Reliable SARS-CoV-2 correlates of protection (COP) are crucial for predicting individual-level risk of infection, estimating population susceptibility, and assessing future epidemic risks.1 However, COP studies are challenging given that blood samples ideally need to be collected close to the time of exposure, which is hard to predict. Thus, most existing SARS-CoV-2 COP estimates are based on vaccine efficacy trial data,2, 3 which include frequent blood sampling and strict infection monitoring and are therefore well suited for this purpose. Yet these trials were conducted before the circulation of highly immune-evasive variants of concern (VOC), and in populations with little previous exposure to SARS-CoV-2, limiting their current relevance. We previously reported how existing acute fever surveillance platforms could be used to monitor population-level temporal changes in SARS-CoV-2 immune markers, and documented that higher antibody levels were associated with lower risk of SARS-CoV-2 infection.4 Here, we build off that previous work to show that routinely collected fever surveillance data analysed using a prospective test-negative design5 can generate rapid and VOC-specific immune COP for symptomatic infection.
As previously described,4 between March 22, 2021, and Aug 17, 2022, we prospectively enrolled 2300 patients aged 2 years and older who presented with undifferentiated acute febrile syndromes across two hospitals in the Dominican Republic. Nasopharyngeal swabs and sera collected at the time of enrolment were tested by real-time PCR (rtPCR) for acute SARS-CoV-2 infection and with the Elecsys platform for total anti-spike antibodies (Roche Diagnostics, Indianapolis, IN, USA), respectively. Of 517 rtPCR-positive samples (22·4% of all samples), 264 with cycle threshold values less than 25 were randomly selected for sequencing using Oxford Nanopore or Illumina platforms. Using a test-negative design that compared antibody levels between VOC sequence-confirmed cases and rtPCR-negative non-cases, we modelled the variant-specific risk of infection by total anti-spike antibody level, controlling for a range of covariates associated or potentially associated with SARS-CoV-2 exposure (figure ). Additional methods are available in the appendix (pp 1–2). Estimates underlying the figure plots are available online.
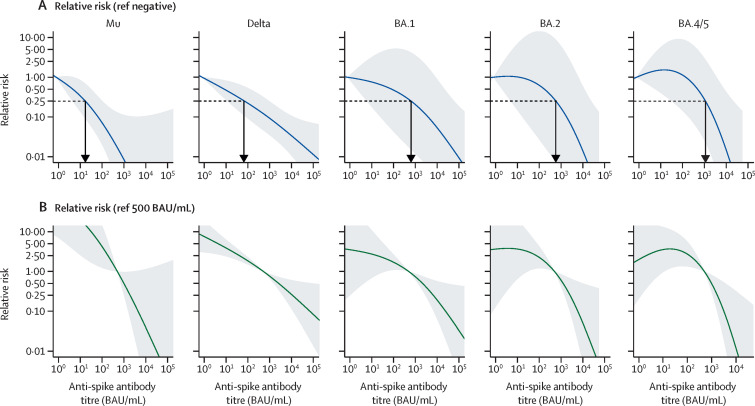
Figure.
Correlates of protection for symptomatic SARS-CoV-2 infection by variants of concern
The plots show binomial generalised additive model covariate adjusted relative risk for real-time PCR (rtPCR) positive test by antibody marker level and stratified by variant of concern. (A) Relative risk of infection scaled to a reference value of 0·79 BAU/mL (manufacturer-defined positive cutoff index of 0·80 BAU/mL). The blue line indicates the regression point estimate with gray shading representing the 95% CI. The horizontal black dashed line indicates 0·25 relative risk and vertical black arrow the total anti-spike value at the 0·25 relative risk intercept, corresponding to an estimated 75% protection against the respective variant. To control for variable risk of pathogen exposure across the study population, covariates that are or might be associated with exposure were included in the model, including age, sex, month of sample collection, number of COVID-19 vaccine doses, days since last vaccine dose, urban versus rural setting, study site, and number of household residents. Given the non-linear relationship between log transformed antibody titre and risk of infection, the antibody titre covariate was modelled using two degrees of freedom. Case samples used in the models were all collected <5 days after symptom onset and were sequence-confirmed except BA.1, which includes all rtPCR-positive cases during the clearly delineated phase of BA.1 transmission. The number of rtPCR-positive/negative study participants per plot are 42/394 (mu), 84/474 (delta), 54/423 (BA.1), 17/288 (BA.2), and 19/288 (BA.4/5). (B) Plots represent the same generalised additive models, but risk of infection is referenced to a total anti-spike antibody titre of 500 BAU/mL. Unadjusted anti-spike antibody levels by rtPCR result and variant are shown in the appendix (p 3). BAU=binding antibody units.
Total anti-spike antibody estimates of 17 (95% CI 4–102), 76 (13–955), 631 (6–60 256), 603 (5–24 547), and 1148 (34–20 893) binding antibody units (BAU)/mL were associated with 75% protection against symptomatic infection with B.1.621 (mu), B.1.617.1 (delta), BA.1 (omicron), BA.2, and BA.4/5 variants, respectively (figure A), with details including estimates for 50%, 60%, 70%, and 80% protection in the appendix (p 3). In addition to estimating the antibody level that corresponds to a specified level of protection, this approach can estimate variant-specific protection that corresponds to specific antibody levels. For example, a cutoff of 100 BAU/mL (ie, the anti-spike antibody level reported through the prospective serology-based Coronavirus Infection Survey that tracks population immune markers in the UK6) is estimated to provide 93% (95% CI 75–98), 77% (46–90), 52% (0–96), 37% (0–97), and 0% (0–85) protection against symptomatic infection for mu, delta, BA.1, BA.2, and BA.4/5 variants, respectively. Additionally, by adjusting the reference antibody value, we can estimate the risk of infection relative to a particular immune marker level, for example a median population immune marker value in a specific country or region, as illustrated for a reference level of 500 BAU/mL (figure B).
Here we report a proof of concept for monitoring variant-specific SARS-CoV-2 COP using existing surveillance infrastructure in the Dominican Republic. However, global networks of acute febrile illness, influenza-like illness, and severe acute respiratory illness surveillance sites exist, which could be leveraged to more rapidly and precisely assess emerging COP. By combining analyses across international surveillance platforms, this approach could provide quick and operationally relevant data to assess population infection risk and guide public health policies for SARS-CoV-2 and, potentially, other emerging pathogens.
EJN is the Principal Investigator on a US Centers for Disease Control and Prevention (CDC)-funded U01 award that funded the study, and CLL, AK, MdSA, WD, and MV have received salary support, consultancy fees, or travel paid through this award. EZG is an employee of the US CDC. CTP, IMS, and RSR are employees of the Ministry of Health and Social Assistance, Dominican Republic, that was subcontracted with funds from the US CDC award. AK is supported by the Wellcome Trust, UK. PJ and KOM declare no competing interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDC.
Supplementary Material
References
- 1.Nilles EJ, Paulino CT, de St Aubin M, et al. SARS-CoV-2 seroprevalence, cumulative infections, and immunity to symptomatic infection—a multistage national household survey and modelling study, Dominican Republic, June–October 2021. Lancet Reg Health Am. 2022;16 doi: 10.1016/j.lana.2022.100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilles EJ, de St Aubin M, Dumas D, et al. Integrated SARS-CoV-2 serological and virological screening across an acute fever surveillance platform to monitor temporal changes in anti-spike antibody levels and risk of infection during sequential waves of variant transmission — Dominican Republic, March 2021 to August 2022. Emerg Infect Dis (in press). [DOI] [PMC free article] [PubMed]
- 5.Dean NE, Hogan JW, Schnitzer ME. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Office for National Statistics Coronavirus (COVID-19) infection survery, UK: 18 November 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/18november2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.