Abstract
Background
The accuracy of alpha‐fetoprotein (AFP) as a diagnostic marker for hepatocellular carcinoma (HCC) is insufficient, and the application of abnormal prothrombin (PIVKA‐II) in HCC is still controversial.
Methods
Serum AFP and PIVKA‐II levels were analyzed in 145 cases of HCC, 57 of benign liver disease, 55 of cholangiocarcinoma and gallbladder carcinoma, 112 of other gastrointestinal tumors with liver metastasis, and 101 healthy controls. Receiver operating characteristic curve and area under the curve were used to evaluate the diagnostic value of AFP and PIVKA‐II for HCC. The changes in serum AFP and PIVKA‐II levels before and after treatment in 47 HCC patients who underwent surgery and 77 who received interventional treatment were used to evaluate treatment efficacy and prognosis in HCC.
Results
The concentrations of AFP and PIVKA‐II in the HCC group were significantly higher than those in other groups (p < 0.01). The diagnostic value of PIVKA‐II in HCC was better than that of AFP, and combined detection improved the diagnostic sensitivity and specificity. The levels of AFP and PIVKA‐II after liver cancer surgery were significantly lower than those before surgery. Elevated levels of PIVKA‐II before surgery predicted disease progression, and patients who remained positive for PIVKA‐II after surgery had worse prognosis than those who became negative after surgery.
Conclusions
Combined detection of AFP and PIVKA‐II is superior to both tests alone. We found that higher serum level of PIVKA‐II indicates more severe HCC, with worse prognosis, while the level of AFP had no correlation with the prognosis.
Keywords: abnormal prothrombin, alpha‐fetoprotein, cancer diagnosis, hepatocellular carcinoma, prognosis
Expression level of serum AFP in each group. Expression level of serum PIVKA‐II in each group. *p < 0.05; **p < 0.01; ***p < 0.001; ns p > 0.05. ROC curves of PIVKA‐II, AFP and PIVKA‐II + AFP in the diagnosis of HCC and benign liver disease. ROC curves of PIVKA‐II, AFP and PIVKA‐II + AFP in the diagnosis of HCC and healthy controls.

1. INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the digestive system. It ranks fifth for morbidity and third for mortality among malignant tumors worldwide, and is a serious threat to health and life. 1 , 2 , 3 The onset of liver cancer is insidious, and most cases are diagnosed as intermediate or advanced, resulting in poor prognosis. Serum alpha ‐fetoprotein (AFP) is one of the main indicators of early screening of liver cancer, and it is recommended that high‐risk groups undergo liver ultrasound and AFP tests every 6 months 4 ;however, the sensitivity and specificity of AFP for HCC diagnosis are only 68.8% and 87.6%, respectively. 5 For patients with AFP‐negative and small tumors, biomarkers with higher sensitivity and specificity are needed to aid diagnosis. Abnormal prothrombin (PIVKA‐II, protein induced by vitamin K absence/antagonist‐II) is a predictive biomarker for AFP‐negative HCC patients. 6 , 7 Since 1984, when Liebman et al. 8 first proposed PIVKA‐II, it has increasingly been used as a specific biomarker for liver cancer. At present, the Japan Society of Hepatology lists PIVKA‐II in its guidelines as an important biological indicator for liver cancer detection. 9 The Chinese Guidelines for Prevention and Treatment of Chronic Hepatitis B 10 recommend PIVKA‐II as an important indicator for diagnosis of HCC, which can be combined with AFP to facilitate early diagnosis. The main treatment methods for liver cancer are surgery, interventional chemotherapy, and radiofrequency ablation, but the radical cure rate is low. Recurrence and metastasis are likely to occur and affect prognosis. AFP and PIVKA‐II are significant in the evaluation of treatment efficacy and prognosis of HCC. Patients with elevated PIVKA‐II are more prone to recurrence and metastasis. 11 , 12 However, there are few reports on the correlation between serum AFP and PIVKA‐II in the prognosis of HCC patients. In this study, we investigated the value of serum AFP and PIVKA‐II in the diagnosis, treatment and prognosis of HCC, and aimed to provide a laboratory basis for their better clinical application.
2. METHODS
2.1. General information
A total of 470 subjects were registered in this retrospective study, including 145 with HCC 57 with benign liver disease (BLD; Among them, 33 cases of hepatic hemangioma, 10 cases of hepatic cyst, 2 cases of hepatic abscess, 4 cases of cirrhosis, 8 cases of hepatic benign tumor), 55 with cholangiocarcinoma and gallbladder cancer (CGC), 112 with other gastrointestinal tumors (colorectal, gastric or duodenum) with liver metastasis, and 101 healthy controls (HCs). HCC was diagnosed by pathology or imaging according to the Standardization for Diagnosis and Treatment of Primary Hepatic Carcinoma (2022 edition). None of the subjects took vitamin K, vitamin antagonists or antibiotics, excluding patients with cholestasis, renal insufficiency and other serious systemic diseases. Incomplete clinical data due to loss of follow‐up and other reasons were not included. The trial was carried out according to the Declaration of Helsinki (revised in 2013). This study was approved by the local Ethics Committee of the Zhejiang Cancer Hospital (IRB‐2022‐389). The flow chart of all subjects is shown in Figure 1. Tables 1 and 2 summarize the baseline characteristics of all subjects and the clinical characteristics in the HCC group.
FIGURE 1.
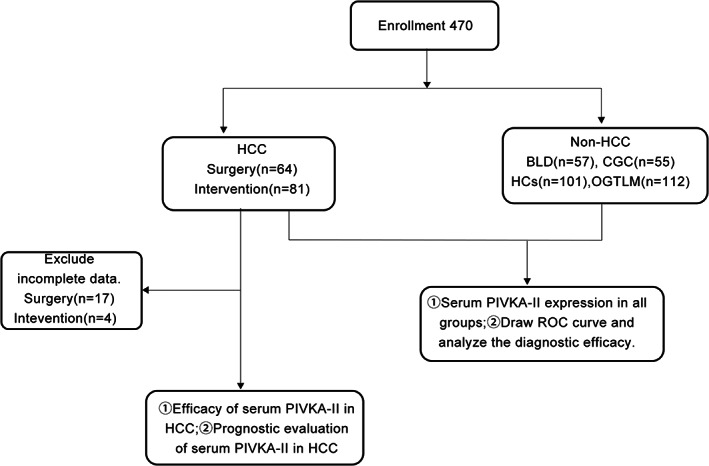
Flowchart of all subjects
TABLE 1.
Baseline characteristics for all study groups
| GROUP | HCC | BLD | CGC | HCs | OGTLM |
|---|---|---|---|---|---|
| N | 145 | 57 | 55 | 101 | 112 |
| Gender n(%) | |||||
| Male | 121(83.4%) | 14(24.6%) | 26(47.3%) | 44(43.6%) | 81(72.3%) |
| Female | 24(16.6%) | 43(75.4%) | 29(52.7%) | 57(56.4%) | 31(27.7) |
| Age(years) | 58.9 ± 10.8 | 48.1 ± 11.6 | 64 ± 10.3 | 39.8 ± 11.3 | 58.7 ± 10.3 |
| PIVKA‐II (mAU/ml) | 1068.54(152.26 ~ 8049.18) | 23.11(19.15 ~ 28.58) | 28.95(19.75 ~ 75.17) | 25.22(21.42 ~ 31.67) | 32.19(25.08 ~ 42.57) |
| AFP (ng/ml) | 52.8(6.15 ~ 1546.6) | 1.9(1.05 ~ 2.85) | 2.4(1.1 ~ 3.9) | 1.7(0.85 ~ 3.0) | 2.8(1.93 ~ 4.9) |
Abbreviations: AFP, alpha‐fetoprotein; BLD, benign liver disease; CGC, cholangiocarcinoma and gallbladder carcinoma; HCC, hepatocellular carcinoma; HCs, healthy controls; N, number of patients; OGTLM, other gastrointestinal tumors with liver metastasis, PIVKA‐II, prothrombin induced by vitamin K absence II.
TABLE 2.
Correlation of serum PIVKA‐II and AFP with clinical characteristics of HCC
| Characteristics | N | PIVKA‐II ≤ 40 mAU/ml | PIVKA‐II >40 mAU/ml | p value | AFP ≤8 ng/ml | AFP > 8 ng/ml | p value |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≤50 | 27 | 4 | 23 | 0.864 | 4 | 23 | 0.209 |
| >50 | 118 | 16 | 102 | 31 | 87 | ||
| Sex | |||||||
| Male | 121 | 13 | 108 | 0.017* | 28 | 93 | 0.529 |
| Female | 24 | 7 | 17 | 7 | 17 | ||
| Tumor size(cm) | |||||||
| ≤5 | 64 | 14 | 50 | 0.012* | 20 | 44 | 0.075 |
| >5 | 81 | 6 | 75 | 15 | 66 | ||
| CNLC stage | |||||||
| I–IIa | 84 | 16 | 68 | 0.031* | 26 | 58 | 0.024* |
| IIb–IV | 61 | 4 | 57 | 9 | 52 | ||
| Tumor number | |||||||
| Single | 99 | 17 | 82 | 0.083 | 27 | 72 | 0.196 |
| Multiple | 46 | 3 | 43 | 8 | 38 | ||
| PVTT | |||||||
| No | 108 | 18 | 90 | 0.086 | 31 | 77 | 0.028* |
| Yes | 37 | 2 | 35 | 4 | 33 | ||
| Lymphatic metastasis | |||||||
| No | 97 | 13 | 84 | 0.846 | 26 | 71 | 0.286 |
| Yes | 48 | 7 | 41 | 9 | 39 | ||
| Hepatitis B | |||||||
| No | 37 | 6 | 31 | 0.080 | 13 | 24 | 0.070 |
| Yes | 108 | 14 | 94 | 22 | 86 | ||
| Hepatic cirrhosis | |||||||
| No | 94 | 12 | 82 | 0.626 | 27 | 67 | 0.080 |
| Yes | 51 | 8 | 43 | 8 | 43 | ||
Abbreviations: AFP, alpha‐fetoprotein; N, number of patients; PIVKA‐II, prothrombin induced by vitamin K absence II; PVTT, portal vein tumor thrombus.
Significant difference (p < 0.05).
2.2. Instrumentation and specimen processing
Alpha‐fetoprotein was measured using the Centaur XP immuno‐chemiluminescence instrument (Siemens), and PIVKA‐II was measured using the Alinity immuno‐chemiluminescence instrument (Laboratories). Fasting blood samples were collected from patients, and the serum was centrifuged at 318 6 g for 10 min (Allegra X‐12; Beckman), and the tests for each index were completed within 2 h. All specimens were free of chyle and hemolysis, and the assays were strictly performed according to the standard operating procedure (SOP) of the project. The manufacturer set the normal values of ≤8 ng/ml for AFP and ≤ 40 mAU/ml for PIVKA‐II.
2.3. Data processing and follow‐up
Sixty‐four patients in the HCC group were followed up at the outpatient clinic or by telephone after discharge. (The deadline is June 31, 2022). We recorded disease control, progression or death within the cut‐off date from the date of first blood collection after admission, and calculated progression‐free survival (PFS). The observation period was from December 1, 2020 to June 31, 2022, and the average follow‐up time was 297 days. There was no loss to follow‐up in this study.
2.4. Statistical methods
Statistical analysis was performed using SPSS version 22.0 (IBM), and metrological data were expressed as mean ± standard deviation (normally distributed data) or median (skewed distribution data). Metrological data were analyzed by t test or rank sum test, and comparison between groups of numerical data was by χ 2 test. p < 0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated. Survival analysis was performed using the Kaplan–Meier method.
3. RESULTS
3.1. Serum AFP and PIVKA‐II levels in HCC patients
In the analysis of the five groups, serum AFP and PIVKA‐II in HCC were significantly higher than those in the other groups (p < 0.01). The levels of PIVKA‐II in the CGC group were significantly higher than those in the BLD and HC groups (p < 0.05), but there was no significant difference between the BLD and HC groups (p > 0.05). As shown in Figure 2a,b.
FIGURE 2.
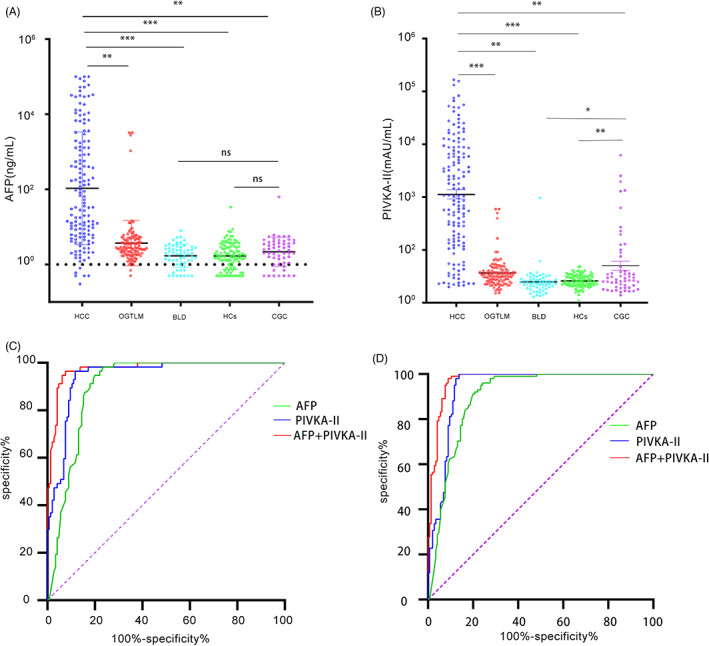
(A) Expression level of serum AFP in each group. (B) Expression level of serum PIVKA‐II in each group. * p < 0.05; ** p < 0.01; *** p < 0.001; ns p > 0.05. (C) ROC curves of PIVKA‐II, AFP and PIVKAII + AFP in the diagnosis of HCC and benign liver disease; (D) ROC curves of PIVKA‐II, AFP and PIVKA‐II + AFP in the diagnosis of HCC and healthy controls. AFP, alpha‐fetoprotein; HCC, hepatocellular carcinoma; PIVKA‐II, prothrombin induced by vitamin K absence II
3.2. Diagnostic performance of AFP and PIVKA‐II in HCC patients
To study the diagnostic value of AFP and PIVKA‐II in HCC patients, ROC curve analysis was performed in the HCC, BLD and HC groups. We compared the diagnostic performance of AFP. PIVKA‐II and AFP + PIVKA‐II. In comparison of HCC and BLD, the AUC of AFP, PIVKA‐II and their combination was 0.903 (0.862_0.944), 0.945 (0.915_0.975) and 0.975 (0.956_0.994), respectively. The optimal cut‐off values for AFP and PIVKA‐II were 5.6 ng/ml and 38.91 mAU/ml, respectively. The diagnostic sensitivity of AFP, PIVKA‐II and their combination was 76.6%,88.3%and 92.4%, and the diagnostic specificity was 98.2%,96.5% and 96.5%, respectively. In comparison of HCC and HCs, the AUC of AFP, PIVKA‐II and their combination were 0.901 (0.861_0.940), 0.938 (0.907_0.970) and 0.972 (0.954_0.991), respectively. The optimal cut‐off values of AFP was 6 ng/ml, and PIVKA‐II was 49.74 mAU/ml. The diagnostic sensitivity of AFP, PIVKA‐II and their combination were 75.9%,86.2% and 91.0%, and the diagnostic specificity was 96%, 100% and 98%, respectively. The results showed that a combination of AFP and PIVKA‐II improved the diagnostic efficiency. As shown in Figure 2c,d.
3.3. Application of AFP and PIVKA‐II for assessment of curative effect in HCC patients
Among 145 HCC patients, AFP and PIVKA‐II levels were detected before and 1 month after treatment. There were 47 patients in the surgery group and 77 in the intervention group. Serum AFP level after surgery was significantly lower than preoperatively (p < 0.05), as was PIVKA‐II (p < 0.01). PIVKA‐II was more sensitive than AFP in assessing the response to surgery (Figure 3). According to the mRESIST criteria, 13 1 month after interventional treatment without any target lesion enhancement was defined as effective. There were 43 patients in the treatment effective group and 34 in the treatment ineffective group. Compared with before surgery, postoperative serum levels of AFP and PIVKA‐II in the effective interventional treatment group were lower (p < 0.05). The preoperative and postoperative differences in the treatment ineffective group were not significant (p > 0.05).
FIGURE 3.
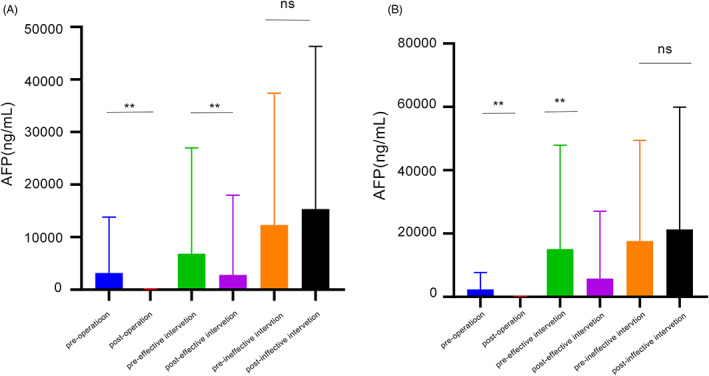
(A) AFP value in each group before& after treatment. (B) PIVKA‐II value in each group before& after treatment. AFP, alpha‐fetoprotein; PIVKA‐II, prothrombin induced by vitamin K absence II
3.4. Evaluation of AFP and PIVKA‐II in prognosis of HCC patients
Sixty‐four patients with HCC surgery were followed up at outpatient clinics or by telephone (cut‐off date June 31, 2022), and PFS was calculated by recording disease control, progression or death between admission and follow‐up. AFP ≤ 8 ng/ml and PIVKA‐II ≤ 40 mAU/ml were defined as the negative group; AFP >8 ng/ml and PIVKA‐II > 40 mAU/ml were defined as the positive group (Figure 4a,b). The patients whose PIVKA‐II remained positive at 1 month after surgery had shorter PFS than those in whom PIVKA‐II became negative after surgery (negative group, mean 448 (95% CI: 165.1–730.8) days; positive group, mean 163 (0.0–418.6) days; log rank p = 0.011). That is, patients who remained positive after surgery had poor prognosis. AFP had no prognostic value in patients (p > 0.05). After interventional therapy, patients who responded to treatment were followed up. AFP and PIVKA‐II levels decreased in six cases, but increased again when HCC relapsed, (Figure 4c,d). Post‐treatment follow‐up detection using serum biomarkers is beneficial for monitoring and prognostic assessment of HCC relapse.
FIGURE 4.
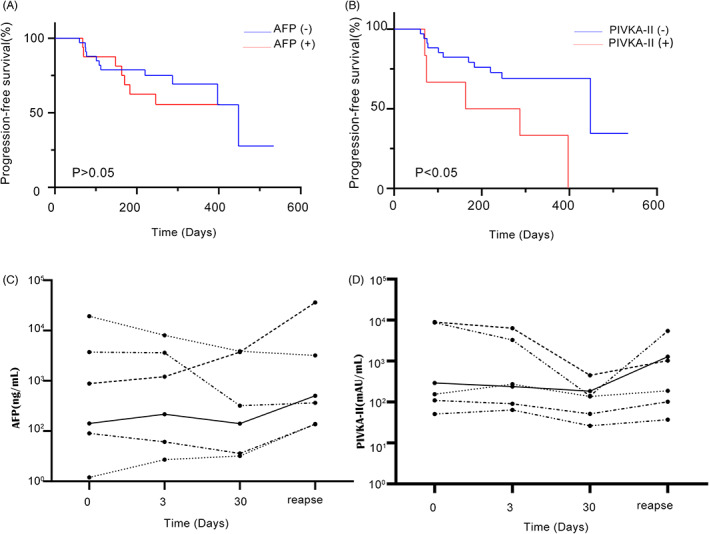
(A) PFS of AFP negative group and AFP positive group after 1 month operation. (B) PFS of PIVKA‐II negative group and PIVKA‐II positive group after 1month operation. (C) Serial AFP changes in 6 patients who responded to treatment after TACE and at the time of HCC relapse. (D) Serial PIVKA‐II changes in 6 patients who responded to treatment after TACE and at the time of HCC relapse. AFP, alpha‐fetoprotein; HCC, hepatocellular carcinoma; PFS, progression‐free survival; PIVKA‐II, prothrombin induced by vitamin K absence II
4. DISCUSSION
Hepatocellular carcinoma is a malignant tumor with high morbidity and mortality. The incidence of liver cancer is gradually increasing worldwide. 14 Abnormal prothrombin (PIVKA‐II; also known as γ‐carboxy prothrombin) is produced in HCC, because of the lack of γ‐carboxylated amino acid residues, produced by insufficient blood coagulation. Normal liver produces prothrombin under vitamin K action, but abnormal prothrombin is produced in patients with vitamin K deficiency or HCC. AFP is mainly synthesized by fetal liver cells and yolk sac, and has a high concentration in fetal blood circulation, but decreases after birth. Sixty to 70 percent of patients with primary liver cancer have elevated AFP levels. AFP is produced by undifferentiated hepatocytes, and is one of the most commonly used biomarkers to detect primary liver cancer. 15 PIVKA‐II is not related to the function of AFP, so it can be used as to supplement diagnosis using AFP. 16 , 17 , 18 AFP shows a transient increase in pregnancy and active hepatitis, which has also been the case for a few secondary liver cancers. 19 In our study, the analysis included five groups, and serum AFP and PIVKA‐II concentrations in HCC patients were significantly higher compared with those in the control groups. ROC curve analysis showed that for comparison between HCC and HCs, the AUC of AFP + PIVKA‐II was 0.972, the sensitivity was 91% and specificity was 98%. PIVKA‐II alone was superior to AFP, and their combined efficacy was better, which is consistent with the literature. 20 , 21 , 22 For comparison of HCC and BLD, AFP + PIVKA‐II had an AUC of 0.975, sensitivity of 92.4% and specificity of 96.5%. This can be used as a differential diagnostic index of HCC and liver metastatic carcinoma and BLD. The level of plasma PIVKA‐II in CGC patients was significantly higher than that in the BLD and HC groups, and this may serve as a potential biomarker for diagnosis of CGC.
The serum half‐life (40–72 h) of PIVKA‐II is shorter than that of AFP (5–7 days), which can better monitor their efficacy in HCC. 23 Judging from the levels of AFP and PIVKA‐II in patients 1 month after surgery, the decrease of PIVKA‐II was significantly lower than that of AFP, and it was more sensitive than AFP in evaluating the efficacy of surgery for HCC. In the evaluation of the effectiveness of interventional therapy, AFP and PIVKA‐II also showed advantages. Serum AFP and PIVKA‐II levels in the effective group after treatment were significantly lower than those before treatment. However, in the ineffective group, the difference before and after treatment was not significant. AFP and PIVKA‐II decreased in six patients with effective interventional therapy under continuous monitoring, but increased again upon relapse. Therefore, AFP and PIVKA‐II can be used to evaluate the curative effect in HCC and are prognostic factors in liver cancer. 24 , 25
The level of PIVKA‐II is associated with early recurrence and is an independent risk factor for recurrence. Active treatment and close monitoring are recommended after liver cancer resection. 26 This study showed that patients whose PIVKA‐II remained positive at 1 month after surgery had a shorter PFS than those whose PIVKA‐II was negative after surgery. Patients with high preoperative PIVKA‐II and those who remained positive for PIVKA‐II postoperatively had poor prognosis, and AFP failed to show any advantage. This confirmed the importance of PIVKA‐II as a biomarker for postoperative prognostic evaluation of liver cancer, which has rarely been reported. PIVKA‐II is a good prognostic biological marker for early monitoring of treatment in HCC patients. 27 Our results provide further support for clinically assisted assessment of liver cancer prognosis. In studies of the prognostic value of HCC surgery, C‐reactive protein/albumin ratio ≥0.037 can also be used as an independent prognostic factor in HCC patients and is superior to other inflammatory indicators. 28 Other studies have performed continuous follow‐up monitoring of serum AFP and PIVKA‐II in HCC patients undergoing liver transplantation. Among the 242 HCC patients followed up, recurrence was found in 41 (16.9%). Almost half of the patients who had recurrence had one or more elevated tumor markers, and elevation of both markers was suggestive of post‐transplantation recurrence. 29 PIVKA‐II was elevated in a small number of patients with intrahepatic cholangiocarcinoma, and the nomogram (including AFP, PIVKA‐II, CA125, CA199 and HBV) based on the study of Si et al. better distinguished HCC from intrahepatic cholangiocarcinoma. 30 PIVKA‐II has made progress in the diagnosis and prediction of other tumors, such as gastric or pancreatic cancer. 31 , 32 , 33 As a serological indicator of liver cancer prognosis, the advantages of PIVKA‐II are gradually being reported.
This study had several advantages over previous studies. First, in previous studies, the three main groups comprised patients with HCC or BLD and healthy individuals, but since PIVKA‐II is also elevated in some other gastrointestinal tumors, there is still some controversy about the diagnostic accuracy of PIVKA‐II. To elucidate the diagnostic role of AFP and PIVKA‐II in HCC, we included 470 subjects, including 145 patients with HCC, 57 with BLD, 55 with CGC, 112 with other gastrointestinal tumors with liver metastases, and 101 HCs. Second, we fully validated the responsiveness of serum AFP and PIVKA‐II in HCC treatment. By dividing patients into surgery and interventional treatment groups, serum AFP and PIVKA‐II could be used to assess the efficacy of HCC treatment, with PIVKA‐II being more sensitive. Third, the correlation between serum AFP and PIVKA‐II expression levels and prognosis in HCC patients has been rarely studied. AFP has been widely accepted as a traditional diagnostic marker, but our study found that PIVKA‐II, another serum marker used as a complementary diagnostic for AFP, was significantly correlated with prognosis 1 month after surgery in HCC treated with surgery, and the higher the PIVKA‐II, the worse the prognosis. In contrast, there was no correlation between the level of AFP and patient prognosis. In order to prolong survival of liver cancer, it is advisable to use serum PIVKA‐II as an indicator for monitoring the prognosis of HCC treatment; because of its advantages of being economic, minimally invasive, and convenient, it can dynamically monitor postoperative patients and evaluate treatment efficacy.
However, there were also some limitations to this study. First, this was a single‐center retrospective study with a small sample size. There was some bias, so more accurate conclusions cannot be drawn. It is necessary to verify the results in a multicenter, large‐sample randomized controlled study. Second, the in‐depth internal related tumor biological mechanisms still need to be studied. Third, we did not follow up longer to dynamically observe the correlation between changes in serum PIVKA‐II after HCC treatment and prognosis. Although serum biomarkers are not widely accepted in clinical practice, they should be recognized for early diagnosis, treatment monitoring, and prognostic analysis.
5. CONCLUSIONS
In summary, serum AFP and PIVKA‐II levels are good biological indicators for diagnosis and assessment of treatment efficacy in HCC patients. Elevation of PIVKA‐II has greater value than AFP in predicting disease progression, and can provide a reference for predicting the survival of patients.
AUTHOR CONTRIBUTIONS
XHX conceived and supervised the research.ST, YYC and YMZ collected specimens. ST and YYC wrote the article and analyzed data. XHX revised the article. All authors read and approved the final article.
FUNDING INFORMATION
This study was supported by Zhejiang medical and health science and technology program: 2021KY553.
CONFLICT OF INTEREST
The authors have declared that no competing interest exists.
DATE AVAILABILITY STATEMENT
Tian S, Chen Y, Zhang Y, Xu X. Clinical value of serum AFP and PIVKA‐II for diagnosis, treatment and prognosis of hepatocellular carcinoma. J Clin Lab Anal. 2023;37:e24823. doi: 10.1002/jcla.24823
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35‐S50. [DOI] [PubMed] [Google Scholar]
- 3. Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42(9):2029‐2041. [DOI] [PubMed] [Google Scholar]
- 4. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417‐422. [DOI] [PubMed] [Google Scholar]
- 5. Xu F, Zhang L, He W, Song D, Ji X, Shao J. The diagnostic value of serum PIVKA‐II alone or in combination with AFP in Chinese hepatocellular carcinoma patients. Dis Markers. 2021;2022:8868370‐8868379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su TH, Peng CY, Chang SH, et al. Serum PIVKA‐II and alpha‐fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J Formos Med Assoc. 2022;121(3):703‐711. [DOI] [PubMed] [Google Scholar]
- 7. Svobodova S, Karlikova M, Topolcan O, et al. PIVKA‐II as a potential new biomarker for hepatocellular carcinoma ‐ a pilot study. In Vivo. 2018;32(6):1551‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des‐gamma‐carboxyprothrombin. Liver Int. 2011;31(1):22‐35. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus‐based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339‐364. [DOI] [PubMed] [Google Scholar]
- 10. Hou J, Wang G, Wang F, et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J Clin Transl Hepatol. 2017;5(4):297‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Y, Li G, Lu Z, Liu Y, Kong J, Liu J. Progression of prothrombin induced by vitamin K absence‐II in hepatocellular carcinoma. Front Oncol. 2021;11:726213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee Q, Yu X, Yu W. The value of PIVKA‐ versus AFP for the diagnosis and detection of postoperative changes in hepatocellular carcinoma. J Interv Med. 2021;4(2):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52‐60. [DOI] [PubMed] [Google Scholar]
- 14. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. [DOI] [PubMed] [Google Scholar]
- 15. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha‐fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214‐2229. [DOI] [PubMed] [Google Scholar]
- 16. Shimada M, Yamashita Y‐i, Hamatsu T, et al. The role of des‐γ‐carboxy prothrombin levels in hepatocellular carcinoma and liver tissues. Cancer Lett. 2000;159(1):87‐94. [DOI] [PubMed] [Google Scholar]
- 17. Park SJ, Jang JY, Jeong SW, et al. Usefulness of AFP, AFP‐L3, and PIVKA‐II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017;96(11):e5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan HLY, Vogel A, Berg T, et al. Performance evaluation of the Elecsys PIVKA‐II and Elecsys AFP assays for hepatocellular carcinoma diagnosis. JGH Open. 2022;6(5):292‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Association for the Study of the liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182‐236. [DOI] [PubMed] [Google Scholar]
- 20. Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early‐stage hepatocellular carcinoma. Hepatology. 2019;69(5):1983‐1994. [DOI] [PubMed] [Google Scholar]
- 21. Huang S, Jiang F, Wang Y, et al. Diagnostic performance of tumor markers AFP and PIVKA‐II in Chinese hepatocellular carcinoma patients. Tumour Biol. 2017;39(6):1010428317705763. [DOI] [PubMed] [Google Scholar]
- 22. Gao J, Song P. Combination of triple biomarkers AFP, AFP‐L3, and PIVAKII for early detection of hepatocellular carcinoma in China: expectation. Drug Discov Ther. 2017;11(3):168‐169. [DOI] [PubMed] [Google Scholar]
- 23. Yuen MF, Lai CL. Serological markers of liver cancer. Best Pract Res Clin Gastroenterol. 2005;19(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 24. Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA‐II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu R, Xiang X, Tan Z, Zhou Y, Wang H, Deng G. Efficacy of PIVKA‐II in prediction and early detection of hepatocellular carcinoma: a nested case‐control study in Chinese patients. Sci Rep. 2016;6:35050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong YM, Cho M, Yoon KT, et al. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol. 2017;39(10):1010428317720863. [DOI] [PubMed] [Google Scholar]
- 27. Payance A, Dioguardi Burgio M, Peoc'h K, et al. Biological response under treatment and prognostic value of protein induced by vitamin K absence or antagonist‐II in a French cohort of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2020;32(10):1364‐1372. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto T, Kitano Y, Imai K, et al. Clinical significance of preoperative inflammation‐based score for the prognosis of patients with hepatocellular carcinoma who underwent hepatectomy. Surg Today. 2022;52(7):1008‐1015. [DOI] [PubMed] [Google Scholar]
- 29. Lee TY, Choi HJ, Ahn J, Hong TH, You YK. Efficacy of tumor markers after liver transplantation In patients with hepatocellular carcinoma. Transplant Proc. 2022;54(2):461‐467. [DOI] [PubMed] [Google Scholar]
- 30. Si YQ, Wang XQ, Pan CC, Wang Y, Lu ZM. An efficient nomogram for discriminating intrahepatic Cholangiocarcinoma from hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:833999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi Y, Inoue T, Fukusato T. Protein induced by vitamin K absence or antagonist II‐producing gastric cancer. World J Gastrointest Pathophysiol. 2010;1(4):129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tartaglione S, Mancini P, Viggiani V, Chirletti P, Angeloni A, Anastasi E. PIVKA‐II: a biomarker for diagnosing and monitoring patients with pancreatic adenocarcinoma. PLoS One. 2021;16(5):e0251656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takano S, Honda I, Watanabe S, et al. PIVKA‐II‐producing advanced gastric cancer. Int J Clin Oncol. 2004;9(4):330‐333. [DOI] [PubMed] [Google Scholar]


