Abstract
The protozoan parasite Entamoeba histolytica causes extensive morbidity and mortality through intestinal infection and amebic liver abscess. Here we show that immunization of gerbils with a single keyhole limpet hemocyanin-coupled 25-mer peptide derived from the 170-kDa subunit of the E. histolytica galactose-binding adhesin is sufficient to confer substantial protection against experimentally induced amebic liver abscesses. Vaccination provided total protection in 5 of 15 immunized gerbils, and abscesses were significantly smaller (P < 0.01) in the remaining vaccinated animals. The degree of protection correlated with the titer of antibodies to the peptide, and results of passive transfer experiments performed with SCID mice were consistent with a role for antibodies in protection. In addition, parenteral or oral vaccination of gerbils with 13-amino-acid subfragments of the peptide N-terminally fused to the B subunit of cholera toxin also significantly inhibited liver abscess formation (P < 0.05). These data indicate that small peptides derived from the galactose-binding adhesin administered by the parenteral or oral route can provide protection against amebic liver abscess and should be considered as components of a subunit vaccine against invasive amoebiasis.
The enteric protozoan Entamoeba histolytica is one of the leading causes of death due to a parasite. It is responsible for an estimated 40 to 50 million cases of dysentery and liver abscess each year, mainly in tropical and subtropical countries (22). As developing countries cannot afford the improvements in sanitation that might prevent the fecal-oral spread of the parasite, amebiasis is presently poorly controlled. Since humans are the only relevant host for E. histolytica it is suggested that an effective vaccination program could potentially eradicate amoebiasis.
Up to now, the development of amoebiasis vaccines is still in its infancy. However, a number of ameba proteins have already been identified as potential vaccine candidates, as these molecules were able to effectively inhibit or prevent amebic liver abscess formation in artificially infected rodents (16, 20, 24). One of these proteins is the galactose- and N-acetylgalactosamine-inhibitable lectin, a membrane-associated surface receptor of the amebae (for a review, see reference 13). This molecule has an unusual complex structure, as it consists of two disulfide-linked light and heavy subunits of 35 and 170 kDa, respectively, which both are inserted into the membrane by separate anchors. In addition, the heavy subunit has an extraordinary high cysteine content of up to 12% within the C-terminal two-thirds of its extracellular region. The lectin appears to play a key role for E. histolytica pathogenicity, as it mediates ameba adherence to host cells, a process which is critical in the pathogenesis of intestinal disease and amebic liver abscess, since amebae efficiently destroy target cells in a strict contact-dependent manner (18).
The purified native galactose- and N-acetylgalactosamine-binding lectin has been used to vaccinate gerbils to protect them against amebic liver abscess (16). Although vaccination was protective in most animals, in others there was evidence for a significant increase in liver abscess size, suggesting that the immune response to the lectin could also exacerbate disease. Recently, by immunization of gerbils using recombinantly expressed sections of the heavy subunit, we have shown that exacerbation of disease is linked to antibodies that bind to the N-terminal domain of the lectin, whereas protection is mainly restricted to the development of an antibody response against a C-terminal cysteine-rich section (170CR2) of the molecule (12). The importance of antibodies for the different outcomes was confirmed by passive transfer experiments with SCID mice. Moreover, indirect evidence suggested that the main protective epitope of 170CR2 is located within a region comprising 25 amino acid residues only (170CR2-PEP5), as in contrast to animals that failed to be protected after vaccination with 170CR2, all of the protected animals had significant titers of antibody to this peptide. Since protection-conferring peptides are of considerable interest for the design of an improved subunit vaccine against invasive amebiasis, we further investigated the vaccine potential of 170CR2-PEP5. Here we report on the results of parenteral vaccination with synthetic 170CR2-PEP5 chemically coupled to keyhole limpet hemocyanin (KLH) as well as of parenteral and oral vaccination with recombinant 170CR2-PEP5-derived peptides fused to the B subunit of cholera toxin (CtxB).
(This article is based in part on doctoral studies carried out by Fareed Khajawa at the Faculty of Medicine, University of Hamburg.)
MATERIALS AND METHODS
Synthetic peptide and KLH-peptide conjugate.
Synthesis of 170CR2-PEP5, a 25-mer peptide derived from the cysteine-rich region of the 170-kDa ameba lectin comprising the sequence NH2-VECASTVCQNDNSCPIIADVEKCNQ, and coupling of the peptide to KLH by glutaraldehyde was performed by Pacemaker Affinity Research (Exeter, United Kingdom).
Expression of CtxB–170CR2-PEP5 fusion proteins.
Complementary sets of oligonucleotides were synthesized encoding either total 170CR2-PEP5 (T), or three overlapping fragments of the peptide designated N, M, or C, and representing the amino-terminal stretch of 13 amino acid residues (NH2-VECASTVCQNDNS), an internal stretch of 15 amino acid residues (NH2-TVCQNDNSCPIIADV), and the carboxy-terminal stretch of 13 amino acid residues (NH2-SCPIIADVEKCNQ), respectively. The oligonucleotides contained EcoRI or NdeI restriction sites, allowing ligation of respective double-stranded fragments into plasmid vector pVA 1542 (5, 6). Subsequently, the resulting ctxb gene fusions (ctxb fused with the T peptide [ctxb+t], ctxb+n, ctxb+m, and ctxb+c) as well as ctxb alone were cloned as EcoRI-BamHI fragments into pINIIIompA2 expression vector (8). Escherichia coli HB101 cells transformed with pINIIIompA2 expression plasmid containing ctxb or the various ctxb gene fusions were grown at 37°C (ctxb, ctxb+n, and ctxb+m) or at 28°C (ctxb+t and ctxb+c) to an optical density (OD) of 0.9 (at 660 nm), and then isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM and cells were grown for an additional 4 and 20 h, respectively. The cells of a 1-liter culture were sedimented and resuspended in 150 ml of sonication buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5]). Subsequently, the bacteria were ultrasonicated on ice at 30 W for 15 min and centrifuged at 10,000 × g, and the supernatant was used for purification of recombinant CtxB or CtxB fusion peptides.
Purification of recombinant proteins.
Purification was performed by affinity chromatography using chicken anti-CtxB antibodies coupled to CnBr-activated Sepharose 4B. Anti-CtxB antibodies were generated by subcutaneous immunization of a chicken with 100 μg of CtxB (Sigma, St. Louis, Mo.) emulsified in complete Freund's adjuvants followed by two booster immunizations after 4 and 7 weeks with the same amount of protein emulsified in incomplete Freund's adjuvants. Four weeks after the final booster immunization, eggs were collected and the antibodies were isolated from the egg yolk according to a standard protocol (17). Ten milligrams of purified chicken anti-CtxB antibodies were coupled to 1 ml of swollen Sepharose gel beads according to the instructions of the manufacturer (Pharmacia). The 10,000 × g supernatant of a 1-liter induced bacterial culture was mixed with 8 ml of anti-CtxB antibodies coupled to Sepharose. The solution was gently mixed at 4°C overnight and then transferred to a 1.5-by-15.0-cm column. Subsequently, the column was washed with sonication buffer until the eluate was free of protein as determined by measuring the A280. Bound proteins were eluted from the column with 1 M glycine, pH 2.5. Eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the presence and purity of recombinant proteins. Fractions of high purity, as revealed by reversed-phase high-performance liquid chromatography were pooled, frozen at −70°C, and lyophilized. For intraperitoneal and oral immunization, proteins were resuspended in phosphate-buffered saline (PBS) and 0.2 M sodium bicarbonate, respectively. The identity of purified proteins was confirmed by Western blotting and N-terminal sequencing using a gas-phase 473A protein sequencer (Applied Biosystems, Foster City, Calif.).
GM1-binding assay and ELISA for detection of anti-CtxB and anti-170CR2-PEP5 antibodies.
Binding of CtxB to immobilized monoganglioside GM1 was determined by enzyme-linked immunosorbent assay (ELISA) using 96-well microtiter plates coated with 2 μg of GM1 (Sigma) in PBS, pH 7.4, at 4°C overnight. After washing with PBS, the wells were blocked with PBS supplemented with 20% fetal calf serum (FCS) at 37°C for 90 min and washed again with PBS, and duplicate samples of serial dilutions of CtxB or CtxB chimeras in PBS were added and incubated at 37°C for 2 h. Following another PBS wash, goat anti-CtxB antibodies (Calbiochem, La Jolla, Calif.) diluted 1:3,000 in PBS supplemented with 20% FCS and 0.05% Tween 20 were added to the wells and incubated at room temperature for 1.5 h at 37°C. Following a PBS wash, peroxidase-labeled rabbit anti-goat immunoglobulin G (IgG) antibodies (DAKO), diluted 1:1,500 in PBS containing 10% FCS and 0.05% Tween 20, were added to the plates, which were then incubated at room temperature for 12.5 h at 37°C. o-Phenylendiamine was added to each well, the reaction was stopped with 2 M H2SO4 after 5 min, and the A495 was measured using an automatic plate reader (MR 5000; Dynatech, Labs Inc., Chantilly, Va.).
For the detection of serum antibodies to CtxB or 170CR2-PEP5, microtiter plates were coated with 5 μg of protein or peptide per ml. The detection of anti-CtxB and anti-170CR2-PEP5 IgA antibodies in gerbil stool samples was performed according to published procedures (23) with minor modifications. Four freshly collected stool pellets were dissolved in 1 ml of PBS containing 0.025 M EDTA and soybean trypsin inhibitor (0.025 mg ml−1; Sigma) and vortexed until a homogenous suspension was achieved. Subsequently, the suspension was centrifuged at 270 × g for 10 min, and the supernatant was collected and centrifuged again at 17,000 × g for 10 min. Finally, 20 μl of 100 mM phenylmethylsulfon (Sigma) was added to 1 ml of the supernatant (centrifuged at 17,000 × g) and stored at −20°C until use. Microtiter plates (96-well; Greiner) were coated overnight at room temperature with CtxB diluted in 0.1 M carbonate buffer, pH 9.5, to a final concentration of 10 μg ml−1. Plates were washed three times with PBS containing 0.05% Tween 20 (PBS-T) and then blocked by incubation at room temperature for 2 h with PBS supplemented with 20% FCS. After washing with PBS-T, 100 μl of stool supernatant diluted 1:4 with PBS-T was added to each well and incubated overnight at room temperature. For the detection of bound IgA, plates were incubated overnight at room temperature with biotin-labeled goat anti-mouse IgA (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.) diluted 1:500 in PBS-T. Following three washes with PBS-T, horseradish peroxidase-streptavidin (Boehringer) in PBS-T was added to each well and incubated for 2 h at room temperature. Plates were washed as described above. The 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Sigma) was then added to each well, and plates were read at an A405 as described above.
Cultivation of parasites.
Trophozoites of the E. histolytica isolate were grown axenically in TYI-S-33 medium (7). Virulence was maintained by gerbil liver passage once per month.
Immunization of rabbit and gerbils.
A New Zealand White rabbit was immunized subcutaneously with 250 μg of KLH-coupled 170CR2-PEP5 emulsified in Freund's adjuvants. Booster immunizations were performed with the same amount of protein using incomplete Freund's adjuvants until an antibody titer of 1:1,000 against the 170-kDa lectin was obtained as determined by ELISA.
Adult female gerbils (Meriones unguiculatus) were immunized intraperitoneally or orally. Intraperitoneal immunization was performed with 50 μg of KLH, KLH-coupled 170CR2-PEP5, or recombinant proteins emulsified with complete Freund's adjuvants followed by two booster immunizations after 14 and 28 days using the same amount of protein emulsified in incomplete Freund's adjuvants. Oral immunization was performed with 100 μg of recombinant proteins diluted in 0.2 M sodium bicarbonate. The antigens were administered intragastrically using a 21-gauge ball-tipped gavage needle. Booster immunizations were performed on days 14 and 28, and in one group of animals an additional oral booster was given on day 90.
Induction of amebic liver abscess in SCID mice and gerbils.
SCID mice were passively immunized by the administration of 200 μl of rabbit immune or preimmune serum intraperitoneally 24 h before challenge. Passively immunized SCID mice and vaccinated gerbils were challenged with the direct hepatic inoculation of 106 or 105 virulent E. histolytica trophozoites, respectively, according to the previously described methods (2, 15). Seven days later, animals were killed, the liver was entirely removed and sectioned, and the weight of abscesses relative to total liver weight was determined.
RESULTS
Vaccine efficacy of 170CR2-PEP5 following active or passive immunization.
Our previous immunization studies in rodents provided indirect evidence that antibodies to a 25-mer peptide (170CR2-PEP5), derived from the cysteine-rich region of the 170-kDa E. histolytica galactose-inhibitable lectin, confer substantial protection against invasive amebiasis. In order to confirm this finding and to assess more directly the vaccine potential of 170CR2-PEP5, a synthetic version of the peptide was chemically coupled to KLH, emulsified in complete or incomplete Freund's adjuvants, and used to immunize adult female gerbils via intraperitoneal injection. Gerbils immunized with KLH emulsified in Freund's adjuvants served as controls. Two independent trials were performed, each comprising 5 or 10 animals. Each animal received 50 μg of KLH or KLH-coupled peptide at day 0, 14, and 28. Subsequently, specific antibodies were determined, and the results indicated that none of the controls but all of the animals immunized with the peptide developed a significant serum IgG antibody titer to recombinant 170CR2 (data not shown). Challenge of these animals by direct liver inoculation of 105 virulent E. histolytica trophozoites showed clear differences between the two groups. As shown in Table 1, all of the 10 sham-immunized controls developed liver abscesses, whereas immunization with 170CR2-PEP5 revealed total protection against liver abscess formation in 5 out of 15 (33.3%) gerbils, as well as significant reduction in the size of abscesses in the remaining animals (P < 0.001). To validate the role of antibodies for the observed protection, passive immunization studies with SCID mice were performed. A polyclonal rabbit antiserum against KLH-coupled 170CR2-PEP5 was transferred into SCID mice 24 h before intrahepatic challenge with 106 virulent E. histolytica trophozoites. Endpoint titration of the antiserum revealed reactivity to 170CR2-PEP5, to recombinant 170CR2, and to purified native ameba lectin at dilutions of up to 1:13,000, 1:800, and 1:1,000, respectively. As shown in Table 1, all of the six infected control animals developed amebic abscesses. In contrast, four out of six (66.7%) of the 170CR2-PEP5-immunized animals were completely protected and the remaining two had very small abscesses, which significantly differed in size from those of the controls (P < 0.004).
TABLE 1.
Protection from amebic liver abscess by active or passive immunization with 170CR2-PEP5 coupled to KLH
| Immunization route | Vaccine group | No. of animals with liver abscess/no. of animals challenged | % Liver abscessed in nonprotected animals (mean ± SD) |
|---|---|---|---|
| Activea | KLH | 10/10 | 8.5 ± 3.2 |
| KLH–170CR2-PEP5 | 10/15 | 2.2 ± 2.3c | |
| Passiveb | Preimmune | 6/6 | 13.8 ± 2.25 |
| Anti-KLH–170CR2-PEP5 | 2/6d | 1.2 ± 0.04e |
Gerbils.
SCID mice.
P < 0.001.
P < 0.03.
P < 0.004.
Expression, purification, and characterization of CtxB–170CR2-PEP5 chimeras.
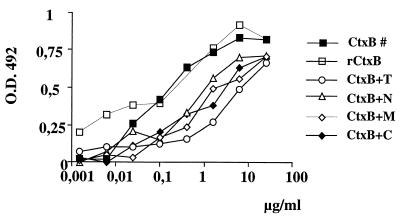
The result that antibodies to the 170CR2-PEP5 peptide were sufficient to confer substantial protection against invasive amoebiasis offered the possibility of generating an oral amoebiasis vaccine based on fusion of the peptide to CtxB. In general, CtxB is taken up from the intestine via its binding to ganglioside GM1 and induces substantial serum IgG and mucosal IgA responses. CtxB can be modified by exchanging the first 10 to 20 N-terminal amino acid residues without the loss of GM1-binding activity. Accordingly, we genetically engineered the gene for CtxB to recombinantly express in E. coli either CtxB alone or one of four different CtxB chimeras containing (i) total 170CR2-PEP5 (CtxB+T), (ii) an N-terminal stretch of 13 amino acid residues of 170CR2-PEP5 (CtxB+N), (iii) a stretch of 15 amino acid residues from the central (middle) part of the peptide (CtxB+M), or (iv) a C-terminal stretch of 13 amino acid residues (CtxB+C). The various recombinant polypeptides were purified from E. coli lysates by affinity chromatography using chicken anti-CtxB antibodies. Identity of each of the purified polypeptides was confirmed by protein sequencing and by Western blotting using rabbit anti-170CR2 as well as goat anti-CtxB antisera. All of the purified recombinant proteins were found to bind to ganglioside GM1. However, depending on the length of the peptide fused to CtxB, binding activity differed between the various molecules. The highest binding activity was found for commercially available, native CtxB and for purified, recombinant CtxB, whereas CtxB+T exhibited the lowest binding activity (Fig. 1).
FIG. 1.
Comparison of binding of native CtxB, recombinant CtxB (rCtxB) and rCtxB-fusion proteins with GM1 ganglioside as measured by ELISA. Serial dilutions of the various proteins were incubated with immobilized GM1 ganglioside bound to an ELISA plate. Binding of proteins was assessed by goat anti-CtxB antibodies and visualized by peroxidase-labeled rabbit anti-goat antibodies, and the OD at A492 was measured.
Immunogenicity and vaccine efficacy of recombinant CtxB–170CR2-PEP5 chimeras after intraperitoneal immunization.
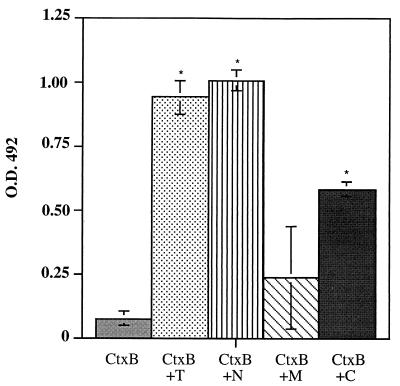
In the first line of experiments, the various CtxB–170CR2-PEP5 fusion proteins were used to immunize gerbils by intraperitoneal injection, in order to determine whether these polypeptides could induce antibody responses to the ameba lectin and whether these antibody responses could confer protection against amebic liver abscess formation. After administration of three doses of 50 μg each of purified, recombinant protein, all animals developed antibodies against CtxB. None of the gerbils immunized with recombinant CtxB alone had antibodies to 170CR2-PEP5. In contrast, sera of nearly all animals immunized with the various recombinant CtxB-170CR2-PEP5 fusion proteins reacted with the ameba polypeptide (Fig. 2). Despite these positive antibody responses, there were significant differences in the ability of each of the peptide vaccines to prevent liver abscess formation in gerbils after intrahepatic challenge with E. histolytica trophozoites (Table 2). All animals immunized with CtxB, CtxB+T, or CtxB+M developed abscesses, whereas gerbils immunized with CtxB+N and CtxB+C showed total protection in 4 out of 11 (36.4%) and 6 out of 11 (54.5%), respectively. Compared to CtxB controls, immunization with each of the various CtxB fusion proteins revealed reduction in size of abscesses. However, the reduction in abscess size was statistically significant only in those gerbils immunized with CtxB+C (P < 0.006).
FIG. 2.
Gerbils intraperitoneally immunized with CtxB-fusion proteins have anti-170CR2-PEP5 serum IgG antibodies. Shown are the IgG anti-170CR2-PEP5 responses in serum samples from CtxB- and CtxB fusion protein-immunized gerbils at day 7 after the final boost as measured by ELISA. Serum samples were measured at dilutions of 1:200. Bars show the mean values ± the standard deviations (error bars) from the respective groups of gerbils as shown in Table 2. Asterisks indicate that the mean OD of CtxB fusion peptide-vaccinated animals is significantly different from the value of CtxB-vaccinated gerbils (P < 0.05).
TABLE 2.
Protection of gerbils from amebic liver abscess by intraperitoneal or oral vaccination with CtxB–170CR2-PEP5 chimeras
| Vaccine route and group | No. of gerbils with liver abscess/no. of gerbils challenged | % Liver abscessed (mean ± SD) |
|---|---|---|
| Intraperitoneal | ||
| CtxB | 9/9 | 12.1 ± 11.2 |
| CtxB+T | 6/6 | 7.4 ± 3.6 |
| CtxB+N | 7/11 | 4.7 ± 5.5 |
| CtxB+M | 5/5 | 7.0 ± 2.0 |
| CtxB+C | 5/11 | 1.4 ± 1.9a |
| Oral | ||
| Trial 1b | ||
| CtxB | 6/6 | 16.0 ± 6.9 |
| CtxB+N | 6/6 | 4.4 ± 2.1 |
| CtxB+C | 6/6 | 6.8 ± 2.6 |
| Trial 2c | ||
| CtxB | 10/10 | 9.5 ± 4.5 |
| CtxB+N | 8/10 | 3.3 ± 3.3d |
| CtxB+C | 7/10 | 2.4 ± 2.3e |
P < 0.006.
Immunization on day 0, 14, and 28.
Immunization on day 0, 14, 28, and 90.
P < 0.006.
P < 0.002.
Immunogenicity and vaccine efficacy of recombinant CtxB–170CR2-PEP5 chimeras after oral vaccination.
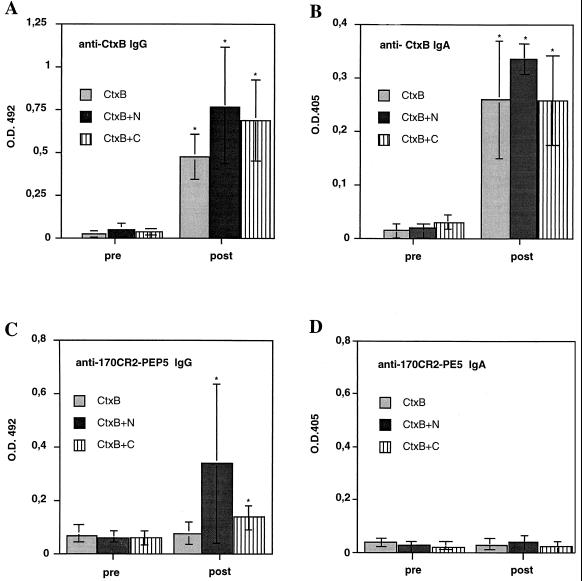
In a second line of experiments, the vaccine potential of orally administered CtxB fusion proteins was investigated. However, only CtxB+N and CtxB+C, the two polypeptides that had revealed some degree of protection after intraperitoneal application, were considered. Animals orally immunized with recombinant CtxB served as controls. Two vaccination schemes were performed. In the first protocol, gerbils were fed three times with 100 μg of the respective antigen at day 0, 14, and 28. In the second protocol, animals received the same amount of antigen but four times at day 0, 14, 28, and 90. Compared to respective pre-immune sera, all animals developed significant serum IgG-antibody titers to CtxB (Fig. 3A). In addition, all of the CtxB+N- and CtxB+C-immunized gerbils but none of the CtxB controls had serum antibodies to 170CR2-PEP5 (Fig. 3C). Although antibody responses to the ameba peptide differed substantially between the various animals, in general, antibody titers to the peptide were higher in CtxB+N-vaccinated gerbils. However, compared to intraperitoneal immunization, serum antibody response to 170CR2-PEP5 was weaker in orally vaccinated gerbils. Interestingly, despite the development of substantial stool IgA antibody responses to CtxB (Fig. 3B), none of the orally immunized animals had measurable IgA antibodies to the ameba peptide (Fig. 3D). Fourteen days after the final booster immunization, orally vaccinated gerbils were challenged by intrahepatic inoculation of E. histolytica trophozoites. Compared to CtxB controls, all CtxB+C- and CtxB+N-immunized gerbils revealed significant reduction in the size of liver abscesses irrespective of the immunization scheme used (Table 2). However, total protection against liver abscess formation was only seen in those gerbils that had received an additional booster immunization at day 90. In addition, there was a direct correlation between antibody titers and degree of protection (r = 0.64). Totally protected animals showed significantly higher titers of antibodies to 170CR2-PEP5 than those vaccinated with the same antigen that developed amebic liver abscesses (P < 0.05).
FIG. 3.
Gerbils orally immunized with CtxB fusion proteins have serum IgG to CtxB (A) and 170CR2-PEP5 (C) as well as stool IgA to CtxB (B) but not to 170CR2-PEP5 (D). Shown are the IgG and IgA anti-CtxB and anti-170CR2-PEP5 responses in serum and stool samples, respectively, from CtxB- and CtxB fusion protein-immunized gerbils before (pre) and 7 days after the final boost (post) as measured by ELISA. Serum samples were measured at dilutions of 1:50, and stool samples were measured at dilutions of 1:4. Bars show the mean values ± the standard deviations (error bars) from the respective groups of gerbils as shown in Table 3. Asterisks indicate that the mean OD of vaccinated animals is significantly different from the value of the respective preimmune sera (P < 0.05).
DISCUSSION
The identification of epitopes inducing protective immune responses is a critical goal in the development of vaccines against a number of pathogens. The objective of this study was to determine whether immunization with a 25-mer peptide (170CR2-PEP5) derived from the 170-kDa subunit of the E. histolytica galactose-inhibitable surface lectin is sufficient to confer protection against invasive amoebiasis using rodent models for amebic liver abscess. Active immunization of gerbils with the KLH-coupled peptide revealed total protection against liver abscess formation in 33% of animals, and the remaining animals had significantly smaller abscesses than did the controls. It is important to note that none of the vaccinated animals developed larger abscesses than did the control gerbils. This contrasts with vaccination studies using the purified native lectin (18) or vaccination with a recombinantly derived peptide from the N-terminal third of the molecule, where abscesses were significantly larger in vaccine failures than in controls (12). Protection was most likely due to anti-170CR2-PEP5 antibodies, as passive transfer of those antibodies into SCID mice provided protection equivalent to that seen by active immunization of the gerbils. These findings are in full agreement with our previous studies, in which immunization with the 115-amino-acid 170CR2 lectin fragment containing the 170CR2-PEP5 sequence provided antibody-mediated protection against amebic liver abscess in gerbils (12). Immunizing with the 170CR2-PEP5 sequence alone did not improve vaccine efficacy, however. This may relate to reduced immunogenicity of the 170CR2-PEP5 fragment or the inability of antibodies raised against the 170CR2-PEP peptide to recognize conformational epitopes present in the full-length lectin. The latter point is consistent with our finding that rabbit antibodies raised by immunization with KLH-coupled 170CR2-PEP5 strongly reacted with the peptide (titers of >1:10,000), while the same antibodies showed maximum titers of 1:1,000 against the purified, native ameba lectin.
The finding that vaccination with a 25-mer peptide conferred significant protection against invasive amoebiasis encouraged us to investigate the vaccine potential of the peptide fused to CtxB. The CtxB subunit mediates holotoxin binding to cell surface ganglioside GM1 (10) but lacks toxin activity. Oral immunization with a given antigen conjugated to CtxB produces greater immune responses than does administration of the antigen without CtxB, coadministration of antigen and CtxB, or oral administration of an antigen fused to a different carrier protein (1, 3, 4, 9, 11, 14, 19, 21). We produced four different fusion proteins containing the entire 25-amino-acid 170CR2-PEP5 sequence fused to CtxB (CtxB+T), the N-terminal 13 peptides of 170CR2-PEP5 fused to CtxB (CtxB+N), the C-terminal 13 peptides fused to CtxB (CtxB+C), and the central 15 amino acids fused to CtxB (CtxB+M). When administered by the oral or parenteral route, each fusion protein induced a significant systemic IgG response to the 170CR2-PEP5 peptide. Despite the induction of relatively high antibody titers to the parent peptide, parenteral immunization with CtxB+T failed to protect gerbils against liver abscess formation. This suggests that the fusion of the full-length peptide to CtxB may have resulted in conformational changes which reduced its ability to induce antibodies capable of conferring protection against amebic liver abscess. In contrast, immunization with either the CtxB+N peptide or the CtxB+C peptide resulted in substantial inhibition of abscess formation. The highest degree of protection was obtained after parenteral administration of the fusion protein containing CtxB fused to the C-terminal 13 amino acids, suggesting that antibodies to this part of the peptide may be critical in inhibiting amebic liver abscess formation. Oral vaccination with either CtxB+N or CtxB+C also proved protective against amebic liver abscess, with protection correlated with the level of antibody titers to the 170CR2-PEP5 peptide. Disappointingly, we did not detect a significant IgA anti-170CR2-PEP5 response in orally vaccinated gerbils. Coadministration of native cholera toxin with the fusion proteins might have increased the mucosal immune response (3, 21). The absence of a consistent rodent model for intestinal amoebiasis precluded studies of whether oral or parenteral vaccination with the peptides could provide protection against intestinal amoebiasis.
In summary, immunization of gerbils with a single KLH-coupled 25-mer peptide derived from the 170-kDa subunit of the E. histolytica galactose-inhibitable lectin was sufficient to confer substantial protection against experimentally induced amebic liver abscesses. The degree of protection correlated with the titer of antibodies to the peptide, and passive transfer experiments performed with SCID mice were consistent with a role for antibodies in the observed protection. We were able to further define protective epitopes by demonstrating that parenteral or oral vaccination with specific 13-mer subfragments of the peptide fused to CtxB also inhibited amebic liver abscess formation. Our data indicate that the 170CR2-PEP5 peptide, and specifically its C-terminal 13 amino acids, should be considered for inclusion in a subunit vaccine against invasive amebiasis.
ACKNOWLEDGMENTS
We thank Bertram Müller-Myhsok for statistical analysis.
This work was supported by the Deutsche Forschungsgemeinschaft (TA 110/5-1), and by NIH grant AI30084 to S.L.S. S. L. Stanley, Jr., is a Burroughs Wellcome Scholar in Molecular Parasitology.
REFERENCES
- 1.Bessen D, Fischetti V. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988;56:2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cieslak P R, Virgin H W, Stanley S L. A severe combined immunodeficient (SCID) mouse model for infection with Entamoeba histolytica. J Exp Med. 1992;176:1605–1609. doi: 10.1084/jem.176.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czerkinsky C, Russel M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dertzbaugh M T, Elson C O. Comparative effectiveness of the cholera toxin B subunit and alkaline phosphatase as carriers for oral vaccines. Infect Immun. 1993;61:48–55. doi: 10.1128/iai.61.1.48-55.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dertzbaugh M T, Marcina F L. Plasmid vectors for constructing translational fusions to the B subunit of cholera toxin. Gene. 1989;82:335–342. doi: 10.1016/0378-1119(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 6.Dertzbaugh M T, Peterson D L, Marcina F L. Cholera toxin-B-subunit gene fusion: structural and functional analysis of the chimeric protein. Infect Immun. 1990;58:70–79. doi: 10.1128/iai.58.1.70-79.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 8.Ghrayeb J, Kimura H, Takahara M, Hsiung H, Masui Y, Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984;3:2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez R A, Sanchez J, Holmgren J, Lopez S, Arias C F. Immunological characterization of a rotavirus-neutralizing epitope fused to the cholera toxin B subunit. Gene. 1993;133:227–232. doi: 10.1016/0378-1119(93)90643-h. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren J. Action of cholera toxin and the prevention and treatment of cholera. Nature. 1981;257:797–798. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit–whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotter H, Zhang T, Seydel K B, Stanley S L, Jr, Tannich E. Identification of an epitope on the Entamoeba histolytica 170-kD lectin conferring antibody-mediated protection against invasive amebiasis. J Exp Med. 1997;185:1793–1801. doi: 10.1084/jem.185.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCoy J J, Mann B J, Petri W A., Jr Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect Immun. 1994;62:3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenzie S J, Harsey J F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984;133:1818–1824. [PubMed] [Google Scholar]
- 15.Meerovitch E, Chadee K. In vivo models of immunity in amebiasis. In: Ravdin J I, editor. Amebiasis. Human infection by Entamoeba histolytica. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 425–437. [Google Scholar]
- 16.Petri W A, Jr, Ravdin J I. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun. 1991;59:97–101. doi: 10.1128/iai.59.1.97-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polson A, Coetzer T, Kruger J, von Maltzahn E, van der Merwe K J. Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunol Investig. 1985;14:323–327. doi: 10.3109/08820138509022667. [DOI] [PubMed] [Google Scholar]
- 18.Ravdin J I, Guerrant R L. The role of adherence in the cytopathic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human red blood cells. J Clin Investig. 1981;86:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russel M W, Wu H. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to cholera toxin B subunit. Infect Immun. 1991;59:4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soong C-J G, Torian B E, Abd-Alla M D, Jackson T F H G, Gatharim V, Ravdin J I. Protection of gerbils from amebic liver abscess by immunization with recombinant Entamoeba histolytica 29-kilodaltan antigen. Infect Immun. 1995;63:472–477. doi: 10.1128/iai.63.2.472-477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura S, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 22.Walsh J A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Cieslak P R, Stanley S L., Jr Protection of gerbils from amebic liver abscess by immunization with a recombinant Entamoeba histolytica antigen. Infect Immun. 1994;62:1166–1170. doi: 10.1128/iai.62.4.1166-1170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Stanley S L., Jr Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect Immun. 1994;62:2605–2608. doi: 10.1128/iai.62.6.2605-2608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]