Abstract
Background
Red ginseng marc, the residue of red ginseng left after water extraction, is rich in dietary fiber. Dietary fiber derived from fruits or vegetables can promote the proliferation of probiotics, and it is a key technology in the food industry to increase the productivity of probiotics by adding growth-enhancing substances such as dietary fiber. In this study, the effect of red ginseng dietary fiber (RGDF) on the growth of probiotic bacterial strains was investigated at the phenotypic and genetic levels.
Methods
We performed transcriptome profiling of Lactiplantibacillus plantarum IDCC3501 in two phases of culture (logarithmic (L)-phase and stationary (S)-phase) in two culture conditions (with or without RGDF) using RNA-seq. Differentially expressed genes (DEGs) were identified and classified according to Gene Ontology terms.
Results
The growth of L.plantarum IDCC3501 was enhanced in medium supplemented with RGDF up to 2%. As a result of DEG analysis, 29 genes were upregulated and 30 were downregulated in the RGDF-treated group in the L-phase. In the S-phase, 57 genes were upregulated and 126 were downregulated in the RGDF-treated group. Among the upregulated genes, 5 were upregulated only in the L-phase, 10 were upregulated only in the S-phase, and 3 were upregulated in both the L- and S-phases.
Conclusions
Transcriptome analysis could be a valuable tool for elucidating the molecular mechanisms by which RGDF promotes the proliferation of L.plantarum IDCC3501. This growth-promoting effect of RGDF is important, since RGDF could be used as a prebiotic source without additional chemical or enzymatic processing.
Keywords: Red ginseng, Dietary fiber, Transcriptome analysis, Lactiplantibacillus plantarum
Graphical abstract

1. Introduction
In the human body, there are between 10 and 100 trillion symbiotic microorganisms. These microbes are ubiquitous not only in the gut but also in the respiratory system, the mouth, and the skin. They interact with the host, causing disease or contributing to health [1,2]. Probiotics such as lactic acid bacteria are live microorganisms beneficial to the host [3]. Probiotics can improve gut health, stimulate the immune system, and have anticancer, anti-cholesterol, anti-diabetes, anti-allergy, and anti-obesity activity [[4], [5], [6], [7]]. Lactiplantibacillus plantarum IDCC3501, a probiotic strain, was isolated from kimchi, a traditional Korean fermented food. The cell-free supernatant of this strain has been shown to reduce the expression of the mRNA of genes related to inflammation, such as TNF-α, IL-1β, and IL-6 in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages [8].
The development of prebiotic food ingredients is of considerable current interest in the functional probiotic food industry. Prebiotics are substrates that are selectively utilized by host microorganisms that confer a health benefit [9]. Fructooligosaccharides (FOS) and galactooligosaccharides (GOS), which dominate the prebiotics, have the ability to increase microflora to enhance health [10]. Recently, in addition to FOS and GOS, naturally occurring prebiotics from fruits and vegetables have also been investigated. The fiber-rich by-products of food processing have been shown to produce positive effects on the gut microbiome.
Ginseng (Panax ginseng Meyer) is the most famous herbal medicine, which has been widely used in Eastern Asia [11]. The root of ginseng plants is steamed and dried to make red ginseng. The types and contents of ginsenosides, the main bioactive constituents of ginseng, are changed during this process [11]. Ginsenosides play important pharmacological roles such as regulation of inflammatory responses [12], suppression of cancer cell proliferation [13], anti-diabetes [14], and anti-aging [15]. Red ginseng is traditionally consumed as a water extract, and the residues are generally discarded. Some research groups have tried to use red ginseng residues as ingredients for food such as crackers, cakes, yackwa, and sausage, since red ginseng by-products still contain ginsenosides, acidic polysaccharide, mineral elements and dietary fiber [[16], [17], [18], [19]]. In particular, it has been reported that dietary fiber (DF), a kind of non-starch polysaccharide, accounts for approximately 32% of general nutritional components of red ginseng residues [20]. DFs are not digested and absorbed in the human gastrointestinal tract, and related to the diversity of gut microbial community [21].
Transcriptome profiling has been used to evaluate the interactions between probiotics and their environments or growth physiology at the molecular level. For example, the characteristics of a culture of a probiotic strain with respect to its carbon source and molecular mechanisms of tolerance toward environmental stress has been studied [22,23].
In this study, the effect of red ginseng dietary fiber (RGDF) on the growth of L.plantarum IDCC3501 was investigated, and its effects upon metabolism during cell proliferation were revealed through transcriptome profiling.
2. Materials and methods
2.1. Preparation and physicochemical composition analysis of dietary fiber-enriched RGDF
The residue remaining after water extraction of red ginseng at 87°C for 24h was provided by Korea Ginseng Corporation (Daejeon, Korea). RGDF was prepared from this red ginseng marc by drying it at 115°C and pulverizing it to 50 mesh.
To confirm the physicochemical characteristics of this RGDF, its ginsenoside and dietary fiber contents were analyzed. The ginsenoside compositions of RGDF were analyzed using Ultra performance liquid chromatography (UPLC) as reported previously [24], and the content of dietary fiber was measured according to McCleary et al [25].
2.2. Bacterial strain and culture conditions
The bacterial strain used in this study, Lactiplantibacillus plantarum IDCC3501, was obtained from Ildong Bioscience (Pyeongtaek, Korea). The bacteria was cultured in MRS broth (BD Difco, Franklin Lakes, NJ, USA) and stored in 25% (w/w) glycerol stock at −80°C. Before the experiments, the strain was pre-cultured in MRS media at 37°C for 18h. To clarify the effect of RGDF on the growth of L.plantarum IDCC3501, MRS supplemented with 0.2–3% RGDF or MRS alone was inoculated with 1% (v/v) pre-culture medium and incubated at 37°C for 18h. To establish a growth curve, the number of viable cell count and the pH value of cultivate were analyzed every 3h during incubation for 27h. The number of viable cells was measured using the standard plate count method. One milliliter of each culture was serially diluted with sterile 0.85% saline, and then these diluted solutions were plated on MRS agar medium and incubated at 37°C for 48h under anaerobic conditions. The numbers of colonies on plates estimated to have between 30 and 300 colonies were counted.
2.3. RNA extraction and sequencing
For RNA extraction, cell pellets were harvested by centrifugation from cultures involving two types of media (with or without RGDF) and two growth phases (logarithmic (L)-phase or stationary (S)-phase). Cell pellets were harvested at 6h to obtain mid-L-phase cells and at 12h for early-S-phase cells. Total RNA from 12 samples, three replicates per experimental group, was extracted according to the instructions of the TRIzol Kit manual (Thermo Fisher Scientific, Waltham, MA, USA). The concentration and purity of the extracted RNA was determined using a BioPhotometer (Eppendorf, Hamburg, Germany). RNA-Seq libraries were constructed using NEBNext® rRNA Depletion Kits (Bacteria) (New England Biolabs, Ipswich, MA, USA) and Illumina TruSeq Stranded Total RNA library kits (Illumina, San Diego, CA, USA). Finally, paired-end sequencing was performed using the Illumina HiseqX Platform.
2.4. Raw data processing and transcriptome analysis
To obtain clean reads, raw reads in FASTQ format were trimmed. In this process, poly N, linkers, and low-quality reads (those with bases of quality value of ≤30) were removed. The clean reads were aligned to the reference genome of L.plantarum IDCC3501 using the Bowtie2 aligner(http://bowtie-bio.sourceforge.net/bowtie2/index.shtml). For transcriptome analysis, the differentially expressed genes (DEGs) were identified and filtered using the R DEseq2 package [26]. The threshold for DEGs was |log2 (fold change)|>1 and Q-value< 0.05 [27]. Gene ontology analysis was performed using Blast2GO software [28], and the NCBI NR database (19.5.22) was used for BLAST searching.
3. Results
3.1. Physicochemical characteristics of RGDF and growth enhancement of L.plantarum IDCC3501 in media supplemented with RGDF
The RGDF used in this study was composed of 314.3 mg/g (approximately 31%) of dietary fiber and 6.63 mg/g of 11 ginsenosides: Rg1, Re, Rf, Rh1, Rg2s, Rb1, Rc, Rb2, Rd, Rg3s, and Rg3r (Table 1). Rg3s (1.73 mg/g) and Rg3r (1.18 mg/g) were the major ginsenosides, and the Re content was the lowest (0.09 mg/g).
Table.1.
Physicochemical Characteristics of Red Ginseng Dietary Fiber
| Components | Amount (mg/g) | |
|---|---|---|
| Ginsenoside | Rg1 | 0.17 |
| Re | 0.09 | |
| Rf | 0.37 | |
| Rh1 | 0.60 | |
| Rg2s | 0.37 | |
| Rb1 | 1.25 | |
| Rc | 0.37 | |
| Rb2 | 0.35 | |
| Rd | 0.15 | |
| Rg3s | 1.73 | |
| Rg3r | 1.18 | |
| Total dietary fiber | 314.3 | |
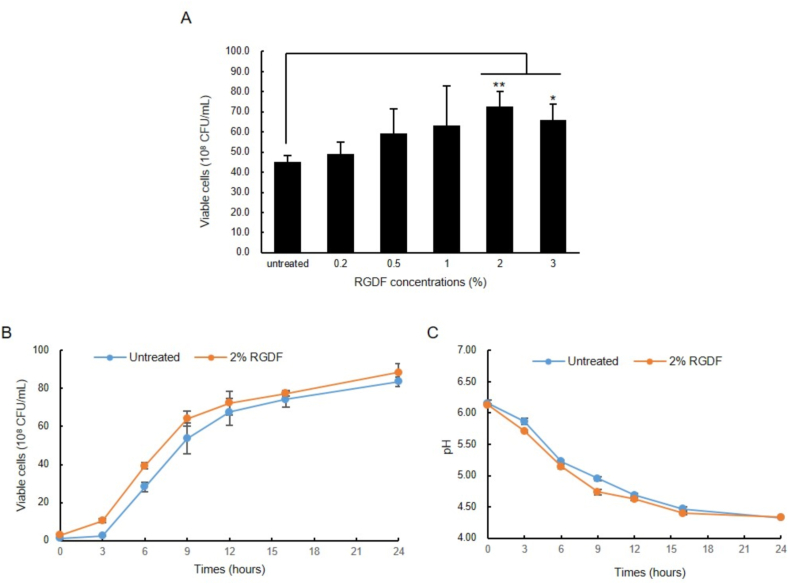
The growth characteristics of L.plantarum IDCC3501 in MRS containing RGDF and the growth phase were monitored for 24h (Fig. 1). The number of viable bacterial cells increased as the RGDF content increased (Fig. 1A). The number of viable bacterial cells cultured with 2% RGDF was approximately 1.6-fold higher than that without RGDF at the end of culture. L.plantarum IDCC3501 grew rapidly during the L-phase (3–9h) with or without RGDF, but the viable cell counts of cultures with RGDF started to increase more quickly than those without RGDF (Fig. 1B).
Fig. 1.
Growth characteristics of L.plantarum IDCC3501 in medium supplemented RGDF. (A) Viable cells and concentration of RGDF supplemented to growth media. Statistical significance was analyzed by one-way analysis of variance (ANOVA). Results are expressed mean ± SD, n = 3, ∗p < 0.05, ∗∗p < 0.01 compared to untreated group. (B) Viable cell count (C) pH value of L.plantarum IDCC3501 in medium supplemented 2% of RGDF.
Over the growth period, the differences in the pH values of the culture medium of cultures in MRS containing 2% of RGDF and those containing MRS only were observed (Fig. 1C).There was no difference in the initial and final pH of culture medium with or without RGDF. However, the pH values during the L-phase were slightly lower for cultures in RGDF (2%)-containing MRS than in those with MRS only.
3.2. RNA sequencing and analysis of DEGs data
To analyze the transcriptional changes in L.plantarum IDCC3501 during growth, we performed RNA sequencing and DEG analysis. The mRNA of L.plantarum IDCC3501 was isolated from 12 cultures (three replicate samples per group) in MRS with and without RGDF at the L-phase and S-phase and then sequenced. As shown in Table 2, we obtained 53,220,054 to 72,404,768 paired-end raw reads per library, and after pre-processing, 5.29 to7.20 gb of clean reads was obtained. The GC content was 45.07% to 46.62%, and the Q20 was over 98%. The clean reads were mapped to the L.plantarum IDCC3501 genome [29]. A total of 3,160 genes were mapped, and an average of 2,666 genes with FPKM ≥ 10 were considered.
Table 2.
Results of RNA Sequencing and Quality Assessment of RNA Samples
| Sample | Raw reads | Raw bases (Gb) | Clean reads | Clean bases (Gb) | Q20 (%) | GC content (%) |
|---|---|---|---|---|---|---|
| Con-L1 | 66,259,650 | 6.69 | 65,607,986 | 6.59 | 98.42 | 45.2 |
| Con-L2 | 55,434,156 | 5.60 | 54,962,178 | 5.52 | 98.57 | 45.35 |
| Con-L3 | 58,930,768 | 5.95 | 58,398,792 | 5.86 | 98.53 | 45.22 |
| Con-S1 | 53,298,506 | 5.38 | 52,863,070 | 5.31 | 98.62 | 45.69 |
| Con-S2 | 61,049,516 | 6.17 | 60,512,276 | 6.08 | 98.54 | 45.74 |
| Con-S3 | 63,169,376 | 6.38 | 62,614,996 | 6.29 | 98.55 | 46.62 |
| RGDF-L1 | 72,404,768 | 7.31 | 71,721,136 | 7.20 | 98.43 | 45.07 |
| RGDF-L2 | 61,624,296 | 6.22 | 61,083,268 | 6.13 | 98.51 | 45.21 |
| RGDF-L3 | 61,332,176 | 6.19 | 60,701,026 | 6.09 | 98.39 | 45.57 |
| RGDF-S1 | 53,220,054 | 5.38 | 52,695,518 | 5.29 | 98.44 | 45.76 |
| RGDF-S2 | 61,740,572 | 6.24 | 61,187,016 | 6.14 | 98.53 | 45.43 |
| RGDF-S3 | 70,835,332 | 7.15 | 70,260,936 | 7.06 | 98.61 | 45.38 |
To analyze DEGs, the samples were divided into two groups. Group A was without RGDF (Con-L) versus with RGDF (RGDF-L) at the L-phase, while group B was without RGDF (Con-S) versus with RGDF (RGDF-S) at the S-phase. The correlations between the three replicates in the same groups were high (R2 > 0.90), indicating that the samples were selected properly (Fig. 2). DEG analysis found that 29 genes were upregulated and 30 genes were downregulated in the RGDF-treated group at the L-phase. We identified 57 upregulated and 126 downregulated genes in the RGDF-treated group at the S-phase (|log2(FC)|>1, Q-value <0.05) (Table 3).
Fig. 2.
Correlations between the three replicates in the same group (red box).
Table 3.
Number of DEGs at Different Normalized log2(FC) Values
| DEGs group | Regulation pattern | Normalized |log2(FC)| |
||||
|---|---|---|---|---|---|---|
| >1 | >2 | >3 | >4 | >5 | ||
| Con_L vs. RGDF_L | Up | 29 | 12 | 4 | 0 | 0 |
| Down | 30 | 20 | 14 | 3 | 1 | |
| Con_S vs. RGDF_S | Up | 57 | 8 | 0 | 0 | 0 |
| Down | 126 | 51 | 25 | 5 | 0 | |
3.3. Gene Ontology analysis of differentially expressed genes
To identify the functions of the DEGs, Gene Ontology (GO) analysis was performed. The DEGs were classified into 10 GO terms at Level 2(Fig. 3). In group A (Con-L vs RGDF-L), three terms were related to Biological Process, two to Cellular Process, and five to Molecular Function. For group B (Con-S vs RGDF-S), five terms were related to Biological Process, two to Cellular Process, and three to Molecular Function. The GO terms for Biological Process in group A included localization (GO:0051179), metabolic process (GO:0008152), and Cellular Process (GO:0009987). For group B, biological regulation (GO:0065007) and regulation of Biological Process (GO:0050789) were added. In the category of Cellular Component, cellular anatomical entity (GO:0110165) and protein-containing complex (GO:0032991) were annotated in groups A and B. For Molecular Function, only three GO terms were identified for group B: catalytic activity (GO:0003824), binding (GO:0005488), and transporter activity (GO:0005215). In addition to group B, two GO terms for Molecular Function were added to group A: ATPase (GO:0016887) and structural molecular activity (GO:0005198).
Fig. 3.
Results of Gene Ontology analysis for DEGs set between (A) Con-L vs. RGDF-L (B) Con-S vs. RGDF-S.
4. Discussion
In this study, we established that RGDF supplementation enhanced the proliferation of L.plantarum IDCC3501 and investigated the molecular mechanisms of this effect, using transcriptome profiling. RGDF, which is used as a growth promoter for L.plantarum IDCC3501, was prepared from the residue of red ginseng after water extraction. Although some of the active ingredients are removed during the extraction process, this material still contains some bioactive components. In a previous study, red ginseng marc was found to have more dietary fiber than wheat flour [18], and we also confirmed that RGDF contains about 30% dietary fiber. The dietary fiber from fruit and vegetable by-products has prebiotic effects on the growth enhancement of lactic acid bacteria and short-chain fatty acid production [30,31]. In some cases, this effect was higher than that of FOS, the best known prebiotic [30]. To evaluate the prebiotic potential of RGDF, the growth of L.plantarum IDCC3501 was measured. As mentioned above, the viable cell counts of L.plantarum IDCC3501 supplemented with 2% of RGDF (7.3 × 109 CFU/mL) was 1.6-fold higher than those of untreated cultures (4.5 × 108 CFU/mL) at the end of cultivation, but the growth of this microbe was no longer promoted when supplemented with 3% of RGDF (Fig. 1A). Ginsenoside in RGDF may have produced an antibacterial effect on L.plantarum. The antimicrobial activities of ginsenoside against gram-positive and gram-negative bacterial strains have been reported [32,33]. This broad-spectrum antibacterial effect would also inhibit the growth enhancement of L.plantarum IDCC3501. The growth curve of L.plantarum IDCC3501 cultured in medium supplemented with 2% RGDF, and the decrease in the pH of the culture medium during cultivation (Fig. 1B and C), indicated that RGDF may be a growth enhancer and is still valuable as a prebiotic. In this study, transcriptome analysis was performed to confirm the molecular mechanism of RGDF as a prebiotic substrate.
We selected two time points for transcriptome analysis during cultivation: 6 and 12h after incubation. These time points represent the middle of the L-phase and the early S-phase in the growth of L.plantarum IDCC3501 (Fig. 1B). Using DEG analysis, 5 out of 29 genes that were upregulated only in RGDF-L and 10 out of 57 genes that were upregulated only in RGDF-S were selected (log2(FC) > 1) (Table 3, Fig. 4). Three genes were upregulated in both the L- and S-phases. During the L-phase of L.plantarum IDCC3501 supplemented with 2% RGDF, the expression of genes involved in the serine catabolism (sdhA, sdhB, and sdaC) was increased. The amino acid serine is an important biomolecule, which is deaminated rapidly to pyruvate and ammonia by serine dehydratase (SDH) (EC 4.3.1.17) [[34], [35], [36]]. The pyruvate originating from serine is transformed to organic acids such as acetate and formate in L.plantarum B3089 [35]. Through this conversion of pyruvate to organic acid, ATP is generated. This would have been the source of the energy contributing to the growth enhancement of L.plantarum IDCC3501 during the L-phase.
Fig. 4.
Venn diagrams show DEGs upregulated (log2(FC) > 1) in RGDF treatment group compare to untreated. Yellow area;only in RGDF-L, blue area;only in RGDF-S, yellow green (overlapped) area; in both RGDF-L and RGDF-S.
In the S-phase, the genes related to fatty acid synthesis and metabolism are upregulated: the acc family (accA, accB, accC, and accD) and the fab family (fabI, fabD, fabF, fabG, fabH, and fabZ) [[37], [38], [39]]. The genes of the acc family (acetyl-CoA carboxylase, EC 6.4.1.2) play a key role in the carbon chain elongation of fatty acids [37,38]. The upregulation of fabG (EC.1.1.1.100) increases the number of unsaturated bonds in fatty acids, which in turn enhances cell membrane fluidity in L.plantarum LIP-1. This increased cell membrane fluidity improves resistance to freeze-drying [37]. Therefore, RGDF in the medium increases the expression of genes in the acc and fab families and is expected to increase the commercial value of lactic acid bacteria in the food industry.
Several genes were upregulated in both the L-phase and S-phase: menH (EC.4.2.99.20), cysE (EC.2.3.1.30), and dapA (EC.4.3.3.7). MenH (2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase, SHCHC synthase) is a menaquinone biosynthetic enzyme. Menaquinone, or vitamin K2, is mainly produced by bacteria and an important electron carrier in the respiratory chain of many microbes. It is synthesized from chorismate [[40], [41], [42]]. MenH, SHCHC synthase, catalyzes a late step in menaquinone synthesis. Based on these results, it is thought that the menaquinone-producing ability of L.plantarum IDCC3501 was also increased by RGDF treatment, because of the enhancement of functionality by the ingestion of this useful microorganism. A gene encoding L-serine O-acetyltransferase (SAT), cysE, is related to cysteine biosynthesis [43], and dapA, encoding 4-hydroxy-tetrahydrodipicolinate synthase (dihydrodipicolinate synthase, DHDPS), is related to lysine biosynthesis [44]. Amino acid metabolism is a fundamental bioprocess for normal living, and therefore, upregulation of this basic pathway might be related to the increase in the number of viable cells.
5. Conclusions
In this study, we found that RGDF could act as a growth enhancer to L.plantarum IDCC3501. Transcriptome analysis could be an optimal tool for elucidating the molecular mechanisms by which RGDF promotes proliferation of L.plantarum IDCC3501. To establish the prebiotic potential of RGDF, it will be necessary to evaluate its influence upon the gut microbiota. Nevertheless, this growth-promoting effect of RGDF is important, since RGDF could be used as a prebiotic source without additional chemical or enzymatic treatment.
References
- 1.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ursell L.K., Metcalf J.L., Parfrey L.W., Knight R. Defining the human microbiome. Nutr Rev. 2012;70(Suppl 1):S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAO/WHO Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Report from FAO/WHO Expert Consultation. 2001;1–4 [Google Scholar]
- 4.Miller L.E., Ouwehand A.C., Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017;30:629–639. doi: 10.20524/aog.2017.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma V., Sharma N., Sheikh I., Kumar V., Sehrawat N., Yadav M., Ram G., Sankhyan A., Sharma A.K. Probiotics and prebiotics having broad spectrum anticancer therapeutic potential: recent trends and future perspectives. Curr Pharmacol Rep. 2021;7:67–79. [Google Scholar]
- 6.Kerry R.G., Patra J.K., Gouda S., Park Y., Shin H.S., Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazloom K., Siddiqi I., Covasa M. Probiotics: how effective are they in the fight against obesity? Nutrients. 2019;11:258. doi: 10.3390/nu11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S.Y., Chae S.A., Bang W.Y., Lee M., Ban O.H., Kim S.J., Jung Y.H., Yang J. Anti-inflammatory potential of Lactiplantibacillus plantarum IDCC 3501 and its safety evaluation. Braz J Microbiol. 2021;52:2299–2306. doi: 10.1007/s42770-021-00603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 10.Walton G.E., Swann J.R., Gibson G.R. Prebiotics. The Prokaryotes. 2013:25–43. [Google Scholar]
- 11.So S.H., Lee J.W., Kim Y.S., Hyun S.H., Han C.K. Red ginseng monograph. J Ginseng Res. 2018;42:549–561. doi: 10.1016/j.jgr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong J., Gwon D., Jang C.Y. Ginsenoside Rg1 suppresses cancer cell proliferation through perturbing mitotic progression. J Ginseng Res. 2022;46:481–488. doi: 10.1016/j.jgr.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan H.D., Kim J.T., Kim S.H., Chung S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu S., Xia H., Guo Y., Qian X., Zou X., Yang H., Yin M., Liu H. Ginsenoside Rb1 retards aging process by regulating cell cycle, apoptotic pathway and metabolism of aging mice. J Ethnopharmacol. 2020;255 doi: 10.1016/j.jep.2020.112746. [DOI] [PubMed] [Google Scholar]
- 16.Park Y.R., Han I.J., Kim M.Y., Choi S.H., Shin D.W., Chun S.S. Quality characteristics of sponge cake prepared with red ginseng marc powder. Korean J Food Cook Sci. 2008;24:236–242. [Google Scholar]
- 17.Zang O.H., Park J., Kim S.H., Lee S.Y., Moon B.K. Quality characteristics of yackwa with red ginseng marc powder. Korean J Food Cook Sci. 2014;30:800–805. [Google Scholar]
- 18.Lee J.Y., Lim T., Kim J., Hwang K.T. Physicochemical characteristics and sensory acceptability of crackers containing red ginseng marc. J Food Sci Technol. 2022;59:212–219. doi: 10.1007/s13197-021-05002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin S.H., Choi W.S. Physiochemical properties of chicken breast sausage with red ginseng marc powder. Food Sci Anim Resour. 2022;42:486–503. doi: 10.5851/kosfa.2022.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S.H., Kim W.J. Study of hongsambak for medicinal foods applications: nutritional composition, antioxidants contents and antioxidative activity. J Physiol Pathol Korean Med. 2006;20:449–454. [Google Scholar]
- 21.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jatuponwiphat T., Namrak T., Nitisinprasert S., Nakphaichit M., Vongsangna W. Integrative growth physiology and transcriptome profiling of probiotic Limosilactobacillus reuteri KUB-AC5. Peer J. 2021;9 doi: 10.7717/peerj.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z.S., Lin C.F., Chen P.W. Transcriptome analysis of Lactobacillus rhamnosus GG strain treated with prebiotic-bovine lactoferrin under a cold environment. J Food Drug Anal. 2021;29:402e418. doi: 10.38212/2224-6614.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H.W., In G., Han S.T., Lee M.W., Kim S.Y., Kim K.T., Cho B.G., Han G.H., Chang I.M. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J Ginseng Res. 2013;37:457–467. doi: 10.5142/jgr.2013.37.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCleary B.V., DeVries J.W., Rader J.I., Cohen G., Prosky L., Mugford D.C., Champ M., Okuma K. Determination of total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study. J AOAC Int. 2010;93:221–233. [PubMed] [Google Scholar]
- 26.Wang L.K., Feng Z.X., Wang X., Wang X.W., Zhang X.G. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;25:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 27.Storey J.D., Tibshirani R. Statistical significance for genome wide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conesa A., Götz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 2008 doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T.Y., Kim Y.H., Moon J.S., Kwon H.S., Choi S.K. Complete genome sequence of probiotic Lactobacillus plantarum IDCC3501 isolated from kimchi. Korean J Microbiol. 2019;55:438–440. [Google Scholar]
- 30.Andrade R.M., Silva S., Costa C.M., Veiga M., Costa E., Ferreira M.S., Gonçalves E.C., Pintado M.E. Potential prebiotic effect of fruit and vegetable byproducts flour using in vitro gastrointestinal digestion. Food Res Int. 2020;137 doi: 10.1016/j.foodres.2020.109354. [DOI] [PubMed] [Google Scholar]
- 31.Massa N.M., Menezes F.N., Albuquerque T.M., Oliveira S.P., Lima M.S., Magnani M., Souza E.L. Effects of digested jabuticaba (Myrciaria jaboticaba (Vell.) Berg) by-product on growth and metabolism of Lactobacillus and Bifidobacterium indicate prebiotic properties. LWT - Food Science and Technology. 2020;131 [Google Scholar]
- 32.Sung W.S., Lee D.G. The Combination effect of Korean red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 2008;31:1614–1617. doi: 10.1248/bpb.31.1614. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Huang Y., Yin G., Wang J., Wang P., Chen Z.Y., Wang T., Ren G. Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother Res. 2019;1–11 doi: 10.1002/ptr.6605. [DOI] [PubMed] [Google Scholar]
- 34.Kriner M.A., Subramaniam A.R. The serine transporter SdaC prevents cell lysis upon glucose depletion in Escherichia coli. Microbiology Open. 2020;9 doi: 10.1002/mbo3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S.Q., Holland R., McJarrow P., Crow V.L. Serine metabolism in Lactobacillus plantarum. Int J Food Microbiol. 2003;89:265–273. doi: 10.1016/s0168-1605(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 36.Fernández M., Zúñiga M. Amino acid catabolic pathways of lactic acid bacteria. Crit Rev Microbiol. 2006;32:155–183. doi: 10.1080/10408410600880643. [DOI] [PubMed] [Google Scholar]
- 37.Jingjing E., Lili M., Zichao C., Rongze M., Qiaoling Z., Ruiyin S., Zongbai H., Junguo W. Effects of buffer salts on the freeze-drying survival rate of Lactobacillus plantarum LIP-1 based on transcriptome and proteome analyses. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126849. [DOI] [PubMed] [Google Scholar]
- 38.Zichao C., Jingjing E., Rongze M., Jingya Z., Yao C., Wang R., Zhang Q., Yang Y., Lia J., Wang J. The effect of aspartic acid on the freeze-drying survival rate of Lactobacillus plantarum LIP-1 and its inherent mechanism. LWT. 2022;155 [Google Scholar]
- 39.Zeng X., Pan Q., Guo Y., Wu Z., Sun Y., Dang Y., Cao J., He J., Pan D. Potential mechanism of nitrite degradation by Lactobacillus fermentum RC4 based on proteomic analysis. J Proteomics. 2019;194:70–78. doi: 10.1016/j.jprot.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Jiang M., Chen X., Guo Z.F., Cao Y., Chen M., Guo Z. Identification and characterization of (1R,6R)-2-Succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase in the menaquinone biosynthesis of Escherichia coli. Biochemistry. 2008;47:3426–3434. doi: 10.1021/bi7023755. [DOI] [PubMed] [Google Scholar]
- 41.Bentley R., Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang M.J., Baek K.R., Lee Y.R., Kim G.H., Seo S.O. Production of vitamin K by wild-type and engineered microorganisms. Microorganisms. 2022;10:554. doi: 10.3390/microorganisms10030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogicevic B., Berthoud H., Portmann R., Bavan T., Meile L., Irmler S. Cysteine biosynthesis in Lactobacillus casei: identification and characterization of a serine acetyltransferase. FEMS Microbiol Lett. 2016;363:fnw012. doi: 10.1093/femsle/fnw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnell R., Oehlmann W., Sandalova T., Braun Y., Huck C., Maringer M. Tetrahydrodipicolinate N-Succinyltransferase and dihydrodipicolinate synthase from Pseudomonas aeruginosa: structure analysis and gene deletion. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031133. [DOI] [PMC free article] [PubMed] [Google Scholar]