Abstract
Background
A wave of the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has rapidly spread in Shanghai, China. Hematological abnormalities have been reported in coronavirus disease 2019 (COVID‐19) patients; however, the difference in hematological parameters between COVID‐19 patients with fever and patients who are febrile from other causes remains unexplored.
Methods
This retrospective cohort study enrolled 663 SARS‐CoV‐2 positive patients identified by RT‐PCR. Clinical parameters, including age, sex, and threshold cycle values of all COVID‐19 patients, and hematological parameters of COVID‐19 patients in the fever clinic were abstracted for analysis.
Results
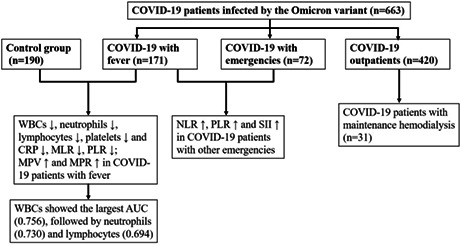
Overall, 60.8% of COVID‐19 patients were male, and the median age was 45 years. Most of COVID‐19 patients were asymptomatic, while 25.8% of patients showed fever and 10.9% of patients had other emergencies. COVID‐19 patients with fever had significantly lower white blood cells (WBCs), neutrophils, lymphocytes, platelets and C‐reactive protein (CRP), and significantly higher monocyte‐to‐lymphocyte ratio (MLR), platelet‐to‐lymphocyte ratio (PLR), mean platelet volume (MPV), and mean platelet volume‐to‐platelet ratio (MPR) levels, compared with those in SARS‐CoV‐2 negative patients with fever from other causes (p < 0.05). Neutrophil‐to‐lymphocyte ratio (NLR), PLR, and systemic inflammatory index (SII) levels were significantly higher in COVID‐19 patients with emergencies (p < 0.05). WBCs showed the best performance with an area under the curve (0.756), followed by neutrophils (0.730) and lymphocytes (0.694) in the diagnosis of COVID‐19 in the fever clinic.
Conclusion
WBCs, neutrophils, lymphocytes, platelets, CRP and MLR, PLR, and MPR may be useful in early diagnosis of COVID‐19 in the fever clinic.
Keywords: coronavirus disease 2019, differential diagnosis, hematological parameters, inflammation, severe acute respiratory syndrome coronavirus 2
This study aimed to investigate the clinical and hematological characteristics of COVID‐19 patients infected by the Omicron variant during the recent coronavirus outbreak in the city of Shanghai, China, from March 1 to May 31, 2022. Hematological parameters including WBCs, neutrophils, lymphocytes, platelets, hs‐CRP and MLR, PLR, and MPR can be used for early diagnosis of COVID‐19 in the fever clinic.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has spread rapidly in more than 200 countries worldwide and has been announced as a public health emergency. The SARS‐CoV‐2 virus evolved continuously since its emergence. In November 2021, the B.1.1.529 variant of SARS‐CoV‐2 was first identified in South Africa and Botswana and named the Omicron variant by the World Health Organization (WHO). 1 , 2
The Omicron variant has a remarkable number of mutations, resulting in higher transmissibility than previous SARS‐CoV‐2 variants. 3 Fortunately, the clinical symptoms of Omicron variant infection are mainly asymptomatic or mild, with a low risk of hospitalization and death. 4 , 5 Currently, the Omicron variant of SARS‐CoV‐2 has overtaken other variants as the dominant transmission strain globally. In late February 2022, a wave of Omicron variant infection suddenly appeared in Shanghai, China. According to the Shanghai Municipal Health Commission, as of May 4, 2022, a total of 601,942 cases have been identified, including 547,056 asymptomatic carriers, and 503 people have died of causes associated with COVID‐19. 6
Hematological parameters play an important role in the early diagnosis of various inflammatory diseases, such as malignancies, 7 , 8 metabolic disorder, 9 and infection. 10 Furthermore, COVID‐19 is associated with increased inflammatory burden. 11 Therefore, hematological abnormalities might be associated with COVID‐19 infection. Previous studies have reported hematological abnormalities in COVID‐19 patients; 12 , 13 however, information on the differences in hematological parameters between COVID‐19 patients with fever and patients who are febrile from other causes remains limited. In addition, these differences in hematological parameters might be used to predict clinical manifestations of COVID‐19 patients and aid in making informed clinical decisions and risk stratification for COVID‐19 patients. Thus, this study aimed to investigate the clinical and hematological characteristics of COVID‐19 patients infected the Omicron variant during the recent coronavirus outbreak in the city of Shanghai, China. We reported this study following the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines. 14 , 15
2. MATERIALS AND METHODS
2.1. Subjects
In this retrospective and cross‐sectional study, we consecutively enrolled a total of 663 COVID‐19 infected patients at the Shanghai Changzheng Hospital, China, from March 1 to May 31, 2022. COVID‐19 infection was diagnosed by real‐time reverse transcription polymerase chain reaction (RT‐PCR) in all cases. Clinical parameters including age, sex, cycle threshold (Ct) value and hematological parameters were abstracted from the electronic medical records. COVID‐19 positive patients who lacked the complete blood count (CBC) test were excluded from subsequent comparisons. Patients with fever who were negative for SARS‐CoV‐2 by RT‐PCR test were collected as a control group. This study was approved by the Ethics Board of the hospital and conducted in accordance with the Declaration of Helsinki. This study had no effect on the subsequent management of patients. There were no adverse events during the study.
2.2. Laboratory tests
Hematological parameters were detected using a Sysmex XN‐9000 automated hematology analyzers (Sysmex Corp., Kobe, Japan). Ratios were calculated as follows: NLR, neutrophil‐to‐lymphocyte ratio; MLR, monocyte‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; MPR, mean platelet volume‐to‐platelet ratio; and systemic inflammatory index (SII), thrombocyte count × neutrophil count/lymphocyte count.
Nasopharyngeal swabs were taken from all specimens for Ct value analysis. Detection was performed by RT‐PCR with the SARS‐CoV‐2 nucleic acid detection kit (Shanghai Zhijiang Biotechnology Co., Ltd.) following the manufacturer's instructions.
2.3. Statistical analysis
The Kolmogorov–Smirnov test was performed to evaluate variable distribution. Continuous variables were presented as median (interquartile range, IQR) and analyzed by nonparametric Mann–Whitney or Kruskal–Wallis test as appropriate. 16 Categorical variables were analyzed by Fisher's exact test or Chi‐square test. The receiver‐operating characteristic (ROC) analysis was performed, and the area under the curve (AUC) was calculated. Optimal cut‐off value, sensitivity and specificity were estimated according to the Youden index. All analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and MedCalc 19.7.2 software (MedCalc Software, Ostend, Belgium). A p value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics of patients infected by the Omicron variant of SARS‐CoV‐2
The flow chart of COVID‐19 patient selection and study design was presented in Figure 1. A total of 663 COVID‐19 patients from March 1 to May 31, 2022 were included, and the demographic and clinical characteristics of all patients were summarized in Table 1. The majority of patients (465/663, 70%) were diagnosed in April, and 403 patients (60.8%) were male. The median age was 45 years (IQR: 31–62 years); 10 patients (1.5%) were children aged <18 years, and 42 patients (6.3%) were aged ≥80 years. Of 663 patients, 31 patients were hemodialysis patients.
FIGURE 1.
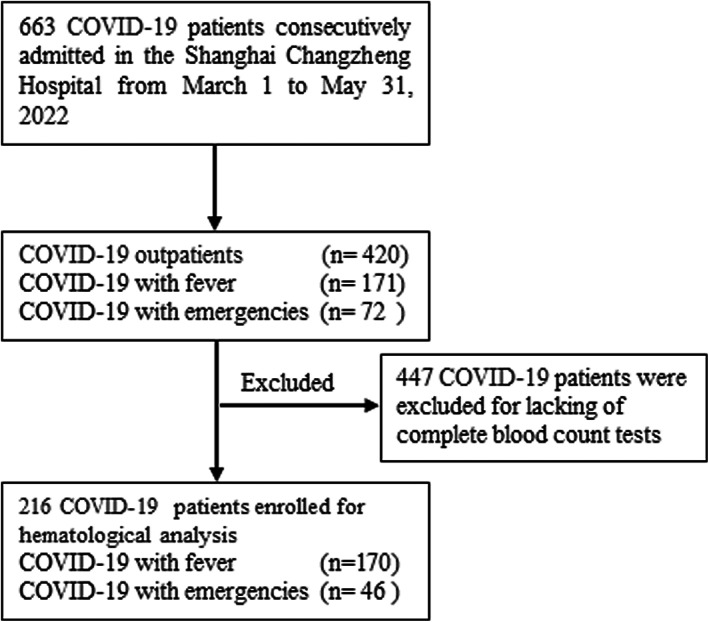
Flow chart of COVID‐19 patient selection.
TABLE 1.
Clinical characteristics of 663 COVID‐19 patients infected with Omicron variant
| Characteristic | Total patients | COVID‐19 outpatients | COVID‐19 with fever | COVID‐19 with emergencies | p‐Value a |
|---|---|---|---|---|---|
| No. (%) | 663 | 420 (63.4) | 171 (25.8) | 72 (10.9) | |
| Distribution, n (%) | |||||
| March | 112 (16.9%) | 94 (22.4%) | 18 (10.5%) | 0 (0) | |
| April | 465 (70.1%) | 297 (70.7%) | 108 (63.2%) | 60 (83.3%) | |
| May | 86 (13.0%) | 29 (6.9%) | 45 (26.3%) | 12 (16.7%) | |
| Sex, n (%) | |||||
| Male | 403 (60.8%) | 296 (70.5%) | 75 (43.9%) | 32 (44.4%) | <0.001 |
| Female | 260 (39.2%) | 124 (29.5%) | 96 (56.1%) | 40 (55.6%) | |
| Age, year | 45 (31–62) | 40 (30–52) | 59 (37–71) | 66 (48–75) | <0.001 |
| Age groups, n (%) | |||||
| <18 years | 10 (1.5%) | 3 (0.7%) | 6 (3.5%) | 1 (1.4%) | |
| 18–49 years | 363 (54.8%) | 291(69.3%) | 54 (31.6%) | 18 (25.0%) | |
| 50–79 years | 248 (37.4%) | 121 (28.8%) | 89 (52.0%) | 38 (52.8%) | |
| ≥80 years | 42 (6.3%) | 5 (1.2%) | 22 (12.9%) | 15 (20.8%) | |
| RT‐PCR Ct value | |||||
| ORF1ab gene | 27.4 (23.1–32.1) | 28.3 (23.5–32.7) | 25.5 (22.4–29.4) | 27.5 (23.2–32.8) | <0.001 |
| N gene | 26.3 (21.8–32.2) | 27.7 (22.5–33.1) | 24.0 (20.6–29.2) | 26.3 (22.1–32.9) | <0.001 |
Comparison of three subgroups. Data are shown as number (%) or median (interquartile range). RT‐PCR, reverse transcription polymerase chain reaction; Ct, cycle threshold.
We further divided all COVID‐19 patients into three subgroups: outpatients, patients with fever, and patients with other emergencies. 420 patients (63.4%) were COVID‐19 outpatients who tested positive for SARS‐CoV‐2 by RT‐PCR tests, and most of them were asymptomatic. There were 171 COVID‐19 patients with a symptom of fever (25.8%) and 72 COVID‐19 patients (10.9%) presented to the emergency department with other emergencies. Significant differences were observed in the gender and age makeup of the three COVID‐19 subgroups (p < 0.001): in COVID‐19 patients with fever, 43.9% were male, and the median age was 59 years; while in COVID‐19 patients with other emergencies, 44.4% were male, and the median age was 66 years. Compared with COVID‐19 outpatients (median age 40 years), COVID‐19 patients with fever or other emergencies were older (median ages 59 and 66 years, respectively, p < 0.001). The Ct values of SARS‐CoV‐2 gene of COVID‐19 patients with fever (25.5 and 24.0) were lower than those of outpatients (28.3 and 27.7, p < 0.01) and patients with other emergencies (27.5 and 26.3, p < 0.05).
3.2. Hematological parameters of patients infected by the Omicron variant of SARS‐CoV‐2
A total of 190 patients with fever who were negative for SARS‐CoV‐2 were collected as a control group. Hematology parameters in each subgroup of COVID‐19 patients and the control group were analyzed. As shown in Table 2, no statistically significant difference was found in gender or age between the control group and COVID‐19 patients with fever (p > 0.05). Compared with the control group, the counts of white blood cells (WBCs), neutrophils, lymphocytes, platelets, and C‐reactive protein (CRP) were significantly lower in COVID‐19 patients with fever (p < 0.01); MLR, PLR, mean platelet volume (MPV), and MPR levels were significantly higher in COVID‐19 patients with fever (p < 0.05). No significant difference was found in red blood cells, hemoglobin levels, or hematocrit between the two groups (p > 0.05), and these parameters were within the normal range, indicating that anemia is not a significant factor in most COVID‐19 patients. Additional comparisons showed that NLR, PLR, and SII levels were significantly higher in COVID‐19 patients with other emergencies, compared with those in COVID‐19 patients with fever (p < 0.05).
TABLE 2.
The hematological profiles of COVID‐19 patients infected by the Omicron variant and control group as indicated
| Variable | Control group (1) | COVID‐19 with fever (2) | COVID‐19 with emergences (3) | p‐Value | |
|---|---|---|---|---|---|
| (n = 190) | (n = 170) | (n = 46) | 1 vs. 2 | 2 vs. 3 | |
| Male/Female | 101/89 | 75/95 | 22/24 | 0.092 | 0.739 |
| Age, year | 57 (34–73) | 59 (37–71) | 68 (57–80) | 0.603 | 0.024 |
| WBC, ×109/L | 9.6 (7.3–12.35) | 6.40 (4.80–8.30) | 6.75 (5.68–9.45) | <0.001 | 0.213 |
| Neutrophils, ×109/L | 7.21 (5.00–9.71) | 4.67 (3.28–6.10) | 4.96 (3.63–8.43) | <0.001 | 0.081 |
| Lymphocytes, ×109/L | 1.44 (0.94–2.04) | 0.93 (0.60–1.40) | 0.77 (0.42–1.25) | <0.001 | 0.086 |
| Monocytes, ×109/L | 0.7 (0.48–1.0) | 0.67 (0.48–0.88) | 0.63 (0.45–0.89) | 0.354 | 0.613 |
| NLR | 4.6 (2.90–8.57) | 4.96 (2.78–8.10) | 6.99 (3.71–12.83) | 0.571 | 0.008 |
| MLR | 0.49 (0.31–0.75) | 0.72 (0.47–1.09) | 0.82 (0.44–1.62) | <0.001 | 0.194 |
| RBC, ×1012/L | 4.65 (4.14–5.06) | 4.58 (4.18–4.99) | 4.41 (4.00–4.70) | 0.647 | 0.03 |
| Hemoglobin, g/L | 138 (125–152) | 136 (123–148) | 136 (123–142) | 0.225 | 0.526 |
| Hematocrit, % | 41.6 (38.05–45.00) | 40.50 (37.20–44.25) | 40.00 (36.73–42.93) | 0.193 | 0.206 |
| MCV, fL | 89.9 (87.3–92.9) | 89.10 (86.30–91.33) | 90.65 (87.65–93.80) | 0.033 | 0.033 |
| MCHC, gL | 334 (326–342) | 333 (323–341) | 334 (329–345) | 0.348 | 0.078 |
| MCH, pg | 29.9 (29.2–31.0) | 29.70 (28.50–30.80) | 30.50 (29.45–31.53) | 0.055 | 0.001 |
| RDW, % | 12.4 (11.9–13.2) | 12.30 (11.90–13.20) | 12.25 (11.90–13.03) | 0.513 | 0.846 |
| Platelets, ×109/L | 200 (158–259) | 176 (145–227) | 202 (153–247) | 0.008 | 0.165 |
| PLR | 140.21 (105.34–204.43) | 184.12 (130.94–293.99) | 258.57 (157.59–449.65) | <0.001 | 0.018 |
| MPV, fL | 9.60 (8.90–10.30) | 9.80 (9.20–10.40) | 10.10 (9.60–10.60) | 0.016 | 0.157 |
| MPR | 0.048 (0.036–0.065) | 0.053 (0.042–0.069) | 0.050 (0.038–0.067) | 0.013 | 0.272 |
| CRP, mg/L | 24.19 (2.19–66.24) | 5.53 (0.98–13.29) | 4.67 (2.76–25.46) | <0.001 | 0.112 |
| SII | 998.35 (593.23–1868.21) | 823.38 (472.68–1702.68) | 1368.57 (682.35–2411.92) | 0.091 | 0.006 |
Note: Values are presented as median (interquartile range) for continuous variables. p‐values with statistical differences were shown in bold.
Abbreviations: WBC, white blood cell; NLR, neutrophil‐to‐lymphocyte ratio; MLR, monocyte‐to‐lymphocyte ratio; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell width distribution; PLR, platelet‐to‐lymphocyte ratio; MPV, mean platelet volume; MPR, mean platelet volume‐to‐platelet ratio; CRP, C‐reactive protein; SII, systemic inflammatory index.
3.3. Performance of hematological parameters in the diagnosis of patients infected by the Omicron variant of SARS‐CoV‐2 in the fever clinic
Since the hematological analysis revealed significant differences between COVID‐19 patients with fever and those with fever from other causes, ROC analysis was then performed. The subgroup of COVID‐19 patients with fever was set as the positive group, and the patients with fever from other causes was set as the negative group. ROC curve was established to analyze the efficacy of various hematological parameters for the diagnosis of COVID‐19 in the fever clinic. As shown in Table 3, the AUC of WBCs was 0.756 (95% confidence interval (CI): 0.706–0.805; p < 0.001); the AUC of neutrophils was 0.730 (95% CI: 0.678–0.782; p < 0.001); the AUC of lymphocytes was 0.694 (95% CI: 0.640–0.749; p < 0.001); the AUC of platelets was 0.581 (95% CI: 0.522–0.640; p = 0.008); the AUC of MPV was 0.574 (95% CI: 0.515–0.633; p = 0.016); the AUC of MLR was 0.660 (95% CI: 0.603–0.716; p < 0.001); the AUC of PLR was 0.643 (95% CI: 0.586–0.700; p < 0.001); and the AUC of CRP was 0.672 (95% CI: 0.615–0.728; p < 0.001). The difference between the AUCs for WBCs and neutrophils was statistically significant (p = 0.0028) (Figure 2). WBCs showed the largest AUC, followed by neutrophils and lymphocytes, indicating that WBCs is the most discriminative hematological parameter for clinical diagnosis of COVID‐19 with fever.
TABLE 3.
The value of hematological parameters in diagnosis of COVID‐19 patients in the fever clinic
| Variable | AUC | Cut off | Sensitivity (%) | Specificity (%) | 95% CI | p‐Value |
|---|---|---|---|---|---|---|
| WBCs, ×109/L | 0.756 | ≤7.25 | 62.9 | 76.7 | 0.706–0.805 | <0.001 |
| Neutrophils, ×109/L | 0.730 | ≤5.07 | 62.4 | 74.6 | 0.678–0.782 | <0.001 |
| Lymphocytes, ×109/L | 0.694 | ≤1.26 | 72.4 | 62.4 | 0.640–0.749 | <0.001 |
| Platelets, ×109/L | 0.581 | ≤242 | 83.5 | 31.7 | 0.522–0.640 | 0.008 |
| MPV, fL | 0.574 | >8.95 | 86.6 | 26.5 | 0.515–0.633 | 0.016 |
| MLR | 0.660 | >0.605 | 64.1 | 64.2 | 0.603–0.716 | <0.001 |
| PLR | 0.643 | >144 | 71.2 | 52.6 | 0.586–0.700 | <0.001 |
| MPR | 0.576 | >0.036 | 88.2 | 26.8 | 0.517–0.635 | 0.013 |
| CRP, mg/L | 0.672 | ≤13.37 | 75.9 | 59.7 | 0.615–0.728 | <0.001 |
Abbreviations: AUC, area under the curve; CI, confidence intervals; WBC, white blood cell; MPV, mean platelet volume; MLR, monocyte‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; MPR, mean platelet volume‐to‐platelet ratio; CRP, C‐reactive protein.
FIGURE 2.
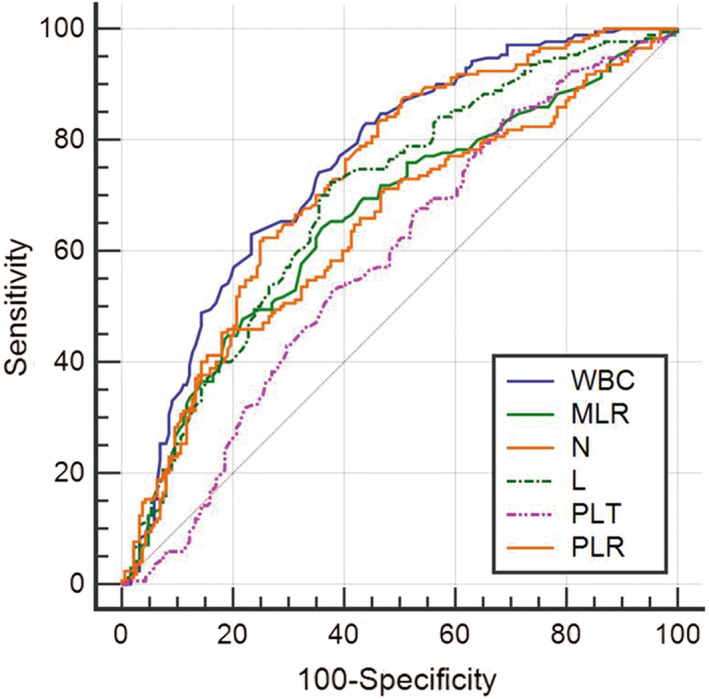
The receiver operating characteristic (ROC) curves of hematological parameters in diagnosis of COVID‐19 patients in the fever clinic. WBC, white blood cell; MLR, monocyte‐to‐lymphocyte ratio; N, neutrophils; L, lymphocytes; PLT, platelets; PLR, platelet‐to‐lymphocyte ratio.
4. DISCUSSION
COVID‐19 is a global pandemic that poses a serious threat to human life and health, particularly for people with underlying diseases and the elderly. Hematological abnormalities have been reported in COVID‐19 patients in some previous studies; 12 however, few studies have compared hematological parameters in COVID‐19 patients with fever versus those with fever from other causes. Given the rapid onset and spread of COVID‐19, early and effective differential diagnosis is critical.
Previous studies have shown that the pathogenicity, severity, and mortality of COVID‐19 decreased during the wide spread of the Omicron variant. 17 In this study, a total of 663 SARS‐CoV‐2 positive patients were included, most of whom were asymptomatic. Furthermore, asymptomatic patients were more likely to be males and middle‐aged adults, while COVID‐19 patients with fever or other emergencies were more likely to be females and elderly. In addition, comparison of Ct values by RT‐PCR showed that the viral load of COVID‐19 patients with fever was higher than those of other subgroups.
In line with other studies, 13 , 18 , 19 we observed that COVID‐19 patients with fever had lower leukocytes, neutrophils, lymphocytes, and platelets and CRP levels compared with those in patients who were febrile from other causes. This might be related to the pathological effects of SARS‐CoV‐2 on the hematological and immune system. 20 Lymphopenia is the most common hematological abnormality in patients with COVID‐19 infection, which is generally considered to be a defective immune response to viral infection. 11 Lymphopenia observed in COVID‐19 may be related to the virus's ability to infect lymphocytes via angiotensin‐converting enzyme 2 (ACE2) receptors, leading to the apoptosis of lymphocytes, and enhanced immune escape ultimately. 21 , 22 The presence of thrombocytopenia has been reported to be associated with the severity of the COVID‐19 and organ failure, and the mechanistic study suggested that platelets take up SARS‐CoV‐2 mRNA might be independent of ACE2. 23
Not only do lymphocytes and platelets played an important role in regulating various inflammatory processes but their ratio may also be an indicator of early inflammation. 24 MLR, PLR, and MPR have been reported as significant predictors for the severity and mortality of COVID‐19. 25 , 26 , 27 In the present study, among patients with fever, MLR, PLR, and MPR levels were significantly higher in COVID‐19 patients than those in patients who were febrile from other causes. These inflammatory indices have been considered as indicators for the diagnosis and progression assessment of various diseases, especially inflammatory diseases. Elevated MLR has been reported to be a feature not only of COVID‐19 infection but also of diabetic kidney injury, 28 non‐alcoholic fatty liver disease (NAFLD), 29 and cancer. 30 , 31 Raised PLR has been also reported in other inflammatory conditions including liver fibrosis, 32 thyroid conditions, 33 , 34 diabetes mellitus, 35 and systemic lupus erythematosus. 36 Increased MPR has been shown to be associated with hepatitis B‐related liver fibrosis, 32 sepsis, 37 and cancer. 38 MPR reflects the proliferation of megakaryocytes and platelet production in the bone marrow. 39 Inflammation in COVID‐19 leads to a decrease in platelet counts, inducing the production of thrombopoietin, which in turn accelerates platelets production. Young platelets released from the bone marrow are usually larger, performed as increased MPV, which in combination with thrombocytopenia, leads to an increase in MPR. 39 , 40
NLR is also known as an inflammatory marker in inflammatory diseases such as inflammatory bowel disease, 41 diabetes mellitus, 42 , 43 cardiac conditions, 44 and thyroiditis. 45 In addition, elevated NLR has been reported to be associated with the severity of COVID‐19. 26 , 46 SII, which relies on thrombocytes, neutrophils and lymphocytes, has been used to define the inflammatory response and predict in‐hospital mortality in COVID‐19 patients. 47 However, in our study, no statistically significant difference was found in NLR or SII between COVID‐19 patients with fever and those with fever from other causes, while a significant increase was found in NLR and SII in COVID‐19 patients with other emergencies. This difference in results might be related to the immune status of the enrolled patients or the excessive inflammatory response caused by complications associated with emergency patients.
In the present study, ROC curve comparison showed that WBCs might be the most valuable hematological parameter for clinical diagnosis of COVID‐19 with fever. Low WBC, neutrophil, and lymphocyte counts, readily available and cost‐effective hematological parameters, could assist clinicians in the early differential diagnosis of COVID‐19 infection in fever clinic. In 2020, some studies have explored machine learning models to predict SARS‐CoV‐2 infection 48 and mortality risk 49 of COVID‐19 based on clinical data. However, these models were trained in moderate or life‐threatening inpatients, which were not applicable to asymptomatic or mild COVID‐19 cases. In the outbreak of COVID‐19 in Shanghai in 2022, most patients with the Omicron variant were asymptomatic or mild with a low risk of hospitalization, leading to little data from CBC testing and other laboratory tests that could be collected. Therefore, it was difficult to perform a machine learning model to predict the infection of Omicron variant based on available laboratory data.
This study had some limitations. Because this was a single‐center retrospective study, the number of COVID‐19 patients in different subgroups was small, so some bias might have occurred. Second, as our hospital was not a designated isolation hospital, this study could only analyze the initial hematological parameters in COVID‐19 patients and could not conduct continuous monitoring of patients during hospitalization or predict the mortality risk stratification. Therefore, further prospective and multi‐center studies are needed to evaluate the prognostic value of these indicators in COVID‐19.
5. CONCLUSIONS
This retrospective study showed that most of COVID‐19 patients infected by the Omicron variant in Shanghai were asymptomatic. Among the COVID‐19 patients with fever, most of them were female, with an older median age and a lower median Ct value. Low leukocytes, neutrophils, platelets, and CRP as well as high MLR, PLR, MPV, and MPR levels were valuable for the differential diagnosis of COVID‐19 patients in the fever clinic. In addition, high NLR and SII values might indicate a high inflammatory status in COVID‐19 patients with other emergency conditions.
FUNDING INFORMATION
This project was supported by National Natural Science Foundation of China (Grant No. 82072371, 82202584), Leading Talents Program of Shanghai Huangpu District (Grant No. 2020‐1‐28), and Foundation of (Grant No. 2021QN37).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Wei T, Li J, Cheng Z, et al. Hematological characteristics of COVID‐19 patients with fever infected by the Omicron variant in Shanghai: A retrospective cohort study in China. J Clin Lab Anal. 2023;37:e24808. doi: 10.1002/jcla.24808
Tingting Wei, Jiangyan Li and Zhuo Cheng contributed equally to this work.
Contributor Information
Hao Wang, Email: whowardwh@163.com.
Lin Zhou, Email: lynnzhou36@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Wang K, Jia Z, Bao L, et al. Memory B cell repertoire from triple vaccinees against diverse SARS‐CoV‐2 variants. Nature. 2022;603(7903):919‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Classification of Omicron (B.1.1.529): SARS‐CoV‐2 Variant of Concern. WHO; 2021. [Google Scholar]
- 3. Guo Y, Han J, Zhang Y, et al. SARS‐CoV‐2 omicron variant: epidemiological features, biological characteristics, and clinical significance. Front Immunol. 2022;13:877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID‐19 omicron wave compared with previous waves. JAMA. 2022;327(6):583‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS‐CoV‐2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X, Zhang W, Chen S. Shanghai's life‐saving efforts against the current omicron wave of the COVID‐19 pandemic. Lancet. 2022;399(10340):2011‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei TT, Wang LL, Yin JR, et al. Relationship between red blood cell distribution width, bilirubin, and clinical characteristics of patients with gastric cancer. Int J Lab Hematol. 2017;39(5):497‐501. [DOI] [PubMed] [Google Scholar]
- 8. Sit M, Aktas G, Ozer B, et al. Mean platelet volume: an overlooked herald of malignant thyroid nodules. Acta Clin Croat. 2019;58(3):417‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kocak MZ, Aktas G, Erkus E, Duman TT, Atak BM, Savli H. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018;28(11):844‐847. [DOI] [PubMed] [Google Scholar]
- 10. Lin SF, Lin HA, Pan YH, Hou SK. A novel scoring system combining modified early warning score with biomarkers of monocyte distribution width, white blood cell counts, and neutrophil‐to‐lymphocyte ratio to improve early sepsis prediction in older adults. Clin Chem Lab Med. 2022;61:162‐172. [DOI] [PubMed] [Google Scholar]
- 11. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rahman A, Niloofa R, Jayarajah U, De Mel S, Abeysuriya V, Seneviratne SL. Hematological abnormalities in COVID‐19: a narrative review. Am J Trop Med Hyg. 2021;104(4):1188‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Usul E, San I, Bekgoz B, Sahin A. Role of hematological parameters in COVID‐19 patients in the emergency room. Biomark Med. 2020;14(13):1207‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng FF, Shen WH, Gong F, et al. Adherence to the standards for reporting of diagnostic accuracy studies (STARD): a survey of four journals in laboratory medicine. Ann Transl Med. 2021;9(11):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu ZD, Zhou ZR, Qian S. How to analyze tumor stage data in clinical research. J Thorac Dis. 2015;7(4):566‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid‐19 severity with omicron variant in South Africa. N Engl J Med. 2022;386(14):1314‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Chen N, Zhao D, Zhang J, Hu Z, Tao Z. Clinical characteristics of COVID‐19 patients infected by the omicron variant of SARS‐CoV‐2. Front Med. 2022;9:912367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmadi E, Bagherpour Z, Zarei E, Omidkhoda A. Pathological effects of SARS‐CoV‐2 on hematological and immunological cells: alterations in count, morphology, and function. Pathol Res Pract. 2022;231:153782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saxena SK, Kumar S, Ansari S, et al. Characterization of the novel SARS‐CoV‐2 omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. 2022;94(4):1738‐1744. [DOI] [PubMed] [Google Scholar]
- 22. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID‐19. Blood. 2020;136(11):1317‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cognasse F, Duchez AC, Audoux E, et al. Platelets as key factors in inflammation: focus on CD40L/CD40. Front Immunol. 2022;13:825892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qu R, Ling Y, Zhang YH, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020;92(9):1533‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fors M, Ballaz S, Ramirez H, et al. Sex‐dependent performance of the neutrophil‐to‐lymphocyte, monocyte‐to‐lymphocyte, platelet‐to‐lymphocyte and mean platelet volume‐to‐platelet ratios in discriminating COVID‐19 severity. Front Cardiovasc Med. 2022;9:822556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghobadi H, Mohammadshahi J, Javaheri N, Fouladi N, Mirzazadeh Y, Aslani MR. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR‐I, and SII) on admission predicts in‐hospital mortality in non‐elderly and elderly COVID‐19 patients. Front Med. 2022;9:916453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kocak MZ, Aktas G, Duman TT, et al. Monocyte lymphocyte ratio As a predictor of diabetic kidney injury in type 2 diabetes mellitus; the MADKID study. J Diabetes Metab Disord. 2020;19(2):997‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohsari M, Moradinazar M, Rahimi Z, Najafi F, Pasdar Y, Shakiba E. New inflammatory biomarkers (lymphocyte and monocyte percentage to high‐density lipoprotein cholesterol ratio and lymphocyte to monocyte percentage ratio) and their association with some cardiometabolic diseases: results from a large Kurdish cohort study in Iran. Wien Klin Wochenschr. 2022;134(17–18):626‐635. [DOI] [PubMed] [Google Scholar]
- 30. Jiang Y, Chen S, Wu Y, et al. Establishment and validation of a novel prognostic model for non‐virus‐related hepatocellular carcinoma. Cancer Cell Int. 2022;22(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu Y, Sun J, Li D, Li Y, Li T, Hu Y. The combined role of PET/CT metabolic parameters and inflammatory markers in detecting extensive disease in small cell lung cancer. Front Oncol. 2022;12:960536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kosekli MA. Mean platelet volume and platelet to lymphocyte count ratio are associated with hepatitis B‐related liver fibrosis. Eur J Gastroenterol Hepatol. 2022;34(3):324‐327. [DOI] [PubMed] [Google Scholar]
- 33. Bala MM, Bala KA. Bone mineral density (BMD) and neutrophil‐lymphocyte ratio (NLR), monocyte‐lymphocyte ratio (MLR), and platelet‐lymphocyte ratio (PLR) in childhood thyroid diseases. Eur Rev Med Pharmacol Sci. 2022;26(6):1945‐1951. [DOI] [PubMed] [Google Scholar]
- 34. Wang W, Tong Y, Sun S, et al. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol. 2022;12:936206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Chen H, Cui S, et al. Platelet‐lymphocyte ratio, neutrophil‐lymphocyte ratio and their dynamic changes with type 2 diabetes mellitus: a cohort study in China. Endocr Res. 2022;47:1‐15. [DOI] [PubMed] [Google Scholar]
- 36. Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372‐376. [DOI] [PubMed] [Google Scholar]
- 37. Velez‐Paez JL, Legua P, Velez‐Paez P, et al. Mean platelet volume and mean platelet volume to platelet count ratio as predictors of severity and mortality in sepsis. PLoS One. 2022;17(1):e0262356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo E, Zhang C, Guo L, et al. Prognostic value of platelet distribution width and mean platelet volume in patients with laryngeal cancer. Future Oncol. 2021;17(9):1025‐1037. [DOI] [PubMed] [Google Scholar]
- 39. Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. 2016;2016:9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Z, Li X, Zhang M, et al. The role of mean platelet volume/platelet count ratio and neutrophil to lymphocyte ratio on the risk of febrile seizure. Sci Rep. 2018;8(1):15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Posul E, Yilmaz B, Aktas G, Kurt M. Does neutrophil‐to‐lymphocyte ratio predict active ulcerative colitis? Wien Klin Wochenschr. 2015;127(7–8):262‐265. [DOI] [PubMed] [Google Scholar]
- 42. Bilgin S, Aktas G, Zahid Kocak M, et al. Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. Aging Male. 2020;23(5):923‐927. [DOI] [PubMed] [Google Scholar]
- 43. Duman TT, Aktas G, Atak BM, Kocak MZ, Erkus E, Savli H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019;19(1):1602‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu X, Cheang I, Xu F, et al. Long‐term prognostic value of inflammatory biomarkers for patients with acute heart failure: construction of an inflammatory prognostic scoring system. Front Immunol. 2022;13:1005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil‐to‐lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras (1992). 2017;63(12):1065‐1068. [DOI] [PubMed] [Google Scholar]
- 46. Khalid A, Ali Jaffar M, Khan T, et al. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS‐COV‐2 infected patients of Pakistan: a retrospective comparative analysis. Hematology. 2021;26(1):529‐542. [DOI] [PubMed] [Google Scholar]
- 47. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts In‐hospital mortality in COVID‐19 patients. Molecules. 2020;25(23):5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang HS, Hou Y, Vasovic LV, et al. Routine laboratory blood tests predict SARS‐CoV‐2 infection using machine learning. Clin Chem. 2020;66(11):1396‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao Y, Cai GY, Fang W, et al. Machine learning based early warning system enables accurate mortality risk prediction for COVID‐19. Nat Commun. 2020;11(1):5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


