Abstract
Objectives
To evaluate humoral and cell-mediated response after three doses of BNT162b2 SARS-CoV-2 vaccine in patients with systemic lupus erythematosus (SLE) treated with Belimumab (BLM).
Methods
SLE patients were vaccinated with three doses of BNT162b2-mRNA vaccine (two-dose primary vaccination, third booster dose after 6 months). The humoral immune response was assessed one and 6 months after the second dose (T1, T2), and 6 months after the booster dose (T3). Serological assay was performed (The Liaison® SARS-CoV-2 TrimericS IgG chemiluminescent). Spike-specific T-cell response was monitored 6 months after the second vaccine dose and the percentage of cytokines producing T cells was assessed by flow cytometry.
Results
Twelve patients [12F; median age 46 years (IQR 8.25); median disease duration 156 months (IQR 188)] were enrolled. At T1, all patients showed seroconversion (median anti-Spike IgG levels 1610 BAU/mL, IQR 1390). At T2––day of the third dose––a significant reduction of median anti-Spike IgG antibodies levels was observed [214 BAU/mL (IQR 94); p = 0.0009]. Anti-Spike IgG were significantly increased at T3, reaching a median value of 1440 BAU/mL (IQR 1316; p = 0.005). Despite declining humoral immunity, almost 60% of patients mounted a virus-specific CD4 + T-cell response 6 months after primary vaccination.
Conclusions
BLM does not impair humoral response to primary BNT162b2 SARS-CoV-2 vaccination. During the follow-up, a decline in antibody levels is evident and the third dose is crucial to increase the specific immune response. Finally, we observed a recall T-cell response to the Spike antigen 6 months after the first vaccination cycle.
Keywords: BNT162b2 SARS-CoV-2 vaccine, systemic lupus erythematosus, Belimumab
Introduction
Since SARS-CoV-2 pandemic spread out in early 2020, the joint effort at world level culminated in the development and the administration of SARS-CoV-2 vaccines. Among numbers of vaccines approved in many countries, mRNA-based vaccines, able to stimulate both humoral and cell-mediated immunity, represented a novelty, being for the first time used for mass immunization.
As recently highlighted by the EULAR, concerns exist about an adequate COVID-19 vaccination protective response in patients taking immunosuppressive treatments. In this regard, a booster vaccine dose, administered 4–6 months after the first course, has been recommended to ensure greater protection against severe COVID-19 disease.1 According to local guidelines, in January 2021, the vaccine campaign against COVID-19 has begun in Italy;2 focusing on BNT162b2-mRNA vaccine, the administration protocol consisted of two doses separated by a 3-week interval. In October 2021, the administration of a third (booster) dose of the mRNA vaccine was authorized by the Italian Ministry of Health, for frail individuals with comorbidities who had received a second dose of vaccine at least 6 months earlier.3
B-cell target therapies, frequently used to treat Systemic Lupus Erythematosus (SLE) patients, may impair immune response to vaccination. This has been extensively demonstrated for Rituximab (RTX), an anti-CD20 chimeric monoclonal antibody specifically targeting B cells. Indeed, its mechanism of action, resulting in B-cell depletion, can influence antibodies production following COVID-19 vaccination, as extensively reviewed by Jena et al.4 Conversely, the few data available for Belimumab (BLM), a recombinant human monoclonal antibody binding the soluble B-lymphocyte stimulator protein (BLyS), suggested a negligible impact on antibody production.5
Since vaccine-induced T-cell response is not routinely assessed, fewer data are available about the impact of B-cell target therapies on the activation of this component of the immune response by COVID-19 vaccines. RTX does not seem to impair T-cell response, while data on BLM are limited.6 Recently, Fabris and colleagues provided data about 17 SLE patients treated with BLM, showing humoral and cellular response to COVID-19 vaccine in most patients.7
To the best of our knowledge, no data are available about the maintenance of vaccine humoral response in SLE patients treated with BLM. For this reason, the first aim of the study was to follow the trajectories of vaccine-induced antibodies in a cohort of BLM-treated SLE patients. Anti-Spike IgG levels were monitored up to 12 months after the first dose of BNT162b2 in patients who received the two-dose primary vaccination and a third booster dose after 6 months. Additionally, T-cell response was assessed immediately before the administration of the third dose.
Methods
Study design
Adult SLE patients diagnosed according to ACR 1997 criteria referring to the Lupus Clinic of Sapienza University of Rome, AOU Policlinico Umberto I (Sapienza Lupus Cohort) and treated with BLM in accordance with current clinical practice were enrolled.8 Demographic and clinical data were collected in a dedicated electronic form and included sex, age, diagnosis, disease duration, and ongoing treatment at the time of vaccination. Written informed consent to use their anonymized data was obtained from patients. The protocol was approved by the Local Ethical Committee (prot. 0501/2021).
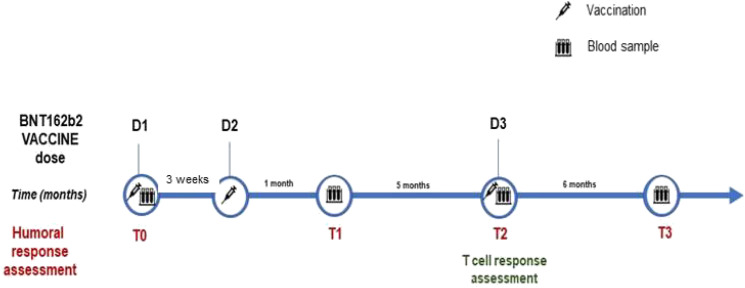
According to the local guidelines for COVID-19 vaccination,2,3 patients were vaccinated with three doses of BNT162b2 vaccine (Pfizer/BioNTech): in detail, two-dose primary course on days 0 and 21 (D1, D2) and an additional booster dose after 6 months (180 days; D3). Blood samples for antibodies assessment were collected from all participants at baseline before vaccination (March–April 2021; T0), 28 days after the completion of the first vaccination cycle (May–June 2021; T1), on the day of the third dose (October 2021; T2), and 180 days after the third dose (April 2022; T3). T-cell response was evaluated 180 days after the first vaccination cycle, on the day of the third dose (T2). Figure 1 summarizes the study protocol.
Figure 1.
Timeline of study protocol. Legend: D = Vaccine dose; T = Time point.
SARS-CoV-2 IgG immunoassays
Blood samples were collected in serum separator tubes (BD Diagnostic Systems, Franklin Lakes, NJ, USA) and centrifuged at room temperature at 1600 rpm for 10 min. Aliquots were transferred to 2 mL polypropylene, screw cap cryotubes (Nunc™, Thermofisher Scientific, Waltham, MA USA) and immediately frozen at −20°C. The Liaison® SARS-CoV-2 TrimericS IgG chemiluminescent assay (DiaSorin, Saluggia VC, Italy), using the trimeric S antigen stabilized in its native form and designed for high throughput in healthcare settings was used. The LIAISON® XL fully automated chemiluminescence analyzer automatically calculates SARS-CoV-2 trimeric S IgG antibody concentrations, expressed as binding antibody units (BAU/mL). The assay range is up to 2080 BAU/mL. According to manufacturer’s instructions, values ≥33.8 BAU/mL were interpreted as positive. Samples that were above the upper limit of the assay were automatically diluted 1:20 and reanalyzed.
PBMC isolation
Whole blood was collected from patients in sodium heparin Vacutainer tubes (BD Biosciences, San Jose, CA, USA). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque PLUS (Cytiva, Marlborough, MA, USA), washed twice with cold wash buffer (2% FCS), and resuspend at 2.0–2.5 × 106 cells/mL in R-10 medium. Alternatively, freshly isolated PBMCs can be frozen in 90% FCS and 10% DMSO and stored in −196°C liquid nitrogen for later experiments.
T cell stimulation assay and cytokine (ICC) detection
A total of 2 × 106 PBMCs were cultured in complete media (RPMI 1640 supplemented with 10% FBS, 2-ME, sodium pyruvate, penicillin, streptomycin, and nonessential amino acids; all from Sigma-Aldrich, Saint Louis MO, USA) at 37°C with 5% CO2 in FACS tubes overnight with 0.6 nmol of each SARS-CoV-2 Spike peptide/mL (Peptivator, Miltenyi, Bergisch Gladbach, Germany). As a positive control, non-specific antigen SEB at 100 ng/mL was added (Sigma-Aldrich). Brefeldin-A (Sigma-Aldrich) was added during the last 18 h of incubation, at 10 μg/mL, to inhibit cellular secretion. After overnight stimulation, PBMCs were stained with Live/Dead Fixable Violet Dead Cell Stain Kit (Thermo Fisher Scientific, Waltham MA, USA) to exclude dead cells from the analyses. Cells were washed twice with FACS buffer and then fixed with BD Cytofix/Cytoperm buffer (BD Biosciences, Franklin Lakes, NJ USA) for 20 min at 4°C. Following fixation and permeabilization, cells were washed twice with 1× BD Perm/Wash buffer and stained with a predetermined optimal concentration of fluorochrome-conjugated Abs: anti-CD3-APC-H7, anti-IL-2-FITC, anti-TNFα PE-Cy7 (all from BD Biosciences), anti-IFN-γ-PerCP-Cy5.5 (Biolegend, San Diego, CA, USA), anti-CD8-APC (eBiosciences, Thermo Fhisher Scientific). Cells were fixed in 200 μL of 1× PBS/formaldehyde (2% v/v) and then acquired by flow cytometry in a Gallios flow cytometer and analyzed using Kaluza software (Beckman Coulter, Brea, CA, USA). Stained samples were acquired with a standard stopping gate set at 50,000 CD3 + T-cells.
Statistical analysis
Data and statistical analyses were done in GraphPad Prism 8.1 (Graph-Pad Software Inc., San Diego, CA), unless otherwise stated. All results are expressed as median (Interquartile Range; IQR) and statistical significance was determined using the non-parametric Friedman test or Wilcoxon signed-rank test (*p < 005, **p < 001). Values of p < 005 and p < 001 were considered significant. T cells data have been calculated as background subtracted data.
Results
Study sample
Twelve SLE patients (12F; median age 46 years, IQR 8.25; median disease duration 156 months, IQR 188) treated with BLM were enrolled. Data about clinical features and concurrent medications are provided in Table 1. In detail, at the time of vaccination, eight patients (66.6%) were treated by low dose glucocorticoids (median dosage 1.875 mg/daily prednisone equivalent, IQR 3.125), and six (50%) by concomitant immunosuppressant drugs.
Table 1.
Clinical disease-related features and concomitant treatment in enrolled patients (N = 12).
| Disease-related features | N (%) |
|---|---|
| Mucocutaneous manifestations | 10 (83.3) |
| Musculoskeletal manifestations | 12 (100) |
| Serositis | 1 (8.3) |
| Kidney involvement | 2 (16.6) |
| Hematological manifestations | 5 (41.6) |
| Neurological manifestations | 2 (16.6) |
| Thrombotic events | 2 (16.6) |
| Immunological abnormalities | N (%) |
| ANA | 12 (100) |
| anti-dsDNA | 12 (100) |
| anti-Sm/RNP | 2 (16.6) |
| Antiphospholipid antibodies | 5 (41.6) |
| Low C3/C4 levels | 6 (50) |
| Concomitant treatments | N (%) |
| Glucocorticoids | 8 (66.6) |
| Hydroxychloroquine | 11 (91.6) |
| Methotrexate | 1 (8.3) |
| Azathioprine | 2 (16.6) |
| Cyclosporine A | 3 (25) |
Anti–SARS-CoV-2 IgG antibody response
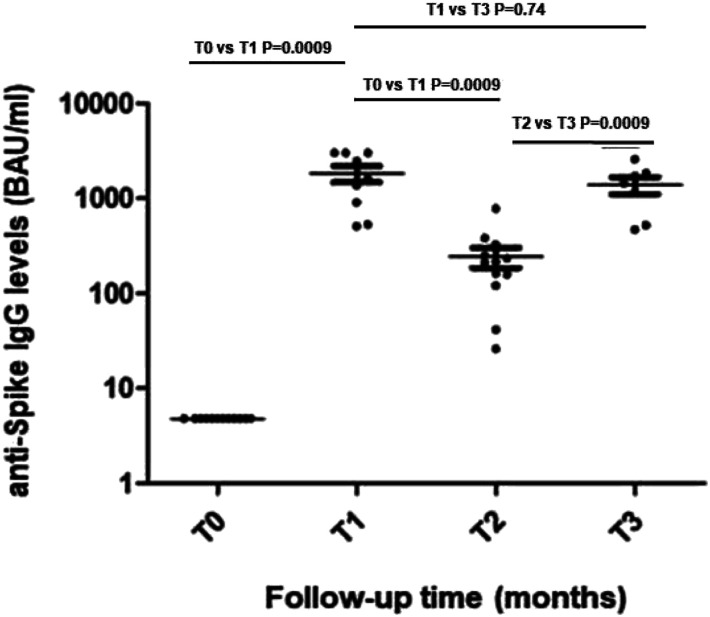
None of the patients had detectable anti-SARS-CoV-2 antibodies at the time of vaccination (T0). At T1, all patients showed seroconversion, as reported in Figure 2. It was possible to monitor longitudinally humoral response in seven patients; in fact, one subject dropped out of the study (due to lack of treatment adherence), while four had a COVID-19 diagnosis between T2 and T3. Serological evaluation performed 6 months after first vaccination cycle (T2), coinciding with the day of third booster dose administration, demonstrated a significant reduction of median anti-Spike IgG levels (1610 BAU/mL [IQR 1390] T1 versus 214 BAU/mL [IQR 94] T2; p = 0.0009). The assessment of humoral response performed after additional 6 months (T3) showed the increase of anti-Spike IgG from 214 BAU/mL [IQR 94] to 1440 BAU/mL [IQR 1316] (p = 0.005), reaching levels like those observed after the first vaccination cycle (Figure 2). Antibody titers of SLE patients completing the study were reported in Table S1.
Figure 2.
Trajectory of humoral response in SLE patients. Legend: Anti-trimeric Spike IgG levels at T0 (before vaccination); T1 (28 days after the first vaccination cycle); T2 (6 months after first vaccination cycle); T3 (180 days after the third dose) are shown. Statistical differences between median IgG levels at different time-points were calculated by Wilcoxon signed-rank test.
T-cell mediated cytokine production in response to SARS-CoV-2 specific stimulations
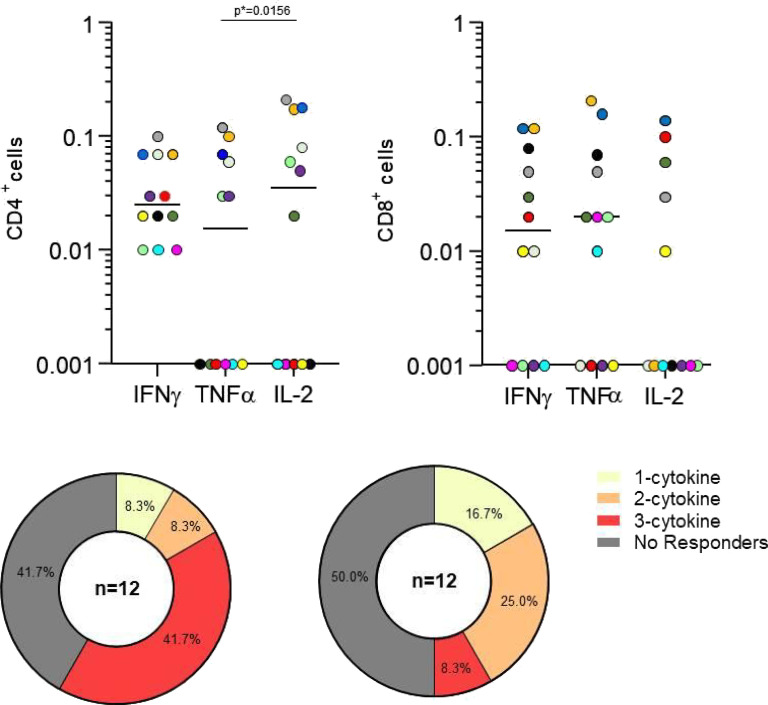
To assess whether COVID-19 vaccine induces SARS-CoV-2-specific T-cell response in BLM-treated patients, we measured by flow cytometry the frequencies of cytokine-producing CD4+ and CD8+ T cells, including IFN-γ, TNF-α, and IL-2, following stimulation with a pool of peptides encompassing the immunogenic domain of the Spike protein. We performed the analysis on PBMCs isolated from 12 SLE patients at T2 (6 months after first vaccination cycle).
A SARS-CoV-2 specific response mediated by both CD4+ and CD8+ T cells was highlighted (Figure 3(a)). In detail, almost 60% of the patients were able to mount a virus-specific CD4+ T-cell response, most of them with a polyfunctional response marked by the simultaneous production of the three cytokines analyzed. We observed a CD8+ T-cell response in almost 50% of the patients; however, among the responders, fewer showed a polyfunctional profile (Figure 3(b)).
Figure 3.
(a) Spike-specific T-cell response 6 months after first vaccination cycle. Different colors indicate different patients. Legend: Percentages of CD4+ and CD8+ T cells producing IFNγ, TNFα, IL2 after Spike stimulation of purified PBMCs are shown. Statistical differences were calculated by Wilcoxon signed-rank test. (b) Functional profile of CD4+ and CD8+ T cells according to the number of cytokines produced (IFNγ, TNFα, IL2). The threshold for a positive T-cell response was set at 0.05% for each cytokine.
Discussion
In the present study, the kinetic of antibodies response in SLE patients treated with BLM and vaccinated with BNT162b2-mRNA was evaluated over a twelve-month period. Overall, BLM treatment does not impact on the ability to respond to vaccination. In particular, we found that a third vaccine dose, administered 6 months after the second dose, increases the specific immune response. Finally, we demonstrated a recall T-cell response to the Spike antigen 6 months after the first vaccination cycle in more than half of patients.
SLE patients are more vulnerable to infections compared to general population predominantly due to the administration of immunosuppressive drugs and glucocorticoids to control disease activity and to prevent chronic damage development.9 Thus, the risk for SARS-CoV-2 infection and its possible complications certainly represent a concern for these patients. The COVID-19 Global Rheumatology Alliance, by analyzing more than 1600 patients, observed as the use of glucocorticoids, the presence of an active disease, or the administration of RTX were associated with more severe COVID-19 outcomes. Other factors such as male gender, age, and comorbidities may worsen the outcome.10,11 Furthermore, immunosuppressive treatments could represent a risk for a reduced response to vaccination. In this regard, the American College of Rheumatology has published specific guidelines suggesting precise timing for immunosuppressive treatments discontinuation before vaccination.12
The immune response to the SARS-CoV-2 vaccine has been deeply investigated in different autoimmune diseases, including SLE: although the available data suggest a humoral response like healthy subjects, some drugs have been clearly associated with a poor response; in particular, B cells target treatment with RTX.13,14
Conversely, few data are available about the efficacy of vaccination in patients treated by BLM. Izmirly and colleagues evaluated the immune response following vaccination in 90 SLE subjects, including 10 BLM-treated. In the latter, a lower humoral response, assessed 2 weeks after the second dose, was observed in 40 patients.14 A larger cohort was evaluated in the study published by Yuki et al., showing that 32 BLM-treated patients had lower seroconversion and neutralizing antibodies positivity in comparison with healthy controls; nonetheless, the authors attributed this result to the concomitant administration of other immunosuppressant drugs, mainly MMF.15 Comparable results have been obtained by the analysis of 22 SLE patients performed by Moyon et al.5 Accordingly, Ruddy and colleagues observed a lower seroconversion rate in MMF treated patients, whereas only 3 patients out of 56 enrolled patients (5%) did not seroconverted after two vaccine shots.16 Similar findings were obtained by Furer and colleagues when evaluating 9 BLM-treated patients.17 Finally, more recent data are emerging about seroconversion after the third vaccine dose in SLE patients treated by BLM. In detail, by evaluating 30 SLE patients, Boedecker-Lips observed a seroconversion in 80% of patients after the second dose, reaching 90% after the third shot.18
Moving on the evaluation of cellular response, as mentioned above, Fabris and colleagues evaluated 17 SLE patients treated by BLM: T-cellular response, assessed by using the interferon-gamma release assay, was observed in 94.1% of BLM-treated patients. Furthermore, the amount of IFNγ and IL-2 released did not differ significantly in comparison with the control group.7 Of note, blood samples were collected three to 4 weeks after first vaccination cycle, thus providing only short term-data.7
In the present study, we focused on antibodies and cellular response after SARS-CoV2 vaccination in SLE patients treated by BLM, the first biological drug approved for the treatment of SLE patients. In view of its increasing use in clinical practice, the availability of information on the immunological response after SARS-CoV-2 vaccine in BLM patients could be extremely interesting.
Our results demonstrated the seroconversion of all the enrolled patients 1 month after the first BNT162b2 vaccine cycle. Then, after a decline of anti-Spike IgG levels, the administration of third vaccine dose was able to restore high antibody levels, maintained up to 6 months after the booster. These results clearly demonstrated the ability of these patients to mount a vaccine-specific humoral immune response.
Of note, these findings represent an extension to data currently available on BLM-treated patients, providing information about the maintenance of humoral response after the third dose. Previous studies provided timely limited data, with the evaluation of immune response only after the first two doses.
Regarding T-cell mediated response, a good IFN production by CD4+ or CD8+ T cells after stimulation for most of tested patients 6 months after the first vaccination cycle was assessed despite BLM treatment.
Our study has some limitations. First, the small number of samples assessed. At the beginning of the vaccination campaign, patients affected by autoimmune diseases were the first to get vaccinated; and thus, some of them were not eligible for the enrollment in the study. Furthermore, in our cohort no patients were taking mycophenolate, previously associated with impaired response to vaccination. Finally, we have not included a control group and this could limit the relevance of our results, especially when considering T-cell response evaluation. Certainly, anti-SARS-CoV-2 serum antibody levels have been largely used as biomarkers of vaccine response both in the general population and in the patients affected by autoimmune diseases. However, low data are available about their role as predictor of protection against infection and more severe disease. Nonetheless, Ahmed and colleagues recently demonstrated that non-responder patients in terms of autoantibodies production were more likely to develop breakthrough infections compare to responder patients.19
In conclusion, we demonstrated that BLM treatment seems do not influence the induction and maintenance of humoral and T-cell mediated response, both detectable in the majority of patients 6 months after the primary vaccination course. Moreover and most important, BLM treatment does not impair the ability of SLE patients to efficiently respond to the administration of the third dose with a marked increase of Spike-specific IgG. Future studies are needed to monitor over time the effect of additional booster doses especially in the context of the emergence of SARS-CoV-2 variants of concerns.
Supplemental Material
Supplemental Material for Evidence of immune response to BNT162b2 COVID-19 vaccine in systemic lupus erythematosus patients treated with Belimumab by Ilaria Schiavoni, Eleonora Olivetta, Francesco Natalucci, Giulio Olivieri, Alessandra Lo Presti, Giorgio Fedele, Paola Stefanelli, Fulvia Ceccarelli and Fabrizio Conti in Lupus
Author contributions: IS and EO: Laboratory analysis, methodology, data analysis, and writing––review and editing. FN, GO, and AL: Data curation, data analysis, and writing––review and editing. GF and PS: Conceptualization, data analysis, and writing––review and editing. FCe and FCo: Conceptualization, methodology, data analysis, and writing––review and editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Alessandra Lo Presti https://orcid.org/0000-0001-7611-5021
Fulvia Ceccarelli https://orcid.org/0000-0001-5026-8783
References
- 1.Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis 2022; 81: 1628–1639. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines of the Strategic Plan on COVID-19 vaccines approved by Parliament . Recommendations for the Organization of the Vaccination Campaign against SARS-CoV-2/COVID-19 and Vaccination Procedure .
- 3.https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=77981&parte=1&serie=null
- 4.Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev 2022; 21: 102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyon Q, Sterlin D, Miyara M, et al. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann Rheum Dis 2022; 81: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieiro Santos C, Calleja Antolin S, Moriano Morales C, et al. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open 2022; 8: e001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabris M, De Marchi G, Domenis R, et al. High T-cell response rate after COVID-19 vaccination in belimumab and rituximab recipients. J Autoimmun 2022; 129: 102827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 9.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 2013; 22: 1286–1294. [DOI] [PubMed] [Google Scholar]
- 10.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. COVID-19 Global Rheumatology Alliance, characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020; 79: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. COVID-19 global rheumatology alliance, factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021; 80: 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis JR, Johnson SR, Anthony DD, et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021; 73: e60–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri C, Ursini F, Gragnani L, et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients’ subgroups. J Autoimmun 2021; 125: 102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izmirly PM, Kim MY, Samanovic M, et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2021; 74: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuki EFN, Borba EF, Pasoto SG, et al. Impact of distinct therapies on antibody response to SARS-CoV-2 vaccine in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2022; 74: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021; 80: 1351–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021; 80: 1330–1338. [DOI] [PubMed] [Google Scholar]
- 18.Boedecker-Lips SC, Claßen P, Kraus D, et al. Belimumab is not associated with COVID-19 mRNA vaccination failure in systemic lupus erythematosus. Rheumatology (Oxford) 2022: keac459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patnaik AP, Rout NK, Ahmed S, et al. Correlation of breakthrough infection during the omicron wave with seropositivity of vaccinated patients undergoing hemodialysis. Cureus 2022; 14: e29296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Evidence of immune response to BNT162b2 COVID-19 vaccine in systemic lupus erythematosus patients treated with Belimumab by Ilaria Schiavoni, Eleonora Olivetta, Francesco Natalucci, Giulio Olivieri, Alessandra Lo Presti, Giorgio Fedele, Paola Stefanelli, Fulvia Ceccarelli and Fabrizio Conti in Lupus