Abstract
Introduction According to the Embryo Protection Act, the selection of embryos with the greatest potential for successful implantation in Germany must be performed in the pronucleus stage. The main aim of this study was to identify morphokinetic parameters that could serve as noninvasive biomarkers of blastocyst quality in countries with restrictive reproductive medicine laws.
Materials and Methods The sample comprised 191 embryos from 40 patients undergoing antagonist cycles for intracytoplasmic sperm injection. Blastocysts were cultured in an EmbryoScope chamber and video records were validated to determine the post-injection timing of various developmental stages, cleavage stages, and blastocyst formation. The Gardner and Schoolcraft scoring system was used to characterize blastocyst quality.
Results Morphokinetic data showed that the zygote diameter and total cytoplasmic volume were significantly different between good and poor blastocysts quality groups, where zygotes, which formed better blastocyst quality, had smaller diameter and smaller total cytoplasmic volume. Zygotes with more rapid pronuclear disappearance developed in better-quality blastocysts. Differences between good- and poor-quality blastocysts were also observed for late-stage parameters and for the spatial arrangement of blastomere where tetrahedral embryos more frequently forming good-quality blastocyst compare to the non-tetrahedral.
Conclusions The study findings could be used to enhance embryo selection, especially in countries with strict Embryo Law Regulations. Further studies, including those in which the implantation potential and pregnancy rate are considered, are warranted to confirm these preliminary results.
Keywords: zygote diameter, embryo morphokinetics, tetrahedral arrangement, blastocyst quality
Zusammenfassung
Einleitung Gemäß dem Embryonenschutzgesetz muss in Deutschland die Selektion von Embryos mit dem größten Potenzial für eine erfolgreiche Einnistung im Vorkernstadium erfolgen. Das Hauptziel dieser Studie war es, die morphokinetischen Parameter zu ermitteln, die in Ländern mit restriktiven reproduktionsmedizinischen Gesetzen als nichtinvasive Biomarker für die Blastozystenqualität dienen könnten.
Material und Methoden Die Untersuchungseinheit bestand aus 191 Embryos von 40 Patientinnen, die sich einem Antagonisten-Protokoll zur Vorbereitung auf intrazytoplasmatische Spermieninjektionen unterzogen. Die gewonnenen Blastozysten wurden in einem EmbryoScope-Inkubator kultiviert, und die Videoaufnahmen wurden validiert, um die Zeitpunkte der verschiedenen Entwicklungsstadien und Spaltungsstadien nach der Injektion und die Blastozystenformation zu bestimmen. Um die Qualität der Blastozysten zu beurteilen, wurde das Gardner/Schoolcraft-Punktesystem verwendet.
Ergebnisse Die morphokinetischen Daten zeigten, dass es signifikante Unterschiede in den Durchmessern der Zygoten und im zytoplasmatischen Gesamtvolumen gab zwischen den qualitativ hochwertigen und den qualitativ minderwertigen Blastozystengruppen. Zygoten, die eine qualitativ hochwertigere Blastozyste bildeten, hatten einen kleineren Durchmesser und ein kleineres zytoplasmatisches Gesamtvolumen. Zygoten mit einem kürzerem Vorkernstadium entwickelten sich zu qualitativ hochwertigeren Blastozysten. Die Unterschiede zwischen qualitativ hochwertigen und qualitativ minderwertigen Blastozysten wurden auch bei Parametern der Spätstadien und bei der räumlichen Anordnung der Blastomere beobachtet, wobei tetrahedral angeordnete Embryos häufiger qualitativ hochwertige Blastozysten bildeten als Embryos ohne tetrahedrale Anordnung.
Schlussfolgerungen Die Ergebnisse dieser Studie könnten zur Verbesserung der Embryonenselektion verwendet werden, besonders in Ländern mit strengen Embryonenschutzgesetzen. Weitere Studien, die auch das Einnistungspotenzial sowie die Schwangerschaftsrate berücksichtigen, sind erforderlich, um diese Ergebnisse zu bestätigen.
Schlüsselwörter: Zygotendurchmesser, embryonale Morphokinetik, tetrahedrale Anordnung, Blastozystenqualität
Introduction
Reproductive medicine is among the most rapidly developing fields, in which the selection of the best embryos for transfer remains a key challenge. In the past two decades, the conventional method of embryo selection for transfer has been based on the critical assessment of morphological parameters (i.e., the number of blastomeres, degree of fragmentation, and blastomere size) during embryonic development 1 . Such morphological evaluations are conducted once a day at a set timepoint, as the frequent removal of embryos from the incubator environment may result in undesired temperature and pH changes in the embryo culture dish 2 . The use of time-lapse imaging enables the continuous monitoring of embryo development without displacement from the regulated and stable incubator conditions 3 . Serial time-lapse imaging also enables morphokinetic monitoring (i.e., the evaluation of embryo quality by the monitoring of event timing and development interval durations), adding another aspect to embryo selection and scoring 4 .
As noted by Alfarawati et al. 5 , most criteria used for the morphological assessment of embryos correlate weakly with in vitro fertilization (IVF) outcomes. Embryo morphology is not always an absolute indicator of implantation potential, especially due to the existence of intra- and inter-observer variability 6 . Abundant data suggest that the precise timing of specific events observed in time-lapse incubators, such as pronuclear formation, early cleavage events, cell cycle intervals, and the synchronicity of cell division, is a more stable and reliable indicator of an embryo’s developmental potential 7 .
Although very well-designed algorithms for the selection of embryos based on morphokinetic parameters on day 3 are available 8 , their application is not possible in countries such as Germany due to the Embryo Protection Act 9 . What is more, besides the EPA, the “Deutsche Mittelweg” is established as well, where embryo selection can be performed to a slightly limited extent. However, still, cryopreservation of cleavage embryos is not permitted in regular cases in which it would affect IVF outcomes. Due to the limited number of embryos that are allowed to be cultured to the blastocyst stage, as well as clinical effectiveness and cost efficiency concerns, the utilization of morphokinetic parameters for the prediction of IVF outcomes in Germany remains disputable 9 10 11 12 .
Based on the aforementioned facts the primary aim of this study was to define morphokinetic parameters that might be useful as noninvasive biomarkers of blastocyst quality in countries with restrictive reproductive medicine laws. In particular, we focused on determining whether the zygote diameter and cytoplasmic volume differ between good- and poor-quality blastocysts.
Materials and Methods
Ethical approval
This retrospective observational study was conducted with time-lapse imaging data from human embryos during in vitro growth. Ethical approval for the study was obtained from the Ethics Committee of Saarland, Germany (reference no. 146/20).
Patient selection
The study population consisted of 40 women undergoing intracytoplasmic sperm injection (ICSI) at Team Kinderwunsch Hannover (Hannover, Germany) in January–March 2020. Included patients were aged 25–40 years and had cycles with at least three mature oocytes. The exclusion criteria were anti-Mullerian hormone (AMH) concentration ≤ 1.0 ng/ml, body mass index < 18 kg/m 2 or > 30 kg/m 2 , oocyte retrieval after the performance of other stimulation protocols (natural, short, or mild IVF cycles), and signs of ovarian hyperstimulation. In addition, cases involving the surgical retrieval of sperm were excluded.
Ovarian stimulation
All patients underwent controlled ovarian stimulation antagonist GnRH protocol treatment where starting doses were based on serum AMH levels, antral follicle counts, or previous responses to ovarian stimulation. Subsequent doses were adjusted according to the monitoring of ovarian responses with serial ultrasound examination and serum estradiol measurement. In each case, a human chorionic gonadotropin (HCG; Ovitrelle; Merck Europe, Darmstadt, Germany) injection was used to trigger final oocyte maturation, and ultrasound-guided ovum retrieval was performed approximately 36 h later.
ICSI, embryo culture, and embryo assessment
The follicles were aspirated and the oocytes were washed and cultured in medium (GM501; Gynemed, Lensahn, Germany) at 37 °C, 20% O 2 , and 6% CO 2 for 3 h before oocyte denudation using hyaluronidase (conc. 80 IU/ml; Gynemed, Lensahn, Germany). Mature oocytes were fertilized using conventional ICSI procedures and placed immediately after injection in sequential culture medium (Cleavage Medium; COOK, Sydney, Australia) in an EmbryoScope chamber (EmbryoSlide; Vitrolife, Sweden) at 37 °C, 5% O 2 , and 6% CO 2 . The culture medium was replaced by performing a half-change with a pre-equilibrated culture medium (Blastocyst Medium; COOK, Sydney, Australia) at 70–72 h after ICSI. On day 5, the blastocysts were classified according to Gardner and Schoolcraft 12 ; grades (1–6) were assigned based on the degree of expansion and hatching status. The inner cell mass (ICM) and trophectoderm (TE) quality of fully developed (grade 3–6) blastocysts were graded. For the ICM, grades A, B, and C corresponded to the presence of many tightly packed cells, several loosely grouped cells, and very few cells, respectively. For TE quality, grades A, B, and C corresponded to the presence of many cells forming a cohesive epithelium, a few cells forming a loose epithelium, and very few large cells, respectively. Good blastocyst quality was defined as a grade of at least 3BB (including 3/4/5AA, AB, BA, or BB).
Time-lapse morphokinetic assessment
Video records were validated to determine the timing of various developmental stages, cleavage stages, and blastocyst formation from the point of ICSI using EmbryoViewer software (Vitrolife). The conduct of ICSI was designated as “time zero” (t0), and all embryo developmental events with the corresponding timing expressed as “hours after ICSI” were evaluated. The following intervals were calculated:
tPNf = the time to pronuclear (PN) fading, defined by the first frame in which the embryo is still in the single-cell stage but pronuclei can no longer be visualized;
t2 = the time to the first cell cleavage;
t3–5 and t8: the times to the first observation of three, four, five, and eight discrete cells, respectively;
TM = the time to morula formation, with no obvious cell boundary; and
tSB = the time to the beginning of cavity formation.
The duration of the embryo cell cycle (ECC), defined as a round of cell division in which the number of blastomeres doubled, was calculated using time-lapse annotation. We defined the duration of the first embryo cell cycle (ECC1) as the interval between the moment of PN fading and the complete separation of the two blastomeres by individual cell membranes (t2–tPNF). The duration of the second embryo cell cycle (ECC2) was defined as the time of the transition from a two-blastomere to a four-blastomere embryo (t4–t2) and that of the third embryo cell cycle (ECC3) was defined as the time of embryo development from four to eight cells (t8–t4; Fig. 1 ). The duration of the transition from the four-cell to the morula stage (TM–t4) was also calculated. Additionally, the synchronicity of the two blastomere divisions in the second cell cycle (S2) was calculated as the time required for the embryo to progress from the three-cell to the four-cell stage (t4–t3), and the synchronicity of the four blastomere divisions in the third cell cycle (S3) was calculated as the time taken for the embryo to progress from the five-cell to the eight-cell stage (t8–t5; Fig. 1 ).
Fig. 1.
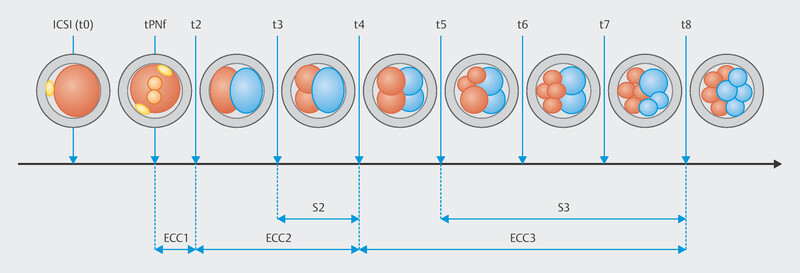
Graphic presentation of embryo development events from sperm injection to the eight-cell stage.
Besides the aforementioned morphokinetics parameters, the zygote diameter, total cytoplasmic volume (TCV) of the zygote, and spatial arrangement of blastomeres in the four-cell stage were also determined. The TCV (n × 10 4 ) was calculated 17 h after injection based on two manually drawn perpendicular diameters. The calculation of TCV used in this study has been described before by Paternot et al. 13 . The spatial arrangement of blastomeres was defined as tetrahedral when the cleavage planes were perpendicular and non-tetrahedral when the planes were parallel.
Data analysis
All variables were analyzed using IBM SPSS software (version 24; IBM Corporation, Armonk, NY, USA). The Mann–Whitney U test was used to compare median values between groups. Continuous nonparametric data are reported as medians and ranges. The chi-square test was used to compare categorical data. Univariate and multiple binary logistic regression analyses were performed with blastocyst quality serving as the dependent variable and the TCV and tPNf serving as independent variables. Receiver operating characteristic analyses were used to calculate areas under the curve (AUCs) characterizing the diagnostic performance of TCV and tPNf. Differences with p ≤ 0.05 were considered to be significant. In addition, since this is a retrospective study, we calculated effect sizes for the main study outcomes to account for study power.
Results
The average age of the patients included in the study was 34.5 ± 4.5 years. In total, 287 oocytes were retrieved. After the exclusion of 23 immature oocytes and 73 oocytes that were not fertilized normally (PN ≠ 2) or did not complete the first division, the sample for the blastocyst development analysis comprised 191 embryos. The cultured embryos were divided into good- and poor-quality blastocyst groups. In addition, the blastocyst formation potential was estimated. The timing of developmental endpoints was compared between embryos that reached the blastocyst stage and those that failed.
Zygote morphokinetic parameters
Of the 191 fertilized oocytes, 113 (59.1%) reached the blastocyst stage; 48 (42.5%) of these blastocysts were of good quality. The zygote diameter was significantly different between good quality and poor-quality blastocyst group where the zygotes with smaller diameter created better quality blastocyst (109.2 [105.6–111.6] vs. 112.1 [105.6–115.2] µm, p < 0.0001). The TCV was also significantly different between the zygotes that developed into a good-quality blastocyst and those that did not. Further analysis indicated that zygotes with smaller TCV formed better blastocyst quality (68.28 [61.65–78.80] × 10 4 vs. 74.55 [61.65–83.86] × 10 4 µm 3 , p < 0.0001; Fig. 2 ). Univariate logistic regression demonstrated that the TCV significantly predicted blastocyst quality (odds ratio [OR] 0.84, 95% confidence interval [Cl] 0.78–0.92, p < 0.0001). Predictive strength was quantified using the area under the curve (AUC) of the receiver operating characteristic (ROC), where the area under the ROC curve was AUC 0.718 ( Fig. 3 ).On average, the tPNf was shorter for embryos that reached the blastocyst stage than for those that failed (22.00 [17–29] vs. 23.00 [18–56] h, p < 0.01; Table 1 ). Moreover, good-quality blastocysts were developed from embryos with shorter time frame of tPNf (21.00 [17–28] vs. 23.00 [18–29] h, p < 0.0001; Fig. 4 ). Univariate logistic regression demonstrated that the tPNf significantly predicted blastocyst quality (OR 074, 95% Cl 0.61–0.88, p < 0.001, AUC 0.699; Fig. 5 ).A multiple binary logistic regression analysis was performed with the parameters that were significant in the univariate logistic regression analysis ( Table 2 ).
Fig. 2.
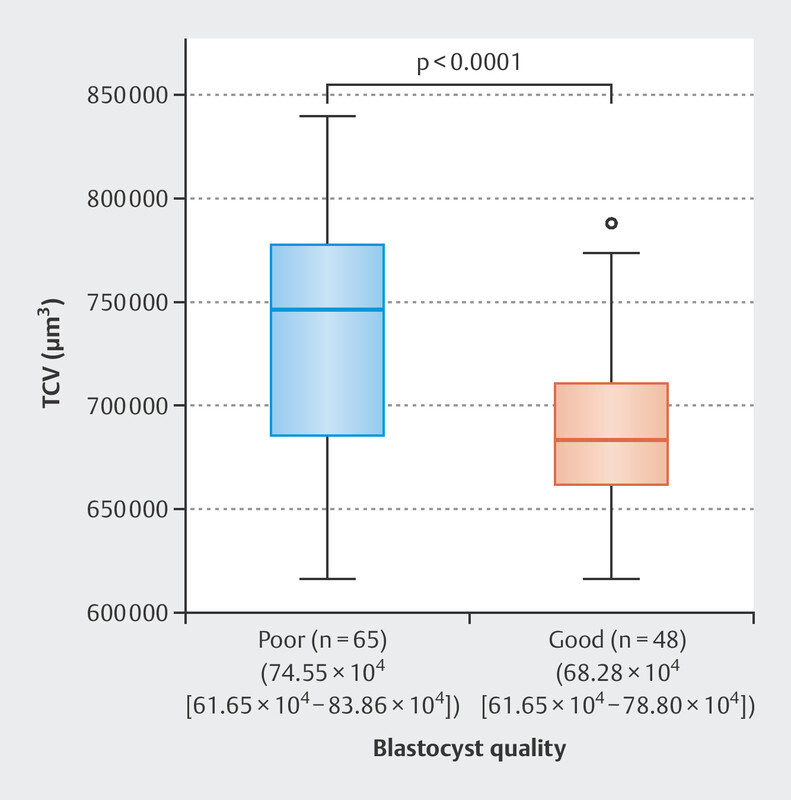
The difference in TCV of zygote between poor (n = 65) and good (n = 48) blastocyst quality groups (74.55 × 10 4 µm 3 [61.65 × 10 4 −83.86 × 10 4 ] vs 68.28 × 10 4 µm 3 [61.65 × 10 4 −78.80 × 10 4 ] retrospectively; p < 0.0001). Results are presented as median (range). p is calculated using the Mann–Whitney U test.
Fig. 3.
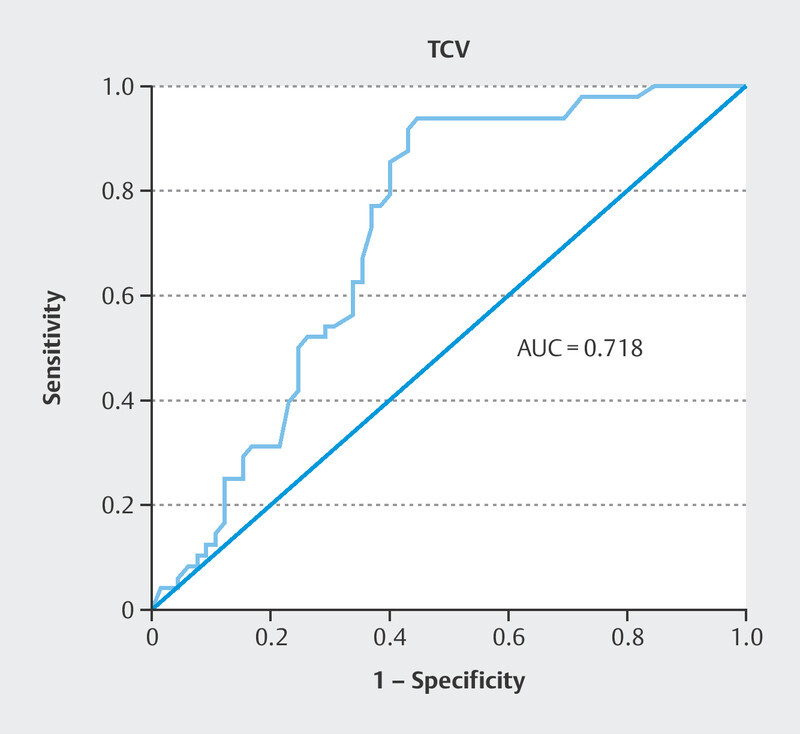
The ROC curve for blastocyst quality prediction by the TCV parameter.
Table 1 Values of morphokinetic parameters for embryos that achieved or did not achieve the blastocysts stage. Differences between groups were calculated for each parameter using the Mann–Whitney U test.
| Parameter | Blastocyst | Non-blastocyst | P-value |
|---|---|---|---|
| * Values are median time in hours. Results are present as median (range). p ≤ 0.05 were considered statistically significant. | |||
| Diameter (µm) | 110.37 (105.6–115.2) | 110.35 (104.9–116.3) | 0.79 |
| TCV (µm 3 ) | 69.12 × 10 4 (60.44 × 10 4 −82.36 × 10 4 ) | 70.83 × 10 4 (61.65 × 10 4 −83.86 × 10 4 ) | 0.49 |
| tPNf* | 22.00 (17–29) | 23.00 (18–56) | 0.004 |
| ECC 1(t2–tPNf)* | 3.00 (1–8) | 3.00 (0–17) | 0.004 |
| ECC 2(t4–t2)* | 13.00 (1–41) | 12.00 (0–29) | 0.93 |
| ECC 3(t8–t4)* | 26.00 (7–55) | 26.00 (10–70) | 0.84 |
| S2 (t4–t3)* | 1.00 (0–41) | 2.00 (0–16) | 0.44 |
| S3 (t8–t5)* | 15.00 (1–53) | 14.00 (0–62) | 0.33 |
| TM* | 89.00 (69–109) | 91.50 (75–114) | 0.66 |
| t4–TM* | 51.00 (31–73) | 54.00 (31–73) | 0.13 |
Fig. 4.
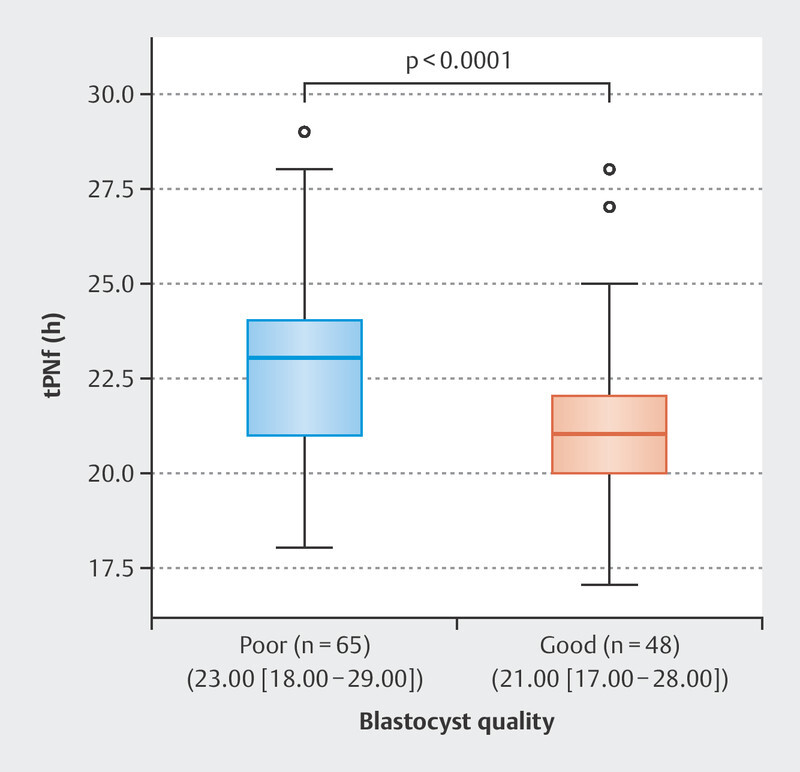
The difference in pronuclear fading time between poor (n = 65) and good (n = 48) blastocyst quality groups (23.00 h [18.00–29.00] vs 21.00 h [17.00–28.00], retrospectively; p < 0.0001). Results are presented as median (range). p is calculated using the Mann–Whitney U test.
Fig. 5.
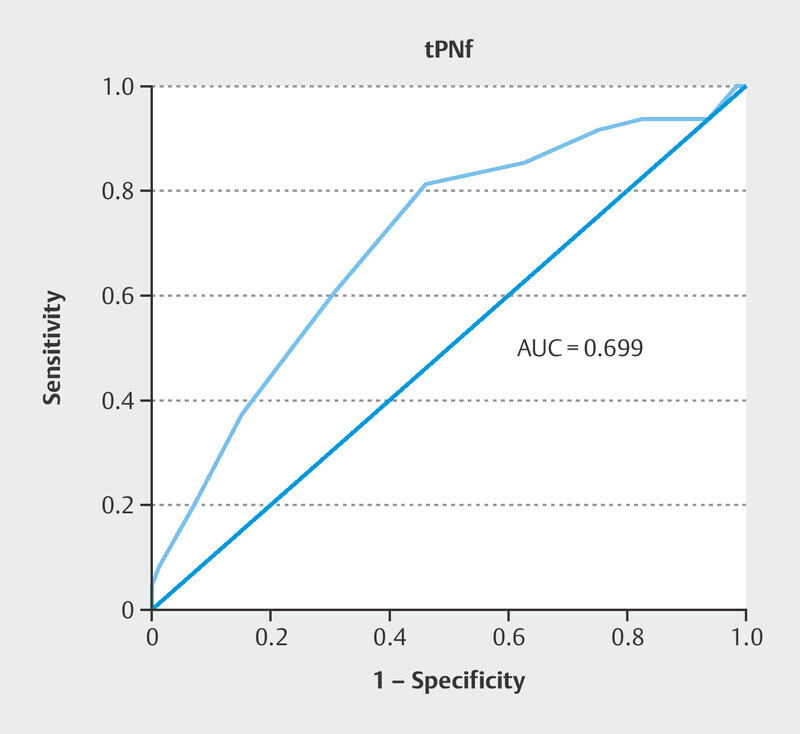
The ROC curve for blastocyst quality prediction by the tPNf parameter.
Table 2 Multiple binary logistic regression analyses in relation to the blastocyst quality.
| Parameter | P-value | Odds Ratio | 95% confidence interval | |
|---|---|---|---|---|
| TCV | 0.0001 | 0.857 | 0.787 | 0.933 |
| tPNf | 0.006 | 0.769 | 0.638 | 0.928 |
Cleavage embryo morphokinetic parameters
The duration of the first cell cycle division (p < 0.01), but not the timing of the second or third cell division, synchronicity, or late-stage cleavage patterns, differed significantly between embryos that formed blastocysts and those that did not ( Table 3 ). The ECC duration and late-stage cleavage patterns (TM, TSB, and TM–t4) differed significantly according to blastocyst quality ( Table 3 ). Out of the eleven evaluated parameters, only synchronicity of the second cell cycle did not statistically differ between good- and poor-quality blastocysts.
Table 3 Timing of embryo morphokinetic events analyzed from good and poor-quality blastocysts group. Differences between groups were calculated for each parameter using the Mann–Whitney U test.
| Parameter | Good-quality blastocyst | Poor-quality blastocyst | P-value |
|---|---|---|---|
| * Values are median time in hours. Results are presented as median (range). p ≤ 0.05 were considered statistically significant. | |||
| Diameter (µm) | 109.2 (105.6–111,6) | 112.1 (105.6–115.2) | 0.001 |
| TCV (µm 3 ) | 68.28 × 10 4 (61.65 × 10 4 −78.80 × 10 4 ) | 74.55 × 10 4 (61.65 × 10 4 −83.86 × 10 4 ) | < 0.001 |
| tPNf* | 21.00 (17–28) | 23.00 (18–29) | 0.004 |
| ECC 1(t2–tPNf)* | 2.00 (1–4) | 3.00 (1–8) | < 0.001 |
| ECC 2 (t4–t2)* | 13.00 (10–26) | 12.00 (0–41) | 0.011 |
| ECC 3 (t8–t4)* | 23.00 (14–41) | 29.00 (7–55) | 0.003 |
| S2 (t4–t3)* | 1.00 (0–13) | 1.00 (0–41) | 0.60 |
| S 3 (t8–t5)* | 8.00 (1–27) | 18.00 (0–53) | < 0.0001 |
| TM* | 85.00 (70–106) | 91.00 (69–109) | < 0.0001 |
| t4–TM* | 48.00 (31–69) | 53.00 (31–73) | 0.004 |
| tSB* | 94.50 (84–118) | 101.00 (77–121) | 0.003 |
Overall, 82.0% (64/78) of embryos with the tetrahedral arrangement reached the blastocyst stage, compared to 43.3% (49/113) of embryos with the non-tetrahedral arrangement (p < 0.0001). In addition, tetrahedral embryos more frequently formed good-quality blastocysts compare to the non-tetrahedral (85.4% [41/48] vs. 14.6% [7/48], p < 0.0001; Fig. 6 ).
Fig. 6.
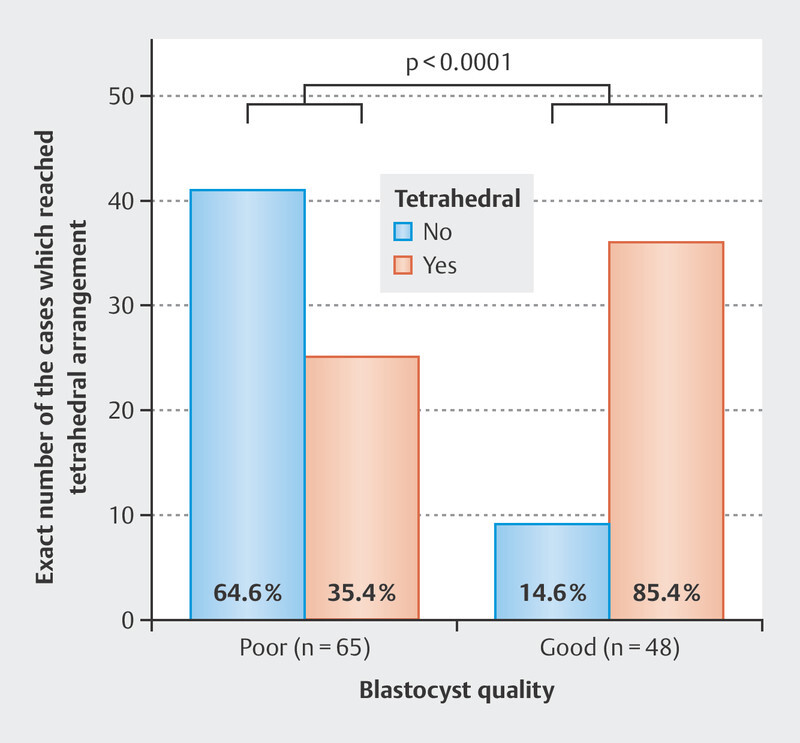
Blastocysts quality according to the tetrahedral arrangement of the embryo. Numbers at the bottom of columns refer to the percent of blastocysts in each category. p is calculated using the chi-square test.
Discussion
The purposes of this study were to assess relationships between embryo morphokinetic parameters and blastocyst quality using time-lapse data, and to determine whether the zygote diameter and TCV are useful for the noninvasive prediction of blastocyst quality.
Morphokinetic parameters differed between embryos that formed blastocysts and those with limited developmental potential, and according to blastocyst quality.
Previously reported time-lapse imaging data enabled us to define several morphokinetic variables (e.g., the timing of cell division and cleavage synchronicity) as markers of embryo quality and implantation potential 2 4 6 14 . However, current morphokinetic selection criteria focus mainly on unique embryo cohorts (i.e., those in the four- and eight-cell stages) 14 15 16 17 and may not be applicable in all clinics, especially in countries with strict reproductive medicine laws. For these reasons, we focused on zygote morphokinetics.
Zygotes with smaller diameters created better-quality blastocysts in this study. Although several studies have evaluated the relationship between oocyte diameter and embryo quality 18 19 , insufficient information is available for human zygotes, despite the accessibility of data acquired during assisted fertilization. To our knowledge, this study is the first to associate the zygote diameter with blastocyst formation and quality. Our findings, applied in combination with existing PN scoring practices 20 21 , might aid embryo selection in Germany. Zygotes with smaller TCVs showed less fragmentation during development, resulting in better blastocyst quality, in this study. Hinidas et al. 22 and Hinidas and Ziebe 23 first reported on the use of sequences of digital images obtained at certain intervals to calculate the degree of fragmentation by comparing the reduction in cytoplasmic volume from the zygote stage to the combined volume of individual blastomeres. Their findings confirmed that the TCV was a predictive biomarker of embryo quality 22 23 . TCVs on days 2 and 3 have been associated significantly with the pregnancy rate 13 24 , and the TCV on day 1 is considered to be important for the evaluation of the implantation potential 25 .
Differences in study designs, aims, and sample sizes prevent comparison of our TCV findings with those reported previously. As the TCV ranges in our good- and poor-quality blastocyst groups were similar, we performed additional tests to confirm the impact of the TCV on blastocyst quality. To our knowledge, this study is among the first to associate the zygote TCV with blastocyst formation and quality.
The tPNf was shorter for embryos that developed into good-quality blastocysts in this study, in line with previously published data 2 6 26 27 28 . A few studies conducted with sizable datasets have confirmed that the choice of culture medium does not affect PN fading 29 30 31 . What is more, obtained results about the impact of tPNf on blastocyst quality were in line with previously published results 27 28 .
More embryos with tetrahedral than with non-tetrahedral arrangements formed good-quality blastocysts in this study, in line with previous reports 32 33 . What is more, compared to our research, Paternot et al. 34 went one step further and reported that the live birth rate was significantly higher with the transfer of tetrahedral embryos than with non-tetrahedral embryo transfer (33% vs. 16%).
The ECC1, ECC2, and ECC3 durations differed significantly between our good- and poor-quality blastocyst groups. The first cell cycle division occurred more rapidly for good-quality blastocysts, in line with previous findings 2 10 . Most embryos divide during a narrow time range in the ECC1, and this range expands in ECC2 and ECC3, reflecting a difference in the cleavage rhythm between good- and poor-quality blastocyst populations, likely due to the onset of embryo gene expression 35 . Only a few studies have involved the analysis of ECC2 and ECC3 impacts on blastocyst quality, and their results are in line with our findings 36 37 .
In contrast to previous findings 2 10 36 37 38 , S2 did not differ between good- and poor-quality blastocysts in this study. One reason for this difference might be the fact that synchronicity of the second cell cycle in patients with endometriosis displayed significant irregularities 39 . Although compared to our research, the study design was different, Fréour et al. 40 noted that smoking affects early embryo morphokinetics. Differences in incubation conditions and patient populations may also have contributed to this inconsistency.
Much research has confirmed the importance of the morphogenetic observation of embryos in the late cleavage stages. In our study, late-stage cleavage patterns (TM, TSB, and TM–t4) differed between groups, in line with previously published findings 2 7 10 35 36 37 38 .
Based on the aforementioned facts, we believe that the findings presented in this research, applied in combination with previously established guidelines 41 42 , might provide a better diagnostic workup for infertility in Germany.
A limitation of this study was the lack of information on implantation and pregnancy outcomes. These aspects should be considered in future research.
Conclusion
The findings of this study confirm our knowledge of the major events occurring in embryo development and the hypothesis that zygote diameter and TCV patterns are associated with blastocyst quality. They support the inclusion of early morphokinetic parameters in embryo evaluation.
Financial Support and Sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Racowsky C, Kovacs P, Martins WP. A critical appraisal of time-lapse imaging for embryo selection: where are we and where do we need to go? J Assist Reprod Genet. 2015;32:1025–1030. doi: 10.1007/s10815-015-0510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai N, Ploskonka S, Goodman LR et al. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;12:54. doi: 10.1186/1477-7827-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhide P, Maheshwari A, Cutting R. Time lapse imaging: is it time to incorporate this technology into routine clinical practice? Hum Fertil (Camb) 2017;20:74–79. doi: 10.1080/14647273.2017.1283068. [DOI] [PubMed] [Google Scholar]
- 4.Faramarzi A, Khalili MA, Micara G et al. Revealing the secret life of pre-implantation embryos by time-lapse monitoring: A review. Int J Reprod Biomed. 2017;15:257–264. [PMC free article] [PubMed] [Google Scholar]
- 5.Alfarawati S, Fragouli E, Colls P et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Sundvall L, Ingerslev HJ, Breth Knudsen U et al. Inter- and intra-observer variability of time-lapse annotations. Hum Reprod. 2013;28:3215–3221. doi: 10.1093/humrep/det366. [DOI] [PubMed] [Google Scholar]
- 7.Chamayou S, Patrizio P, Storaci G et al. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet. 2013;30:703–710. doi: 10.1007/s10815-013-9992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrie A, Homburg R, McDowell G et al. Examining the efficacy of six published time-lapse imaging embryo selection algorithms to predict implantation to demonstrate the need for the development of specific, in-house morphokinetic selection algorithms. Fertil Steril. 2017;107:613–621. doi: 10.1016/j.fertnstert.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Beier HM, Beckman JO. Implications and consequences of the German Embryo Protection Act. Hum Reprod. 1991;6:607–608. doi: 10.1093/oxfordjournals.humrep.a137389. [DOI] [PubMed] [Google Scholar]
- 10.Kirkegaard K, Kesmodel US, Hindkjær JJ et al. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–2651. doi: 10.1093/humrep/det300. [DOI] [PubMed] [Google Scholar]
- 11.Minasi MG, Colasante A, Riccio T et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31:2245–2254. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 12.Gardner DK, Schoolcraft WB. Carnforth: Parthenon Press; 1999. In vitro Culture of human Blastocysts; pp. 378–388. [Google Scholar]
- 13.Paternot G, Debrock S, D’Hooghe T et al. Computer-assisted embryo selection: a benefit in the evaluation of embryo quality? Reprod Biomed Online. 2011;23:347–354. doi: 10.1016/j.rbmo.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Yoon HJ, Jang JM et al. Evaluation of human embryo development in in vitro fertilization- and intracytoplasmic sperm injection-fertilized oocytes: A time-lapse study. Clin Exp Reprod Med. 2017;44:90–95. doi: 10.5653/cerm.2017.44.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciray HN, Aksoy T, Goktas C et al. Time-lapse evaluation of human embryo development in single versus sequential culture media–a sibling oocyte study. J Assist Reprod Genet. 2012;29:891–900. doi: 10.1007/s10815-012-9818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freour T, Dessolle L, Lammers J et al. Comparison of embryo morphokinetics after in vitro fertilizationintracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99:1944–1950. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 17.Hojnik N, Vlaisavljević V, Kovačič B. Morphokinetic Characteristics and Developmental Potential of In Vitro Cultured Embryos from Natural Cycles in Patients with Poor Ovarian Response. Biomed Res Int. 2016;2016:4.286528E6. doi: 10.1155/2016/4286528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassil R, Casper RF, Meriano J et al. Can Oocyte Diameter Predict Embryo Quality? Reprod Sci. 2021;28:904–908. doi: 10.1007/s43032-020-00306-3. [DOI] [PubMed] [Google Scholar]
- 19.Romão GS, Araújo MCPM, de Melo AS et al. Oocyte diameter as a predictor of fertilization and embryo quality in assisted reproduction cycles. Fertil Steril. 2010;93:621–625. doi: 10.1016/j.fertnstert.2008.12.124. [DOI] [PubMed] [Google Scholar]
- 20.Scott L. The biological basis of non-invasive strategies for selection of human oocytes and embryos. Hum Reprod Update. 2003;9:237–249. doi: 10.1093/humupd/dmg023. [DOI] [PubMed] [Google Scholar]
- 21.Zollner U, Zollner KP, Steck T et al. Pronuclear scoring. Time for international standardization. J Reprod Med. 2003;48:365–369. [PubMed] [Google Scholar]
- 22.Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. Hum Reprod. 2004;19:288–293. doi: 10.1093/humrep/deh070. [DOI] [PubMed] [Google Scholar]
- 23.Hnida C, Ziebe S. Total cytoplasmic volume as biomarker of fragmentation in human embryos. J Assist Reprod Genet. 2004;21:335–340. doi: 10.1023/b:jarg.0000045473.80338.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paternot G, Debrock S, De Neubourg D et al. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum Reprod. 2013;28:627–633. doi: 10.1093/humrep/des427. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, De Neubourg D, Debrock S et al. Selecting the embryo with the highest implantation potential using a data mining-based prediction model. Reprod Biol Endocrinol. 2016;14:10. doi: 10.1186/s12958-016-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman LR, Goldberg J, Falcone T et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275–285. doi: 10.1016/j.fertnstert.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Minasi MG, Greco P, Varricchio MT et al. The clinical use of time-lapse in human-assisted reproduction. Ther Adv Reprod Health. 2020;14:2.633494120976921E15. doi: 10.1177/2633494120976921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs C, Nicolielo M, Erberelli R et al. Correlation between morphokinetic parameters and standard morphological assessment: what can we predict from early embryo development? A time-lapse-based experiment with 2085 blastocysts. JBRA Assist Reprod. 2020;24:273–277. doi: 10.5935/1518-0557.20190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basile N, Morbeck D, García-Velasco J et al. Type of culture media does not affect embryo kinetics: a time-lapse analysis of sibling oocytes. Hum Reprod. 2013;28:634–641. doi: 10.1093/humrep/des462. [DOI] [PubMed] [Google Scholar]
- 30.Costa-Borges N, Bellés M, Meseguer M et al. Blastocyst development in single medium with or without renewal on day 3: a prospective cohort study on sibling donor oocytes in a time-lapse incubator. Fertil Steril. 2016;105:707–713. doi: 10.1016/j.fertnstert.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Desai N, Yao M, Richards EG et al. Randomized study of G-TL and global media for blastocyst culture in the EmbryoScope: morphokinetics, pregnancy, and live births after single-embryo transfer. Fertil Steril. 2020;114:1207–1215. doi: 10.1016/j.fertnstert.2020.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Desai N, Gill P. Blastomere cleavage plane orientation and the tetrahedral formation are associated with increased probability of a good-quality blastocyst for cryopreservation or transfer: a time-lapse study. Fertil Steril. 2019;111:1159–1168. doi: 10.1016/j.fertnstert.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Cauffman G, Verheyen G, Tournaye H. Developmental capacity and pregnancy rate of tetrahedral- versus non-tetrahedral-shaped 4-cell stage human embryos. J Assist Reprod Genet. 2014;31:427–434. doi: 10.1007/s10815-014-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paternot G, Debrock S, De Neubourg D et al. The spatial arrangement of blastomeres at the 4-cell stage and IVF outcome. Reprod Biomed Online. 2014;28:198–203. doi: 10.1016/j.rbmo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Wong CC, Loewke KE, Bossert NL et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 36.Dal Canto M, Coticchio G, Mignini Renzini M et al. Cleavage kinetics analysis of human embryos predicts development to blastocyst and implantation. Reprod Biomed Online. 2012;25:474–480. doi: 10.1016/j.rbmo.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Cetinkaya M, Pirkevi C, Yelke H et al. Relative kinetic expressions defining cleavage synchronicity are better predictors of blastocyst formation and quality than absolute time points. J Assist Reprod Genet. 2015;32:27–35. doi: 10.1007/s10815-014-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz M, Garrido N, Herrero J et al. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25:371–381. doi: 10.1016/j.rbmo.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Boynukalin FK, Serdarogullari M, Gultomruk M et al. The impact of endometriosis on early embryo morphokinetics: a case-control study. Syst Biol Reprod Med. 2019;65:250–257. doi: 10.1080/19396368.2019.1573275. [DOI] [PubMed] [Google Scholar]
- 40.Fréour T, Dessolle L, Lammers J et al. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99:1944–1950. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 41.Toth B, Baston-Büst DM, Behre HM et al. Diagnosis and therapy before assisted reproductive treatments. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Register Number 015–085, February 2019)—part 1, basic assessment of the woman. Geburtshilfe Frauenheilkd. 2019;79:1278–1292. doi: 10.1055/a-1017-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wischmann T, Borkenhagen A, Dorn A et al. Psychosomatically oriented diagnostics and therapy for fertility disorders. Guideline of the DGPFG (S2k-Level, AWMF Registry Number 016/003, December 2019) Geburtshilfe Frauenheilkd. 2021;81:749–768. doi: 10.1055/a-1341-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]


