Abstract
Background
Although many studies have evaluated the efficacy and pharmacokinetics of Korean Red Ginseng (KRG) components (Rg1, Rb1, Rg3, Rd, etc.), few have examined the in vivo pharmacokinetics of the radiolabeled components. This study investigated the pharmacokinetics of ginsenosides and their metabolite compound K (CK), 20(s)-protopanaxadiol (PPD), and 20(s)-protopanaxatriol (PPT) using radioisotopes in rat oral administration.
Methods
Sprague-Dawley rats were dosed orally once with 10 mg/kg of the tritium(3H) radiolabeled samples, and then the blood was collected from the tail vein after 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, 48, 96, and 168 h. Radioactivity in the organs, feces, urine, and carcass was determined using a liquid scintillation counter (LSC) and a bio-imaging analyzer system (BAS).
Results and conclusion
After oral administration, as the 3H-labeled ginsenosides were converted to metabolites, Cmax and half-life increased, and Tmax decreased. Interestingly, Rb1 and CK showed similar values, and after a single oral administration of components, the cumulative excretion ratio of urine and feces was 88.9%–92.4%. Although most KRG components were excreted within 96–168 h of administration, small amounts of components were detected in almost all tissues and mainly distributed to the liver except for the digestive tract when observed through autoradiography. This study demonstrated that KRG components were distributed to various organs in the rats. Further studies could be conducted to prove the bioavailability and transmission of KRG components to confirm the mechanism of KRG efficacy.
Keywords: Korean Red Ginseng, Ginsenosides, Pharmacokinetics, Radioisotopes, Autoradiography
Graphical abstract
1. Introduction
Ginseng, which belongs to the genus Panax, family Araliaceae, is a perennial, indigenous plant with a long history of traditional use [1]. The ginseng components are divided into non-saponin fractions (proteins, peptides, nitrogen compounds, lipid-soluble compounds, polysaccharides, flavonoids, and fatty acids) and ginsenoside fractions [[2], [3], [4], [5], [6]]. The manufacturing process of Korean Red Ginseng (KRG), which involves steaming and drying the roots of fresh ginger without removing its epidermis, was registered with the International Organization for Standardization and internationally certified in April 2017 (ISO 19610). Fresh ginseng and KRG have different chemical profiles due to changes in their principal constituents during preparation. These changes occur in ginsenosides and polysaccharides, leading to arginine-fructose-glucose, maltol, and panaxytriol. Ginsenosides are the principal constituents of KRG, and 43 different types of ginsenosides have been characterized to date [[6], [7], [8]]. Ginsenosides may be classified into protopanaxadiols (ginsenoside Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, 20(S)-protopanaxadiol and Compound K), protopanaxatriols (ginsenoside Re, Rg1, Rg2, and Rh1, 20(S)-protopanaxatriol), and oleanane groups according to their aglycone moieties. Of these, ginsenosides Rg1, Rb1, and Rg3 are used as marker substances of KRG.
When absorbed into the body, the major ginsenosides, such as Rg1, Rb1, Rg3, are subjected to stepwise deglycosylation in the intestines and converted to the metabolites compound K (CK), 20(S)-protopanaxadiol (PPD), and 20(S)-protopanaxatriol (PPT) [9]. Past studies on the efficacy, content, and structure of ginsenosides in KRG mention that ginsenoside Rg1 and Rb1, the major hydrophilic ginsenosides, have antioxidative, anti-inflammatory, vasorelaxant, and angiogenic properties [[10], [11], [12], [13], [14]]. In addition, ginsenoside Rg3, a minor hydrophobic ginsenoside, has anti-metastatic and anticarcinogenic properties [15,16]. Among the pharmacological effects of CK, PPD, and PPT, CK has anticancer, anti-inflammatory, antidiabetic, and hepatoprotective effects [17], while PPD has anticarcinogenic [18] and anti-inflammatory [19] effects. In addition, PPT has recently exhibited antitumor effects [20] by inhibiting angiogenesis, as well as anti-stress [21], anti-inflammatory [22], antidiabetic [23] effects.
Pharmacokinetic studies of various ginsenosides have shown diverse results depending on the method of analysis, administered dose, method of administration, and species. In general, orally administered ginsenosides are degraded by digestive enzymes or intestinal bacteria, producing several metabolites [24]. The digestive tract mainly absorbs Rg1 and Rb1, their bioavailability is reduced to extremely low levels after oral and intravenous administration, and they are rapidly removed from the blood [25]. Rg1 has a shorter half-life than Rb1 due to its lower degree of binding to plasma protein [26]. However, Rg3 is almost completely metabolized after oral administration, and only 1% of the administered dose can be detected in the feces, even though it is not detected in the blood after oral administration [27].
The absolute oral bioavailability of PPD was around 20.7%–36.8%, suggesting that it may vary with different solubilizers [28]. For PPT, oral bioavailability was also reported to be very low (approximately 3.69%), and despite unclear reasons for low bioavailability until now, extensive metabolism in the intestine and liver is suggested to be a causative factor [29]. Pharmacokinetic studies of KRG components have been used to measure the amounts of ginsenosides in them. Their results show that metabolites were found in some organs after administering KRG or ginsenosides. No pharmacokinetic studies have dealt with the KRG components by utilizing each of the body's organs. In addition, researchers have used different methods of extracting ginsenosides and their metabolites from each organ, so obtaining accurate measurements of their amounts is limited. Therefore, this study intends to accurately measure the in vivo absorption of the major ginsenosides and their metabolites in all the body organs through multiple simultaneous radioisotope analyses.
2. Materials and methods
2.1. Test substances
Ginsenoside Rb1, Rg1, and Rg3s, CK, PPD, PPT labeled with radioisotope 3H (tritium) were used for this study and provided by RC TRITECH (Teufen, Switzerland). The specific radioactivity levels of 3H-labeled Rg1, Rb1 and Rg3s were 636, 62.9, 96.2 GBq/mmol (794.0, 56.7, and 122.5 Mbq/mg), while CK, PPD, and PPT were 1.37,1.51 and 1.71 TBq/mmol (2.20, 3.28 and 3.59 GBq/mg), respectively (Fig. 1). The Korea Ginseng Corporation provided unlabeled ginsenoside Rg1, Rb1, Rg3s, CK, PPD, and PPT samples.
Fig. 1.
Chemical structures of [3H]-labeled (A) ginsenosides Rb1, (B) Rg1, (C) Rg3s, (D) Compound K(CK), (E) 20(s)-protopanaxadiol (PPD), and (F) 20(s)-protopanaxatriol (PPT).
2.2. Experimental animals
This experiment was conducted according to the Guidelines for Animal Experimentation of Sekisui Medical Co., Ltd (Tokyo, Japan). Sprague-Dawley Crl:CD (SD) rats 7 weeks of age and weighing approximately 251.5–290.6 g (Charles River Laboratories Japan, Inc., Yokohama, Japan) were used. The rats were fed with rodent diets (MF, Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. Environmental conditions were set around 20°C-25.1 °C, and 41%–63% relative humidity. Before the study, the animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee.
2.3. Preparation of dose formulation and administration method
Saline solution (JP standard, Otsuka Pharmaceutical Factory, Tokushima, Japan) and 0.5% methylcellulose (For Biochemistry, Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) were used as vehicle solutions. The labeled ginsenosides (Rb1, Rg1, Rg3s) or metabolites (CK, PPD PPT) before the solvent was evaporated. Afterward, the residue was dissolved or suspended with each vehicle solution to prepare a 1 mg dosing formulation. The rats were divided into six groups, with three rats in each. The groups were orally administered dosing formulations of 7.4 MBq/kg (radioactivity dose) and 10 mg/kg (dose).
2.4. Measurement of radioactivity (dry method)
The sample was dried at 40 °C for 24 h or more before being combusted in a sample oxidizer, and the generated 3H2O was absorbed in 12 mL of the scintillator MONOPHASE S (PerkinElmer, Waltham, MA, USA). The radioactivity in the sample was measured using a liquid scintillation counter (LSC, 2500 TR, PerkinElmer, Waltham, MA, USA).
2.5. Radioactivity concentration measurement in plasma
Blood was collected from the tail veins after administration using a capillary tube and centrifuged (8000×g, 4 °C, 5 min) to separate plasma. A plasma aliquot of 100 μL was collected into a filter paper cup for combustion, and the radioactivity in the sample was measured using the dry method. The radioactivity in each sample was measured using an LSC for 2 min and expressed as ng eq./mL.
2.6. Radioactivity measurements in urine, feces, and carcasses
After administration, urine and feces were collected at 0, 24, 48, 72, 96, 120, 144, and 168 h. The cage was rinsed with distilled water at each sampling period, and the cage rinse was combined as a urine sample. The radioactivity in approximately 0.5 g was measured using the LSC after adding 10 mL of the scintillator Hionic-Fluor cocktail (PerkinElmer, Waltham, MA, USA). Each fecal sample was diluted to 100 mL with distilled water and homogenized. An aliquot of approximately 0.5 g fecal homogenate was dissolved in 2 mL of tissue solubilizer Soluence-350 (PerkinElmer, Waltham, MA, USA) by heating, and radioactivity was measured using an LSC after adding a 10 mL scintillator Hionic-Fluor cocktail. At 96 h after administration, the animals were euthanized by inhalation of carbon dioxide. Each carcass was dissolved in 500 mL of 2 mol/L sodium hydroxide solution (guaranteed grade, Nacalai Tesque, Kyoto, Japan) and 50 mL of toluene (guaranteed grade, Kanto Chemical, Tokyo, Japan) by refluxing with heating. The total volume was finally diluted to 1000 mL with water and homogenized. Radioactivity in 0.5 mL was measured using an LSC after adding a 10 mL scintillator Hionic-Fluor cocktail, and excretion was expressed as a percentage (%) of the administered dose.
2.7. Whole-body autoradiography
The rats were euthanized by carbon dioxide inhalation and frozen in a dry ice-acetone mixture. The frozen carcass was embedded in 4% w/v CMC-Na on a microtome stage and cut into 30 μm sections with a cryomicrotome. The sections were freeze-dried and covered with a 4-μm thick protective membrane (Diafoil, Mitsubishi Chemical Corporation, Tokyo, Japan). The sections without protective membranes were exposed to an imaging plate (TYPE BAS TR2040, Fujifilm, Tokyo, Japan) for 72 h. The radioactivity in organs was converted to the photo-stimulated luminescence per unit area (PSL/mm2) and subtracted with the background radioactivity corresponding to the assay area. When the radioactivity was not detected visually, the result was expressed as N.S. (Not specified).
2.8. Tissue distribution of radioactivity
The rats were euthanized by exsanguination from the abdominal aorta while anesthetized after administration before tissue and organ harvesting. Pieces of tissues or organs were weighed, and the radioactivity in the sample was measured by LSC using the dry method.
2.9. Data analysis
Pharmacokinetic parameters of radioactivity concentrations in plasma were calculated by non-compartmental analysis model using Phoenix WinNonlin Ver. 6.4 (Certara, NJ, USA). WinNonlin automatically set the time points used for the calculation of HL_Lambda_z.
2.10. Statistical analysis
All pharmacokinetic parameters are expressed as the mean value ± standard deviation (SD) of the measurements obtained with three animals using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Radioactivity concentration measurement in plasma
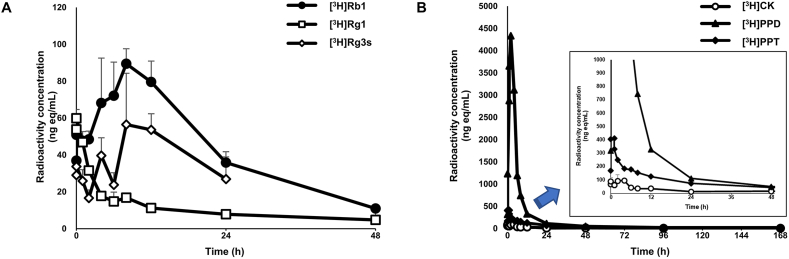
The radioactivity concentrations and pharmacokinetic parameters in plasma after oral administration of [3H]Rb1, [3H]Rg1, [3H]Rg3s, [3H]CK, [3H]PPD, and [3H]PPT are shown. The Cmax (ng eq/mL), Tmax(h), and AUC (ng eq∙h/mL) of each component are also described in Table 1; Fig. 2.
Table 1.
Pharmacokinetic parameters of [3H]-labeled (A) ginsenoside Rb1, Rg1, Rg3s, CK, PPD, and PPT after a single oral administration to a fasted male rat.
| Cmax (ng eq./mL) | Tmax (h) | AUC0-last (ng eq.·h/mL) | t1/2 (h) | |
| G-Rb1 | 94.58 ± 2.88 | 6.67 ± 2.31 | 2100 ± 100 | 12.9 ± 0.8 |
| G-Rg1 | 59.96 ± 4.6 | 0.42 ± 0.14 | 484 ± 83 | 23.8 ± 4.1 |
| G-Rg3s | 64.95 ± 17.15 | 10.7 ± 2.31 | 1130 ± 430 | NC |
| CK | 115.94 ± 30.48 | 2.17 ± 1.76 | 2060 ± 930 | 59.1 ± 53.8 |
| PPD | 4369.17 ± 633.18 | 1.83 ± 0.29 | 28400 ± 2400 | 40.9 ± 26.6 |
| PPT | 427.04 ± 276.09 | 1 ± 0.5 | 7770 ± 470 | 56.9 ± 3 |
Data are expressed as the mean values ± SD (n = 3).
NC: Not calculated.
Fig. 2.
Radioactivity concentrations in plasma after a single oral administration of [3H]-labeled (A) ginsenoside Rb1, Rg1, Rg3s and (B) CK, PPD, PPT to a fasted male rat (n = 3 per group). The rats were orally administered dosing formulations of 7.4 MBq/kg (radioactivity dose) and 10 mg/kg (ginsenoside Rg1, Rb1, Rg3s, CK, PPD, and PPT doses) once.
After oral administration, a significant increase was observed in the radioactivity concentration of all components in plasma. The [3H]Rg1 concentration increased rapidly after administration and reached the maximum concentration (Cmax) within 25 min. In addition, the Cmax and AUC of [3H]PPD were measured to be 4369.2 ng eq/mL and 28,400 ng eq∙h/mL, respectively, which was the highest among all components. Meanwhile, [3H]CK, [3H]PPD, and [3H]PPT tended to have higher Cmax and AUC of plasma radioactivity concentration compared to [3H]Rb1, [3H]Rg1, and [3H]Rg3s, and the time to reach Tmax was relatively short. However, there was no difference in Cmax and AUC when compared with [3H]Rb1 and [3H]CK.
3.2. Radioactivity measurement in urine, feces, and carcass
The cumulative excretion of radioactivity in urine, feces, and residual radioactivity in carcasses after a single oral administration of [3H]Rb1, [3H]Rg1, [3H]Rg3s, [3H]CK, [3H]PPD, and [3H]PPT are shown in Table 2.
Table 2.
Cumulative excretion of radioactivity in urine, feces, and residual radioactivity. in carcass after an oral administration of [3H]-labeled Ginsenoside Rb1, Rg1, Rg3s (A), CK, PPD, PPT (B) to the fasted male rats (n = 3/group). The rats were orally administered dosing formulations of 7.4 MBq/kg (radioactivity dose) and 10 mg/kg (ginsenoside Rg1, Rb1, Rg3s, CK, PPD, and PPT doses) once.
| Cumulative excretion of radioactivity (% of dose) |
||||
|---|---|---|---|---|
| Time (h) | G- Rb1 | G-Rg1 | G- Rg3s | |
| (A) | ||||
| Urine | 0–24 | 0.2 ± 0 | 1 ± 0.2 | 0.3 ± 0 |
| 48 | 0.3 ± 0.1 | 1.2 ± 0.2 | 0.4 ± 0.1 | |
| 72 | 0.3 ± 0.1 | 1.3 ± 0.2 | 0.5 ± 0.1 | |
| 96 |
0.3 ± 0.1 |
1.4 ± 0.2 |
0.5 ± 0.1 |
|
| Feces | 0–24 | 87.3 ± 1.5 | 84.7 ± 1.6 | 75.5 ± 11.7 |
| 48 | 91.5 ± 0.7 | 90.3 ± 1 | 88.1 ± 3.8 | |
| 72 | 91.9 ± 0.7 | 90.8 ± 0.8 | 91 ± 1.6 | |
| 96 |
92 ± 0.7 |
90.8 ± 0.8 |
91.9 ± 0.3 |
|
| Carcass |
96 |
0.4 ± 0.1 |
1 ± 0.3 |
0.7 ± 0.5 |
| Recovery |
92.7 ± 0.6 |
93.2 ± 0.5 |
93.2 ± 0.4 |
|
| Time (h) |
CK |
PPD |
PPT |
|
| (B) | ||||
| Urine | 0–24 | 0.3 ± 0.1 | 1.5 ± 0.6 | 2.6 ± 0.1 |
| 48 | 0.4 ± 0.2 | 1.9 ± 0.7 | 3.2 ± 0.1 | |
| 72 | 0.5 ± 0.2 | 2.2 ± 0.8 | 3.8 ± 0.2 | |
| 96 | 0.5 ± 0.2 | 2.5 ± 0.8 | 4.2 ± 0.2 | |
| 120 | 0.6 ± 0.2 | 2.7 ± 0.9 | 4.4 ± 0.1 | |
| 144 | 0.6 ± 0.3 | 2.8 ± 0.9 | 4.7 ± 0.2 | |
| 168 |
0.6 ± 0.3 |
3 ± 1 |
4.9 ± 0.2 |
|
| Feces | 0–24 | 88.5 ± 1.8 | 79.9 ± 4.1 | 80.5 ± 2.3 |
| 48 | 91.6 ± 1.2 | 87.2 ± 0.4 | 83.2 ± 0.3 | |
| 72 | 91.9 ± 1.1 | 87.8 ± 0.6 | 83.6 ± 0.2 | |
| 96 | 91.9 ± 1.1 | 88 ± 0.6 | 83.8 ± 0.2 | |
| 120 | 92 ± 1 | 88.2 ± 0.6 | 83.9 ± 0.3 | |
| 144 | 92 ± 1 | 88.3 ± 0.6 | 84 ± 0.3 | |
| 168 |
92 ± 1 |
88.4 ± 0.6 |
84.2 ± 0.2 |
|
| Carcass |
168 |
0.5 ± 0.2 |
2.3 ± 0.4 |
3 ± 0.5 |
| Recovery | 93.2 ± 0.7 | 93.6 ± 0.7 | 92.1 ± 0.5 | |
Data are expressed as mean values ± SD (n = 3).
After oral administration of [3H]Rb1, [3H]Rg1, and [3H]Rg3s, the cumulative excretion of the radioactivity in urine compared to dose was 0.3%, 0.5%, and 1.4%, respectively. The measured radioactivity was 92%, 92%, and 91% in feces and 0.4%, 0.7%, and 1.0% in the cadavers. On the other hand, the radioactivity of the metabolites [3H]CK, [3H]PPD, and [3H]PPT were 0.6%, 3.0%, and 4.9% in the urine. The measurements were 92%, 88%, and 84% in feces and 0.5%, 2.3%, and 3.0% in the cadavers. In addition, compared to [3H]Rb1-, [3H]Rg1-, [3H]Rg3s, and [3H]CK-forms in which sugars are bound, [3H]PPD and [3H]PPT, which is a structure without sugar bound to it, showed slightly higher cumulative excretion in urine and body residue when administered. These results confirmed that the ginsenosides and metabolites were mainly excreted in feces after administration, and the radioactivity recovery was about 92% or 93%.
3.3. Whole-body autoradiogram and tissue distribution of radioactivity
The whole-body autoradiograms in tissues after an oral administration of [3H]Rb1, [3H]Rg1, [3H]Rg3s, [3H]CK, [3H]PPD, and [3H]PPT to fasted male rats (n = 1/group) at a dose of 10 mg/kg are shown in Fig. 3. The radioactivity concentrations in tissues after a single oral administration to fasted male rats (n = 3/group) are shown in Fig. 4.
Fig. 3.
Whole-body radioluminogram after a single oral administration of [3H]-labeled (A) ginsenosides Rb1, (B) Rg1, (C) Rg3s, (D) CK, (E) PPD, and (F) PPT to a fasted male rat (n = 1 per group). The rats were orally administered dosing formulations of 7.4 MBq/kg (radioactivity dose) and 10 mg/kg (ginsenoside Rg1, Rb1, Rg3s, CK, PPD, and PPT dose) once.
Fig. 4.
Radioactivity concentrations in tissues (A) after 8 h of a single oral administration of [3H]-labeled ginsenoside Rb1, Rg1, Rg3s and (B) after 24 h of oral administration of [3H]-labeled CK, PPD, PPT to a fasted male rat (n = 3 per group). The rats were orally administered dosing formulations of 7.4 MBq/kg (radioactivity dose) and 10 mg/kg (ginsenoside Rg1, Rb1, Rg3s, CK, PPD, and PPT doses) once.
After measuring the intensity of radioactivity labeled with tritium components administered to rats, [3H]Rb1, [3H]Rg1, and [3H]Rg3s were detected in the gastric and small intestinal contents 1 h after administration. Small amounts were also detected in the large intestine for 24 h [3H]CK and [3H]PPT were mainly detected in the liver and intestines, and [3H]PPD was detected in the liver, pancreas, kidneys, heart, and adrenal glands, but none were detected after 24 h. While measuring radioactivity concentrations in the tissues, [3H]Rb1, [3H]Rg1, [3H]Rg3s, [3H]CK, and [3H]PPT mostly had high concentrations in the large and small intestines, liver, and stomach. However, in terms of [3H]CK and [3H]PPT, the concentration rapidly decreased as time passed. In comparison, [3H]PPD showed relatively high concentrations in tissues compared to other components 24 h after administration.
4. Discussion
Ginseng is extensively used as a form of medicine due to the pharmacological efficacy of ginsenoside [30]. Among the 150 different types of Rb1, Rb2, Rc, Rd, Re, and Rg1 constitute more than 90% of all ginsenosides in ginseng. However, Rb1, Rg1, Rg3, Rd, Re, Rh1, and Rh2 are the most studied [31]. When absorbed into the body, these ginsenosides are metabolized by gut microbiota. The sugar moiety attached to the ginsenosides is first disconnected before diol-ginsenosides become CK with a glucose unit attached to C20, where a sugar moiety is removed. They then turn into PPD when further metabolized, which causes the glucose to be separated. Triol-ginsenosides lose all sugar moieties, and only the PPT skeleton remains [32]. The radioisotope measurement method was not widely used because the handling of isotopes is difficult. In addition, since the synthesis of radioisotope derivatives requires a high level of effort and advanced equipment, it was mainly performed only in cases with materials where their development was confirmed or has already entered the development stage. Hence, its use in the drug discovery stage is limited. However, in vivo pharmacokinetics through radiolabeled compounds has the advantage of high sensitivity due to its low biological background [33]. In this study, the measurement of radioisotope concentrations was used to determine the absorption of Rg1, Rb1, and Rg3, which were the standards for KRG and their metabolites through more sophisticated and accurate in vivo analysis. In addition, some studies have shown the multiple pharmacological effects of Rb1, Rg1, and Rg3 on neuronal, cardiovascular, and immune systems, as well as their anticarcinogenic and antidiabetic activities. Rg1 has a shorter half-life than Rb1, which can be attributed to the lower degree of binding to plasma protein than Rb1. In past studies, Rg3 was not detected in the blood after oral administration, and Rg1 and Rb1 are mainly absorbed by the digestive tract [34]. In contrast, this study showed that Rb1 had a shorter half-life than Rg1 (12.9 ± 0.8 h vs. 23.8 ± 4.1 h). Regarding the plasma radioactivity concentration of KRG components, the concentrations of the aglycone moieties were higher than that of the ginsenosides. PPD increased rapidly about 2 h after administration, and Cmax reached 4369.17 ng eq/mL, the highest among all components.
The Rb1, Rg1, and Rg3s analysis showed that 0.3% or more of Rb1, 1.4% or more of Rg1, and 0.5% or more of Rg3s were absorbed into the blood. The radioactivity derived from Rb1, Rg1, and Rg3s were mainly excreted through the feces. Radioactivity recovery at 96 h was 93% of the dosing preparation, and the remainder was excreted via expired air. The percentage distributions of Rb1 radioactivity in all tissues were 0.03% of the dose or less at 1 h and 0.21% of the dose or less 8 h after administration. The percentage distribution of radioactivity of Rg1 in all tissues was 1.70% of the dose or less at 1 h, and 0.26% of the dose or less at 8 h after administration.
Meanwhile, the percentage distribution for Rg3s was 0.06% of the dose or less at 1 hand 0.11% of the dose or less at 8 h after administration. These results indicated that radioactivity derived from Rb1, Rg1, and Rg3s was not absorbed well. In the tissue distribution study, radioactive Rg1 was detected in almost all tissues and mainly distributed to the kidney and liver. Another tissue distribution study showed that the body slowly absorbed radioactivity of Rb1 and Rg3s because they were detected in numerous organs 8 h after administration compared to measurements obtained 1 h after administration. Similar to other studies, in this study, Rg1 and Rg3 were mainly located in the liver [35,36].
Some previous studies have reported that ginsenosides can be hydrolyzed in the gastrointestinal tract to produce their metabolites, including PPT and PPD, all of which have been reported to be more absorbable than the original saponins [37]. It has been reported that Rb1, Rb2, Rc, and Rd, the major KRG components, are metabolized to become CK by lactic acid bacteria and gut microbiota before being hydrolyzed to PPD, the final metabolite of PPD-type ginsenosides [38,39]. According to previous animal pharmacokinetic studies, the bioavailability of PPD ranged from 20.7% to 48.12%, while that of PPT reached 3.69%. These differences are thought to have resulted from different experimental methods, solubilizers, and analytical instruments [40,41].
This study showed that minute amounts of the ginsenosides and aglycons were absorbed in the rat's body and distributed to 20 organs. In addition, this study confirmed the in vivo absorption, distribution, and excretion patterns of ginsenosides through a more accurate radioisotope analysis than traditional methods. This study demonstrated that, although in very small amounts, ginsenosides present in KRG were absorbed in the rat's body and distributed to various organs, indicating that the ginsenosides could affect the absorption and distribution of other KRG components. Ginsenosides absorbed in various organs can exert various effects. However, the absorption and delivery of the non-ginsenoside component present in KRG may be affected, likely to be absorbed and distributed. Further studies suggest that proving the bioavailability and transmission of non-ginsenosides may be a basis for confirming the mechanism of KRG efficacy.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank Sekisui Medical Co., LTD. (Japan) for their technical assistance on the use of radioisotopes for the study's analysis. This study did not receive funding.
References
- 1.Park H.J., Kim D.H., Park S.J., Kim J.M., Ryu J.H. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36(3):225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis R., Wake G., Court J.A., Pickering A.T., Kim Y.C., Perry E.K. Non-ginsenoside nicotinic activity in ginseng species. Phytother Res. 1999;13:59–64. doi: 10.1002/(SICI)1099-1573(199902)13:1<59::AID-PTR423>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka N., Tanaka O., Shibata S. Chemical studies on the oriental plant drugs. XXVIII. Saponins and sapogenins of ginseng; Stereochemistry of sapogenin of ginsenoside Rb1, Rb2 and Rc. Chem Pharm Bull. 1972;20:1212–1216. doi: 10.1248/cpb.20.1212. [DOI] [Google Scholar]
- 4.Karikura M., Miyase T., Tanizawa H., Taniyama T., Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem Pharm Bull (Tokyo) 1991;39(9):2357–2361. doi: 10.1248/cpb.39.2357. [DOI] [PubMed] [Google Scholar]
- 5.Christensen L.P. Chapter 1 Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee S.M., Bae B.-S., Park H.-W., Ahn N.-G., Cho B.-G., Cho Y.-L., Kwak Y.-S. Characterization of Korean Red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:382–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin B.-K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiao L., Zhang X., Wang M., Li B., Liu Z., Liu S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohydr Polym. 2014;114:567–573. doi: 10.1016/j.carbpol.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Liu H.F., Yang J.L., Du F.F., Gao X.M., Ma X.T., Huang Y.H., Xu F., Niu W., Wang F.Q., Mao Y., et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.-K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res. 2013;37:451–456. doi: 10.5142/jgr.2013.37.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan C., Huo Y., An X., Singh G., Chen M., Yang Z., Pu J., Li J. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vasc Pharmacol. 2012;56:150–158. doi: 10.1016/j.vph.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Shi A.-W., Gu N., Liu X.-M., Wang X., Peng Y.-Z. Ginsenoside Rg1 enhances endothelial progenitor cell angiogenic potency and prevents senescence in vitro. J Int Med Res. 2011;39:1306–1318. doi: 10.1177/147323001103900418. [DOI] [PubMed] [Google Scholar]
- 13.Wang N., Wan J.-B., Chan S.-W., Deng Y.-H., Yu N., Zhang Q.-W., Wang Y.-T., Lee S.M.-Y. Comparative study on saponin fractions from Panax notoginseng inhibiting inflammation-induced endothelial adhesion molecule expression and monocyte adhesion. Chin Med. 2011;6:37. doi: 10.1186/1749-8546-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim T.-H., Lee S.-M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y., Lu X., Xiang F.-L., Lui E.M.K., Feng Q. North American ginseng protects the heart from ischemia and reperfusion injury via upregulation of endothelial nitric oxide synthase. Pharmacol Res. 2011;64:195–202. doi: 10.1016/j.phrs.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki M., Yoo Y.C., Matsuzawa K., Sato K., Saiki I., Tono-oka S., Samukawa K., Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)- ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 17.Yang X.-D., Yang Y.-Y., Ouyang D.-S., Yang G.-P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. 2015;100:208–220. doi: 10.1016/j.fitote.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhu G.Y., Li Y.W., Tse A.K., Hau D.K., Leung C.H., Yu Z.L., Fong W.F. 20(S)-Protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur J Pharmacol. 2011;668:88–98. doi: 10.1016/j.ejphar.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee W.-M., Kim S.-D., Kim K.-S., Song Y.-B., Kwak Y.-S., Cho J.-Y., Park H.-J., Oh J.-W., et al. Protopanaxadiol modulates LPS-induced inflammatory activity in murine macrophage RAW264.7 cells. J Ginseng Res. 2006;30:181–187. doi: 10.5142/JGR.2006.30.4.181. [DOI] [Google Scholar]
- 20.Chen X.J., Zhang X.J., Shui Y.M., Wan J.B., Gao J.L. Anticancer activities of protopanaxadiol and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016:5738694. doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh H.A., Kim D.-E., Choi H.J., Kim N.J., Kim D.-H. Anti-stress effects of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol in immobilized mice. Biol Pharm Bull. 2015;38:331–335. doi: 10.1248/bpb.b14-00669. [DOI] [PubMed] [Google Scholar]
- 22.Kim D.Y., Ro J.Y., Lee C.H. 20(S)-protopanaxatriol inhibits release of inflammatory mediators in immunoglobulin E-mediated mast cell activation. J Ginseng Res. 2015;3:189–198. doi: 10.1016/j.jgr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han K.L., Jung M.H., Sohn J.H., Wang J.K. Ginsenoside 20Sprotopanaxatriol (PPT) activates peroxisome proliferator-activated receptor γ (PPARγ) in 3T3-L1 adipocytes. Biol Pharm Bull. 2006;29:110–113. doi: 10.1248/bpb.29.110. [DOI] [PubMed] [Google Scholar]
- 24.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31(8):1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 25.Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenosides Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 26.Ling Y., Yon L., L C.-X. Metabolism and pharmacokinetics of ginsenosides. Asian J Pharmacokinet Pharmacodyn. 2006;6:103–120. [Google Scholar]
- 27.Qian T., Cai Z., Wong R.N.S., Mak N.K., Jiang Z.-H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Biomed Appl. 2005;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Han M., Chen J., Chen S., Wang X. Development of a UPLC-ESI-MS/MS assay for 20(S)-protopanaxadiol and pharmacokinetic application of its two formulations in rats. Anal Sci. 2010;26:749–753. doi: 10.2116/analsci.26.749. [DOI] [PubMed] [Google Scholar]
- 29.Kong L.-T., Wang Q., Xiao B.-X., Liao Y.-H., He X.-X., Ye L.-H., Liu X.-M., et al. Different pharmacokinetics of the two structurally similar dammarane sapogenins, protopanaxatriol and protopanaxadiol, in rats. Fitoterapia. 2013;86:48–53. doi: 10.1016/j.fitote.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.-J., Zhang D., Yang D.-C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33:717–735. doi: 10.1016/j.biotechadv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.H., Kim J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang E.J., Kim T.H., Shin K.C., Oh D.K. Complete conversion of all typical glycosylated protopanaxatriol ginsenosides to aglycon protopanaxatriol by combined bacterial β-glycosidases. Amb Express. 2018;8(1):8. doi: 10.1186/s13568-018-0543-1. Published 2018 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong B., Chen H., Han J., Xie Q., He J., Bai K., Dong Y., Yi R. A study of 11-[³H]-Tetrodotoxin absorption, distribution, metabolism and excretion (ADME) in adult sprague-dawley rats. Mar Drugs. 2017;15:159. doi: 10.3390/md15060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L., Yong L., Liu C.-X. Metabolism and pharmacokinetics of ginsenosides. Asian J Pharmacokinet Pharmacodyn. 2006;6:103–120. [Google Scholar]
- 35.Bae S.H., Park J.B., Zheng Y.F., Jang M.J., Kim S.O., Kim J.Y., et al. Pharmacokinetics and tissue distribution of ginsenoside Rh2 and Rg3 epimers after oral administration of BST204, a purified ginseng dry extract, in rats. Xenobiotica. 2014;44:1099–1107. doi: 10.3109/00498254.2014.929192. [DOI] [PubMed] [Google Scholar]
- 36.Won H.-J., Kim H.I., Park T., Kim H., Jo K., Jeon H., et al. Non-clinical pharmacokinetic behavior of ginsenosides. Brain Res Bull. 2019;43(3):354–360. doi: 10.1016/j.jgr.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa H., Sung J.H., Benno Y. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:346–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- 38.Park S.-E., Na C.-S., Yoo S.-A., Seo S.-H., Son H.-S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J Ginseng Res. 2017;41:36–42. doi: 10.1016/j.jgr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42:255–263. doi: 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren H.C., Sun J.G., Wang G.J., A J.Y., Xie H.T., Zha W.B., et al. Sensitive determination of 20(S)-protopanaxadiol in rat plasma using HPLC-APCIMS: application of pharmacokinetic study in rats. J Pharm Biomed Anal. 2008;48:1476–1480. doi: 10.1016/j.jpba.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 41.Kong L.T., Wang Q., Xiao B.X., Liao Y.H., He X.X., Ye L.H., et al. Different pharmacokinetics of the two structurally similar dammarane sapogenins, protopanaxatriol and protopanaxadiol, in rats. Fitoterapia. 2013;86:48–53. doi: 10.1016/j.fitote.2013.01.019. [DOI] [PubMed] [Google Scholar]