Mucosal immunity has a pivotal role in protection from respiratory viral infections.1 The current authors have showed substantial protection from omicron infection by high concentrations of nasal mucosal SARS-CoV-2 WT spike immunoglobulin-A (M-IgA) over a 4-week screening period.2 A sharp increase in M-IgA concentrations following BA.1 or BA.2 breakthrough infection in triple vaccinated health-care workers was also observed.2 Here, we present follow-up data with prospectively collected omicron infection rates and systemic and mucosal antibody concentrations from the same cohort (appendix pp 7–9, 12–14).
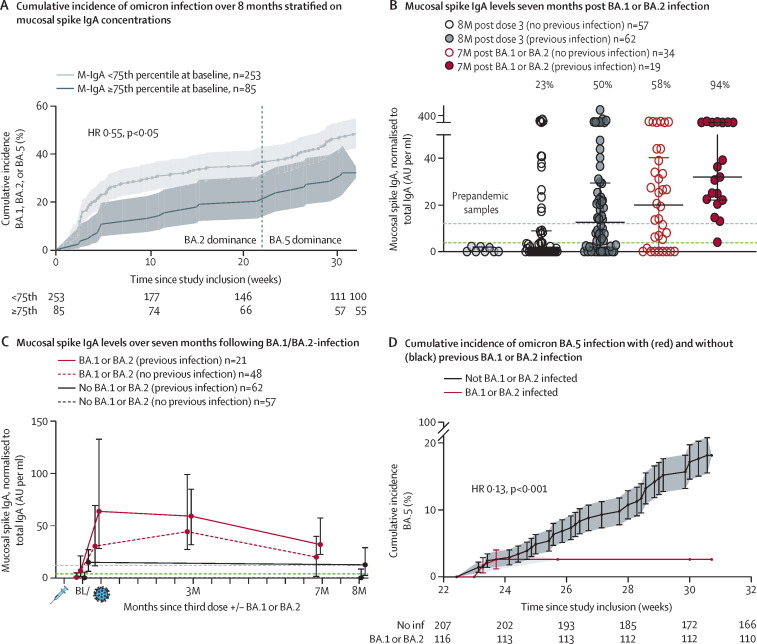
The association between M-IgA concentrations at the 75th percentile or higher at enrolment and a reduced risk of symptomatic BA.1, BA.2, or BA.5 breakthrough infection remained over an 8-month follow-up period, with a hazard ratio (HR) of 0·55 (95% CI 0·35–0·87), much due to the initial risk difference (figure A ). Serum WT spike-specific IgG (S-IgG) concentrations waned over 8 months following a third vaccine dose in all study participants (appendix p 10), concurrent with previous data.3 However, concentrations of nasal M-IgA in participants with previous SARS-CoV-2 infection, but without omicron breakthrough infection, remained above the amount associated to 65% protection2 over the 8-month study period (figure C). This finding suggests a long-lasting mucosal immunity evoked by SARS-CoV-2 infection.
Figure.
Duration and protection by mucosal immune responses
(A) the cumulative incidence of BA.1, BA.2, and BA.5 breakthrough infection over the 8-month follow-up period, stratified on baseline (ie, 5 weeks after third vaccine dose) M-IgA concentrations above or below the 75th percentile. HRs from Cox regression model. (B) Individual M-IgA concentrations 7 months after BA.1 or BA.2 breakthrough infection in 53 individuals that became infected with BA.1 or BA.2 early during the study period. 119 individuals without breakthrough infection 8 months after third vaccine dose and eight pre-pandemic samples are shown as reference. The horizontal green dashed line indicates the cut-off level (mean + 3SD of pre-pandemic samples) and the horizontal grey dashed line indicates the upper 75th percentile of all samples at baseline (ie, the M-IgA concentration associated with protection against BA.1 or BA.2 breakthrough infection).2 Percentages on top of each column indicate the proportion of study participants with M-IgA concentrations remaining above the upper 75th percentile of all samples at baseline (ie, the concentration associated with protection against BA.1 or BA.2 breakthrough infection).2 Black horizontal lines represent median and IQR. (C) Median with IQR of M-IgA concentrations over the 7-month follow-up after BA.1 or BA.2 breakthrough infection in individuals with (red lines) and without (dashed red lines) previous WT or delta infection. Individuals without breakthrough infection are shown as reference. The horizontal green dashed line indicates the cut-off level (mean + 3 standard deviation of pre-pandemic samples) and the horizontal grey dashed line indicates the upper 75th percentile of all samples at baseline (ie, the M-IgA concentration associated with 65% protection against BA.1 or BA.2 breakthrough infection).2 (D) the cumulative incidence of BA.5 breakthrough infection over a 10-week period with BA.5 dominance in triple-vaccinated health-care workers with and without previous BA.1 or BA.2 infection. HRs from Cox regression model. HR=hazard ratio.
We next followed systemic and mucosal immune responses in participants that had a BA.1 or BA.2 breakthrough infection during the screening study. 7 months following breakthrough infection, S-IgG concentrations waned to be lower than at baseline (appendix p 10). As previously shown,4 serological responses were lower among participants with a history of SARS-CoV-2 infection before breakthrough infection compared with those without and the difference remained over the 7-months follow-up (appendix p 10). Whether these findings reflect immune imprinting after previous infection5 or a hampered systemic viral replication due to stronger and more rapid mucosal immune responses2 needs further investigation. Interestingly, although nasal M-IgA concentrations waned, they remained above the protective threshold2 in 94% of participants with previous SARS-CoV-2 WT or delta infection and in 58% of previously SARS-CoV-2-naive participants (figure B). In line with this, and in agreement with recent population-based data,6, 7 BA.1 and BA.2 infections were strongly protective against subsequent BA.5 infection in this cohort, with a HR of 0·13 (95% CI 0·04–0·44; figure D).
To assess whether M-IgA in nasal samples originated in the mucosa, we correlated M-IgA to mucosal spike-specific secretory IgA in nasal samples, and M-IgA to spike-specific IgA in serum. Concentrations of M-IgA correlated stronger to mucosal secretory IgA in nasal samples (r=0·9, p<0·001) than to spike-specific IgA in serum (r=0·64, p<0·001) (appendix p 11). Although a spillover from the circulation cannot be ruled out, these results indicate a mucosal origin of nasal IgA.
These findings highlight the key role of antigen presentation at the mucosa and support a protective effect of mucosal immunity for up to 8 months. Whether nasal or oral vaccines can elicit mucosal immune responses and protection similar to those following natural infection in mRNA-vaccinated individuals, will be an important aspect of ongoing clinical trials.
We declare no competing interests.
Supplementary Material
References
- 1.Focosi D, Maggi F, Casadevall A. Mucosal vaccines, sterilizing immunity, and the future of SARS-CoV-2 virulence. Viruses. 2022;14:187. doi: 10.3390/v14020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havervall S, Marking U, Svensson J, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N Engl J Med. 2022;387:1333–1336. doi: 10.1056/NEJMc2209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilboa M, Regev-Yochay G, Mandelboim M, et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw Open. 2022;5:e2231778. doi: 10.1001/jamanetworkopen.2022.31778. e2231778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom K, Marking U, Havervall S, et al. Immune responses after omicron infection in triple-vaccinated health-care workers with and without previous SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:943–945. doi: 10.1016/S1473-3099(22)00362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds CJ, Pade C, Gibbons JM, et al. Immune boosting by B.1.1.529 (omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377 doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malato J, Ribeiro RM, Leite PP, et al. Risk of BA.5 infection among persons exposed to previous SARS-CoV-2 variants. N Engl J Med. 2022;387:953–954. doi: 10.1056/NEJMc2209479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Protective effect of previous SARS-CoV-2 infection against omicron BA.4 and BA.5 subvariants. N Engl J Med. 2022;387:1620–1622. doi: 10.1056/NEJMc2209306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.