background:
Pharmacological treatments for opioid use disorder are essential, life-saving medications, yet successful induction of them and long-term retention on them is limited in many settings. Induction into opioid agonist treatment (OAT) features the highest risk of mortality throughout the treatment course, and greatest risk of discontinuation. We aimed to identify determinants of completing OAT induction and, among those completing induction, time to OAT discontinuation in British Columbia (BC), Canada.
Methods:
We conducted a retrospective study using linked population-level health administrative databases to capture all individuals in BC receiving at least one OAT dispensation from January 1, 2008, to September 30, 2018. We constructed covariates capturing client demographics, clinical history, and characteristics of the treatment episode and the primary prescribing physician. We estimated a two-part model to identify determinants of the probability of completing induction using a generalized linear mixed model with logit link and the time to OAT discontinuation among those completing induction using a Cox proportional hazards frailty model.
Results:
We observed 220,474 OAT episodes (73.9% initiated with methadone, 24.7% with buprenorphine, and 1.4% with slow-release oral morphine) among 45,608 individuals over the study period. Less than 60% of all OAT episodes completed induction (59.0% for methadone episodes, 56.7% for buprenorphine/naloxone, 41.0% for slow-release oral morphine) and half of all episodes that completed induction reached the minimum effective dosage (51.0% for methadone episodes [60 mg/day], 48.2% for buprenorphine/naloxone [12 mg/day], 59.4% for slow-release oral morphine [240 mg/day]). In multiple regression analysis, the adjusted odds of completing induction with buprenorphine improved over time, exceeding that of methadone in 2018: 1.46 (1.40, 1.51). For those who completed induction, buprenorphine use was associated with shorter times to discontinuation throughout the study period, but the estimated rate of discontinuation decreased over time (adjusted hazard ratio, vs. methadone in 2008: 2.50 (2.35, 2.66); in 2018: 1.79 (1.74, 1.85)).
Conclusion:
We found low rates of completing OAT induction and, for those who did complete it, low rates of reaching the minimum effective dose.
Keywords: Opioid agonist treatment, Opioid use disorder, Treatment retention, Treatment induction
1. Introduction
Evidence-based treatment for opioid use disorder (OUD) entails time-unlimited treatment using opioid agonists and partial agonists, such as methadone and buprenorphine/naloxone (American Society of Addiction Medicine, 2015; Bruneau et al., 2018; Connery, 2015; Government of British Columbia, 2017). Long-term retention in opioid agonist treatment (OAT) is associated with reduced rates of drug use, hospitalization, criminal activity, and mortality in observational studies (Nosyk et al., 2010; Peles et al., 2008). However, induction and long-term retention in OAT remains a challenge, and clients who enter into treatment with OAT often follow a pattern of repeated engagement, disengagement, and re-engagement (Nosyk et al., 2009; Socías et al., 2020). Specifically, in British Columbia (BC), Canada, the risk of mortality after discontinuing OAT has increased 2.1-fold compared with periods on OAT before the introduction of fentanyl in the illicit drug supply in 2012 and rose to 3.4-fold in 2016–2018 (Pearce et al., 2020).
Prior studies have identified a range of factors associated with prolonged OAT retention (Nosyk et al., 2009), but a dearth of research exists on factors that determine successful induction, defined as titrating up to a therapeutic dose that fully suppresses withdrawal symptoms. Critically, risk of mortality on OAT is highest in the first two weeks of treatment (Cornish et al., 2010; Degenhardt et al., 2009; Nosyk et al., 2015; Sordo et al., 2017). As clinical management, including medication dosing, differs during induction and subsequent maintenance (American Society of Addiction Medicine, 2015;Canadian Research Initiative in Substance Misuse, 2018; Government of British Columbia, 2017), the field needs information about factors that influence the likelihood of completing induction and, separately, factors influencing long-term retention.
In BC, guidelines recommended methadone treatment prior to July 2017 (Canadian Research Initiative in Substance Misuse, 2018; Government of British Columbia, 2017). BC's clinical guidelines recommend starting doses below 20 mg for OAT-naïve clients and below 40 mg otherwise, with dose increases of 5 to 10 mg every 5 days (Canadian Research Initiative in Substance Misuse, 2018; College of Physicians and Surgeons of British Columbia, 2014; Government of British Columbia, 2017). In July 2017, buprenorphine-naloxone replaced methadone as the first line treatment, in part due to its lower overdose mortality risk while engaged in treatment and a faster induction schedule. Buprenorphine-naloxone can be quickly titrated to a therapeutic dosage within 1–3 days, with suggested dose increases of 2 mg at a time and multiple increases possible in a single day (American Society of Addiction Medicine, 2015; Canadian Research Initiative in Substance Misuse, 2018; Centre for Addiction and Mental Health, 2011; Government of British Columbia, 2017; Vancouver Coastal Health & Providence Health Care, 2015). While buprenorphine/naloxone is possible to titrate quickly, slow or inadequate induction schedules applied in practice may increase discontinuations during the induction period, and evidence exists of poorer retention in treatment after a stabilization dose is reached compared with methadone (Commonwealth of Australia, 2014; Jacobs et al., 2015).
The most recent systematic review of randomized controlled trials reported no difference in retention between methadone and buprenorphine-naloxone at high fixed doses (Johnson et al., 1992; Johnson et al., 2000; Mattick et al., 2014; Pani et al., 2000), but worse outcomes for buprenorphine/naloxone in clinical trials at low fixed doses (Mattick et al., 2014), flexible dose trials (Mattick et al., 2003) and in observational studies (Burns et al., 2009a; Burns et al., 2014; Pinto et al., 2010). Evidence on dosing and induction in real-world settings will help to advance clinical guidance to improve OAT retention through induction and long-term maintenance.
The objective of this study is to identify determinants of completing induction into OAT and, for those who do, determinants of time to OAT discontinuation. We employ a population-level linked administrative database capturing all individuals accessing OAT in BC, Canada, to satisfy these objectives.
2. Methods
2.1. Study population
We conducted a retrospective study based on a linkage of seven provincial health administrative databases to capture all individuals receiving at least one OAT dispensation in BC, Canada, from 01/01/2008 to 30/09/2018. We used the PharmaNet database (BC Ministry of Health creator, 2018a) to identify OAT dispensations for directly observed doses and OAT prescriptions for take-home doses during the follow-up period. The study used prescription data from BC PharmaNet database (capturing medication dispensations), the Discharge Abstract Database (BC Ministry of Health creator, 2018b) (DAD; records of hospitalizations), Medical Services Plan (BC Ministry of Health creator, 2018c) (MSP; physician billing records), BC Vital Statistics (BC Vital Statistics Agency [creator], 2018) (capturing deaths and their underlying cause), BC Provincial Corrections (Ministry of Public Safety and Solicitor General (PSSG) [creator], 2018) (records of entry into incarcerations and releases to community), Perinatal Care Database (Perinatal Services BC [creator], 2018) (capturing maternal/infant care and outcomes), and NACRS Database (BC Ministry of Health creator, 2018d) (capturing ED visits). The government linked the databases deterministically using a unique, individual-level personal health number (Government of British Columbia).
2.2. Study setting
All BC residents are required to enroll in the provincial single-payer health insurance plan, the British Columbia Medical Services Plan (MSP), and those with active MSP enrollment are eligible for income-dependent “Pharmacare” insurance coverage for prescribed OAT medications. There are currently multiple medications available for OAT in BC. Methadone and buprenorphine/naloxone are available in private clinical practices, private specialty clinics, and community health centers (CHCs) (Government of British Columbia, 2017). Methadone was the only available medication until BC introduced buprenorphine/naloxone in 2008; however, the government required physicians to receive authorization through an exemption and training to prescribe either medication. Injectable OAT with diacetylmorphine became available only through “special access” for a limited number of BC clients in May 2016 (Government of Canada, 2016). The government lifted the requirement to receive an exemption to prescribe buprenorphine/naloxone in July 2016, making it more widely accessible (College of Physicians and Surgeons of British Columbia, 2016). In June 2017 new guidelines recommended buprenorphine/naloxone as the first line OAT medication and supported the use of slow-release oral morphine as a second-line treatment option. In May 2018, the government removed the requirement for authorization to prescribe methadone (College of Physicians and Surgeons of British Columbia, 2018). Finally, the government formally approved hydromorphone in May 2019 as a second injectable OAT medication to treat severe OUD (Health Canada, 2019).
2.3. Constructing continuous OAT episodes
Consistent with provincial guidelines on the duration of missed doses that would prompt a reversion back to the starting dose (Government of British Columbia, 2017), the study defined an OAT episode as having no missed doses over 6 days for buprenorphine/naloxone and 5 days for methadone or slow-release oral morphine. The study considered two outcomes for each episode. We measured whether an episode completed induction, defined as reaching the end of a 2-week period with no dose increases, and for those who completed induction, we considered the time to discontinuation of the OAT episode from the end of the 2-week stable period. The study team chose a 2-week period because, although methadone suggests a minimum of 5-day gap between dose increases, providers should increase the gap for individuals at higher risk of opioid toxicity (Government of British Columbia, 2017). The summary statistics for gaps between dose increases reflects this requirement for increased gaps between doses (Supplementary appendix, Table A6), as methadone had a median gap of 10 days between dose increases. Thus, the 2-week period allows for dose increases 10 days apart with an observation period to confirm that participants completed induction. The study excluded episodes with any injectable OAT at any point in the episode duration.
2.4. Covariates
We constructed a range of covariates capturing client demographics and clinical history, characteristics of the treatment episode, and characteristics of the primary prescribing physician. The study captured a client's clinical history through their administrative health records from January 1, 1996, until the beginning of each OAT episode. Client demographics, measured at the beginning of each OAT episode, included age, sex, chronic disease score (CDS) (Clark et al., 1995), any prior evidence of unstable housing (as indicated via ICD9/10 codes), incarceration status (never incarcerated, previously incarcerated but currently in the community, currently incarcerated), any prior evidence of social assistance (indicated by the PharmaCare plan type in medication dispensations), any hospital or emergency department contact in the past 7 days, HIV and HCV status, prior alcohol use disorder or other substance use disorders, previous mental health conditions, history of chronic pain, history of tobacco use, and whether the individual resided in a rural region (defined by their local health area) (British Columbia Ministry of Health, 2016). We used ICD-9/10 codes attached to physician billing and hospitalization records to define each comorbid condition (Supplementary appendix; Table A3). The study team also constructed a covariate to indicate whether the dosage at the end of induction reached the minimum effective maintenance dosage (12 mg/day for buprenorphine/naloxone; 60 mg/day for methadone; 240 mg/day for slow-release oral morphine). The study also included a measurement for the year an episode was initiated to control for time trends, as well as an interaction between the initiation year and the medication received, as medication types may have different time trends.
The research team identified prescribers through the PharmaNet database via de-identified ID. We assigned a primary prescriber to each OAT episode on the basis of that prescriber being referenced in the majority of dispensation records prior to the end of induction. We also considered the prescriber referenced on the first dose for each OAT episode. We constructed measures for years of OAT prescribing experience (<1 year, 1–5, 5–10, ≥10 years), client load (number of OAT clients in the past year), and whether treatment was delivered at a CHC (indicated by the absence of fee-for service claims in MSP records, as prescribers working in these settings are reimbursed through the provincial alternative payment plan) (Government of British Columbia, 2018).
2.5. Statistical analysis
The study team first provided summary statistics on the duration of continuous OAT episodes and Kaplan-Meier curves stratified by the medication received. We then implemented a two-part model. First, the study considered the probability of completing induction using a generalized linear mixed model with a binomial distribution and a logit link. Second, for those episodes that completed induction we applied a proportional hazards gamma frailty model (Sargent, 1998) to assess the association between the covariates and the duration of continuous retention in OAT from the end of the 2-week period, accounting for the dependence between the lengths of individuals' repeated episodes as well as the unobserved heterogeneity. The study assessed the assumption of proportionality for all covariates via Schoenfeld residual plots (Grambsch & Therneau, 1994). The covariates that the study considered in the final analysis were measured at the start of the OAT episode for the first model and measured at the end of the induction period for the second. The team transformed the estimates into odds ratios for the first model and hazard ratios for the second model. The team interpreted adjusted odds ratios above 1 as a positive client outcome (i.e., higher odds of completing induction), whereas we interpreted hazard ratios above 1 as a negative client outcome (i.e., faster time to discontinuation). As a sensitivity analysis, and to better compare results to a prior analyses on a similar linked database (Nosyk et al., 2009), we reconsidered the episode definition as a period of treatment with no gaps larger than 30 days. The research team executed data analysis using SAS 9.4 and R version 3.5.0 with package survival (Therneau, 2021).
3. Results
3.1. Summary statistics
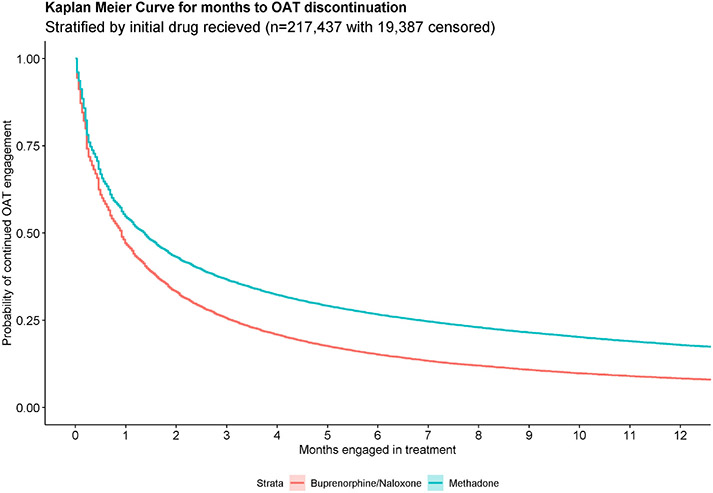
The study identified a total of 220,474 episodes from 45,608 individuals during the study period, representing 111,339 person-years on OAT. Nearly two-thirds of the population of individuals accessing OAT were male (65.9%), most initiated OAT between the ages of 20 and 40 (60.3%), and the majority resided in an urban setting (90.0%) (Table 1). Most OAT episodes were initiated with methadone (n = 162,990; 73.9%), while 54,518 (24.7%) began with buprenorphine/naloxone and 3062 (1.4%) were initiated with slow-release oral morphine (Table 2). Less than 60% of episodes completed induction (59.0% for episodes initiated with methadone, 56.7% for buprenorphine/naloxone and 41.0% for slow-release oral morphine). Half of all episodes that completed induction began the maintenance phase at the minimum effective dosage (51.0% for episodes initiated with methadone, 48.2% for buprenorphine/naloxone, and 59.4% for slow-release oral morphine), and two-thirds reached the minimum effective dosage overall (68.6% for methadone, 63.2% for buprenorphine/naloxone, and 72.3% for slow-release oral morphine). The median years of OAT experience of the initiating prescriber was 10.1 for methadone-initiated episodes, 8.1 for buprenorphine/naloxone, and 4.7 for slow-release oral morphine. The 12-month retention rates were 18.1% for episodes initiated with methadone, 7.9% for buprenorphine/naloxone, and 8.9% for slow-release oral morphine. Fig. 1 shows the differences in retention between methadone and buprenorphine/naloxone, where the initial slope of the Kaplan-Meier curve is steeper for episodes initiated with buprenorphine/naloxone.
Table 1.
Explanatory variables for people with opioid use disorder engaged in opioid agonist treatment (n = 45,608), British Columbia, Canada, 2008–2018. The summary statistics are on individuals' first treatment episode captured after 2008.
| Explanatory variable | Median (IQR) | No. | % |
|---|---|---|---|
| Individual characteristics | |||
| Female sex | 15,576 | 34.2 | |
| Age at treatment entry, years | |||
| <20 | 1176 | 2.6 | |
| 20-<30 | 13,541 | 29.7 | |
| 30-<40 | 13,945 | 30.6 | |
| 40-<50 | 9083 | 19.9 | |
| 50-<60 | 5696 | 12.5 | |
| ≥60 | 2167 | 4.8 | |
| Clark chronic disease score | 1.99 (1.20–3.04) | ||
| Ever indication of unstable housing | 1835 | 4.0 | |
| Ever evidence of social assistance | 27,507 | 60.3 | |
| Living area | |||
| Rural | 4577 | 10.0 | |
| Urban | 41,031 | 90.0 | |
| Other comorbidities/diagnoses | |||
| Alcohol use disorder | 10,822 | 23.9 | |
| Other substance use disorder | 31,217 | 68.5 | |
| Human immunodeficiency virus | 1253 | 2.8 | |
| Hepatitis C virus | 2263 | 5.0 | |
| Mental health condition | 33,509 | 73.5 | |
| History of chronic pain | 28,902 | 63.4 | |
| History of tobacco use | 12,780 | 28.0 | |
| Incarceration history | |||
| Never | 30,578 | 67.1 | |
| Released over 3 years prior | 5974 | 13.1 | |
| Release within past 3 years | 6714 | 14.7 | |
| Currently incarcerated | 2342 | 5.1 | |
| Death during follow up | 8203 | 21.7 | |
| Death while on treatment | 790 | 2.1 |
Range of Clark chronic disease scores observed: 0–9.4.
Table 2.
Explanatory variables for all opioid agonist treatment episodes (n = 220,474) captured in British Columbia, Canada, 2008–2018. The summary statistics are on characteristics of all episodes coming from 45,608 individuals, stratified by the initial medication received.
| Medication received at initiation | |||
|---|---|---|---|
| Methadone (n = 162,990) |
Buprenorphine/ naloxone (n = 54,447) |
Slow-release oral morphine (n = 3037) |
|
| No. (%) | |||
| Initial prescriber's characteristics | |||
| OAT client loada | 123 (62–210) | 147 (68–264) | 104 (49–223) |
| Years OAT prescribing experiencea | 10.1 (4.6–15.1) | 8.1 (2.9–16.0) | 4.6 (1.7–12.1) |
| CHC prescriber | 11,637 (7.1%) | 3463 (6.4%) | 460 (15.1%) |
| Treatment characteristics | |||
| Episode duration (days)a | 40 (9–183) | 27 (7–84) | 18 (5–63) |
| 3-month retentionb | 57,862 (36.7%) | 12,742 (25.2%) | 579 (25.9%) |
| 12-month retentionb | 25,837 (18.1%) | 3094 (7.9%) | 29 (8.9%) |
| Censored | 13,139 (8.1%) | 5109 (9.5%) | 606 (20.0%) |
| Minimum effective daily dose ever being prescribed | 82,179 (50.4%) | 29,638 (54.4%) | 1574 (51.8%) |
| Episode number | |||
| 1 | 22,909 (14.1%) | 12,621 (23.2%) | 208 (6.8%) |
| 2 | 17,593 (10.8%) | 8118 (14.9%) | 201 (6.6%) |
| 3 | 14,478 (8.9%) | 5983 (11.0%) | 194 (6.4%) |
| 4 | 12,147 (7.5%) | 4593 (8.4%) | 189 (6.2%) |
| 5 | 10,425 (6.4%) | 3593 (6.6%) | 202 (6.7%) |
| ≥6 | 85,438 (52.4%) | 19,539 (35.9%) | 2139 (10.4%) |
| Completed induction | 96,147 (59.0%) | 30,867 (56.7%) | 1244 (41.0%) |
| Initial maintenance dosage above minimum effective dose | 48,875 (50.8%c) | 15,059 (48.8%c) | 738 (59.3%c) |
| Ever reached minimum effective dose | 65,984 (68.6%c) | 19,508 (63.2%c) | 899 (72.3%c) |
Abbreviations: OAT: opioid agonist treatment, CHC: community health center. Italicized: reporting among those who completed induction.
Median (IQR).
For 3 m/12 m retention rates, only episodes initiated before June 30th 2018/Sept 30th 2017 were included.
Percentage of episodes that completed induction.
Fig. 1.
Kaplan Meier Curve for discontinuation times of all opioid agonist treatment episodes (n = 220,474) from 45,608 individuals in British Columbia, Canada, from January 1st 2008 to September 30th 2018, stratified by the initial medication received.
3.2. Factors associated with completed induction
We found that compared to individuals who had never been incarcerated, individuals who initiated treatment while incarcerated had increased odds of completing induction (Table 3; adjusted odds ratio: 1.28, 95% CI: 1.21, 1.36); however, individuals who initiated treatment in the community but had an incarceration history (0.75 (0.73, 0.78)) were less likely to finish induction.
Table 3.
Results from the generalized linear model with a logit link for the probability of completing induction for 220,570 episodes from 45,608 individuals in British Columbia, Canada, 2008–2018.
| Explanatory variable | Odds ratio | 95% CI |
|---|---|---|
| Year | 0.91 | (0.90, 0.91) |
| Individual characteristics | ||
| Male | Reference | |
| Female | 0.77 | (0.74, 0.79) |
| Age | ||
| <40 | Reference | |
| ≥40 | 1.58 | (1.53, 1.63) |
| CDS | ||
| <4 | Reference | |
| ≥4 | 1.11 | (1.06, 1.15) |
| Living area | ||
| Urban | Reference | |
| Rural | 1.54 | (1.47, 1.62) |
| Ever indication of unstable housing | ||
| No | Reference | |
| Yes | 0.77 | (0.73, 0.81) |
| Incarceration status | ||
| Never incarcerated | Reference | |
| Previously incarcerated, but currently in community | 0.75 | (0.73, 0.78) |
| Currently incarcerated | 1.28 | (1.21, 1.36) |
| Recent ED or hospital discharge | ||
| No | Reference | |
| Yes | 1.17 | (1.12, 1.21) |
| Ever evidence of social assistance | ||
| No | Reference | |
| Yes | 0.93 | (0.90, 0.96) |
| Other comorbidities/diagnoses | ||
| Alcohol use disorder | 0.86 | (0.83, 0.89) |
| Other substance use disorder | 0.97 | (0.93, 1.01) |
| Human immunodeficiency virus | 1.07 | (0.99, 1.16) |
| Hepatitis C virus | 1.14 | (1.08, 1.21) |
| Mental health condition | 1.25 | (1.20, 1.31) |
| Chronic pain | 1.14 | (1.10, 1.18) |
| History of tobacco use | 1.07 | (1.04, 1.10) |
| Treatment characteristics | ||
| Initiation medication at first year of follow-up (2008) | ||
| Methadone | Reference | |
| Buprenorphine/naloxone | 0.20 | (0.18, 0.22) |
| Initiation medication for mid-point year of follow-up (2013) | ||
| Methadone | Reference | |
| Buprenorphine/naloxone | 0.54 | (0.52, 0.56) |
| Initiation medication for last year observed (2018) | ||
| Methadone | Reference | |
| Buprenorphine/naloxone | 1.46 | (1.40, 1.51) |
| Slow-release oral morphinea | 0.70 | (0.63, 0.78) |
| Episode number | ||
| 1 | Reference | |
| 2 | 1.27 | (1.22, 1.31) |
| 3–4 | 1.22 | |
| 5–6 | 1.14 | (1.18, 1.26) |
| ≥7 | 1.10 | (1.09, 1.18) |
| Initial prescriber characteristics | ||
| Years of OAT prescribing experience | ||
| <1 | Reference | (1.06, 1.14) |
| 1–5 | 0.87 | (0.83, 0.91) |
| 5–10 | 0.97 | (0.93, 1.02) |
| >10 | 1.22 | (1.17, 1.28) |
| OAT client load | ||
| Q1 | Reference | |
| Q2 | 1.01 | (0.97, 1.05) |
| Q3 | 0.96 | (0.93, 1.00) |
| Q4 | 1.07 | (1.03, 1.11) |
| CHC prescriber | ||
| No | Reference | |
| Yes | 0.63 | (0.60, 0.66) |
| Estimated variance | 1.05 |
Abbreviations: CDS: Clark chronic disease score, OAT: opioid agonist treatment, CHC: community health center.
Slow-release oral morphine was introduced in 2017, and thus not included in estimates prior to 2017.
Compared to individuals whose initiating prescriber was inexperienced (<1 year of OAT prescribing), having a prescriber with 1–5 years of experience was associated with a lower probability of completing induction (0.87 (0.83, 0.91)); however, individuals whose initiating prescriber had more than 10 years of OAT prescribing experience were more likely to complete induction (1.22 (1.17, 1.28)). Finally, OAT episodes initiated within CHCs had substantially lower odds of completing induction (0.63 (0.60, 0.66)).
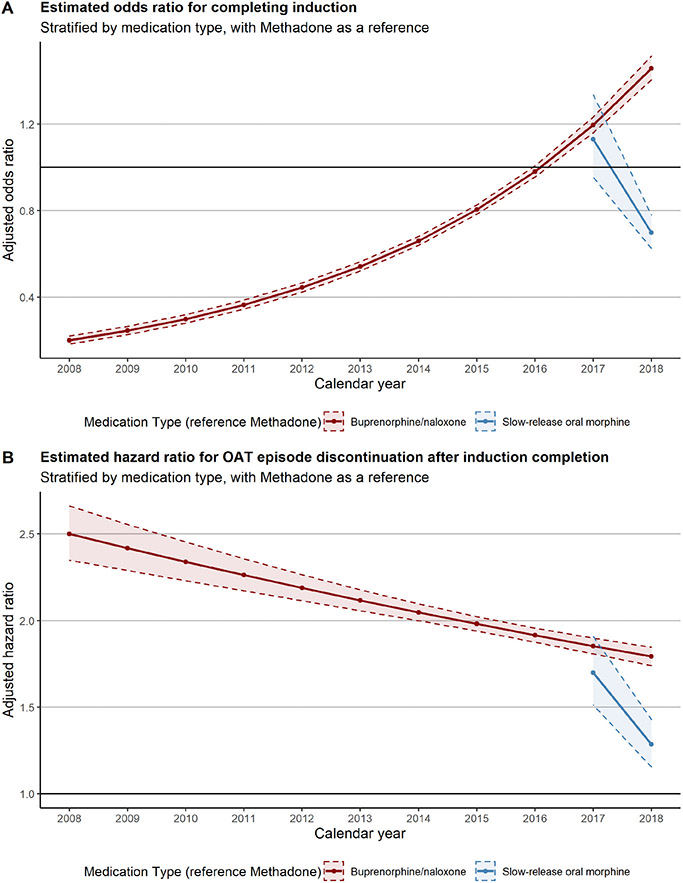
We also found that compared to 1st attempts, individuals had higher odds of completing induction in their 2nd to 6th attempts at initiating OAT (Table 3), though this benefit dissipated in subsequent attempts. Last, compared to methadone, individuals who started on buprenorphine/naloxone had lower odds of completing induction up to 2018, when the adjusted odds of completion exceeded that of methadone: 1.46 (1.40, 1.51) (Fig. 2A).
Fig. 2.
Adjusted estimates for the odds ratio for probability of completing induction and the hazard ratio for OAT discontinuation after completing induction, both stratified by medication type.
3.3. Factors associated with sustained retention after completing induction
Among individuals completing OAT induction, higher levels of comorbidity, measured by the Charlson comorbidity index, were associated with longer times to discontinuation (Table 4). Individuals with concurrent substance use disorder (adjusted hazard ratio: 0.81 (0.79, 0.84)), HIV/AIDS (0.94 (0.89, 0.98)), hepatitis-C virus (0.94 (0.91, 0.98)) mental health conditions (0.79 (0.77, 0.81)), chronic pain (0.92 (0.90, 0.94)), and tobacco use (0.96 (0.94, 0.97)) had longer times to discontinuation; however, having a concurrent alcohol use disorder was associated with shorter times to discontinuation (1.03 (1.01, 1.05)). Individuals with a history of unstable housing had shorter OAT episodes (1.20 (1.17, 1.24)). Compared to individuals without any incarceration records, individuals in the community but with an incarceration history had shorter times to discontinuation (1.24 (1.22, 1.27)), as did individuals who were currently incarcerated when completing the induction phase (1.15 (1.10, 1.19)). However, the Schoenfeld residual plots (provided in the Supplemental appendix) indicated an inconsistent effect for currently incarcerated individuals with different OAT durations. To address this, we removed the incarceration history covariate, which had a minimal impact on the remaining covariates (Supplementary appendix, Table A11).
Table 4.
Results from the cox proportional hazard model with a gamma frailty for the duration of continuous retention in opioid agonist treatment among 122,173 episodes from 37,785 clients who received opioid agonist treatment and completed induction in British Columbia, Canada, 2008–2018.
| Explanatory variable | Hazard ratio |
95% CI |
|---|---|---|
| Year of initiation | 0.99 | (0.99, 1.00) |
| Individual characteristics | ||
| Male | Reference | |
| Female | 1.07 | (1.05, 1.10) |
| Age | ||
| <40 | Reference | |
| ≥40 | 0.85 | (0.83, 0.87) |
| CDS | ||
| <4 | Reference | |
| ≥4 | 0.95 | (0.92, 0.97) |
| Living area | ||
| Urban | Reference | |
| Rural | 1.09 | (1.05, 1.12) |
| Ever indication of unstable housing | ||
| No | Reference | |
| Yes | 1.20 | (1.17, 1.24) |
| Incarceration status | ||
| Never | Reference | |
| Previously incarcerated, but currently in community | 1.24 | (1.22, 1.27 |
| Currently incarcerated | 1.15 | (1.10, 1.19) |
| Ever evidence of social assistance | ||
| No | Reference | |
| Yes | 0.89 | (0.87, 0.91) |
| Other comorbidities/diagnoses | ||
| Alcohol use disorder | 1.03 | (1.01, 1.05) |
| Other substance use disorder | 0.81 | (0.79, 0.84) |
| Human immunodeficiency virus | 0.94 | (0.89, 0.98) |
| Hepatitis C virus | 0.94 | (0.91, 0.98) |
| Mental health condition | 0.79 | (0.77, 0.81) |
| Chronic pain | 0.92 | (0.90, 0.94) |
| History of tobacco use | 0.96 | (0.94, 0.97) |
| Treatment characteristics | ||
| Medication at the end of induction for first year observed (2008) | ||
| Methadone | Reference | |
| Buprenorphine/naloxone | 2.50 | (2.35, 2.66) |
| Medication at the end of induction for mid-point year of follow-up (2013) | ||
| Methadone | Reference | |
| Buprenorphine/naloxone | 2.12 | (2.06, 2.18) |
| Medication at the end of induction for last year observed (2018) | ||
| Methadone | Reference | |
| Buprenorphine/naloxone | 1.79 | (1.74, 1.85) |
| Slow-release oral morphinea | 1.28 | (1.16, 1.43) |
| Maintenance dose at or above minimum effective dose | ||
| No | Reference | |
| Yes | 0.79 | (0.78, 0.8) |
| Episode number | ||
| 1 | Reference | |
| 2 | 1.15 | (1.12, 1.17) |
| 3–4 | 1.20 | (1.17, 1.23) |
| 5–6 | 1.23 | (1.20, 1.26) |
| ≥7 | 1.35 | (1.32, 1.39) |
| Primary prescriber characteristics | ||
| Years of OAT prescribing experience | ||
| <1 | Reference | |
| 1–5 | 1.01 | (0.98, 1.05) |
| 5–10 | 1.02 | (0.99, 1.06) |
| >10 | 1.02 | (0.99, 1.05) |
| OAT client load | ||
| Q1 | Reference | |
| Q2 | 0.97 | (0.95, 1.00) |
| Q3 | 1.01 | (0.99, 1.04) |
| Q4 | 1.08 | (1.06, 1.11) |
| CHC prescriber | ||
| No | Reference | |
| Yes | 1.32 | (1.28, 1.37) |
| Estimated frailty variance | 0.38 |
Abbreviations: CDS: Clark chronic disease score, OAT: opioid agonist treatment, CHC: community health center.
Slow-release oral morphine was introduced in 2017, and thus not included in estimates prior to 2017.
Furthermore, successive attempts at treatment had statistically significantly shorter times to discontinuation. In comparison to 1st episodes, 2nd episodes had a hazard ratio of 1.15 (1.12, 1.17), which increased with later episodes (3–4: 1.20 (1.17, 1.23), 5–6: 1.23 (1.130, 1.26), ≥7: 1.35 (1.32, 1.39)). Among those completing induction, receiving treatment from a CHC was associated with shorter times to treatment discontinuation (1.32 (1.28, 1.37)). When the dose at the beginning of the maintenance stage was at or above the minimum effective dosage, we observed longer treatment durations (0.79 (0.78, 0.80)). Those completing induction on methadone had longer times to discontinuation throughout the study period, though the relative hazard declined over time (Fig. 2B).
Last, our sensitivity analysis (Supplementary appendix, Table A6) produced similar results for most covariates, with the exception of the effect of the calendar year of initiation and the episode count number, which indicated longer times to discontinuation in successive attempts at treatment. In the sensitivity analysis, the hazard ratio for 2nd episodes was not significant, with an estimate of 1.01 (0.98, 1.04), but later episodes had significantly lower estimates (3–4: 0.94 (0.914, 0.97), 5–6: 0.92 (0.89, 0.96)).
4. Discussion
In this population-based study of OAT retention in BC, Canada, we found that more than 40% of episodes did not complete induction and, for those that did, only half began the maintenance stage at or above the minimum effective dose. Further, only two-thirds reached the minimum effective dose at any point in their episode. We also found that the adjusted odds ratio of completing induction for those starting on buprenorphine/naloxone started out 80% lower compared to methadone but increased to 46% higher by the end of the study period. Once titrated, buprenorphine/naloxone started with a discontinuation hazard that was 2.5 times higher compared to methadone; however, this difference decreased to 1.8 times higher discontinuation hazard for buprenorphine/naloxone by the end of the study period.
Our findings indicate the distinction of these outcomes is clinically important given the exceptionally high discontinuation rate observed during induction, the low rate of reaching the minimum effective dose—which other population-level evidence confirms (Office of the Provincial Health Officer, 2017)—as well as the distinct induction processes and challenges associated with each medication (American Society of Addiction Medicine, 2015; Canadian Research Initiative in Substance Misuse, 2018; Government of British Columbia, 2017). A dearth of evidence exists exploring motivations to discontinue OAT. In one qualitative study of individuals on buprenorphine/naloxone, the most common reason for discontinuing treatment within six months was a conflict or challenge with program policies, expectations, or staff; only 4% reported that they left the program because they felt they had completed their buprenorphine treatment regimen successfully (Gryczynski et al., 2014). Further research should investigate motivations for discontinuations, as our study indicates that current dosing and induction schedules may not be meeting clients' needs.
The greater likelihood of completing induction on methadone then buprenorphine/naloxone prior to 2016, while adjusting for unobserved confounding, is counterintuitive given the relatively shorter induction period associated with buprenorphine/naloxone (Government of British Columbia, 2017). Prior evidence suggests that different dosing trajectories influence one-week retention rates and that those who start on a moderate dose and then shift to a high dose are three times less likely to drop out in the first seven days compared with those who start and remain on low doses (Jacobs et al., 2015). As our results adjusted for unobserved individuals' baseline characteristics, this difference cannot be explained by a selection effect for buprenorphine/naloxone versus methadone. However, in 2016 buprenorphine/naloxone started to have a higher likelihood of completing induction than methadone. We also found that initiating prescribes who had more than 10 years of OAT prescribing experience were more likely to complete induction. Together, with the effect change for buprenorphine/naloxone, these results suggest that more prescribing experience may be associated with a better understanding of dosing trajectories for buprenorphine/naloxone that promotes retention. Unlike methadone, which is a full opioid agonist, buprenorphine/naloxone is a partial agonist with a ceiling effect at maximum dosage (Government of British Columbia, 2017). Evidence on buprenorphine/naloxone's effectiveness for OUD treatment precedes the introduction of fentanyl into the illicit drug supply and we do not know whether it holds the same effects in this context.
We additionally found that among those who completed induction, only about half began the maintenance phase at or above the minimum effective dose, and reaching the minimum effective dose was associated with a 20% lower discontinuation rates on average. This rate held for buprenorphine/naloxone even with our modest minimum effective dose definition of 12 mg based on the BC guidelines (Government of British Columbia, 2017). American Society of Addiction Medicine and Ireland's Health Service Executive guidelines recommend a minimum of 16 mg (American Society of Addiction Medicine, 2015; Health Service Executive, 2017), aligning with evidence from experimental and observational settings that suggests higher doses (≥16 mg) promote better retention outcomes (Mattick et al., 2014). Along with our finding that buprenorphine/naloxone is associated with shorter times to discontinuation, low rates of reaching the minimum effective dose add additional insight—controlling for covariates and distinguishing between induction and later retention—into an emerging trend in the literature that buprenorphine/naloxone retention outcomes differ in practice from what might be expected under ideal circumstances (Bell et al., 2009; Burns et al., 2009a; Burns et al., 2009b; Burns et al., 2014; Government of British Columbia, 2017; Pinto et al., 2010; Proctor et al., 2014). Our results indicate that dosing is a clinically relevant characteristic. Assessing the comparative effectiveness of treatment regimens with methadone and buprenorphine/naloxone—fully accounting for confounding by indication at baseline and over time—is a critical next step in this line of research to inform accurate clinical guidance (Hernan & Robins, 2016; Piske et al., 2020).
Several other findings in our analysis are worth noting. First, we observed that individuals with a higher chronic disease score had longer times to discontinuation—which is consistent with a previous analysis on the same population (Nosyk et al., 2009)—and they had a greater likelihood of completing the induction period. The study derived chronic disease scores from prescription records (Clark et al., 1995), which better capture treated chronic diseases than all chronic diseases. This finding suggests a positive effect from health care engagement among individuals receiving treatment for multiple comorbidities. We also found that diagnosed mental health conditions were associated with lower discontinuation rates, which we hypothesize represents a similar treatment engagement effect. Mental health conditions are highly prevalent among individuals with substance use disorders (Currie et al., 2005; Goldner et al., 2014; Quello et al., 2005; RachBeisel et al., 1999; Seal et al., 2012; Sullivan et al., 2006) and in our sample 73% of all individuals initiating OAT had a diagnosed mental disorder. The study observed the opposite effect for having an alcohol use disorder, potentially reflecting greater client complexity as well as limited guidance on concurrent clinical management of both conditions (BC Centre on Substance Use, 2019; Government of British Columbia, 2017).
Second, roughly one-third of our sample had been or was currently incarcerated at OAT initiation. Those currently incarcerated had 31% higher odds of completing induction, which suggests that incarceration may offer a conducive setting for the induction phase of treatment, and may remove certain barriers faced in community-based settings (e.g., logistical barriers associated with daily dispensations in community and ease of accessing illicit alternatives). The success of induction during incarceration provides more support to have OAT accessible in correctional facilities, which in BC is available to incarcerated individuals with OUD (BC Mental Health and Substance Use Services, 2021), but is often underutilized elsewhere. However, discontinuation rates for those currently incarcerated were higher compared to those never incarcerated. When assessing the assumption of proportionality with Schoenfeld residual plots, we found that for people in episodes lasting up to 30 days, incarceration had a positive effect on retention, but for longer episodes it had a negative effect, suggesting incarceration status may be a time-varying confounder. Therefore, while incarceration may be a good setting to initiate treatment, our results indicate potential long-term retention challenges, which we hypothesize relates to post-release disruptions to care during transitions back to the community. Sentences or detainments are often short-term (Reitano, 2017) and numerous studies have noted a pattern of recidivism and churn in and out of correctional facilities (Yukhnenko et al., 2019). Our findings underscore a need for improvements in continuity of care processes between settings (Green et al., 2018), particularly since previous research has demonstrated an elevated risk of mortality upon release from correctional facilities (Binswanger et al., 2007; Binswanger et al., 2013; Degenhardt et al., 2014; Groot et al., 2016; Kouyoumdjian et al., 2016). Following recommendations from the College of Family Physicians of Canada (The College of Family Physicians of Canada, 2016) and the World Health Organization (World Health Organization, 2014), BC transitioned oversight of health care delivery in correctional facilities from Corrections BC to the Provincial Health Services Authority in 2017 (PHSA). PHSA has taken steps to improve coordination of care, with all clients on OAT during incarceration now receiving a prescription at discharge and connection to a community physician to continue treatment, which build on process improvements initiated by Corrections BC (BC Mental Health and Substance Use Services, 2020).
Third, we found that individuals with a history of unstable housing had lower odds of completing induction and higher subsequent rates of discontinuation, which is consistent with other research outlining medical engagement challenges for the unstably housed (Havens et al., 2009; Hunter et al., 2015; Milloy et al., 2012; Sajatovic et al., 2006). Our finding of higher discontinuations and lower odds of completing induction at community health centers (CHCs)—which are low-threshold services aiming to improve chronic disease management and provide comprehensive care—similarly reflects greater levels of instability within the client base; any interpretation of these findings should acknowledge this context, as CHCs provide a level of accessibility that reaches client who may not otherwise engage in care. The deleterious impacts of housing instability, benefits of treating comorbid conditions, and evidence of unique needs for individuals experiencing incarceration or complex comorbidities such as alcohol use disorder all serve to reiterate the importance of care models that reduce barriers to client success in treatment. Improving social stability, through housing stability, comprehensive care, and transitional supports, are important aspects of comprehensive care models for OAT clients and are increasingly being funded in similar populations in other jurisdictions (Dombrowski et al., 2018; Griffin et al., 2020).
Finally, our finding that later treatment episodes were shorter on average runs contrary to findings from an earlier study on the same population (Nosyk et al., 2009). This discrepancy resulted from the OAT episode discontinuation rule; when we applied the same 30-day discontinuation rule in sensitivity analysis, our results were consistent with the prior study. While no consensus exists in the literature on what duration of absence constitutes a “discontinuation”, the duration that we used was based on a newly developed clinical guideline for the province of BC and acknowledges an elevated risk of mortality within the first week of discontinuing treatment (Government of British Columbia, 2017; Pearce et al., 2020).
4.1. Limitations
Several limitations exist in this analysis. First, as in any observational study, we could not rule out the potential for unmeasured confounding, though the Cox proportional hazards frailty model is designed to control for unmeasured baseline confounding. Nevertheless, our inferences cannot be interpreted as causal. Second, the administrative nature of the data used in this study may be subject to misclassification for history of unstable housing and tobacco history. Specifically, tobacco history is mostly identified through medication dispensations indicated for nicotine cessation products and administrative records for unstable housing has previously shown to have a low sensitivity (Peterson et al., 2015). Finally, the analysis takes place in the context of BC's low-threshold care model, where most forms of OAT (including both methadone and buprenorphine/naloxone) can be prescribed by primary care physicians and dispensed through community-based pharmacies (Gostin et al., 2017; Office of the Provincial Health Officer, 2017). Individuals attempting to access OAT in other settings may experience additional barriers to care (e.g., high costs and low availability), which could limit this study's generalizability.
5. Conclusion
In conclusion, in a population-level study of 45,608 individuals, we found low rates of completing OAT induction, low rates of reaching the minimum effective dose, and shorter episodes for those who did not reach the minimum effective dose. Buprenorphine/naloxone performed worse than methadone on both completing induction and subsequent long-term retention even after controlling for individual and clinical characteristics. Evidence on the comparative effectiveness of OAT medications—fully accounting for the effects of dosing and induction periods—will help to advance the clinical guidance on OAT administration.
Supplementary Material
Supported by:
Health Canada Substance Use and Addictions Program award no. 1819-HQ-000036 and the National Institutes of Health/National Institute on Drug Abuse award no. R01DA050629. The funding agreements ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
Disclaimer
All inferences, opinions, and conclusions drawn in this study are those of the authors, and do not reflect the opinions or policies of the Data Steward(s).
CRediT authorship contribution statement
Megan Kurz: Methodology, formal analysis, data curation, writing – original draft, writing – review & editing, visualization
Jeong Eun Min: Methodology, writing – review – editing, data curation
Laura M Dale: Writing – original draft, writing – review & editing
Bohdan Nosyk: Conceptualization, methodology, supervision, funding acquisition.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsat.2021.108647.
References
- American Society of Addiction Medicine. (2015). National practice guideline for the use of medications in the treatment of addiction involving opioid use. Journal of Addiction Medicine; 9(5), 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BC Centre on Substance Use. (2019). Provincial guideline for the clinical management of high-risk drinking and alcohol use disorder. In. Vancouver, BC. [Google Scholar]
- BC Mental Health and Substance Use Services. (2020). Correctional health services. http://www.bcmhsus.ca/our-services/health-services-for-people-in-custody/correctional-health-services.
- BC Mental Health and Substance Use Services. (2021). Correctional health services. Retrieved July 19 from http://www.bcmhsus.ca/our-services/health-services-for-people-in-custody/correctional-health-services.
- BC Ministry of Health [creator]. (2018a). PharmaNet. British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- BC Ministry of Health [creator]. (2018b). Discharge abstract database (hospital separations). British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- BC Ministry of Health [creator]. (2018c). Medical services plan (MSP) payment information file. British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- BC Ministry of Health [creator]. (2018d). National ambulatory care reporting system (NACRS). British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- BC Vital Statistics Agency [creator]. (2018). Vital statistics deaths. British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- Bell J, Trinh L, Butler B, Randall D, & Rubin G (2009). Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction, 104, 1193–1200. 10.1111/j.1360-0443.2009.02627.x [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, & Stern MF (2013). Mortality after prison release: Opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine, 159(9), 592–600. 10.7326/0003-4819-159-9-201311050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, & Koepsell TD (2007). Release from prison—a high risk of death for former inmates. New England Journal of Medicine, 356(2), 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Columbia Ministry of Health. (2016). B.C. Health System strategy geographic service areas. Retrieved from https://www2.gov.bc.ca/assets/gov/health/about-bc-s-healthcare-system/health-priorites/geographic-service-areas.docx.
- Bruneau J, Ahamad K, Goyer M-È, Poulin G, Selby P, Fischer B, & Wood E (2018). Management of opioid use disorders: A national clinical practice guideline. Canadian Medical Association Journal, 190(9), E247–E257. 10.1503/cmaj.170958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L, Gisev N, Larney S, Dobbins T, Gibson A, Kimber J, & Degenhardt L (2014). A longitudinal comparison of retention in buprenorphine and methadone treatment for opioid dependence in New South Wales, Australia. Addiction, 110, 646–655. 10.1111/add.12834 [DOI] [PubMed] [Google Scholar]
- Burns L, Randall D, Hall WD, Law M, Butler T, Bell J, & Degenhardt L (2009a). Opioid agonist pharmacotherapy in New South Wales from 1985 to 2006: Patient characteristics and patterns and predictors of treatment retention. Addiction, 104(8), 1363–1372. [DOI] [PubMed] [Google Scholar]
- Burns L, Randall D, Hall WD, Law M, Butler T, Bell J, & Degenhardt L (2009b). Opioid agonist pharmacotherapy in new SouthWales from 1985 to 2006: Patient characteristics and patterns and predictors of treatment retention. Addiction, 104, 1363–1372. 10.1111/j.1360-0443.2009.02633.x [DOI] [PubMed] [Google Scholar]
- Canadian Research Initiative in Substance Misuse. (2018). CRISM national guidelines for the clinical management of opioid use disorder. In. [Google Scholar]
- Centre for Addiction and Mental Health. (2011). Buprenorphine/naloxone for opioid dependence: Clinical practice guideline.. In. [Google Scholar]
- Clark D, Von Korff M, Saunders K, Baluch W, & Simon G (1995). A chronic disease score with empirically derived weights. Medical Care, 33(8), 783–795. 10.1097/00005650-199508000-00004 [DOI] [PubMed] [Google Scholar]
- College of Physicians and Surgeons of British Columbia. (2014). Methadone maintenance program: Clinical practice guideline. In. [Google Scholar]
- College of Physicians and Surgeons of British Columbia. (2016). Important notice regarding Suboxone®. https://www.cpsbc.ca/important-notice-regarding-suboxone%C2%AE.
- College of Physicians and Surgeons of British Columbia. (2018). Practice standard: Prescribing methadone. [Google Scholar]
- Commonwealth of Australia. (2014). National guidelines for medication assisted treatment of opioid dependence. [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry, 23(2), 63–75. [DOI] [PubMed] [Google Scholar]
- Cornish R, Macleod J, Strang J, Vickerman P, & Hickman M (2010). Risk of death during and after opiate substitution treatment in primary care: Prospective observational study in UK general practice research database. BMJ, 341, Article c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Patten SB, Williams JV, Wang J, Beck CA, El-Guebaly N, & Maxwell C (2005). Comorbidity of major depression with substance use disorders. The Canadian Journal of Psychiatry, 50(10), 660–666. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Larney S, Kimber J, Gisev N, Farrell M, Dobbins T, & Butler T (2014). The impact of opioid substitution therapy on mortality post-release from prison: Retrospective data linkage study. Addiction, 109(8), 1306–1317. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Randall D, Hall W, Law M, Butler T, & Burns L (2009). Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug and Alcohol Dependence, 105(1–2), 9–15. [DOI] [PubMed] [Google Scholar]
- Dombrowski JC, Ramchandani M, Dhanireddy S, Harrington RD, Moore A, & Golden MR (2018). The Max Clinic: Medical care designed to engage the hardest-to-reach persons living with HIV in Seattle and King County,Washington. 32(4), 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner EM, Lusted A, Roerecke M, Rehm J, & Fischer B (2014). Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: Systematic review and meta-analyses. Addictive Behaviors, 39(3), 520–531. [DOI] [PubMed] [Google Scholar]
- Gostin LO, Hodge JG Jr., & Noe SA (2017). JAMA, 318(16), 1539–1540. 10.1001/jama.2017.13358 [DOI] [PubMed] [Google Scholar]
- Government of British Columbia. Personal health identification. https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/msp/bc-residents/personal-health-identification.
- Government of British Columbia. (2017). A guideline for the clinical management of opioid use disorder. Retrieved from http://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/bc_oud_guidelines.pdf.
- Government of British Columbia. (2018). Alternative payments program. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/physician-compensation/alternative-payments-program.
- Government of Canada. (2016). Health Canada to propose regulatory change to enable consideration of applications under the special access programme to facilitate treatment of chronic relapsing opioid dependence. Retrieved Jan 21 from https://www.canada.ca/en/health-canada/news/2016/05/health-canada-to-propose-regulatory-change-to-enable-consideration-of-applications-under-the-special-access-programme-to-facilitate-treatment-of-chronic-relapsing-opioid-dependence.html.
- Grambsch PM, & Therneau TM (1994). Proportional hazards tests and diagnostics based on weighted residuals. Biometrika, 81(3), 515–526. 10.2307/2337123 [DOI] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, Marshall BD, Alexander-Scott N, Boss R, & Rich JD (2018). Postincarceration fatal overdoses after implementing medications for addiction treatment in a statewide correctional system. JAMA Psychiatry, 75(4), 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A, Dempsey A, Cousino W, Avery L, Phillips H, Egwim E, & Cheever L (2020). Addressing disparities in the health of persons with HIV attributable to unstable housing in the United States: The role of the Ryan white HIV/AIDS program. PLoS Medicine, 17(3), Article e1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot E, Kouyoumdjian FG, Kiefer L, Madadi P, Gross J, Prevost B, & Persaud N (2016). Drug toxicity deaths after release from incarceration in Ontario, 2006–2013: Review of Coroner's cases. PLoS One, 11(7), Article e0157512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell S, Jaffe J, O’Grady K, Olsen Y, & Schwartz R (2014). Leaving buprenorphine treatment: Patients' reasons for cessation of care. Journal of Substance Abuse Treatment, 46(3), 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Latkin CA, Pu M, Cornelius LJ, Bishai D, Huettner S, & Strathdee SA (2009). Predictors of opiate agonist treatment retention among injection drug users referred from a needle exchange program. Journal of Substance Abuse Treatment, 36(3), 306–312. [DOI] [PubMed] [Google Scholar]
- Health Canada. (2019, May 15). Government of Canada approves new treatment options for opioid use disorder and supports research, treatment and harm reduction projects in Ontario. https://www.canada.ca/en/health-canada/news/2019/05/government-of-canada-approves-new-treatment-options-for-opioid-use-disorder-and-supports-research-treatment-and-harm-reduction-projects-in-ontario.html. In.
- Health Service Executive. (2017). Clinical guidelines for opioid substitution treatment. [Google Scholar]
- Hernan M, & Robins J (2016). 183, 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CE, Palepu A, Farrell S, Gogosis E, O’Brien K, & Hwang SW (2015). Barriers to prescription medication adherence among homeless and vulnerably housed adults in three Canadian cities. Journal of Primary Care & Community Health, 6(3), 154–161. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Ang A, Hillhouse MP, Saxon AJ, Nielsen S, Wakim PG, & Blaine JD (2015). Treatment outcomes in opioid dependent patients with different buprenorphine/naloxone induction dosing patterns and trajectories. The American Journal on Addictions, 24(7), 667–675. 10.1111/ajad.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, & Bigelow GE (2000). A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. The New England Journal of Medicine, 343(18), 1290–1297. 10.1056/NEJM200011023431802 [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, & Fudala PJ (1992). A controlled trial of buprenorphine treatment for opioid dependence. JAMA, 267(20), 2750–2755. [PubMed] [Google Scholar]
- Kouyoumdjian FG, Kiefer L, Wobeser W, Gonzalez A, & Hwang SW (2016). Mortality over 12 years of follow-up in people admitted to provincial custody in Ontario: A retrospective cohort study. CMAJ Open, 4(2), E153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Ali R, White JM, O'Brien S, Wolk S, & Danz C (2003). Buprenorphine versus methadone maintenance therapy: A randomized double-blind trial with 405 opioid-dependent patients. Addiction, 98(4), 441–452. 10.1046/j.1360-0443.2003.00335.x [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2014). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence, 10.1002/14651858.CD002207.pub4 [DOI] [Google Scholar]
- Milloy M-J, Kerr T, Bangsberg DR, Buxton J, Parashar S, Guillemi S, & Wood E (2012). Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting. AIDS Patient Care and STDs, 26(1), 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Public Safety and Solicitor General (PSSG) [creator]. (2018). BC corrections dataset. British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- Nosyk B, MacNab YC, Sun H, Fischer B, Marsh DC, Schechter MT, & Anis AH (2009). Proportional hazards frailty models for recurrent methadone maintenance treatment. American Journal of Epidemiology, 170(6), 783–792. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Marsh DC, Sun H, Schechter MT, & Anis AH (2010). Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. Journal of Substance Abuse Treatment, 39(1), 22–31. 10.1016/j.jsat.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Nosyk B, Min J, Evans E, Li L, Liu L, Lima V, & Montaner J (2015). The effects of opioid substitution treatment and highly active antiretroviral therapy on the cause-specific risk of mortality among HIV-positive people who inject drugs. Clinical Infectious Diseases, 61(7), 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Provincial Health Officer. (2017). BC opioid substitution treatment system performance measures 2014/15 - 2015/16. [Google Scholar]
- Pani PP, Maremmani I, Pirastu R, Tagliamonte A, & Gessa GL (2000). Buprenorphine: A controlled clinical trial in the treatment of opioid dependence. Drug and Alcohol Dependence, 60(1), 39–50. 10.1016/s0376-8716(99)00140-4 [DOI] [PubMed] [Google Scholar]
- Pearce LA, Min JE, Piske M, Zhou H, Homayra F, Slaunwhite A, & Nosyk B (2020). Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: Population based retrospective cohort study. BMJ, 338. 10.1136/bmj.m772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Linzy S, Kreek M, & Adelson M (2008). One-year and cumulative retention as predictors of success in methadone maintenance treatment: A comparison of two clinics in the United States and Israel. Journal of Addictive Diseases, 27(4), 11–25. 10.1080/10550880802324382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perinatal Services BC [creator]. (2018). British Columbia perinatal data registry. British Columbia Ministry of Health; [publisher]. Data Extract. MOH (2018). http://www.health.gov.bc.ca/data/. In. [Google Scholar]
- Peterson R, Gundlapalli AV, Metraux S, Carter ME, Palmer M, Redd A, & Fargo JD (2015). Identifying homelessness among veterans using VA administrative data: Opportunities to expand detection criteria. PLoS One, 10(7), Article e0132664. 10.1371/journal.pone.0132664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, & Holland R (2010). The SUMMIT trial: A field comparison of buprenorphine versus methadone maintenance treatment. Journal of Substance Abuse Treatment, 39(4), 340–352. 10.1016/j.jsat.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Piske M, Thomson T, Krebs E, Hongdilokkul N, Bruneau J, Greenland S, & Nosyk B (2020). Comparative effectiveness of buprenorphine-naloxone versus methadone for treatment of opioid use disorder: a population-based observational study protocol in British Columbia, Canada. BMJ Open, 10, e036102. 10.1136/bmjopen-2019-036102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SL, Copeland AL, Kopack AM, Herschman PL, & Polukhina N (2014). A naturalistic comparison of the effectiveness of methadone and two sublingual formulations of buprenorphine on maintenance treatment outcomes: Findings from a retrospective multisite study. Experimental and Clinical Psychopharmacology, 22, 424–433. 10.1037/a0037550 [DOI] [PubMed] [Google Scholar]
- Quello SB, Brady KT, & Sonne SC (2005). Mood disorders and substance use disorder: A complex comorbidity. Science & Practice Perspectives, 3(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RachBeisel J, Scott J, & Dixon L (1999). Co-occurring severe mental illness and substance use disorders: A review of recent research. Psychiatric Services, 50(11), 1427–1434. [DOI] [PubMed] [Google Scholar]
- Reitano J (2017). Adult correctional statistics in Canada, 2015/2016. ON: Statistics Canada Ottawa. [Google Scholar]
- Sajatovic M, Valenstein M, Blow FC, Ganoczy D, & Ignacio RV (2006). Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disorders, 8(3), 232–241. [DOI] [PubMed] [Google Scholar]
- Sargent DJ (1998). A general framework for random effects survival analysis in the cox proportional hazards setting. Biometrics, 54(4), 1486–1497. [PubMed] [Google Scholar]
- Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, & Neylan TC (2012). Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA, 307(9), 940–947. [DOI] [PubMed] [Google Scholar]
- Socías ME, Dong H, Wood E, Brar R, Richardson L, Hayashi K, & Milloy M (2020). Trajectories of retention in opioid agonist therapy in a Canadian setting. International Journal of Drug Policy, 77, Article 102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, & Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ, 357, Article j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Zhang L, Unützer J, & Wells KB (2006). Association between mental health disorders, problem drug use, and regular prescription opioid use. Archives of Internal Medicine, 166(19), 2087–2093. [DOI] [PubMed] [Google Scholar]
- The College of Family Physicians of Canada. (2016). Position statement on health care delivery. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiv90rdy4_tAhXRHjQIHeiyBfoQFjAGegQIBRAC&url=https%3A%2F%2Fwww.cfpc.ca%2FuploadedFiles%2FDirectories%2FCommittees_List%2FHealth%252520Care%252520Delivery_EN_Prison%252520Health.pdf&usg=AOvVawl3MAOiK13aOOENqOc5JGLz.
- Therneau TM (2021). Package 'survival'. In. [Google Scholar]
- Vancouver Coastal Health, & Providence Health Care.. (2015). A guideline for clinical management of opioid addiction. In. [Google Scholar]
- World Health Organization. (2014). Prisons and health. [Google Scholar]
- Yukhnenko D, Sridhar S, & Fazel S (2019). A systematic review of criminal recidivism rates worldwide: 3-Year update. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.