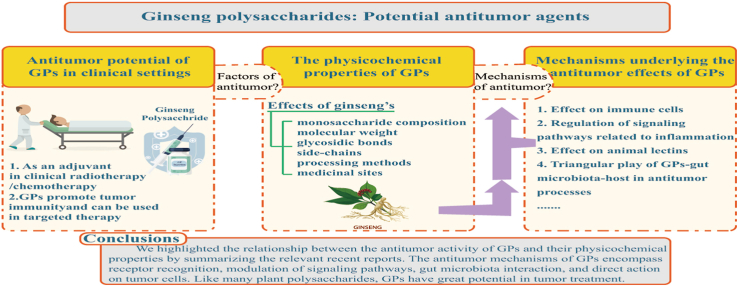
Abstract
As a famous herbal medicine in China and Asia, ginseng (Panax ginseng C. A. Meyer) is also known as the "King of All Herbs" and has long been used in medicine and healthcare. In addition to the obvious biological activities of ginsenosides, ginseng polysaccharides (GPs) exhibit excellent antitumor, antioxidant stress, and immunomodulatory effects. In particular, GPs can exert an antitumor effect and is a potential immunomodulator. However, due to the complexity and diversity in the structures and components of GPs, their specific physicochemical properties, and underlying mechanisms remain unclear. In this article, we have summarized the factors influencing the antitumor activity of GPs and their mechanism of action, including the stimulation of the immune system, regulation of the gut microbiota, and direct action on tumor cells
Keywords: Ginseng polysaccharides, antitumor, immunomodulator, immune system, gut microbiota
Graphical abstract
1. Introduction
Polysaccharides, a type of natural polymer, are usually composed of more than ten monosaccharides arranged in straight or branched chains bounded through glycosidic bonds, with molecular weights reaching up to thousands or even millions of Daltons. According to their origin, these can be classified as animal polysaccharides, plant polysaccharides, or fungal polysaccharides [1]. Traditionally, polysaccharides were thought of as non-degradable molecules which could not be absorbed by the human system owing to the complexity of their macromolecular structures and were thus separated and discarded [2]. However, in the 1960s, scientists have discovered that the plant polysaccharides extracted from bamboo exert potential antitumor effects [3]. Since then, several plant polysaccharides with antitumor and immunomodulatory activities have been discovered and even investigated in clinical settings, for example, Fucoidan [4,5], Lycium barbarum polysaccharides [6], Astragalus polysaccharides [7], Ganoderma lucidum Polysaccharide [8], and ginseng polysaccharides (GPs) [9].
As a famous herbal medicine, ginseng has been widely used for thousands of years. Its active ingredients include saponins, polysaccharides, volatile oils, amino acids, and organic acids among others. Research on ginsenosides is relatively complete and strong evidence for their physiological functions has been demonstrated. However, polysaccharides, the most abundant among ginseng components (approximately 40% in total), have not been adequately studied [10]. GPs were first isolated by a group of researchers in 1966 [11], and since then progress in studies on GPs has been gradual. The rich physiological activities of GPs have been slowly "uncovered". Several experiments have demonstrated the antitumor, immunomodulatory, antioxidant [12], and antibacterial [13] effects of GPs. The GP Injection has also been gradually introduced in clinical practice as adjuvant treatment for malignant tumors and has been proven to yield better efficacy [14].
Usually, subtle differences in molecular weight and monosaccharide conformations that are exhibited may have tremendous impacts on the antitumor activity of GPs. For instance, GPs can significantly activate macrophages and NK cells in tumor-bearing mice, enhance immune function, and inhibit lung metastasis of colon26-M3.1 cells [15]. In addition, GPs can be used as an adjuvant in the treatment of Lewis lung cancer (LLC) and B16F10 melanoma, along with PD-1/PD-L1 monoclonal antibodies. As a result, GPs strongly enhance the sensitivity of tumor-bearing mice towards monoclonal antibodies and inhibit tumor metastases. The underlying mechanism is significantly related to the alteration of the gut microbiota [16]. The active ingredients of GPs have been shown to exert direct effects on tumor cells, including HepG2 [17], whereby they affect the proliferation, migration, and apoptosis of these cells .
Therefore, in this review, we have focused on the biological activities of GPs and reviewed the mechanisms underlying the immunomodulatory and antitumor effects of GPs, to provide a reference for further research on the antitumor applications of GPs.
2. Antitumor potential of GPs in clinical settings
2.1. Adjuvant effects of GPs in clinical radiotherapy/chemotherapy
The results of current clinical studies suggest that GPs can be used as effective active substances in the adjuvant-based treatment of tumors, wherein the main mechanism of action may be related to improving the immune function of the patients.
In a clinical trial conducted in China, 131 patients with nasopharyngeal carcinoma (NPC) were divided into radiotherapy and radiotherapy combined with GPs groups, parallelly in a randomized manner. In the combination group, 12 mg of GPs were administered intravenously daily for three-seven days before radiotherapy and until the end of this treatment regime. The primary foci and the status of the lymph nodes in the neck were compared between the two groups. Relative to the GPs group, the patients in the combination group were found to have smaller primary foci, fewer metastatic lymph nodes, and significant increases in the activities of the T cells, NK cells, and LAK cells, after treatment. Moreover, no toxic side-effects were observed [18]. In addition, several clinical studies on GPs for the treatment of non-small cell lung cancer (NSCLC) have been ongoing in the last decades. The clinical study on the adjuvant treatment of NSCLC with GPs in Hubei, China, comprised 80 patients who were parallelly and randomly assigned to different treatment groups. In the observation group, GPs were administered intravenously twice daily in the two courses of treatment along with chemotherapy. The result suggested a significant increase in the overall efficiency and clinical benefits to these patients. Alleviation of the adverse effects of radiotherapy, including reduced white blood cell count, decreased platelets, anemia, gastrointestinal symptoms, and hair loss [19] was also reported. Consistently, in a clinical study in Nanjing, China, 96 patients with NSCLC were divided equally into two groups. The control group underwent dendritic cell therapy by thoracoscopy, while the observation group was simultaneously administered GP injection at 0.5 mg/kg four times a week, for 30 days. The FACT-L score, Th1 cytokine (INF-γ, IL-2), and Th1/Th2 ratio (INF-γ/IL-4, IL-2/IL-5) were markedly higher in the observation group relative to those in the control group after treatment. This suggested that as compared to dendritic cell therapy alone, the combination treatment could significantly improve the immune function of patients [20]. Similarly, results from two other clinical trials for chemotherapy in combination with GPs show that patients who are intravenously administered GPs, have a higher quality of life and immune capacity [21].
The rapidly expanding study on key functions of GPs in several cancers such as NPC and NSCLC has revealed its promising therapeutic value on enhancing the cancer care and improving the quality of life for patients without obvious side-effect. However, the recommended dose of GP Injection is obscure limited to the number and quality of literatures involved. Besides, the different drug injection strategies also make the selection of dose and course of treatment vary from person to person. More research and clinical trials should be considered to shed further light on the efficacy and concentration of it.
2.2. GPs promote tumor immunity and can be used in targeted therapy
Unlike the clinical uses of GPs, which are currently limited to an adjuvant, along with radiotherapy/chemotherapy in cancer patients, experimental pharmacological studies continually enrich the knowledge on GPs. It's an excellent and promising immunostimulant with anti-inflammatory [22], anti-oxidative stress [23], and antitumor properties, and can enhance the efficacies of tumor immunotherapy and targeted therapy.
An article published in May 2021 shows that GPs can enhance the antitumor efficacy of the anti-PD-1/PD-L1 immunotherapy [16]. These investigators show that in LLC-bearing mice, the combination therapy of GPs (200mg/kg) administered by gavage for 24 days and PD-1 monoclonal antibody (250ug/mouse) every third day could significantly enhance the tumor immunotherapeutic effects of PD-1 monoclonal antibody and remarkably prolong the survival of these mice. Moreover, GPs remold the sensitivity of PD-1 monoclonal antibody-treated non-responding mice to treatment with immune checkpoint blockade (ICB) and increase the ratio of CD8+/CD4+ cells. In this process, GPs reshape the gut microbiome, thereby producing specific microbial metabolites which intermediate the communication between microbiota and immune cells, thus strengthening the ICB treatment. Weather GPs can be used as a dietary supplement through regulating gut microbiome for NSCLC patients to improve immunotherapy efficacy is of great further concerned.
Recent studies also show that GPs can target the T cells to treat tumors by affecting their apoptosis and activation [24]. The direct effects of GPs on inhibiting the proliferation and apoptosis of tumor cells [25], and weakening their migration and invasion abilities [26] have also been proven. These reports have provided strong support for the assumption that GPs can be used in targeted therapy in tumor immunity.
3. Effects of the physicochemical properties of GPs on their antitumor activities
3.1. Effects of monosaccharide composition, molecular weight, glycosidic bonds, and side-chains
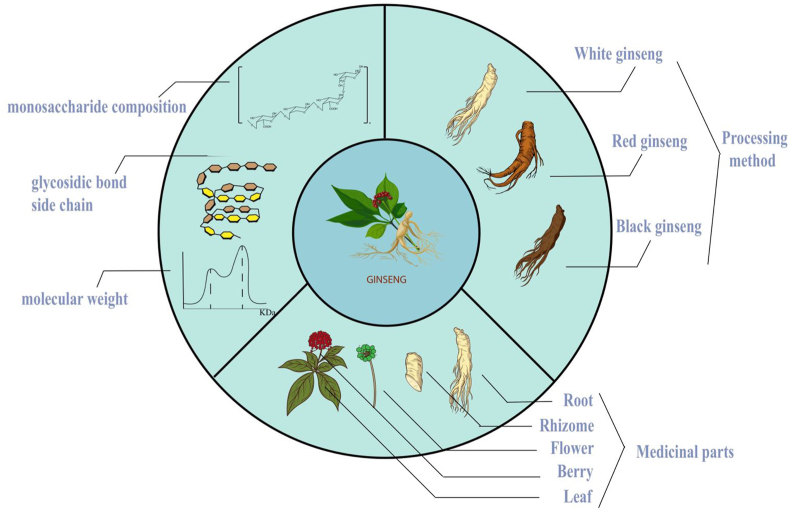
GPs have diverse compositions and complex structures. According to the composition of monosaccharides, GPs can be divided into two main groups as follows: ginseng neutral polysaccharides and ginseng acidic pectins [9]. Neutral polysaccharides are mainly composed of glucan and arabinogalactan, while pectins are polysaccharides rich in galacturonic acid. An in vitro culture of mouse spleen lymphocytes treated with neutral and acidic polysaccharides showed a strong stimulatory effect at low concentrations (25 mg/L), while treatment with acidic sugar showed a better stimulatory effect at high concentrations (400 mg/L) (Song Lihua, Wang Hongmei et al. 2012). PGPW1 (Glc: Gal: Man: Ara = 3.3: 1.2: 0.5: 1.1) and PGP2a (Gal: Ara: Glu: GlaA = 3.7: 1.6: 0.5: 5.4), two monosaccharides with different antitumor GPs fractional composition, were purified by different extraction and isolation methods. These inhibit the cellular proliferative activity and migration while promoting apoptosis of HGC-27 cells [25].
Depending on their molecular weights, the isolated GPs also have different structural compositions chemically, suggesting that the utilization of polysaccharides is closely related to the length of the polymer [27]. Two fractions, ginseng berry polysaccharide extract (GBPE, molecular weight 11-20 kDa) and ginseng berry polysaccharide portion (GBPP, molecular weight 76 kDa), have been isolated and purified from the ginseng berry polysaccharides. These exhibit different physicochemical properties, with GBPE having better antitumor activity and GBPP exerting a stronger anti-inflammatory effect [28]. The ginseng neutral polysaccharide (GPN) is hydrolyzed into two extracts, GPNE-I (molecular weight 80.3 kDa) and GPNE-II (molecular weight 31.5 kDa). GPNE-I is a heteropolysaccharide (38.17% branching) comprising the glucose structural-domain and arabinogalactans, AG-I and AG-II, whereas GPNE-II is a glucan (50.78% branching) with a (1→ 4)-α-d-Glcp backbone and a substitution at O-6 on each of the two residues; (1→ 3)-α-d-Glcp and (1→ 6)-α-d-Glcp located at the two branches. The differential molecular weights and branching side-chains result in varied physiological activities, as evidenced by GPNE-I being able to efficiently stimulate proliferation of the lymphocytes [29].
3.2. Effect of processing methods
As fresh products are difficult to store at room temperature for longer periods, different processing methods have been invented and they also exert varied effects on the products. Different ginseng products, including white, red, and black ginseng sold in the market [30], have been examined in this context; reportedly, after steaming, the starch content of fresh ginseng reduces significantly. Maltose is formed in large quantities, and the content of amino sugars such as arginine-fructose-glucose (AFG) and arginine-fructose (AF) in red ginseng significantly increases due to the Millard reaction. Acidic polysaccharides, an immunologically active component in red ginseng, which is three times more abundant than that in white ginseng, are found in the main root (7.5%) and lateral roots (4.7%); in the white ginseng, 0.63% is found in the main root and 0.86% in the lateral roots [31]. Black ginseng, which is made by drying white or red ginseng several times, is found to have further less polysaccharide content [32]. A previous study reports that the oligosaccharide content in white ginseng is 1.4 times higher than that in red ginseng and 119 times higher relative to that in black ginseng, while the pectin content in red ginseng is 44 times higher than that in white ginseng and 18.3 times higher than that in black ginseng [33]. Differences in the polysaccharide contents in each ginseng product result in the extraction of varying polysaccharide fractions, which may account for their differential pro-immune activities and antitumor effects. Regretfully, hardly any articles obtain an overall evaluation among these ginseng products now. Apart from ginsenoside,the antitumor activities of different polysaccharide fractions deserve to be investigated too.
3.3. Effect of medicinal sites
Owing to the scarcity and preciousness of ginseng, researchers have attempted to expand its utilization. The distribution of GPs varies within the tissue parts [34]. Ginseng roots have the highest content of polysaccharides, with the proportion of neutral polysaccharides (16.09%) exceeding that of acidic pectins (5.58%) [35]. Although the ginseng rhizomes contain less neutral (4.37%) and acidic polysaccharides (3.51%), the polysaccharides in the rhizomes above-ground, have a better antioxidant capacity as evidenced by the results of the DDPH method [36]. The polysaccharide composition of ginseng leaves is similar to that of the roots, consisting mainly of pectins and heteropolysaccharides; these also exhibit a good pro-immune activity [15]. Ginseng berries mainly contain pectin components, with higher levels of arabinose and galactose as compared to other monosaccharides [37]. The acidic polysaccharide content in ginseng flowers (55.2%) is significantly higher than that of neutral polysaccharides (10.9%) [38]. The less abundant neutral polysaccharides are a less branched (1→4)-β-D-galactan and a significantly branched (1→6)-β-D-galactan, which can activate macrophages and enhance the immune functions [39] Fig. 1.
Fig. 1.
The relationship between physicochemical properties and activity of GPs. The different monosaccharide compostiton, molecular weight, glycosidic bonds and side-chains of GPs are responsible for its diverse activities; The alterations of GPs in several ginseng products depending on their processing methods also exert influence on its antitumor activities; The utilization of medicinal sites vary GPs from parts to parts, too.
4. Mechanisms underlying the antitumor effects of GPs
As biomacromolecules, the mechanisms by which GPs exert anticancerous and immunomodulatory effects remain unclear. Two routes including injection and oral are used to administer GP currently. After separation and purification, GPs can be injected into the human body. GPs can affect the immune cells, activate the immune system, regulate the inflammatory signaling pathways, and bind to several lectins [9]. Additionally, GPs can facilitate a subtle interaction between microbiota, thereby exerting its influence on the organism.
Gut microbiota is an abundant group of organisms in the intact large bowel, which approximately include 800 different species [40]. The microbiota has diverse functions and complex structures, thereby constituting a “micro ecological organ” in the organism. The gut microbiota participates in several physiological processes, which includes the energy metabolism [41] and immunomodulation [42], thereby impacting the occurrence and development of tumors. The majority of the oral intake of GPs, except starch, cannot be absorbed by the small intestine due to the lack of corresponding digestive enzymes. When the polysaccharide components reach the large intestine, the microbiota “encounter” them, and subsequently, the mutual interaction begins [27].
4.1. Study on the mechanisms of action of GPs as an immunomodulator
4.1.1. Effect on immune cells
In a previous study which aimed at demonstrating the immunomodulatory and antitumor activities of the neutral GPs, the Sarcoma-180 (S180) tumor-bearing mice were daily orally administrated both 5-Fluorouracil (25mg/kg) and different doses of neutral GPs (25, 50, 75, 100, or 100mg/kg) for 10 consecutive days [43]. The results show that the combination could significantly improve the tumor inhibition rate and reduce the burden on tumor-bearing mice. Meanwhile, GPs can facilitate the recovery of the killing ability of NK cells inhibited by the 5-FU and stimulate the production of NO and TNF-α [43]. In an immunosuppressive model constructed using cyclophosphamide, mice were administered GPs at a concentration of 200 or 400mg/kg, for 10 consecutive days. Relative to the cyclophosphamide model group, the cytotoxicity and proportion of NK cells in the whole blood, and the levels of expression of perforin and granzyme in the NK cells in the Cy + low-dose GP and Cy + high-dose GP groups enhance significantly [44]. The non-saponin fractions with rich polysaccharides of red ginseng also show immune-promoting activity, which in turn increases plasma cell count in the spleen and inhibits the melanoma lung metastases in rats [45]. In combination with the αPD-1 monoclonal antibody (mAb) (250μg/mouse), GPs significantly increase the ratio of CD8+/CD4+ T cells in the spleen, blood, and tumor tissues of mice, along with an increase in the production of functional cytokines, including IFN-γ, TNF-α, and GZMB. Simultaneously, GPs can also enhance the efficacy of tumor immunotherapy by decreasing the number of Treg cells.
Consistent with the aforementioned results, Lee, Park et al., show that a polysaccharide fraction, GBPP-I, isolated from ginseng berry significantly inhibits lung metastases in melanoma, and this effect can be attributed to the activation of the macrophages and NK cells [46]. Other ginseng berry polysaccharides, GBPE and GBPP, suppress chemically-induced (AOM/DSS) pro-inflammatory cytokine levels and inhibit the differentiation of Th1 and Treg cells, thus reducing the tumor load in mice. In vitro, two preparations from berries also show anti-proliferative effects on HCT-116 and HT-29 cells [28]. Moreover, Shin, Hwang et al. show that the polysaccharides extracted from ginseng leaves also serve as an immune adjuvant for preventing cancer metastasis through the activation of the NK cells and other immune-related cells including the macrophages [47].
In addition to berries and leaves, a neutral polysaccharide fraction (WGFPN) isolated from ginseng flowers has proven its potential as an immunomodulatory agent as it is shown to enhance immunity in cyclophosphamide (CTX)-induced immunosuppressed mice in vivo and activate RAW264.7 macrophages in vitro by increasing macrophage phagocytosis, releasing NO, and secreting TNF-α, IL-6, IFN-γ, and IL-1β [39].
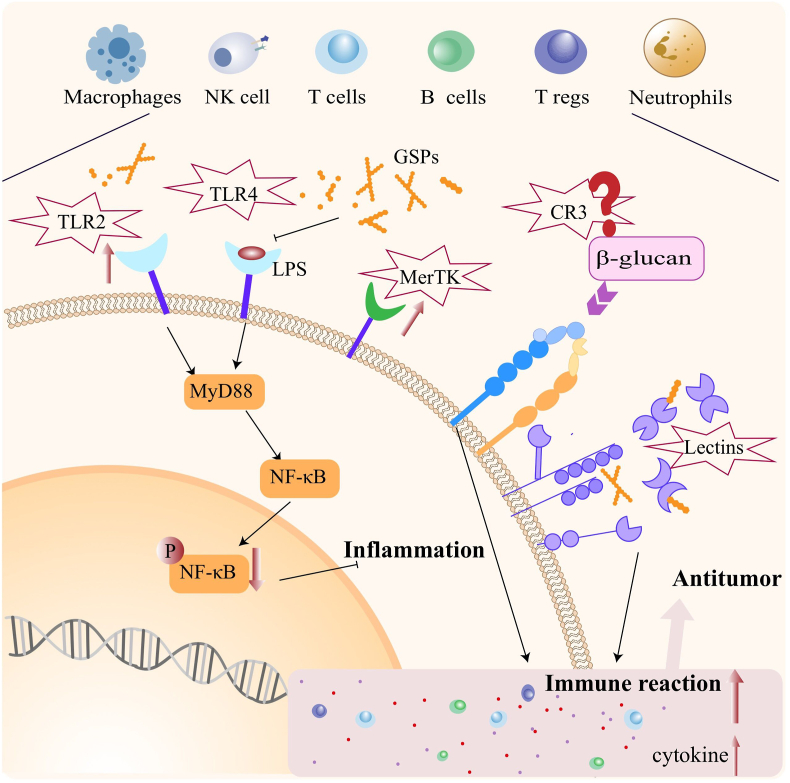
In conclusion, GPs can exert dramatic effects on several immune cell types, causing them to secrete different cytokines or increase their killing activity. The question therefore is, what is the underlying molecular mechanism? Studies show that CR3 (CD11b / CD18 and α M β 2-integrin (Mac-1), the first β-Glucan receptor that participates in innate immune cell recognition), is highly expressed in macrophages, neutrophils, NK cells, and some activated T/B cells [48]. This makes the binding of β-Glucan with neutrophils and monocytes, specific and dose-dependent, thereby playing important roles in the recognition and utilization of the plant polysaccharides [49]. Therefore, the effect of GP on CR3 is a potential mechanism of regulation of immune cells.
4.1.2. Regulation of signaling pathways related to inflammation
Toll-like receptor (TLR) is a transmembrane pattern recognition receptor [50]. When these encounter pathogen-associated molecular patterns (PAMPs), they activate several downstream signaling pathways and stimulate the production of a series of inflammatory mediators, which play an important role in regulating the innate immunity [51]. TLR2/4 are pattern recognition receptors, mainly expressed in myelogenous cells [52], and are the binding sites of natural active components such as polysaccharides and alkaloids [53]. Usually, TLR2 works through the myeloid differentiation factor 88 (MyD88)-dependent TLR signaling pathway, which mediates the transcription of AP-1 controlled by the NF-κB signaling pathway and mitogen-activated protein kinases (MAPKs) [54]. In addition to the MyD88 pathway, TLR4 receptor can also recognize LPS, other plant polysaccharides, and signal through the TIR domain-containing adaptor (TRIF)-dependent pathway [55], thereby activating the NF-κB signaling pathway, as well as, autophagy [56].
Relevant experimental verification confirms that GPs can regulate TLR2/4-mediated inflammatory signaling pathways. For example, GPs inhibit the TLR4-P38-MAPK–NF–κB inflammatory signaling axis activated by LPS in the DSS-induced colitis rat model, which in turn regulates the gut microbiota and reduces the levels of TNF-α, IL-8, and IL-1β in the intestinal inflammatory environment. Moreover, GPs also reverse the phosphorylation of mTOR and alleviate autophagy [57].
In a previous study RAW264.7 cells were cultured in vitro and treated with three doses (100, 250, or 500 ug/ml) of ginseng enzymatic hydrolysate (GEH) extracted from the oligosaccharides derived from ginseng marc polysaccharides, After 24 hours, GEN increased the transcription levels and protein expressions of TLR2 and MERTK in a dose-dependent manner, resulting in the activation of phagocytosis of macrophages and stimulating the production of proinflammatory cytokines (TNF-α and IL-6) and the anti-inflammatory cytokine, IL-10 [58]. MERTK is a TYRO3/AXLl/MER (TAM) receptor tyrosine kinase, known for crucially regulating inflammation [59]. Previous studies show that mice lacking MERTK/AXL/TYRO3 suffer from autoimmune diseases, including rheumatoid arthritis, and the pathogenesis is closely related to chronic inflammation [60].
Taken together, the diverse components of GPs can regulate the inflammation-related signaling pathways such as the TLR2/4 axis. Additionally, the effects of GPs on macrophage membrane surface receptor, MerTK, may also be a potential mechanism of action of regulation of inflammation and innate immunity.
4.1.3. Effect on animal lectins
Lectin is a protein found in plants, animals, and microorganisms. It can specifically recognize and bind to sugars without any structural modifications [55]. Based on the main structure and function of animal lectin, it can be mainly divided into five, the C-type (Ca2+ required), P-type (mannose-6-phosphate binding), S-type (galactose lectin, β-galactoside-specific), I-type (immunoglobulin-like), and pentamers (Ca2+ ligands, including C-reactive protein, which plays a crucial role in the immune system) [61]. M-type, L-type, chitinase type, and F-type lectins have also been reported. [62]. Animal lectins participate in many biological processes, including cellular transport, immune regulation, and prevention of autoimmune reactions [63]. As a recognition molecule in the immune system, the main functions of lectin are direct defense (antibody and/or similar to complement), recognition and transportation in the immune system, regulation of immunity, and prevention of autoimmunity [64,65].
With extensive studies on structures of GPs, researchers conclude that they consist of arabinogalactan, pectin, glucose, fructose, maltose, sucrose, and other kinds of sugars [66]. Animal lectins can recognize glycan structures such as galactose, glucose, rhamnose, and mannose [67]. The aggregation of various lectins leads to the gathering of simple binding sites of the lectin, thereby inducing the affinity towards oligosaccharides and enhancing the recognition and binding of GPs [68]. For example, galectin-3 (Gal-3), an S-type lectin, can mediate cell adhesion, regulate inflammation, induce T cell activation, and apoptosis, thereby playing an important role in the tumor immunity [69]. Xue, Zhao et al. show that several pectin components isolated from GPs significantly inhibit the growth of Sarcoma-180 tumors. Mechanistically, ginseng pectins selectively inhibit T cell apoptosis induced by galectin-3 without affecting the T cell activation. Moreover, pectin components can also promote T cell proliferation and production of IL-2, which is expected to provide a new avenue for studies on the antitumor mechanisms of GPs [24]. Cui, Wang et al. also show that the three kinds of rhamnogalacturonan I (RG-I) type pectin extracted from ginseng flowers have rich side chains and show strong binding to galectin-3; the corresponding KD values are 4.9μM, 0.71μM, and 0.24μM, respectively [38]. These results indicate the potential of GPs as animal lectin receptor inhibitors for tumor therapy Fig. 2.
Fig. 2.
The mechanisms of action of GPs as an immunomodulator. GPs play a fundamental role for antitumor effects in modulating the function of immunes cells such as NK cells, T cells, macrophages and neutrophils. The modes underlying are associated with the expression of CR3 in immune cells, regulation of Inflammatory pathways (NF-κB) and activation of animal lectins (galectin-3).
4.2. Triangular play of GPs-gut microbiota-host in antitumor processes
4.2.1. Metabolic transformation of GPs by gut microbiota
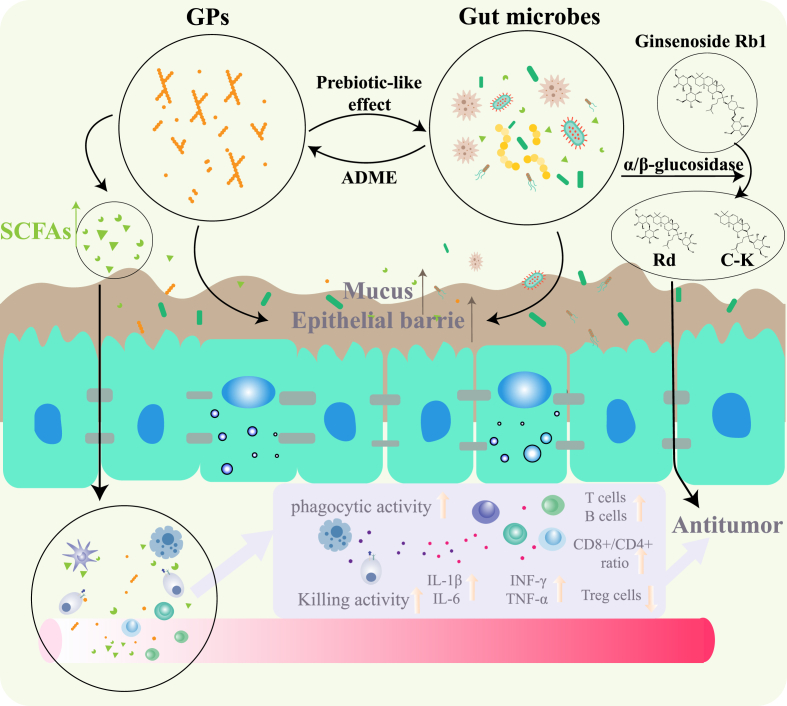
As an important way for the hosts to digest and absorb nutrients, the gut microbiota produces a series of endogenous metabolites which can be used by the human body [70]. Probiotics in the gut microbiome, including Bifidobacteria and Lactobacillus, can produce glycosidases, such as β-glucosidase, rhamnosidase, and xylanase, which can degrade dietary fibers. Moreover, the gut microbes produce short-chain fatty acids through glycolysis and the pentose phosphate pathway [71].
Short-chain fatty acids produced in the colon can be absorbed through the intestinal capillaries and are transported into the hepatic portal vein, thereafter used as nutrients. Additionally, these short-chain fatty acids can maintain the acidic environment of the intestine, intestinal health, and influence metabolism and intestinal immunity; these are also implicated in cancers [72]. For example, acetic acid, propionic acid, and butyric acid are usually enriched in the cecum and proximal colon. They are the main sources of energy for the intestinal epithelial cells and contribute to the protection of the integrity of the intestinal barrier system [73], furthermore, inhibiting inflammation and tumor [74].
The different components of GPs play regulatory roles in the production of SCFAs after hydrolysis, hydration, and degradation, all of which are mediated by gut microbes. For instance, after long-term administration of GPs, the contents of acetic acid, isobutyric acid, and butyric acid in the colon of rats increase significantly, along with the abundance of probiotics, in particular the butyric acid-producing bacteria, such as Firmicutes [75]. Huang, Liu et al. show that intragastric administration of GPs combined with PD-1 monoclonal antibody can significantly change the abundance of short-chain fatty acids in the intestine of tumor-bearing mice, in particular, of valeric acid [16]. Yang, Han et al. also report that when piglets when fed on food containing 800mg/kg GPs, the probiotics in the colon, namely Lachnospiraceae and Anaerostipes, significantly increase the abundance of different short-chain fatty acids, including acetic acid, isobutyric acid, and butyric acid [76].
After entering the systemic circulation, SCFAs can inhibit histone deacetylases (HDACs), thereby activating the G protein-coupled receptors. Acting as energy substrates and signaling molecules, SCFAs profoundly affect the physiological activities of the host [73]. Chiefly, these are transported into the cells through transporters (MCT1/4 and SMCT1/2) or by passive diffusion [77]. Moreover, the receptors of SCFAs include GPR41, GPR43, GPR109A, olfr-78 (murine) / OR51E2 (homo sapiens), PPARc, and AHR among others. In cancer treatment, SCFAs show their strong ability to promote the production of cytotoxic T lymphocytes, thereby affecting the functions of the chimeric antigen receptor T cells and regulating metabolism and epigenetics of effector T cells [78]. For instance, sodium butyrate supplementation in mice with liver metastasis due to colorectal cancer can increase the proportion of NKT and Th17 cells while reducing Tregs, to improve the antitumor immune responses [79]. Therefore, the transformation and metabolism of GPs by gut microbiota is an essential means to promote the absorption and utilization of their effective components.
4.2.2. Prebiotic-like effects of GPs
Moreover, as dietary fibers, GPs exert prebiotic-like effects on the gut microbiota [80]. According to the biochemical reactions of the microbes and their effects on the host, these can be divided into three types: probiotics (dominant bacteria in the body), conditional pathogens, and pathogenic bacteria [81]. The imbalance in gut microbiota often promotes the development of colorectal cancer and other intestinal diseases, thus worsening liver and breast cancers. Finally, this imbalance has an essential impact on tumor treatment and drug activity. Fortunately, GPs can increase/inhibit the growth of some specific bacteria, thereby changing the structure of the gut microbiome and enhancing the activity of metabolic enzymes produced by the bacteria [82].
As an acidic heteropolysaccharide, soluble dietary fibers of ginseng (ginseng-SDF, 8.98% content) extracted from its residue significantly improved the growth performance and serum immunoglobulin (IgA, IgM, and IgG) levels in rats [75]. GPs change the structure of the gut microbiota and increase the abundance of probiotics such as Lactobacillus. Additionally, the production of butyric acid in the cecum is enhanced [75,83].
GPs also play a vital role in the triangular play under various challenging conditions through the modulation of the gut microbiota. In a mouse model of diarrhea induced by lincomycin, ginseng neutral polysaccharides can significantly regulate the gut microbiota. With the increase in Lactobacillus, Lactococcus, and Streptococcus and decrease in Firmicum, the intestinal mucosal injury is also alleviated [84]. The effects of GPs on microbes in combination with PD-1 monoclonal antibody in a mouse model of Lewis lung cancer (LLC), the antitumor ability of GPs is well-reflected on tumor-bearing. After 24 days of administration, the tumor metastasis markedly decreases and the immune function is significantly enhanced. Additionally, the abundance of two specific Bacteroides increases after treatment of GPs, which changes the sensitivity of no-response to PD-1 immune checkpoint therapy in the mice [16].
4.2.3. Effects of GPs on absorption, distribution, metabolism and excretion (ADME) of ginseng active components
Gut microbiota exhibits different functions and varied metabolic capacities for sugars, proteins, and lipids [85]. Studies show that the ability to metabolize ginsenoside largely depends on the composition and structure of the gut microbiota. The abundance of Bifidobacterium GM1 plays a key role in the transformation of ginsenoside Rb1 to Rd [86]. Changing the structure of the gut microbiota through prebiotic intervention can result in alteration of the metabolism of other components in the intestine in an effect to enhance the utilization of the specific components and the efficacy of the targeted therapy [87]. The research conducted by the Chinese Academy of Traditional Chinese Medicine shows that when Sprague Dawley rats were administered with ginsenoside Rb1 after a two-week prebiotic intervention with fructooligosaccharide, galactooligosaccharide, or fibersol-2, the composition and structure of gut microbiota of SD rats changed significantly. The prebiotics promotes the proliferation of certain bacterial strains by glycoside hydrolysis (Prevotella), thus increasing the bioavailability of the ginsenoside, Rb1, and its metabolite ginsenoside, C–K [88]. Kim, Yoo et al. show that the oral administration of soluble prebiotic fiber, NUTRIOSE, can stimulate the intestinal bacterial metabolic conversion of ginsenoside Rb1 to ginsenoside Rd in an effect to improve the absorption of the latter. The underlying mechanism is correlated to improved fecal α/β-glucosidase activities of some bacteria [89]. Similarly, as a prebiotic with rich structures and diverse components, GPs can also promote the ADME of ginseng active components in organisms by regulating the gut microbiota.
In the DSS-induced experimental model of colitis, rats were orally administered GPs for a week and on day 7 all rats received an oral dose of Rb1. The results suggested that GPs could alleviate DSS-induced colitis-like symptoms and modulate the imbalance in levels of Lactobacillus, Bacteroides, and other bacteria to promote the production of deglycosylation metabolism of ginsenoside Rb1 and increase the exposure of the secondary saponin system. Additionally, the β-glucosidase activity in the intestine improved after treatment. When Caco-2 cells were cultured with both GPs and ginsenoside Rb1 in vitro, the GPs could promote the metabolism of ginsenoside Rb1 and accelerate cellular transport. Finally, the transformation of ginsenoside Rd, F2, and CK were markedly enhanced [90]. Consistent with the above-mentioned findings, Zhou, Xu et al., found that when in an acute cold stress model, rats were orally administered GPs (200mg/kg) daily for 14 consecutive days and i.g. administered ginsenoside solution (500 mg/kg) on the last day, GPs could rebuild the balance in the organism by influencing the gut microbiota through an increase in the abundance of Lactobacillus and Bacteroides. Moreover, the metabolism and absorption of specific ginsenosides in the intestine were dramatically boosted [91].
GPs can affect the in vivo effects of ginsenosides, an active antitumor component in the ginseng [92], which fully highlights the importance of GPs in promoting the absorption of effective components of ginseng and their vital role in complex interplay of the gut microbiome, tumor and host Fig. 3.
Fig. 3.
Complex interplay among GPs, gut microbiota and host. By transforming the GPs in the host's digestive system, the microbiota, especially some probiotics, degrade dietary fibers and produce short-chain fatty acids, thus enhancing the immune system and curing the tumor. GPs also play a vital role in modulating the structure of gut microbiota through its prebiotic-like effects, which promotes the absorption, distribution, metabolism and excretion of other ginseng active components simultaneously.
4.3. Other mechanisms underlying the antitumor effects of GPs
4.3.1. Regulation of apoptosis and cell cycle
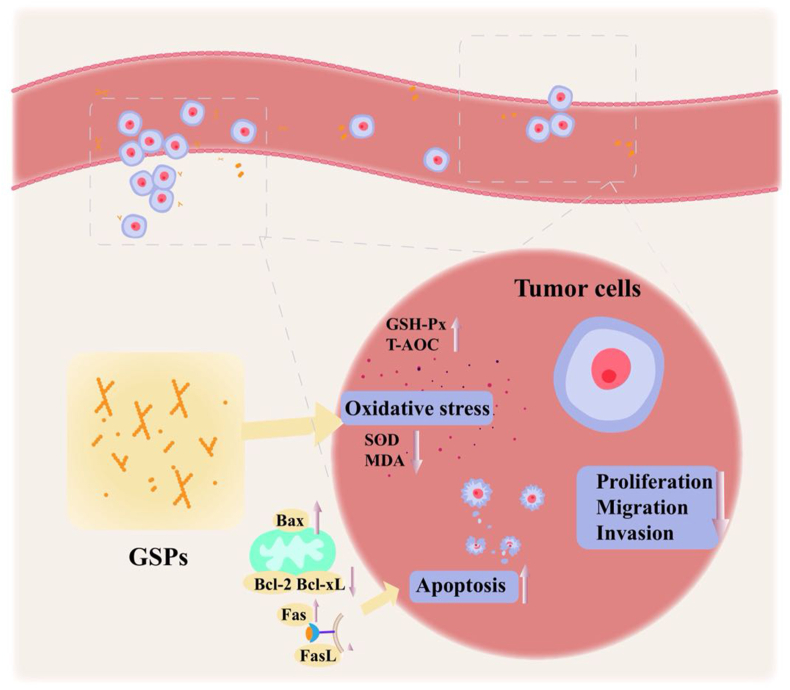
To date, three different cascades have been shown to initiate apoptosis, namely mitochondrial pathway, endoplasmic reticulum pathway, and death receptor pathway [93]. PGP2a, an acidic polysaccharide derived from the ginseng root, shows dose-dependent inhibition of proliferation of HGC-27 cells and can promote apoptosis of tumor cells. In the mitochondrial pathway, the expressions of caspases 3, caspases 9, Bax, and PARP are activated, along with the downregulation of Bcl-2 and Bcl-xl levels. The expressions of Fas and FasL increase in the exogenous apoptosis pathway mediated by the death receptor signaling. Moreover, the cell cycle of HGC-27 cells is blocked in the G2 / M phase (46.9% vs. 14.7%) [25]. Similarly, GBPP and GBPE extracted from the ginseng berry polysaccharides can also block the cell cycle in HCT116 and HT29 cells by arresting them in the G2 / M phase in vitro, to inhibit the cellular proliferation [28].
4.3.2. Regulation of the oxidative stress
Several studies implicate oxidative stress in many human diseases, including inflammatory disorders and cancer. Excess levels of reactive oxygen species (ROS) are closely correlated to the occurrence and development of tumors [94]. As reported previously, GPs exert antioxidant stress effects as evidenced by improved glutathione peroxidase (GSH-Px) levels and total antioxidant capacity (T-AOC), along with a decrease in the superoxide dismutase (SOD) and malondialdehyde (MDA) in the serum of rats [75]. Additionally, the SOD levels in serum of weaned piglets also reduce after the administration of basal diet supplemented with 800 mg/kg GPs [95]. However, whether GPs ought to regulate oxidative stress in the process of exerting antitumor effects, a potential antitumor mechanism remains unclear.
4.3.3. Inhibition of migration and invasion of tumor cells
Among the ten hallmarks of cancer, limitless replicative potential, tissue invasion, and metastases have remained the prime focus of researchers. If drugs can effectively inhibit tumor cell proliferation and invasion, they can exert a substantial beneficial impact on the cancer treatment [96]. The findings of in vitro experiments suggest that the antitumor polysaccharide component, PGPW1, in the ginseng root significantly inhibits the proliferation of T24 cells and the migration of HGC-27 cells. Furthermore, PGPW1 suppresses the EMT processes of HGC-27 cells by upregulating the level of E-cadherin and downregulating the levels of N-cadherin and vimentin, in an effect to hinder the invasion of tumor cells [26] Fig. 4
Fig. 4.
Other antitumor mechanisms of GPs. GPs might inhibit tumorigenesis via regulating the apotosis, modulating oxidative stress and weakening the migration and invasion of tumor cells.
5. Conclusions and perspectives
As an indispensable active ingredient, polysaccharide plays a key role in the treatment via traditional Chinese medicine (TCM). Accumulating intensive research on polysaccharides used in TCM suggests that several polysaccharides show excellent biological activities and thus have promising application prospects, such as GPs, astragalus polysaccharides [97], Lycium barbarum polysaccharide [6], Ganoderma lucidum polysaccharide [8], Hirsutella sinensis polysaccharide [98], and aloe polysaccharides [99]. Ginseng, the “king of herbs” in TCM, has great therapeutic and healthcare effects. To date, it has widely been used in antitumor, immune therapy, and antifatigue treatments. Notably, several studies show that polysaccharides are an important material basis of ginseng's antitumor effects. Therefore, this review systematically summarizes the antitumor-related effects of GPs reported in recent years and deeply analyzes their antitumor efficacy and mechanisms of action.
GP injection has been listed in many Asian countries. In clinical settings, it is commonly used as an adjuvant in radiotherapy and chemotherapy for the treatment of malignant tumors. Results show that it can effectively alleviate adverse reactions, including hypoimmunity and gastrointestinal side-effects of radiotherapy/chemotherapy, thereby improving the immunity and quality of life of the patients. Moreover, the experimental findings on the antitumor effects of GP have blossomed in recent times. Particularly, the contribution of Huang, Liu et al. [16] strongly suggests that GPs potentiate the antitumor effects of αPD-1 mAb by enhancing CD8+ T cell functions and reducing the suppressive effects of Tregs, by reshaping the structure of gut microbiota and tryptophan metabolism. Clearly, the current evidence is not sufficient enough to determinate the clinical dosage of GPs for cancer treatment, and so is the molecular mechanism behind the targeted therapy in tumor immunity.
As macromolecular compounds, the activity of plant polysaccharides including GPs is closely related to their physicochemical properties. Herein, we report that GPs can now be extracted from ginseng roots, rhizomes, flowers, leaves, and berries due to the expansion of utilization and research on TCM sources. Considering the different parts of ginseng, the biological activities tend to be altered, including the antitumor effects. Moreover, given the necessity of medicinal material storage or curative effects, ginseng is more likely to be processed by specific methods. The immune activities of several ginseng products, such as white ginseng, black ginseng, and red ginseng, are different. Taken together, the primary causes of the above-mentioned differences can be attributed to changes in side-chains, molecular weights, and glycosidic bonds in polysaccharides in the processes of extraction and processing. The monosaccharide composition of various sugars also can contribute.
Finally, we have summarized the mechanisms underlying the antitumor effects of GPs and the main references have been listed in Table 1 and Table 2. A valuable tonic in TCM, GPs can significantly improve the innate and acquired immunity of patients. Based on the present research findings, GPs promote NK cell killing activity, macrophage phagocytosis, and increase the proportion of CD8+T cells. These functions may be related to the binding of some components of the polysaccharides to CR3 on the surface membrane of immune cells. Moreover, GPs possess the ability to regulate the inflammatory responses mediated by the toll-like receptors and can be recognized by some lectin receptors to stimulate the activity of immune cells. However, further studies are needed to confirm these relationships. Apart from directly influencing immune cells, GPs interact with the intestinal microbes after entering the digestive system, resulting in a triangular play among GPs, gut microbiota, and host immunity. SCFAs such as butyrate and valeric acid produced by GPs which have been metabolized are significantly increased and are beneficial for intestinal health and tumor immunotherapy. GPs also regulate the structure of the gut microbiota and increase the abundance of some probiotics, such as Lactobacillus and Bifidobacteria, thus influencing the metabolism of drugs or improving the bioavailability of other effective components in ginseng. In tumor cells, GPs can also directly exert antitumor effects by inducing apoptosis, inhibiting invasion, or regulating tumor cell oxidative stress. Although the exact function of GPs in regulating oxidative stress needs further validation. Consequently, GPs have the strong ability to regulate the processes of cancer and other diseases.
Table 1.
Summary of Main Adjuvant Effects of GPs From Clinical Trials
| Country | GPs formulation | Dose and route of administration | Experimental objects | Type of clinical trials | Observation indicators | Outcomes | Adverse reactions | References | |
|---|---|---|---|---|---|---|---|---|---|
| China | GP injection (product of the Pharmaceutical Factory of Shenyang University of Pharmacy, batch number 001001) | 12 mg for intravenous dripping, starting from 3-7 days before RT till ending RT | 131 cases of NPC (RT-GP group, n = 64, aged 25-65 years; control group, n = 67, aged 26-63 years) | randomized, parallel,placebo-controlled | Status of primary carcinoma and cervical lymph node | CR rate of local focus: 96.6% vs.93.3%; | No evident toxic-adverse reaction | [25] | |
| CR rate of cervical metastatic lymph node: 85.7% vs.78.0% | |||||||||
| CR rate shown by CT: 60.3% vs.51.7% | |||||||||
| T-lymphocyte subsets activity (%) | T3: pre-intervention: 53.3 ± 9.2% vs.52.6 ± 10.8%; post-intervention: 60.9 ± 8.7% vs.45.7 ± 8.9% | ||||||||
| T4: pre-intervention: 40.7 ± 7.6% vs.41.3 ± 8.5%; post-intervention: 43.5 ± 6.8% vs.35.6 ± 9.2% | |||||||||
| T8: pre-intervention: 27.3 ± 4.8% vs.27.2 ± 7.3%; post-intervention: 28.7 ± 5.1% vs.28.3 ± 7.8% | |||||||||
| NK cell activity (%) | pre-intervention: 26.8 ± 8.2% vs.28.8 ± 9.8%; post-intervention: 34.6 ± 8.7% vs.24.4 ± 8.8% | ||||||||
| LAK cell activity (%) | pre-intervention: 14.3 ± 6.1% vs.15.2 ± 6.3%; post-intervention: 23.8 ± 8.3% vs.13.3 ± 5.2% | ||||||||
| China | GP injection (bought from Newcrest pharmaceutical Co., Ltd., Jilin, China) | 4ml for intravenous dripping, twice a day for 6-8 weeks | 80 patients diagnosed with NSCLC (Treatment group, n = 40, 59.3 ± 8.1 years old; Control group, n = 40, 60.1 ± 7.5 years old) | randomized, parallel,placebo-controlled | Total effective rate and clinical benefit rate (%) | Total effective rate: 60.0% vs.45.0%; Clinical benefit rate: 85.0% vs.77.5% | Not mentioned | [26] | |
| Incidence of adverse reactions (%) | Leukopenia: 55% vs.80%; Thrombocytopenia: 40% vs.65%; anemia: 50%vs.65%; Intestinal injury:72.5% vs.87.5%; alopecia:57.5% vs.72.5% | ||||||||
| Korea | Y-75(Ginsan) | oral administrated, 3g twice a day for 14weaks | healthy older adults,50-75 years old (Y-75 group, n = 36; placebo control, n = 36) | randomized, placebo-controlled, parallel, double-blind | NK cell activity (%) | Pre-intervention: 38.5 ± 14.11% vs.40.6 ± 13.4%; Post-intervention:52.0 ± 13.7% vs.40.8% ± 14.6% (week 8);53.9 ± 13.0% vs.41.6 ± 15.5% (week 14) | Total numbers: 14 vs.5; Gastrointestinal disease: 3 vs.2; Infection: 3 vs.1; Musculoskeletal and connective disease: 2 vs.1 | [101] | |
| Macrophages phagocytic activity | Pre-intervention:19632.1 ± 5227.1 vs.20150.4 ± 6620.4;Post-intervention:24585.3 ± 7597.2 vs.21615.4 ± 5662.6(week 8);27366.1 ± 7772.3 vs.21913.5 ± 6455.8(week 14) | ||||||||
| Monocyte-derived mediators (pg/ml) | TNF-α: pre-intervention: 2297.8 ± 1551.6 vs.2322.9 ± 1861.9; post-intervention: 3174.8 ± 1694.5 vs.2286.4 ± 1769.3(week 8); 3319.5 ± 1886.8 vs.2360.3 ± 1552.6(week 14); IL-12: not significantly altered | ||||||||
| China | GP injection (bought from Shanxi Pude pharmaceutical Co., Ltd., Shanxi, China) | Injection under the thoracoscope, 0.5 mg/kg, once a week, 4 times for 30 days | 96 patients diagnosed with NSCLC (Treatment group, n = 48, 33-66 years old; Control group, n = 48, 32-65 years old) | randomized, parallel,placebo-controlled | FACT-L scores | significantly altered | No evident toxic-adverse reaction | [27] | |
| Expression of Th1 and Th2 cytokines (%) | Th1: INF-γ: pre-intervention: 32.46 ± 9.65% vs.34.13 ± 9.24%; post-intervention: 75.35 ± 12.86% vs.65.36 ± 10.21%; IL-2: pre-intervention: 24.12 ± 6.98% vs.25.15 ± 6.14%; post-intervention: 62.42 ± 17.97% vs.51.56 ± 9.48% | ||||||||
| Th2: IL-4: pre-intervention: 47.88 ± 9.67% vs.49.54 ± 9.26%; post-intervention: 32.54 ± 8.32% vs.39.34 ± 9.12%; IL-5: pre-intervention: 61.34 ± 14.29% vs.62.58 ± 12.14%; post-intervention: 43.37 ± 11.32% vs.53.25 ± 12.37% | |||||||||
| China | GP injection (bought from Changchun Boao Biochemical Pharmaceutical Co., Ltd., Changchun, China) | 4ml for intravenous dripping, twice a day for 12 weeks | 130 patients diagnosed with NSCLC (Treatment group, n = 65, 60.8 ± 7.9 years old; Control group, n = 65, 59.4 ± 7.7 years old) | randomized, parallel,placebo-controlled | Total effective rate (%) | 58.5% vs.41.5% | Not mentioned | [28,29] | |
| T-lymphocyte subsets activity (%) | CD3: pre-intervention: 58.7 ± 7.3% vs.58.4 ± 6.9%; post-intervention: 70.1 ± 8.0% vs.62.3 ± 7.5% | ||||||||
| CD4: pre-intervention: 32.5 ± 5.6% vs.31.9 ± 5.3%; post-intervention: 44.2 ± 6.1% vs.35.9 ± 5.9% | |||||||||
| CD8: pre-intervention: 38.7 ± 6.9% vs.38.9 ± 7.0%; post-intervention: 34.2 ± 6.1% vs.36.9 ± 6.5% | |||||||||
| CD4/CD8: pre-intervention: 0.83 ± 0.20 vs.0.81 ± 0.15; post-intervention: 1.21 ± 0.27 vs.0.91 ± 0.19 | |||||||||
| Adverse reactions | Myelosuppression and gastrointestinal reaction significantly decreased | ||||||||
| Quality of life score | Pre-intervention:53.1 ± 10.8 vs.52.7 ± 11.2; Post-intervention: 71.5 ± 12.9 vs.64.2 ± 12.2 | ||||||||
| China | GP injection (bought from Changchun Boao Biochemical Pharmaceutical Co., Ltd., Changchun, China) | 18mg for intravenous dripping, once a day for 60 Days | 108 patients diagnosed with NSCLC (Treatment group, n = 60, 72.4 ± 1.9 years old; Control group, n = 48, 78.2 ± 1.4 years old) | randomized, parallel,placebo-controlled | Total effective rate (%) | 56.7% vs.33.3% | Not mentioned | [28] | |
| T-lymphocyte subsets activity (%) | CD3: pre-intervention: 54 ± 7% vs.52 ± 10%; post-intervention: 65 ± 10% vs.54 ± 10% | ||||||||
| CD4: pre-intervention: 32 ± 7% vs.31 ± 7%; post-intervention: 41 ± 8% vs.35 ± 9% | |||||||||
| CD8: pre-intervention: 36 ± 8% vs.35 ± 9%; post-intervention: 34 ± 8% vs.35 ± 7% | |||||||||
| CD4/CD8: pre-intervention: 0.83 ± 0.12 vs.0.84 ± 0.16; post-intervention: 0.85 ± 0.09 vs.0.83 ± 0.24 | |||||||||
| Serum immunoglobulin (g/ml) | IgA: pre-intervention: 1.26 ± 0.11 vs.1.28 ± 0.10%; post-intervention: 1.39 ± 0.10% vs.1.30 ± 0.12% | ||||||||
| IgG: pre-intervention: 9.3 ± 1.8 vs.9.2 ± 1.9%; post-intervention: 11.2 ± 2.1% vs.10.2 ± 2.0% | |||||||||
| IgM: pre-intervention: 0.91 ± 0.10 vs.0.94 ± 0.19%; post-intervention: 1.72 ± 0.17% vs.1.42 ± 0.17% | |||||||||
| Quality of life score | Pre-intervention: 40 ± 12 vs.40 ± 13; Post-intervention:42 ± 11 vs.45 ± 15 (month 1);35 ± 8 vs.40 ± 12 (month 3) | ||||||||
Table 2.
Summary of Main Antitumor Activities of GPs From Experimental Pharmacology Studies
| Physicochemical characterizations (molar ratios) | Experimental subjects | Dosage or concentration | Parameter(s) | Main findings | References |
|---|---|---|---|---|---|
| purified ginseng polysaccharide (PGPW1) (Glc: Gal: Man: Ara = 3.3: 1.2: 0.5: 1.1) | T24 cell; HGC-27 cell | 50, 100 and 200 ug/ml | Antiproliferative activity; Cytotoxic effects; cellular migration and invasion; | inhibited the proliferation of T24 cells from 21.23% at 50 g/ml to 67.3% at 200 g/ml; increased the LDH levels; Inhibited both cells' migration and invasion | [34,35] |
| purified ginseng polysaccharide (PGP2a) (Gal: Ara: Glu: GlaA = 3.7: 1.6: 0.5: 5.4) | HGC-27 cell | 25, 50, 100, 200 and 400 ug/mL | cell proliferation; cell apoptosis and cell cycle; | inhibited the cells proliferation; cell apoptotic↑; G2/M phase↑; Bcl-2↓; Bax/Bcl2↑; | [33] |
| ginseng polysaccharides (GPs) | BALB/c mice | 200 and 400 mg/kg (oral gavage for 10 days) | NK cell cytotoxicity; NK cell proportion | NK cell cytotoxicity and proportion in the whole blood significantly increased; perforin↑, granzyme B↑ | [54] |
| GPs (M.W. 11605 kDa, Tople Company, Guangdong, China) extracted from the roots with hot water precipitated by 80% ethanol and deproteinated using the Sevag method | Landrace sows (at day 90 of gestation); piglets | 100, 200, 400mg/kg for 28 days; 800mg/kg for 28 days | body weights of piglets; milk of sows; blood samples; antibody levels; enzymes activities; cytokines |
body weights significantly improved at 100, 200mg/kg; IgG and specific swine fever antibody levels improved; SOD↑, GSH-Px↑, MDA↓; IL-2↑, IL-6↓, TNF-α↑, IFN-γ↑ | [[94], [103]] |
| ginseng berry polysaccharide extract (GBPE); ginseng berry polysaccharide portion (GBPP) |
Male C57BL/6J ob/ob mice; HCT-116 and HT-29 cells | vivo: 50, 150mg/kg, oral gavage once a day for 15 consecutive days; vitro: 100, 200ug/ml; vitro: 60, 120, 250 ug/ml | DAI score; inflammatory cytokines; tumorigenesis; cell apoptosis and cell cycle; | reduce the DAI score; IL-1α↓, IL-1β↓, IL-6↓, G-CSF↓, tumor size↓ and number↓; inhibited the HCT-116 and HT-29 cells in vivo; Th1↓; Treg↓; G2/M phase↑ | [37,57] |
| GPs from leaves (M.W. 10.2 kDa; 6.4 % neutral sugars, 39.8 % uronic acids, 3.8 % Kdo-like materials) | BALB/c mice; colon 26-M3.1 carcinoma cell; | vivo: 4,20,100,500 μg/mouse; 5 mice/group(i.v.); vitro: 10, 100 ug/ml | cytokines; tumor metastasis; NK cell activity; antigen-specific antibody production | inhibited lung metastasis; TNF-α↑; IL-12↑ in PEMs; a high level of LDH; NK cell activity↑; OVA-specific cytokine production: serum IgG1↑, IgG2b↑, IL-2↑, IFN-γ↑, GM-CSF↑, IL-10↑; T lymphocyte proliferation↑; lgE↓ | [21,58] |
| ginseng berry polysaccharide portion fractions (GBPP-I: 89.1% neutral sugars, 10.4% uronic acid; GBPP-II: 61.3% neutral sugars, 16.1% uronic acid; GBPP-III: 52.9% neutral sugar, 13.0% uronic acid) | BALB/c mice, B16-BL6 melanoma cells | vivo: 10, 50, 100μg/mouse (p.o. for 15 days); vitro: 10,50,100μg/ml | Anticomplementary Activity, Macrophage Cytotoxicity; Cytokine Production; NK-Mediated Tumor Cytotoxicity; Granzyme | GBPP-I: exhibit anticomplementary activities; IL-6↑, IL-12↑, TNF-α↑; Granzyme B↑; Antimetastatic Activity↑ | [56] |
| non-saponin fractions with rich polysaccharide (NFP) (arginine-fructose-glucose: 11.63 mg/g; acidic polysaccharide: 438.08 mg/g) | SD rat; BALB/C mice; C57BL/6J mice | SD rat: 150mg/kg for 8 weeks (oral gavage); C57BL/6J and BALB/C mice: 75, 150, 300 mg/kg(oral gavage) | IgM Antibody-Producing Cells; Antimetastatic Potential | thymus weight↑, plasma cell↑ at 150mg/kg; increased inhibition of lung metastasis | [55] |
| Neutral water-soluble polysaccharides from ginseng flowers (WGFPN, M.W.11.0kDa, Gal, 78%, Ara, 14.3% Glc, 5.2% Man, 2.5%) | BALB/c mice | vivo: 25, 50, 100mg/kg/day for 14 days; vitro: 50, 100, 200 ,400ug/ml | Immunomodulatory activity | TNF-α↑, IL-6↑, IL-1β↑, IFN-γ↑ in RAW264.7 cells; body weight↑, spleen↑, thymus indices↑ | [48] |
| Pectins: WGPA-UD (GalA: 24.6%, Rha: 10.8%, Gal: 30.8%, Ara: 20.6%), RG-I-4(Gal: 33.8%, Rha: 21.8%, Gal: 19.5%, Ara: 9.2%), MCP (GalA: 85%, Rha: 1.6%%, Gal: 9.3%, Ara: 4%) and P-galactan (GalA: 11.3%, Rha: 6.1%, Gal: 70% and Ara: 10.0%) | Male ICR mice; Jurkat cells; S-180 cell | vivo: i.p.10mg/kg for 10 days; vitro:0.1, 0.5, 2 mg/ml | Gal-3-mediated T cell activation and apoptosis; Tumor growth | CD69↑; IL-2↓(treated with MCP); c-caspase-3↓(treated with WGPA-UD and RG-I-4); PI3K↑(treated with P-galactan); inhibit tumor growth(WGPA-UD and RG-I-4); | [32] |
| ginseng-SDF (fucose: galactose: galacturonic acid: glucose: glucuronic acid: D-Glucosamine: mannose: rhamnose: xylose = 8.20: 14.50: 2.31: 58.03: 0.25: 2.12: 2.84: 0.38: 11.37) | SD rat | 200, 400, and 800 mg/kg (oral gavage for 15 days) | growth performance of rats; antioxidant status; immune-related factors; cecal health | significant increase in feed intake; MDA↓; GSH-Px↑; T-AOC↑; IGF-1↑; IGF-2↑; IgM↑; IgG↑; | [[93], [104]] |
| ginseng polysaccharides (GPs) | C57BL/6J mice; humanised PD-1 knock-in (HuPD-1) mice | 200mg/kg, Daily oral treatment for 24 days | immunity; cytokines; antitumor effect | CD8+/CD4+ ratio↑; IFN-γ↑; TNF-α↑; GZMB↑; FoxP3+ regulatory T (Treg) cells↑ | [22] |
To date, no strong published evidence elucidates the antitumor mechanism or target of GPs. Due to the complexity and diversity of TCM components, it is essential to screen and modify the antitumor components with stable structure, safety parameters, and high biological activity to further expand the scope of clinical application of GPs or even TCM. In addition, as dietary polysaccharides, GPs are closely related to the gut microbiota. Further research on the interaction between microbes and GPs will be on the fore. In recent years, the study on the immunomodulatory activities of GPs also indicates their distinctive advantages in antitumor therapy. Escaping immune surveillance is one of the fourteen hallmarks of a tumor [96] and immunotherapy tends to utilize inhibition through ICBs, such as PD-1 / PD-L1 monoclonal antibody to activate the acquired immunity of the body. However, the efficiency and sensitivity of ICB therapy remain unsatisfactory. Monotherapy is only effective in 20 ∼ 30% of the patients and one of the key factors is that the innate immune system is not activated [100]. The regulation of GPs on several immune cell types and cytokines in innate immunity may trigger the development of GPs as potential immunotherapy and antitumor drugs.
Taken together, we highlighted the relationship between the antitumor activity of GPs and their physicochemical properties by summarizing the relevant recent reports. The antitumor mechanisms of GPs encompass receptor recognition, modulation of signaling pathways, gut microbiota interaction, and direct action on tumor cells. Like many plant polysaccharides, GPs have great potential in tumor treatment. However, it is still necessary to clarify and verify the antitumor mechanisms underlying the effects of GPs in large-scale clinical trials. We believe that with further research, the mechanisms underlying the effects of GPs in various systems will be revealed, and this would facilitate their use in the treatment of human diseases worldwide.
Author contributions
ZW & YL: Conceptualization and design the review, review final version approval, and Funding acquisition. RT & KL: bibliographic research, Writing – original draft, Investigation. GZ, YX, HH, & YZ: Writing – review & editing, Supervision. All authors contributed to the article and approved the submitted version.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (82004124, 81961128020, and 81973734), China Postdoctoral Science Foundation (2020M671551), Natural Science Foundation of Jiangsu Province (BK20200154), Jiangsu Province Traditional Chinese Medicine Leading Talents Program (SLJ0229), The Open Project of Chinese Materia Medica First-Class Discipline of Nanjing University of Chinese Medicine (2020YLXK20), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22-2013 and KYCX22-2039).
Contributor Information
Ruizhi Tao, Email: taoruizhi0612@163.com.
Keqin Lu, Email: lkq1075006596@163.com.
Gangfan Zong, Email: 3399176974@qq.com.
Yawen Xia, Email: zhizhi0831@163.com.
Hongkuan Han, Email: hanhongkuan@126.com.
Yang Zhao, Email: y.zhao@njucm.edu.cn.
Zhonghong Wei, Email: wzh1225@njucm.edu.cn.
Yin Lu, Email: luyingreen@njucm.edu.cn.
References
- 1.Yu Y., Shen M., Song Q., et al. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review [J] Carbohydr Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Xie J.H., Jin M.L., Morris G.A., et al. Advances on bioactive polysaccharides from medicinal plants [J] Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S60–S84. doi: 10.1080/10408398.2015.1069255. [DOI] [PubMed] [Google Scholar]
- 3.Sugayama J., Kamasuka T., Takada S., et al. On the anticancer active polysaccharide prepared from bamboo grass [J] J Antibiot. 1966;19(3):132–136. [PubMed] [Google Scholar]
- 4.Corso C.R., Mulinari Turin DE Oliveira N., Moura Cordeiro L., et al. Polysaccharides with antitumor effect in breast cancer: a systematic review of non-clinical studies [J] Nutrients. 2021;13(6) doi: 10.3390/nu13062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Xing M., Cao Q., et al. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies [J] Mar Drug. 2019;17(3) doi: 10.3390/md17030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao Z., Deng Q., Zhou W., et al. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? [J] Pharmacol Ther. 2021 doi: 10.1016/j.pharmthera.2021.107921. [DOI] [PubMed] [Google Scholar]
- 7.Ding G., Gong Q., Ma J., et al. Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice [J] Cancer Sci. 2021;112(10):4050–4063. doi: 10.1111/cas.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo C., Guo D., Fang L., et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon [J] Carbohydr Polym. 2021;267 doi: 10.1016/j.carbpol.2021.118231. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B., Lv C., Lu J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: a review of isolation, structures, and bioactivities [J] Int J Biol Macromol. 2019;133:324–336. doi: 10.1016/j.ijbiomac.2019.03.229. [DOI] [PubMed] [Google Scholar]
- 10.Wang N., Wang X., He M., et al. Ginseng polysaccharides: a potential neuroprotective agent [J] J Ginseng Res. 2021;45(2):211–217. doi: 10.1016/j.jgr.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ovodov Y.S., Solov'Eva T.F. Polysaccharides of panax ginseng [J] Chem Nat Comp. 1966;2(5):243–245. [Google Scholar]
- 12.Jiao R., Liu Y., Gao H., et al. The anti-oxidant and antitumor properties of plant polysaccharides [J] Am J Chin Med. 2016;44(3):463–488. doi: 10.1142/s0192415x16500269. [DOI] [PubMed] [Google Scholar]
- 13.Kachur K., Suntres Z.E. The antimicrobial properties of ginseng and ginseng extracts [J] Exp Rev Anti-infect Ther. 2016;14(1):81–94. doi: 10.1586/14787210.2016.1118345. [DOI] [PubMed] [Google Scholar]
- 14.Li N., Yu X., Yu Q.H., et al. Research progress on stability of polysaccharides in traditional Chinese medicine [J] Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J Chin Mater Medica. 2019;44(22):4793–4799. doi: 10.19540/j.cnki.cjcmm.20190916.309. [DOI] [PubMed] [Google Scholar]
- 15.Shin M.S., Hwang S.H., Yoon T.J., et al. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation [J] Int J Biol Macromol. 2017;103:1327–1333. doi: 10.1016/j.ijbiomac.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 16.Huang J., Liu D., Wang Y., et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy [J] Gut. 2021 doi: 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia H., Zhao B., Zhang F., et al. Extraction, structural characterization, and anti-hepatocellular carcinoma activity of polysaccharides from panax ginseng meyer [J] Front Oncol. 2021;11 doi: 10.3389/fonc.2021.785455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie F.Y., Zeng Z.F., Huang H.Y. [Clinical observation on nasopharyngeal carcinoma treated with combined therapy of radiotherapy and ginseng polysaccharide injection] [J] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(5):332–334. [PubMed] [Google Scholar]
- 19.Li Liping. Liu Jinsong Effect of ginseng polysaccharide assisted GP chemotherapy on non-small cell lung cancer. J] J Hunan Univ Trad Chin Med. 2013;33(12):36–37. [Google Scholar]
- 20.Ma J., Liu H., Wang X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer [J] J Trad Chin Med = Chung I Tsa Chih Ying Wen pan. 2014;34(6):641–645. doi: 10.1016/s0254-6272(15)30076-5. [DOI] [PubMed] [Google Scholar]
- 21.Tu Xuesong, Huang Fuen, Qu Guangqiao, et al. Effects of ginseng compound polysaccharide on immune function and quality of life in elderly patients with advanced non-small cell lung cancer. J] Med Rev. 2016;22(8):1659–1661. + 64. [Google Scholar]
- 22.Xu M., Chen Q., Fan R., et al. Anti-inflammation effect of small molecule oligopeptides prepared from panax ginseng C. A. Meyer in rats [J] Molecules. 2019;24(5) doi: 10.3390/molecules24050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu R., Chen Q.H., Ren J.W., et al. Ginseng (panax ginseng meyer) oligopeptides protect against binge drinking-induced liver damage through inhibiting oxidative stress and inflammation in rats [J] Nutrients. 2018;10(11) doi: 10.3390/nu10111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue H., Zhao Z., Lin Z., et al. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis [J] Carbohydr Polym. 2019;219:121–129. doi: 10.1016/j.carbpol.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Li C., Tian Z.N., Cai J.P., et al. Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer [J] Carbohydr Polym. 2014;102:103–109. doi: 10.1016/j.carbpol.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Cai J.P., Wu Y.J., Li C., et al. Panax ginseng polysaccharide suppresses metastasis via modulating Twist expression in gastric cancer [J] Int J Biol Macromol. 2013;57:22–25. doi: 10.1016/j.ijbiomac.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Tuncil Y.E., Xiao Y., Porter N.T., et al. Reciprocal prioritization to dietary glycans by gut bacteria in a competitive environment promotes stable coexistence [J] mBio. 2017;8(5) doi: 10.1128/mBio.01068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C.Z., Hou L., Wan J.Y., et al. Ginseng berry polysaccharides on inflammation-associated colon cancer: inhibiting T-cell differentiation, promoting apoptosis, and enhancing the effects of 5-fluorouracil [J] J Ginseng Res. 2020;44(2):282–290. doi: 10.1016/j.jgr.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B., Zhang N., Feng Q., et al. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity [J] Int J Biol Macromol. 2019;123:713–722. doi: 10.1016/j.ijbiomac.2018.11.140. [DOI] [PubMed] [Google Scholar]
- 30.Guo M., Shao S., Wang D., et al. Recent progress in polysaccharides from Panax ginseng C. A. Meyer [J] Food Funct. 2021;12(2):494–518. doi: 10.1039/d0fo01896a. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.M., Bae B.S., Park H.W., et al. Characterization of Korean red ginseng (panax ginseng meyer): history, preparation method, and chemical composition [J] J Ginseng Res. 2015;39(4):384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metwaly A.M., Lianlian Z., Luqi H., et al. Black ginseng and its saponins: preparation, phytochemistry and pharmacological effects [J] Molecules. 2019;24(10) doi: 10.3390/molecules24101856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan J.Y., Fan Y., Yu Q.T., et al. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng [J] J Pharm Biomed Anal. 2015;107:89–97. doi: 10.1016/j.jpba.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q.L., Chen Y.J., Zhou S.S., et al. Laser microdissection hyphenated with high performance gel permeation chromatography-charged aerosol detector and ultra performance liquid chromatography-triple quadrupole mass spectrometry for histochemical analysis of polysaccharides in herbal medicine: ginseng, a case study [J] Int J Biol Macromol. 2018;107(Pt A):332–342. doi: 10.1016/j.ijbiomac.2017.08.162. [DOI] [PubMed] [Google Scholar]
- 35.Sun L., Wu D., Ning X., et al. α-Amylase-assisted extraction of polysaccharides from Panax ginseng [J] Int J Biol Macromol. 2015;75:152–157. doi: 10.1016/j.ijbiomac.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Chen F., Huang G. Antioxidant activity of polysaccharides from different sources of ginseng [J] Int J Biol Macromol. 2019;125:906–908. doi: 10.1016/j.ijbiomac.2018.12.134. [DOI] [PubMed] [Google Scholar]
- 37.Lee D.Y., Park C.W., Lee S.J., et al. Anti-cancer effects of panax ginseng berry polysaccharides via activation of immune-related cells [J] Frontiers in Pharmacology. 2019;10:1411. doi: 10.3389/fphar.2019.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui L., Wang J., Huang R., et al. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3 [J] Int J Biol Macromol. 2019;128:459–467. doi: 10.1016/j.ijbiomac.2019.01.129. [DOI] [PubMed] [Google Scholar]
- 39.Cui L., Chen L., Yang G., et al. Structural characterization and immunomodulatory activity of a heterogalactan from Panax ginseng flowers [J] Food Res Int. 2021;140 doi: 10.1016/j.foodres.2020.109859. [DOI] [PubMed] [Google Scholar]
- 40.Forster S.C., Kumar N., Anonye B.O., et al. A human gut bacterial genome and culture collection for improved metagenomic analyses [J] Nat Biotechnol. 2019;37(2):186–192. doi: 10.1038/s41587-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J., Wang K., Wang X., et al. The role of the gut microbiome and its metabolites in metabolic diseases [J] Protein Cell. 2021;12(5):360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamada N., Seo S.U., Chen G.Y., et al. Role of the gut microbiota in immunity and inflammatory disease [J] Nat Rev Immunol. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 43.Ni W., Zhang X., Wang B., et al. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil [J] Journal of Medicinal Food. 2010;13(2):270–277. doi: 10.1089/jmf.2009.1119. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y., Guo M., Feng Y., et al. Effect of ginseng polysaccharides on NK cell cytotoxicity in immunosuppressed mice [J] Exp Ther Med. 2016;12(6):3773–3777. doi: 10.3892/etm.2016.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y.Y., Kim S.W., Youn S.H., et al. Biological effects of Korean red ginseng polysaccharides in aged rat using global proteomic approach. [J] Mol. 2020;25(13) doi: 10.3390/molecules25133019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee D.Y., Park C.W., Lee S.J., et al. Immunostimulating and antimetastatic effects of polysaccharides purified from ginseng berry [J] The American Journal of Chinese Medicine. 2019;47(4):823–839. doi: 10.1142/s0192415x19500435. [DOI] [PubMed] [Google Scholar]
- 47.Hwang S.H., Shin M.S., Yoon T.J., et al. Immunoadjuvant activity in mice of polysaccharides isolated from the leaves of Panax ginseng C.A. Meyer [J] Int J Biol Macromol. 2018;107(Pt B):2695–2700. doi: 10.1016/j.ijbiomac.2017.10.160. [DOI] [PubMed] [Google Scholar]
- 48.Erdei A., Lukácsi S., Mácsik-Valent B., et al. Non-identical twins: different faces of CR3 and CR4 in myeloid and lymphoid cells of mice and men [J] Semin Cell Dev Biol. 2019;85:110–121. doi: 10.1016/j.semcdb.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 49.VAN Bruggen R., Drewniak A., Jansen M., et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles [J] Mol Immunol. 2009;47(2–3):575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Brennan J.J., Gilmore T.D. Evolutionary origins of toll-like receptor signaling [J] Mol Biol Evol. 2018;35(7):1576–1587. doi: 10.1093/molbev/msy050. [DOI] [PubMed] [Google Scholar]
- 51.Brubaker S.W., Bonham K.S., Zanoni I., et al. Innate immune pattern recognition: a cell biological perspective [J] Annual Review of Immunology. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurt-Jones E.A., Popova L., Kwinn L., et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus [J] Nature Immunology. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 53.Sahasrabudhe N.M., Beukema M., Tian L., et al. Dietary fiber pectin directly blocks toll-like receptor 2-1 and prevents doxorubicin-induced ileitis [J] Front Immunol. 2018;9:383. doi: 10.3389/fimmu.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda K., Akira S. Toll-like receptors [J] Current Protocols in Immunology. 2015;109:14. doi: 10.1002/0471142735.im1412s109. 2.1-.2.0. [DOI] [PubMed] [Google Scholar]
- 55.VAN Dammes E.J., Fouquaert E., Lannoo N., et al. Novel concepts about the role of lectins in the plant cell [J] Adv Exp Med Biol. 2011;705:271–294. doi: 10.1007/978-1-4419-7877-6_13. [DOI] [PubMed] [Google Scholar]
- 56.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity [J] Biochem Biophys Res Commun. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 57.Wang D., Shao S., Zhang Y., et al. Insight into polysaccharides from panax ginseng C. A. Meyer in improving intestinal inflammation: modulating intestinal microbiota and autophagy [J] Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.683911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo J.Y., Choi J.W., Lee J.Y., et al. Enzyme hydrolysates of ginseng marc polysaccharides promote the phagocytic activity of macrophages via activation of TLR2 and mer tyrosine kinase [J] Journal of Microbiology and Biotechnology. 2018;28(6):860–873. doi: 10.4014/jmb.1801.01003. [DOI] [PubMed] [Google Scholar]
- 59.Linger R.M., Keating A.K., Earp H.S., et al. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer [J] Adv Cancer Res. 2008;100:35–83. doi: 10.1016/s0065-230x(08)00002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothlin C.V., Carrera-Silva E.A., Bosurgi L., et al. TAM receptor signaling in immune homeostasis [J] Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabius H.J. Animal lectins [J] European Journal of Biochemistry. 1997;243(3):543–576. doi: 10.1111/j.1432-1033.1997.t01-1-00543.x. [DOI] [PubMed] [Google Scholar]
- 62.Anderson K., Evers D., Rice K.G. Springer Berlin Heidelberg; Berlin, Heidelberg: 2008. Structure and function of mammalian carbohydrate-lectin interactions [M]//FRASER-REID B O, TATSUTA K, THIEM J. Glycoscience: chemistry and chemical biology; pp. 2445–2482. [Google Scholar]
- 63.Kilpatrick D.C. Animal lectins: a historical introduction and overview [J] Biochimica et biophysica acta. 2002;1572(2–3):187–197. doi: 10.1016/s0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 64.Dunphy J.L., Balic A., Barcham G.J., et al. Isolation and characterization of a novel inducible mammalian galectin [J] The Journal of Biological Chemistry. 2000;275(41):32106–32113. doi: 10.1074/jbc.M003739200. [DOI] [PubMed] [Google Scholar]
- 65.Irjala H., Johansson E.L., Grenman R., et al. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium [J] J Exp Med. 2001;194(8):1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youn S.H., Lee S.M., Han C.K., et al. Immune activity of polysaccharide fractions isolated from Korean red ginseng [J] Molecules (Basel, Switzerland) 2020;25(16) doi: 10.3390/molecules25163569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy R., Murphy P.V., Gabius H.J. Multivalent carbohydrate-lectin interactions: how synthetic chemistry enables insights into nanometric recognition. J]. Molecules (Basel, Switzerland) 2016;21(5) doi: 10.3390/molecules21050629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weis W.I., Drickamer K. Structural basis of lectin-carbohydrate recognition [J] Annual Review of Biochemistry. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 69.Lis H., Sharon N. Lectins: carbohydrate-specific proteins that mediate cellular recognition [J] Chem Rev. 1998;98(2):637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 70.Mohajeri M.H., Brummer R.J.M., Rastall R.A., et al. The role of the microbiome for human health: from basic science to clinical applications [J] Eur J Nutr. 2018;57(Suppl 1):1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan S., Wei P.C., Chen Q., et al. Functional and structural characterization of a β-glucosidase involved in saponin metabolism from intestinal bacteria [J] Biochem Biophys Res Commun. 2018;496(4):1349–1356. doi: 10.1016/j.bbrc.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Gu F., Borewicz K., Richter B., et al. Vitro fermentation behavior of isomalto/malto-polysaccharides using human fecal inoculum indicates prebiotic potential [J] Mol Nutr Food Res. 2018;62(12) doi: 10.1002/mnfr.201800232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh A., DE Vadder F., Kovatcheva-Datchary P., et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites [J] Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 74.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer [J] Nat Rev Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 75.Hua M., Liu Z., Sha J., et al. Effects of ginseng soluble dietary fiber on serum antioxidant status, immune factor levels and cecal health in healthy rats [J] Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130641. [DOI] [PubMed] [Google Scholar]
- 76.Yang C.M., Han Q.J., Wang K.L., et al. Astragalus and ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets [J] Front Physiol. 2019;10:418. doi: 10.3389/fphys.2019.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao Y., Cai X., Fei W., et al. The role of short-chain fatty acids in immunity, inflammation and metabolism [J] Crit Rev Food Sci Nutr. 2020:1–12. doi: 10.1080/10408398.2020.1854675. [DOI] [PubMed] [Google Scholar]
- 78.Luu M., Visekruna A. Microbial metabolites: novel therapeutic tools for boosting cancer therapies [J] Trends Cell Biol. 2021;31(11):873–875. doi: 10.1016/j.tcb.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Ma X., Zhou Z., Zhang X., et al. Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice [J] Cell Biol Toxicol. 2020;36(5):509–515. doi: 10.1007/s10565-020-09518-4. [DOI] [PubMed] [Google Scholar]
- 80.Caleffi E.R., Krausová G., Hyršlová I., et al. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots [J] Int J Biol Macromol. 2015;80:392–399. doi: 10.1016/j.ijbiomac.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 81.Zimmermann M., Zimmermann-Kogadeeva M., Wegmann R., et al. Mapping human microbiome drug metabolism by gut bacteria and their genes [J] Nature. 2019;570(7762):462–467. doi: 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma W., Nguyen L.H., Song M., et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men [J] Genome Med. 2021;13(1):102. doi: 10.1186/s13073-021-00921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hua M., Sun Y., Shao Z., et al. Functional soluble dietary fiber from ginseng residue: polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity [J] J Food Biochem. 2020;44(12) doi: 10.1111/jfbc.13524. [DOI] [PubMed] [Google Scholar]
- 84.Li S., Qi Y., Chen L., et al. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea [J] Int J Biol Macromol. 2019;124:931–937. doi: 10.1016/j.ijbiomac.2018.11.271. [DOI] [PubMed] [Google Scholar]
- 85.Vieira-Silva S., Falony G., Darzi Y., et al. Species-function relationships shape ecological properties of the human gut microbiome [J] Nat Microbiol. 2016;1(8) doi: 10.1038/nmicrobiol.2016.88. [DOI] [PubMed] [Google Scholar]
- 86.Dong W.W., Xuan F.L., Zhong F.L., et al. Comparative analysis of the rats' gut microbiota composition in animals with different ginsenosides metabolizing activity [J] J Agric Food Chem. 2017;65(2):327–337. doi: 10.1021/acs.jafc.6b04848. [DOI] [PubMed] [Google Scholar]
- 87.Murga-Garrido S.M., Hong Q., Cross T.L., et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. [J]. Microbiome. 2021;9(1):117. doi: 10.1186/s40168-021-01061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X., Chen S., Duan F., et al. Prebiotics enhance the biotransformation and bioavailability of ginsenosides in rats by modulating gut microbiota [J] J Ginseng Res. 2021;45(2):334–343. doi: 10.1016/j.jgr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim K.A., Yoo H.H., Gu W., et al. Effect of a soluble prebiotic fiber, NUTRIOSE, on the absorption of ginsenoside Rd in rats orally administered ginseng [J] J Ginseng Res. 2014;38(3):203–207. doi: 10.1016/j.jgr.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen H., Gao X.J., Li T., et al. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism [J] J Ethnopharmacol. 2018;216:47–56. doi: 10.1016/j.jep.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 91.Zhou S.S., Xu J., Zhu H., et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction [J] Sci Rep. 2016;6 doi: 10.1038/srep22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H., Zheng Y., Sun Q., et al. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies [J] J Nanobiotechnology. 2021;19(1):322. doi: 10.1186/s12951-021-01062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins [J] Nat Rev Mol Cell Biol. 2019;20(3):175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Habtetsion T., Ding Z.C., Pi W., et al. Alteration of tumor metabolism by CD4+ T cells leads to TNF-α-dependent intensification of oxidative stress and tumor cell death [J] Cell Metab. 2018;28(2):228–242. doi: 10.1016/j.cmet.2018.05.012. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang K., Zhang H., Han Q., et al. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide [J] J Anim Physiol Anim Nutr (Berl) 2020;104(4):1096–1105. doi: 10.1111/jpn.13244. [DOI] [PubMed] [Google Scholar]
- 96.Hanahan D. Hallmarks of cancer: new dimensions [J] Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.Cd-21-1059. [DOI] [PubMed] [Google Scholar]
- 97.Hong Y., Sheng L., Zhong J., et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice [J] Gut Microbes. 2021;13(1):1–20. doi: 10.1080/19490976.2021.1930874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu T.R., Lin C.S., Chang C.J., et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis [J] Gut. 2019;68(2):248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 99.Liu C., DU P., Guo Y., et al. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota [J] Carbohydr Polym. 2021;261 doi: 10.1016/j.carbpol.2021.117874. [DOI] [PubMed] [Google Scholar]
- 100.Patel S.A., Minn A.J. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies [J] Immunity. 2018;48(3):417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho Y.J., Son H.J., Kim K.S. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of ginseng polysaccharide (Y-75) [J] J Transl Med. 2014;12:283. doi: 10.1186/s12967-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang K., Zhang H., Han Q., et al. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide [J] J Anim Physiol Anim Nutr (Berl) 2020;104(4) doi: 10.1111/jpn.13244. [DOI] [PubMed] [Google Scholar]
- 104.Hua M., Sun Y., Shao Z., et al. Functional soluble dietary fiber from ginseng residue: Polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity [J] J Food Biochem. 2020;44(12) doi: 10.1111/jfbc.13524. [DOI] [PubMed] [Google Scholar]