Abstract
Background
In the 2022 mpox (monkeypox) outbreak, 79,000 global cases have been reported. Yet, limited dermatologic data have been published regarding lesion morphology and progression.
Objective
The objective of this study was to characterize skin lesion morphology, symptomatology, and outcomes of mpox infection over time.
Methods
The American Academy of Dermatology/International League of Dermatological Societies Dermatology COVID-19, Mpox, and Emerging Infections Registry captured deidentified patient cases of mpox entered by health care professionals.
Results
From August 4 to November 13, 2022, 101 cases from 13 countries were entered, primarily by dermatologists (92%). Thirty-nine percent had fewer than 5 lesions. In 54% of cases, skin lesions were the first sign of infection. In the first 1-5 days of infection, papules (36%), vesicles (17%), and pustules (20%) predominated. By days 6-10, pustules (36%) were most common, followed by erosions/ulcers (27%) and crusts/scabs (24%). Crusts/scabs were the predominant morphology after day 11. Ten cases of morbilliform rash were reported. Scarring occurred in 13% of the cases.
Limitations
Registry-reported data cannot address incidence. There is a potential reporting bias from the predilection to report cases with greater clinical severity.
Discussion
These findings highlight differences in skin findings compared to historical outbreaks, notably the presence of skin lesions prior to systemic symptoms and low overall lesion counts. Scarring emerged as a major possible sequela.
Key words: general dermatology, global health, immunocompromised, infectious disease, international health, medical dermatology, monkeypox, mpox, skin lesions, vaccine, viral infection, virus
Capsule Summary.
-
•
This international, registry-based study highlights variations in clinical course and morphologic lesion progression during the 2022 mpox (monkeypox) outbreak in contrast to prior outbreaks.
-
•
Early detection and treatment are crucial for minimizing disease burden. Dermatologists play key roles in detection, especially given novel morphology and progression noted during this outbreak.
Introduction
The monkeypox virus (mpox) is a zoonotic, double-stranded DNA virus of the Orthopoxvirus genus. Mpox infection has similar skin manifestations to smallpox but is less severe, self-limited with lower case-fatality rates.1 Prior to May 2022, mpox was described as a zoonotic and traveler-associated infection, with few cases reported outside endemic countries.2 Over 79,000 cases of mpox infections in 103 nonendemic countries have been reported since May 2022.3 , 4 In July 2022, the World Health Organization declared the ongoing outbreak a “Public Health Emergency of International Concern.”5
Human-to-human transmission occurs via direct contact with skin lesions, respiratory droplets, and, less commonly, through fomites.6 Mpox viral DNA has been detected in a replication-competent form that may support sexual transmission, though not yet confirmed.7 , 8 The incubation period of mpox ranges from 5 to 24 days.7 Additional evidence of possible presymptomatic transmission has also emerged.9 , 10
Classic mpox begins with a prodrome of fever, fatigue, and lymphadenopathy, followed by skin lesions, predominantly on the face.7 Lesions typically progress from umbilicated papule to pustule to crusted scab prior to re-epithelialization and resolution.11 Abscesses and mucocutaneous lesions were rarely reported prior to the current outbreak.
Studies from the 2022 mpox outbreak have reported notable distinctions in transmission dynamics and clinical presentation in nonendemic countries.9 , 10 The previously reported prodrome of fever/lymphadenopathy/myalgia has not always followed the expected timeline and may appear simultaneously or after cutaneous symptoms.6 , 12 , 13 Skin lesions have been noted commonly in the anogenital region, as frequently as 73%.6 Instead of lesions traveling in uniform crops, new lesions developed well into the disease course.12 Novel symptoms during this outbreak included rectal pain, sore throat, penile edema, and a high frequency of mucocutaneous lesions.6 , 12 , 14 In immunosuppressed patients, more severe and atypical, necrotic skin lesions have occurred.15
Many affected patients in 2022 are living with HIV (41%) and/or had a concomitant sexually transmitted infection (29%). Nearly all mpox infections (98%) occurred among gay or bisexual men who reported recent sexual activity (95%).6
While case reports and clinical studies continue to emerge, few have reported more specifically on the dermatologic features of mpox infection, including morphologic progression and associated symptomatology. Efforts to increase early recognition are important for timely treatment and stigma reduction.
Our objective was to characterize clinical mpox symptoms, timeline, skin lesion morphology over time, affected population, hospitalization, treatment, and outcome of patients reported to the American Academy of Dermatology (AAD) and International League of Dermatological Societies (ILDS) Dermatology COVID-19, Monkeypox, and Emerging Infections Registry.
Methods
Data collection
The registry was established in March 2020 to collect information on the cutaneous manifestations of COVID-19 and COVID-19 vaccine reactions, as a collaboration between the AAD and ILDS. The registry was expanded on August 4, 2022, to become the “AAD/ILDS Dermatology COVID-19, Monkeypox, and Emerging Infections Registry,” the same day that the United States declared mpox to be a public health emergency. The expanded registry accepts cases of mpox and mpox/smallpox vaccine reactions.
Case entry is open to health care workers only, including nurses, residents, and other medical providers. All data are inputted to the registry via REDCap online survey platform. De-identified cases are accepted globally, and data entry is not restricted to AAD/ILDS members. The Massachusetts General Brigham Institutional Review Board exempted this study as not human subject research. All data were analyzed using Stata, version 16 (StataCorp, LLC).
Survey development
The mpox infection module of the registry collects data on patient demographics, exposure to mpox, symptomatology, morphology of cutaneous reaction(s), timing, duration of symptoms, diagnosis, and treatments. “Unknown” was an available option when applicable. The survey questionnaire was developed by a panel of experts in dermatology and infectious disease.
Detailed skin lesion morphology
Skin lesions were classified into papule(s), vesicle(s) or blister(s), pustules, erosion(s), ulcer(s), crust, abscess, or morbilliform rash. Registrants were asked for timing of initial onset of each type of lesion, as well as the time periods over which each type of lesion continued to be present.
Results
Patient and reporter population
One hundred one cases of mpox infection across 13 nonendemic countries were reported to the AAD/ILDS registry between August 4 and November 13, 2022; of which, 97% were male with a median age of 35 (IQR 31, 44) (Table I ). Ninety two percent of the patients were reported by dermatologists and were submitted by health professionals from Italy (19%), Germany (16%), Spain (16%), and the United States (32%). Most patients reported to the registry were White (62%), followed by Hispanic/Latino (20%), and Black/African American (11%).
Table I.
Characteristics of cases reported for mpox infection to the AAD/ILDS Registry
| Characteristic | Total (N = 101) |
|---|---|
| Reporter | |
| Dermatologist | 93 (92.1%) |
| Other physician | 2 (2.0%) |
| Podiatrist | 1 (1.0%) |
| Other medical professional | 5 (4.9%) |
| Patient age, y, median (IQR) | 35 (31,44) |
| Patient sex, male, n (%) | 98 (97.0%) |
| Patient race/ethnicity, n (%)∗ | |
| White | 63 (62.4%) |
| Black/African American | 11 (10.9%) |
| Hispanic/Latino | 20 (19.8%) |
| Asian | 3 (3.0%) |
| Other | 2 (2.0%) |
| Missing | 3 (3.0%) |
| Patient geographic region | |
| North America | 32 (31.7%) |
| Europe | 60 (59.4%) |
| Spain | 17 (16.8%) |
| Italy | 18 (24.0%) |
| Germany | 17 (16.8%) |
| Asia | 5 (5.0%) |
| Latin America and the Caribbean | 2 (2.0%) |
| Africa | 2 (2.0%) |
AAD/ILDS, American Academy of Dermatology/International League of Dermatological Societies.
Question not asked of all participants.
Exposure and diagnosis
Thirty-two respondents (32%) reported a known mpox exposure. Most respondents described their exposure as a sexual contact (26 of 32, 26% of total). The majority engaged in same sex sexual behavior (87%) and group sex activity (27%), defined as sexual activity between greater than 2 people, at a festival, group sex event, or sex party.
The median time from exposure to onset of symptoms among patients for which an exposure was identifiable was 7 days (IQR 7, 10). The median time from first symptom (day 0) to the day of diagnosis was 5 days (IQR 4, 9). All but 2 patients had polymerase chain reaction–confirmed diagnoses; one patient was diagnosed on biopsy while the other was based on clinical suspicion.
Clinical presentation at sign/symptom onset
Over half (54%) the cases submitted to the registry reported skin lesions or rash as the very first sign/symptom of infection, with others reporting fever (16%), general malaise (9%), sore throat (8%), or rectal pain (7%) (Table II ). Onset of skin lesions or rash most often occurred in the initial phases of infection. For patients presenting with skin lesions as their first sign/symptom, most (43 of 61 patients) had exclusively skin lesions. Eighty-five percent of the patients reported skin lesions within the first 3 days of sign/symptom onset.
Table II.
Cutaneous and systemic symptoms of mpox infection at symptom onset and total duration of illness
| Symptoms | Total (N = 101) |
|---|---|
| Initial symptoms (day 0) | |
| Skin lesions or rash∗ | 55 (54.5%) |
| Intra-oral or throat lesions† | 1 (1.0%) |
| Lymphadenopathy | 3 (3.0%) |
| Fever | 16 (15.8%) |
| General malaise (fatigue, chills, myalgia) | 9 (8.9%) |
| Sore throat | 8 (7.9%) |
| Rectal pain | 7 (6.9%) |
| Ocular/Ophthalmic symptoms | 1 (1.0%) |
| Edema | 1 (1.0%) |
| Number of lesions at symptom onset | |
| 1 | 20 (19.8%) |
| 2-5 | 52 (51.5%) |
| 6-20 | 20 (19.8%) |
| 21-50 | 5 (5.0%) |
| Unknown | 4 (4.0%) |
| Anatomic site of mucocutaneous lesions | |
| Oral cavity | 11 (10.9%) |
| Tonsils | 3 (3.0%) |
| Throat | 0 (0%) |
| Soft palate | 1 (1.0%) |
| Total number of lesions – throughout duration of infection | |
| 1 | 10 (9.9%) |
| 2-5 | 29 (28.7%) |
| 6-25 | 38 (37.6%) |
| 26-50 | 17 (16.8%) |
| 51-100 | 3 (3.0%) |
| 101+ | 1 (1.0%) |
| Edema location | |
| Peri-orbital | 2 (2.0%) |
| Face‡ | 8 (7.9%) |
| Perirectal/perianal | 4 (4.0%) |
| Scrotal/penile | 12 (11.9%) |
| Extremities | 4 (4.0%) |
| Symptoms during course of illness | |
| Skin lesions or rash | 99 (98.0%) |
| Intraoral or throat lesions | 15 (14.9%) |
| Fever | 65 (64.4%) |
| Chills | 21 (20.8%) |
| Myalgia | 18 (17.8%) |
| Arthralgia | 10 (9.9%) |
| Fatigue | 39 (38.6%) |
| Sore throat | 21 (20.8%) |
| Cough | 5 (5.0%) |
| Headache | 19 (18.8%) |
| Lymphadenopathy | 52 (51.5%) |
| Proctitis | 17 (16.8%) |
| Rectal pain | 16 (15.8%) |
| Penile edema | 5 (5.0%) |
| Other | 8 (7.9%) |
Patients also reported to have eye pain, abdominal pain and vomiting, conjunctivitis, penile pain, limb edema.
Any location on the body including groin, anal/perianal or lips.
Specified as lesions inside mouth or in the throat.
Includes the lips, excludes the eyes.
On the day of skin lesion appearance, 20% had a single lesion, whereas 52% had 2-5 lesions and 20% had 6-20 lesions. Seventy patients developed initial skin lesions either in the genito-inguinal area (44%) or the peri-anal/anal area (26%). Of the remaining cases (n = 29), most frequently reported areas included the face (16%), lips (5%), or arms/hands (4%). Two patients in the registry were reported to present initially with a generalized, morbilliform rash, nonspecific to one anatomic area.
General clinical presentation
All patients developed mucocutaneous lesions, and nearly all patients (98%) had skin manifestations (Table II). Common systemic symptoms reported throughout the course of illness included fever (64%), lymphadenopathy (52%), fatigue (39%), proctitis (17%), sore throat (21%), headache (19%), and rectal pain (16%). Edema was reported in 25 (25%) cases, most commonly of the face (8 of 23) and the scrotal or penile areas (12 of 23). Anatomically, symptoms most often progressed throughout the course of infection to involve the genitals, anus/peri-anal area, face, and extremities.
Lesion morphology
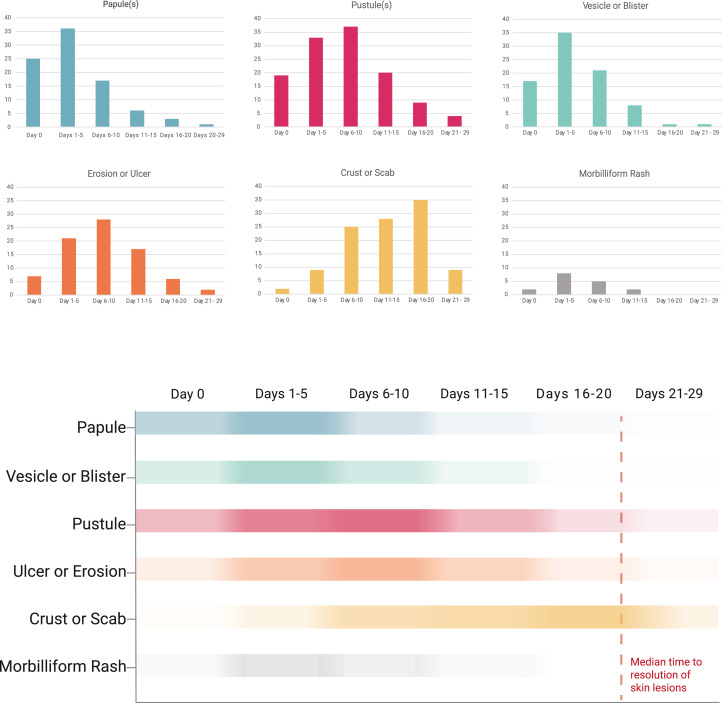
The most common skin lesion morphologies and secondary characteristics reported included papules, vesicles/blisters, pustules, erosions/ulcers, and crust/scabs (Fig 1 and Supplement 1, available via Mendeley at https://doi.org/10.17632/5xcwrr87gn.1). On day 0, the most common skin morphologies were papules (25%), vesicles (17%), or pustules (20%). On days 1-5, lesions were papules (36%), vesicles (36%), pustules (39%), or erosions/ulcers (21%). By days 6-10, pustules (36%) were most common, followed by erosions/ulcers (27%) and crusts/scab (24%). After day 11, crust and scabs were predominant. Overall, pustules were the most common morphology (24%).
Fig 1.
Cutaneous morphologies of skin lesions from patients reported with mpox infection to the AAD/ILDS Registry. ∗Color intensity indicates frequency of skin morphology at designated time period. AAD/ILDS, American Academy of Dermatology/International League of Dermatological Societies.
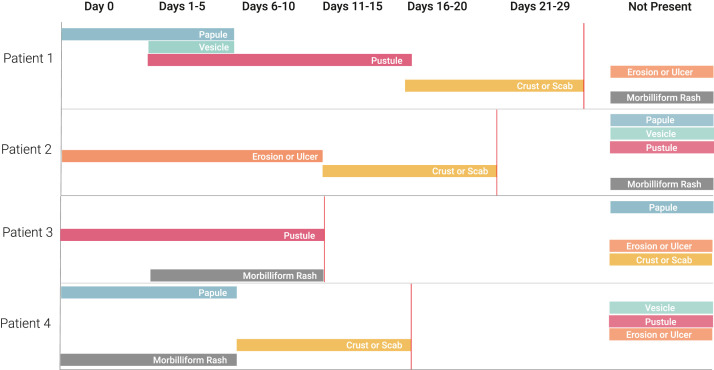
While morphologic transitions observed herein mirror historical reports overall, on the individual patient level, lesion progression did not always follow this sequence. For instance, some patients experienced multiple morphologies at the same time or skipped from initial morphologies such as a papule to a later stage morphology such as an ulcer, without a vesicle or pustule in between (Figs 2 and 3 ).
Fig 2.
Exemplar individual patient skin morphologies over time among select registry cases. ∗All cases included in Figure 2 were resolved at time of reporting.
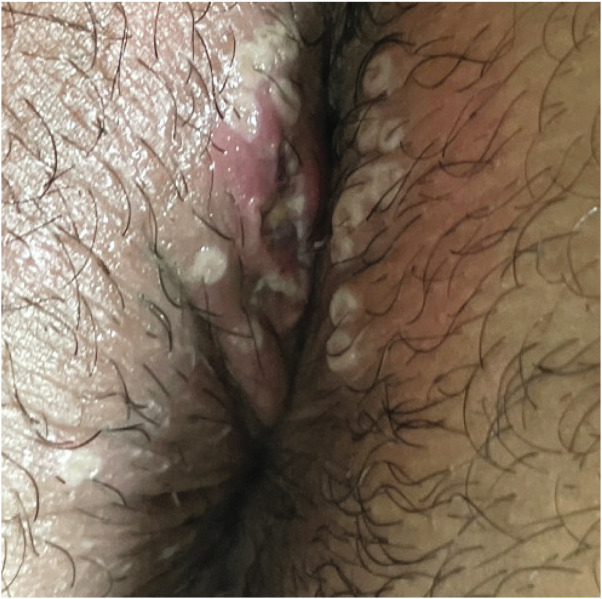
Fig 3.
Morphology of pseudopustular mpox skin lesions “pseudopustular donuts” (for additional images, see Supplement 1, available via Mendeley at https://doi.org/10.17632/5xcwrr87gn.1).
Other less frequent morphologies included abscesses (n = 4) and morbilliform rash (n = 10). Morbilliform rash occurred in the range of days 0 to 15 (frequently on days 1-5).
Medical history and hospitalization
Seven patients had been vaccinated for smallpox/mpox; 4 were known to have received the vaccine prior to mpox exposure/infection and the remaining 3 were vaccinated either after exposure or after infection.
Many cases (38%) had a concurrent sexually transmissible infection at the time of mpox infection including gonorrhea (17%), syphilis (14%), and chlamydia (7%) (Supplement 2, available via Mendeley at https://doi.org/10.17632/5xcwrr87gn.1). Seven patients (7%) had herpes simplex virus active infection. Thirty-eight percent of the registry cohort was documented as having a history of HIV. No other causes of immunosuppression were reported.
Twenty-one patients required hospitalization (21%). Patients were hospitalized for skin rash/lesions (n = 6), health system isolation protocol (n = 5), sore throat/oral lesions (n = 4), rectal pain (n = 3), sepsis (n = 1), malaise (n = 1), and other (n = 1).
Treatment and resolution
Resolution of infection was defined as the time at which complete re-epithelialization of the lesion-affected area has occurred. Of the total registry cases, 86 patients were reported to have reached resolution of skin infection at the time of entry. The median time to resolution was 20 days (IQR 14, 22). Of the 86 patients who had resolved infection, 11 (13%) had visible scarring after resolution, reported between 1 and 4 months after infection.
A quarter (25%) of the patients reported to the registry received tecovirimat (TPOXX) for mpox infection, all from the United States (Supplement 2, available via Mendeley at https://doi.org/10.17632/5xcwrr87gn.1). Other frequently reported treatments included intravenous or oral antibiotics (9%), topical medications including antiseptics, topical antibiotics, and topical analgesics (11%), and/or oral pain medications (7%). No patients received cidofovir, brincidofovir, intravenous pain medication, or vaccinia globulin.
Discussion
In this registry-based study, we report clinical symptoms and morphologic evolution of 101 mpox cases across 13 non-endemic countries from the AAD/ILDS Dermatology COVID-19, Monkeypox, and Emerging Infections Registry. This multinational study highlights the importance of dermatologic assessment in early recognition and treatment of mpox infection as skin or mucocutaneous lesions were the initial clinical sign in the majority of cases. Additionally, low lesion counts and involvement of the peri-anal or genito-inguinal regions create potential for under recognition or misdiagnosis. A unique feature of this work was case entry by dermatologists (92%), which adds specificity to morphologic reporting. Scarring, a finding in 13% of the registry cases, highlights both the need for further investigation on this potential long-term sequela and the continued role of dermatologists in care of the affected patients.
Studies conducted during the 2022 outbreak of mpox have highlighted key differences in clinical presentation of skin lesions compared to prior outbreaks and endemic mpox.14 , 16 In concert with other studies in the current epidemic, most registry cases presented with initial skin lesions in the genito-inguinal (44%) or peri-anal (26%) region. However, this study's inclusion of the progression of skin lesions provides novel evidence of morphologic changes differing from previously reported lesion progression: in some cases, skin lesions skipped morphologic phases, for example progression from papule to ulcer, and included multiple lesion types at any one point during the illness. Skin lesions also appeared prior to onset of typical prodromic signs and symptoms in 54% of reported cases and, upon initial presentation, 82% had fewer than 20 total lesions. In 20% of the cases, a single lesion was the initial presenting skin sign and 72% had fewer than 5 lesions, emphasizing a need for high clinical suspicion in those presenting with isolated lesions who are part of high-risk groups. Total lesion count over the disease course similarly demonstrated relatively low lesion counts, with 77% of reported cases with 20 or fewer lesions. Despite reports of fewer lesions during the illness course as compared to prior studies, the frequency of hospitalization was higher, with 21% of reported cases requiring hospitalization.12 , 17
In patients who reached clinical resolution, a significant proportion (13%) experienced residual scarring in areas of lesion development- an outcome underemphasized in the current literature.18 Scarring has potential implications when considering the stigmatization and discrimination associated with mpox infections and the effects on physical and mental health in this patient population. In collaboration with the CDC, the AAD has released provider and patient-facing recommendations on caring for skin lesions and how to reduce the risk of scarring.19
The largest burden of mpox in the 2022 outbreak that has been reported in gay, bisexual, and other men who have sex with men reflected similarly in our registry data (87%).4 , 20 It is important for clinicians treating sexual and gender diverse patients with mpox infections to recognize the potential for compounded stigma and work alongside patients to minimize the risk of scarring. Further investigations are needed to identify methods of reducing scarring in lesions of varying morphology and anatomic location as seen in the current outbreak.
An additional morphology noted in this study that has been only rarely reported in prior studies is morbilliform rash.6 , 14 Several possible explanations exist. This may be an immune response to viral infection, a result of virally infected skin, or a combination of both. Morbilliform eruptions are seen in other viral eruptions such as measles or parvovirus, though generally less typical of pox viruses. Less likely would be a morbilliform drug eruption since several patients had not yet been exposed to oral medications at the time of rash development.
This study is limited by the constraints of registry-reported data, which cannot estimate the prevalence or incidence, nor can it ensure accuracy or uniformity of health care–provider input. Additionally, as reflected in a higher frequency of hospitalization, there is a potential for preferential reporting of more severe or clinically evident mpox cases, especially those with cutaneous manifestations. In terms of lesion characterization, the registry did not capture presence or absence of umbilication. Also, “pseudopustule” was not included as a possible lesion morphology.
The largest global burden of mpox reported cases in the current outbreak lies in the United States, representing over one-third of cases worldwide21; this distribution is mirrored in the registry-reported data (32%, the United States.). In the US population, race and ethnicity data indicate that Black or African American patients are disproportionally affected.20 Registry-reported cases were primarily in White patients (62%). Concerted efforts to capture cases in Black or African American patients would more accurately reflect the clinical course and associated outcomes during the current outbreak.
These findings reinforce deviations in skin findings in the current mpox outbreak compared to prior mpox outbreaks – notably the presence of skin lesions prior to the onset of systemic illness and the presence of fewer than 20 skin or mucocutaneous lesions overall. Our study adds detail regarding the morphology of skin lesions during the 2022 outbreak, quantifying patients experiencing atypical progression of lesions and/or the involvement of multiple morphologies simultaneously. Case entry was completed primarily by dermatologists, supporting lesion evaluation and reporting. Scarring after mpox infection affects a large proportion of reported cases and warrants further investigation to reduce negative long-term outcomes.
While the current outbreak trends downward, transmission continues. Vaccine coverage remains sub-optimal, especially in racial/ethnic minority populations and in low- and middle-income countries.10 Previously endemic countries in central and west Africa have, at the time of writing, no access to Jynneos/Imvanex vaccine. As the virus continues to circulate and we prepare for possible future surges, we must remain vigilant in evaluating patients for subtle and atypical presentations to interrupt mpox virus transmission.
Conflicts of interest
Esther Freeman, Klint Peebles, Misha Rosenbach, Terrence Cronin and George Hruza are members of the AAD Ad Hoc Task Force to Create Monkeypox Content. Esther Freeman is the Principal Investigator of the AAD/ILDS Dermatology Registry for COVID-19, Monkeypox, and Emerging Infections, and serves on the WHO Living Monkeypox Atlas and the WHO Monkeypox Guidelines Committee. Kieron Leslie is a dermatology consultant for the ACTG Study: Tecovirimat For Human Monkeypox Virus (STOMP) and is the Lead Dermatologist on the WHO Living Monkeypox Atlas. Alexander Stratigos is the immediate past President of the EADV. Mark Kaufmann is the President of the AAD. Terrence Cronin is President-elect of the AAD. Lars French is the President of the ILDS. Henry W. Lim and Claire Fuller are Board members of the ILDS. Lindy Fox is a Board member of the AAD.
Footnotes
Funding sources: The AAD/ILDS Dermatology Registry for COVID-19, Monkeypox, and Emerging Infections is supported by a grant from the ILDS and by in-kind support from the AAD.
IRB approval status: Reviewed by Massachusetts General Hospital Institutional Review Board, deemed not human subjects research.
References
- 1.Bunge E.M., Hoet B., Chen L., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. Plos Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titanji B.K., Tegomoh B., Nematollahi S., Konomos M., Kulkarni P.A. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9(7) doi: 10.1093/ofid/ofac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC Centers for Disease Control and Prevention: 2022 U.S. map & case count. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- 4.WHO . World Health Organization [WHO]; 2022. 2022 Monkeypox Outbreak: Global Trends. 04 November 2022. [Google Scholar]
- 5.WHO WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. 2022. https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern
- 6.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 7.Guarner J., Del Rio C., Malani P.N. Monkeypox in 2022-what clinicians need to know. JAMA. 2022;328(2):139–140. doi: 10.1001/jama.2022.10802. [DOI] [PubMed] [Google Scholar]
- 8.CDC . Centers for Disease Control [CDC]; 2022. Science Brief: Detection and Transmission of Monkeypox Virus. CDC. gov, (DHCPP) DoH-CPaP. [Google Scholar]
- 9.Ward T., Christie R., Paton R.S., Cumming F., Overton C.E. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ. 2022;379 doi: 10.1136/bmj-2022-073153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman E.E., Abbott S., Kurpiel B., Okwor T. The dynamics of monkeypox transmission. BMJ. 2022;379:o2504. doi: 10.1136/bmj.o2504. [DOI] [PubMed] [Google Scholar]
- 11.Basgoz N., Brown C.M., Smole S.C., et al. Case 24-2022: a 31-year-old man with perianal and penile ulcers, rectal pain, and rash. N Engl J Med. 2022;387(6):547–556. doi: 10.1056/NEJMcpc2201244. [DOI] [PubMed] [Google Scholar]
- 12.Patel A., Bilinska J., Tam J.C.H., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catala A., Clavo Escribano P., Riera J., et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022;187(5):765–772. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]
- 15.Miller M.J., Cash-Goldwasser S., Marx G.E., et al. Severe monkeypox in hospitalized patients - United States, August 10-October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(44):1412–1417. doi: 10.15585/mmwr.mm7144e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarín-Vicente E.J., Alemany A., Agud-Dios M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira-Júnior J.M., Tenório M.D.L., dos Santos Caduda S., Santana R.R.R., Martins-Filho P.R. Reasons for hospitalization of patients with monkeypox: a quantitative evidence synthesis. Infection. 2022 doi: 10.1007/s15010-022-01937-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.CDC Key characteristics for identifying monkeypox 2022. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- 19.American Academy of Dermatology Association Monkeypox: caring for skin 2022. https://www.aad.org/member/clinical-quality/clinical-care/monkeypox/treatment
- 20.CDC . Centers for Disease Control [CDC]; 2022. Monkeypox Cases by Age and Gender, Race/Ethnicity, and Symptoms. [Google Scholar]
- 21.CDC 2022 Monkeypox Outbreak Global Map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html